Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ascites is an abnormal fluid collection within the fetal peritoneal cavity and is often the first finding in hydrops fetalis. Ascites is present in 85% of cases of nonimmune hydrops fetalis. Isolated fetal ascites indicates there is fluid accumulation in the abdominal cavity without involvement of other body cavities or subcutaneous edema.
Hydrops fetalis occurs in approximately 1:1700–3000 pregnancies ; the incidence of isolated ascites is much lower.
Free fluid in the abdomen could represent
A transudate, owing to increased fluid leaking from the fetal capillary beds without adequate lymphatic return.
An exudate, as an inflammatory response to infection or malignancy.
Leaked urine, from an overdistended or ruptured bladder.
Meconium, from perforated bowel producing meconium peritonitis.
Chylous fluid, due to peritoneal lymphangiectasia (Waldmann disease) or lymphatic hypoplasia.
Common etiologies for isolated fetal ascites are listed in Box 24.1 .
Chylous lymphangiectasia
Meconium peritonitis
Cystic fibrosis
Biliary atresia
Fetal arrhythmia
Congenital heart defects
Ruptured bladder or kidney
Bladder outlet obstruction
Ruptured fetal ovarian cyst
TORCH infections, particularly CMV
Parvovirus B19
Syphilis
Rh incompatibility
Atypical red cell antigen incompatibility (e.g., Kell isoimmunization)
Nonimmune hydrops fetalis
Fetal-maternal hemorrhage
Inborn errors of metabolism
Aneuploidy
Fetal neoplasia
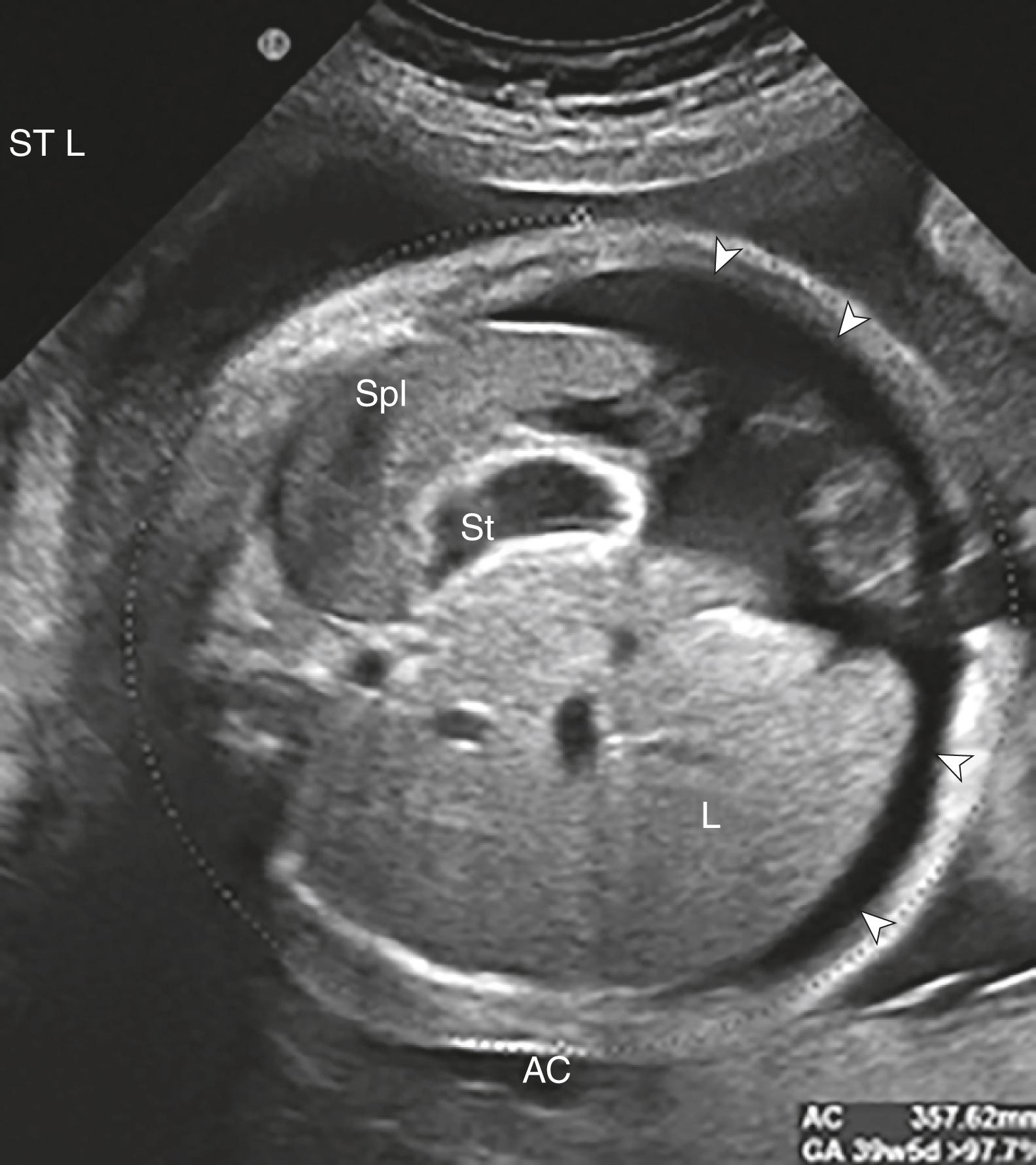
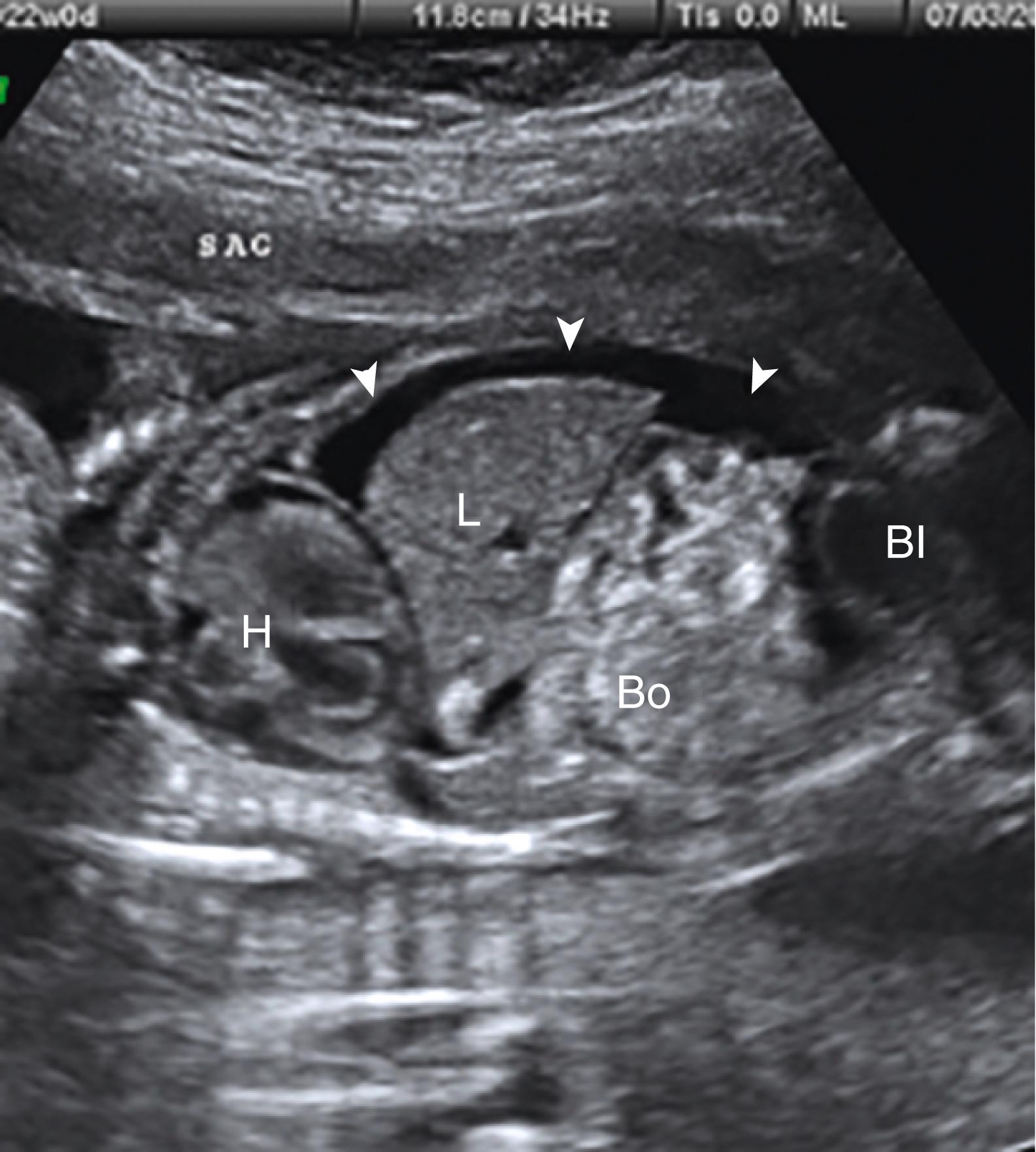
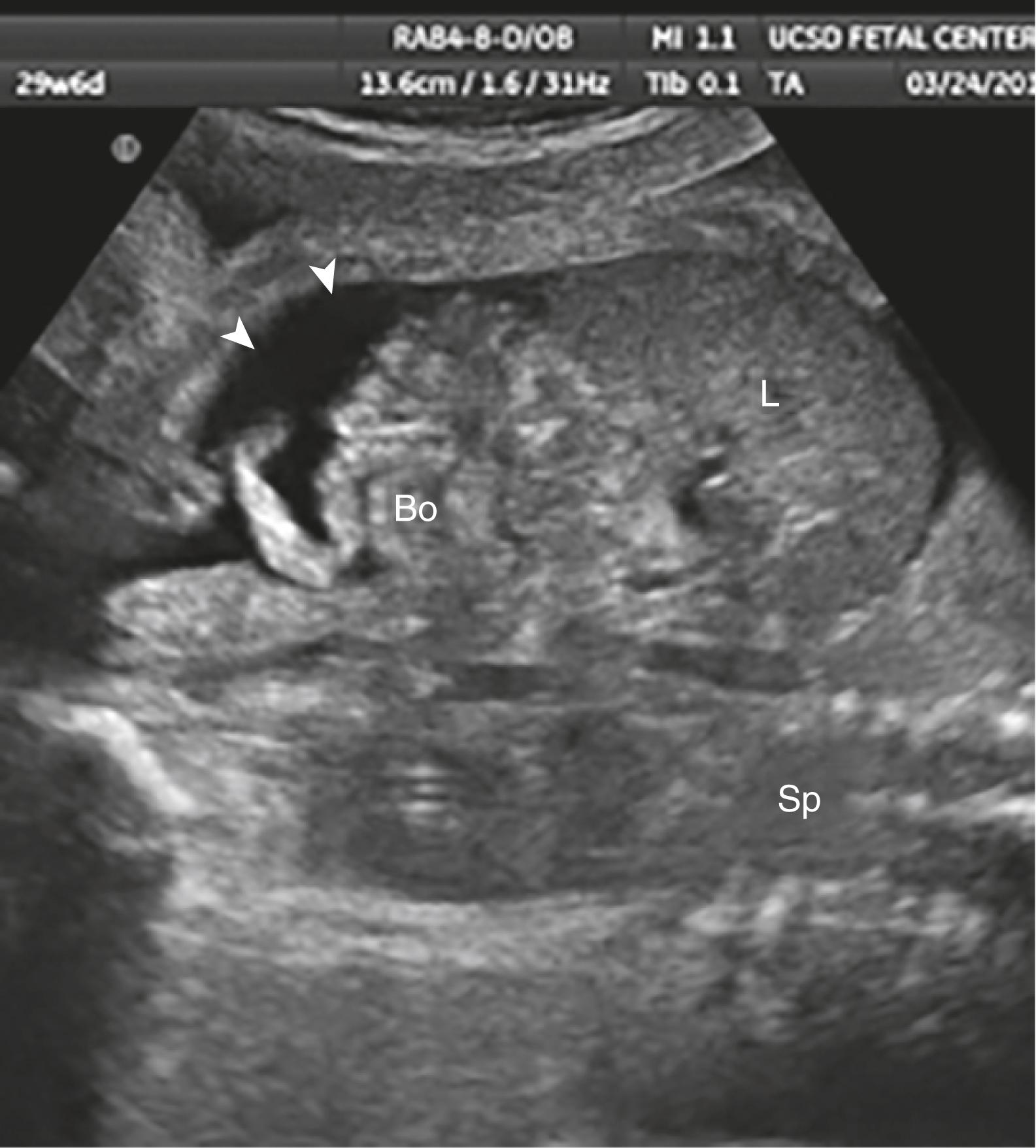
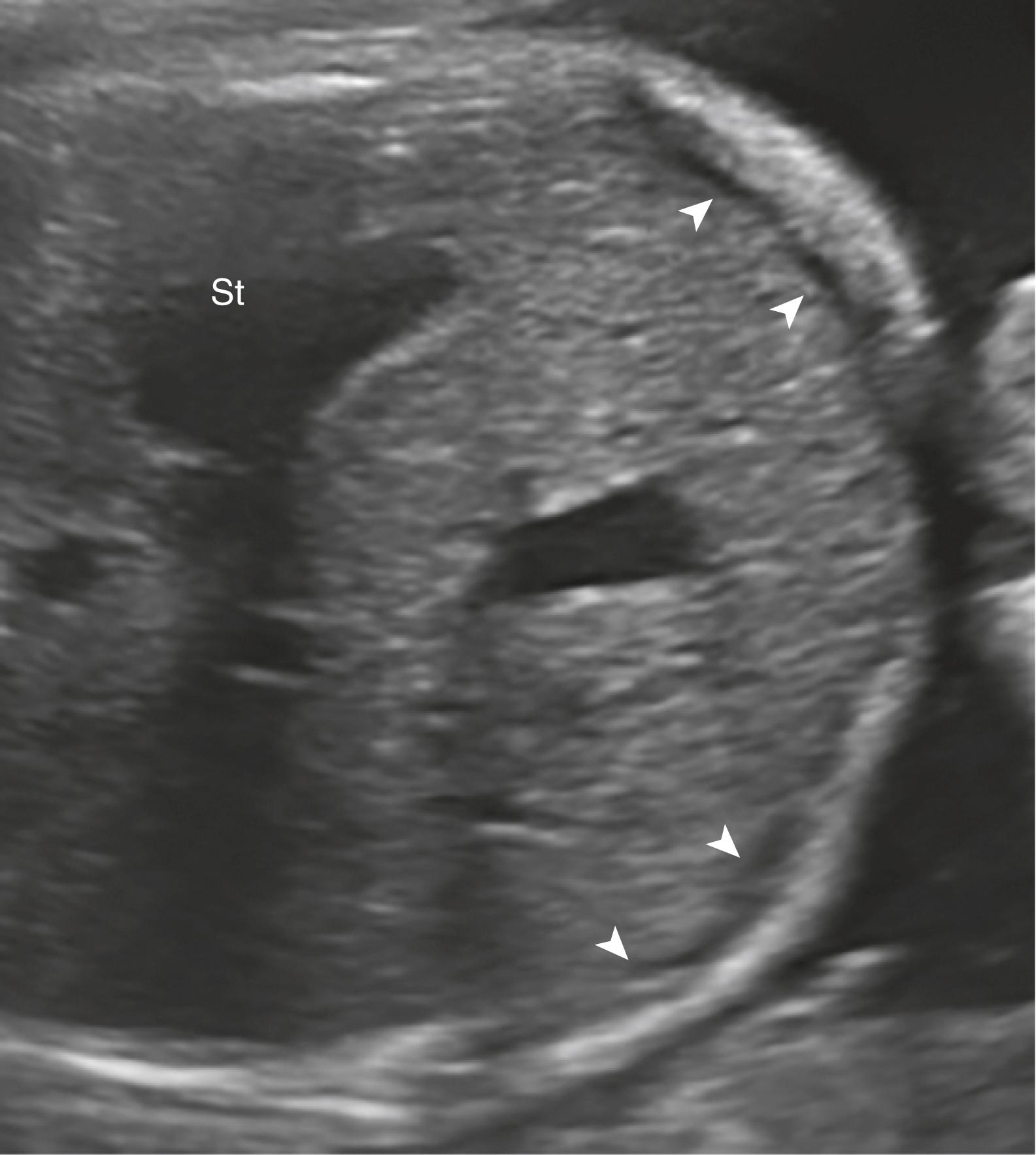
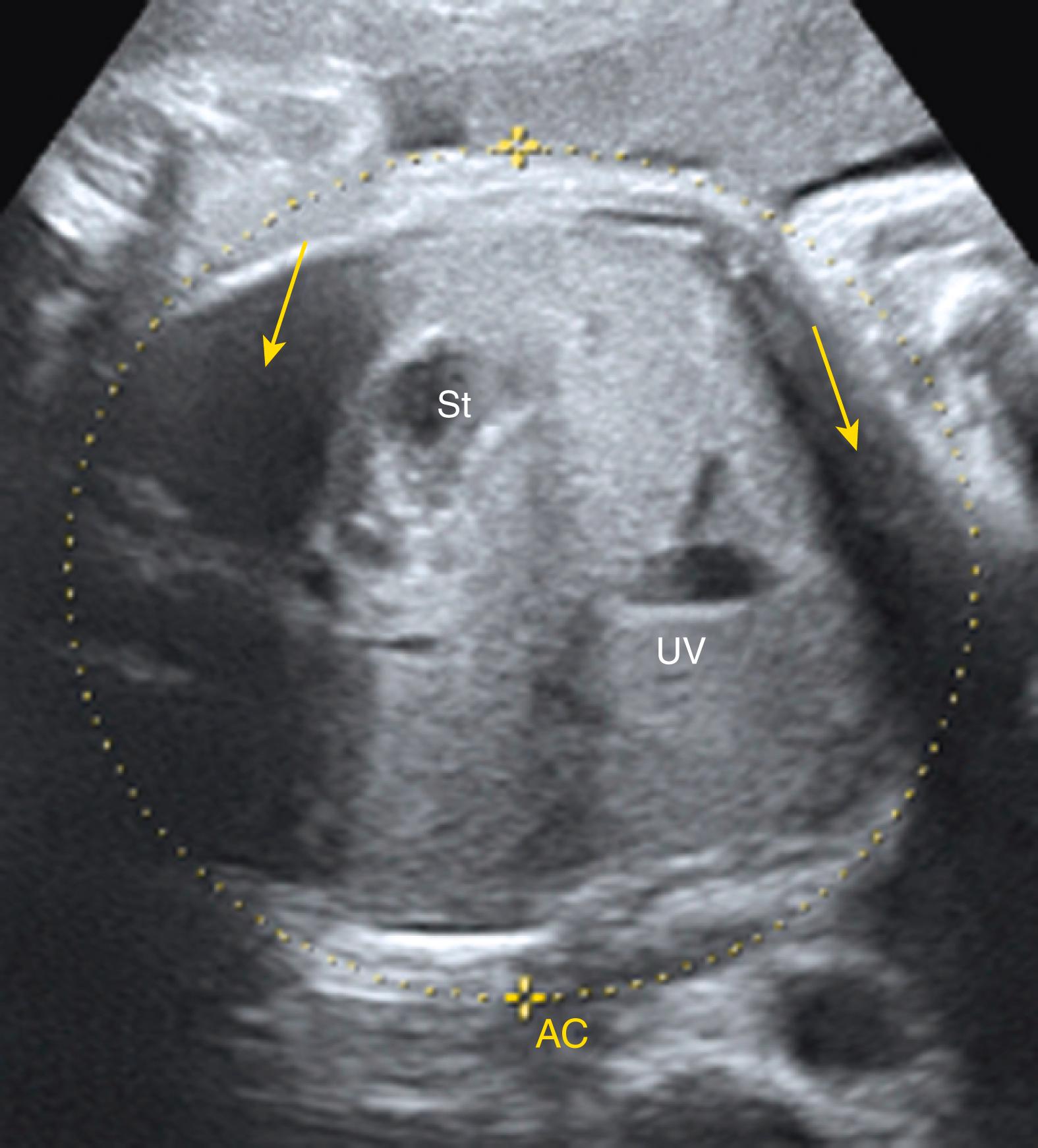
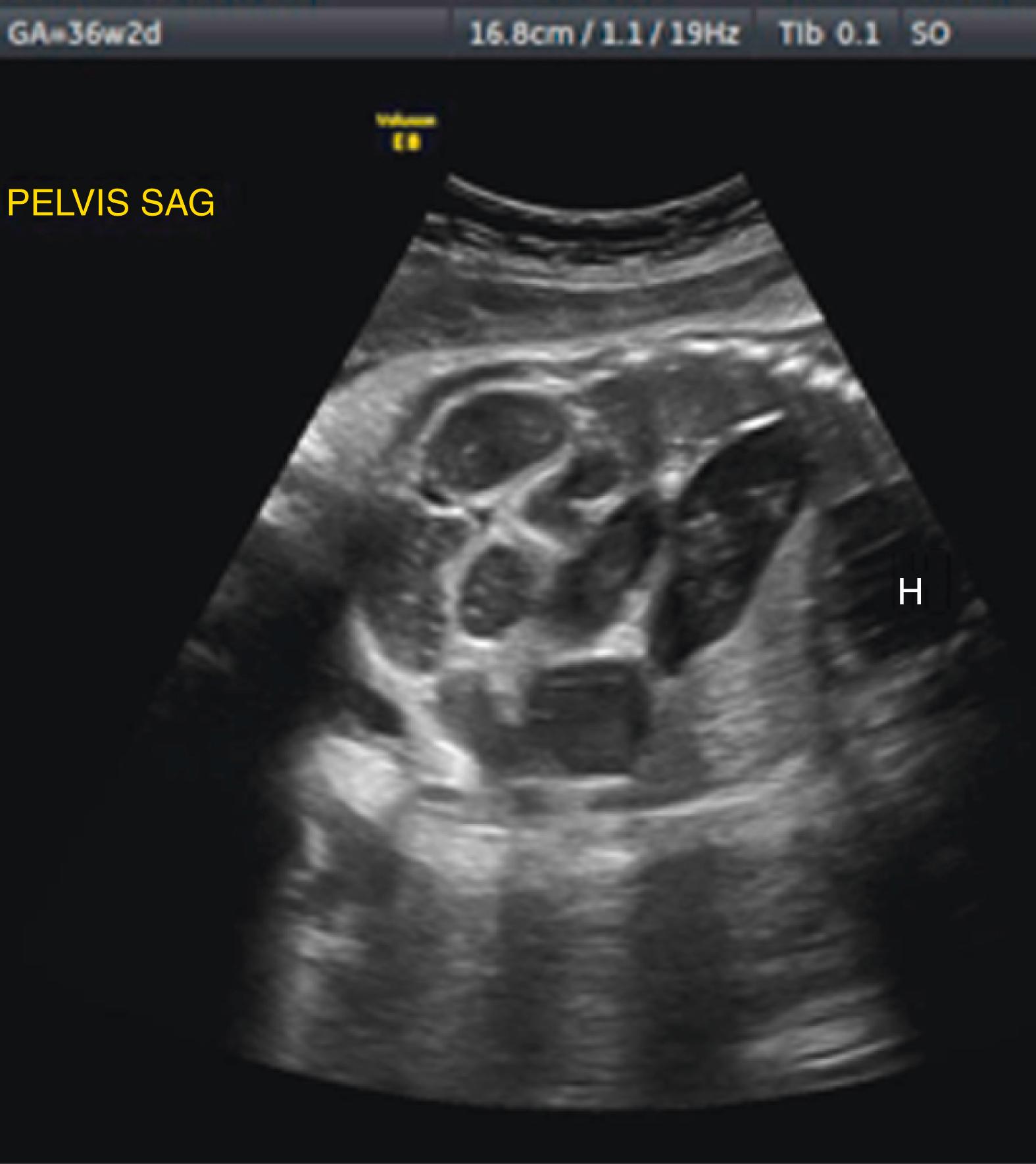
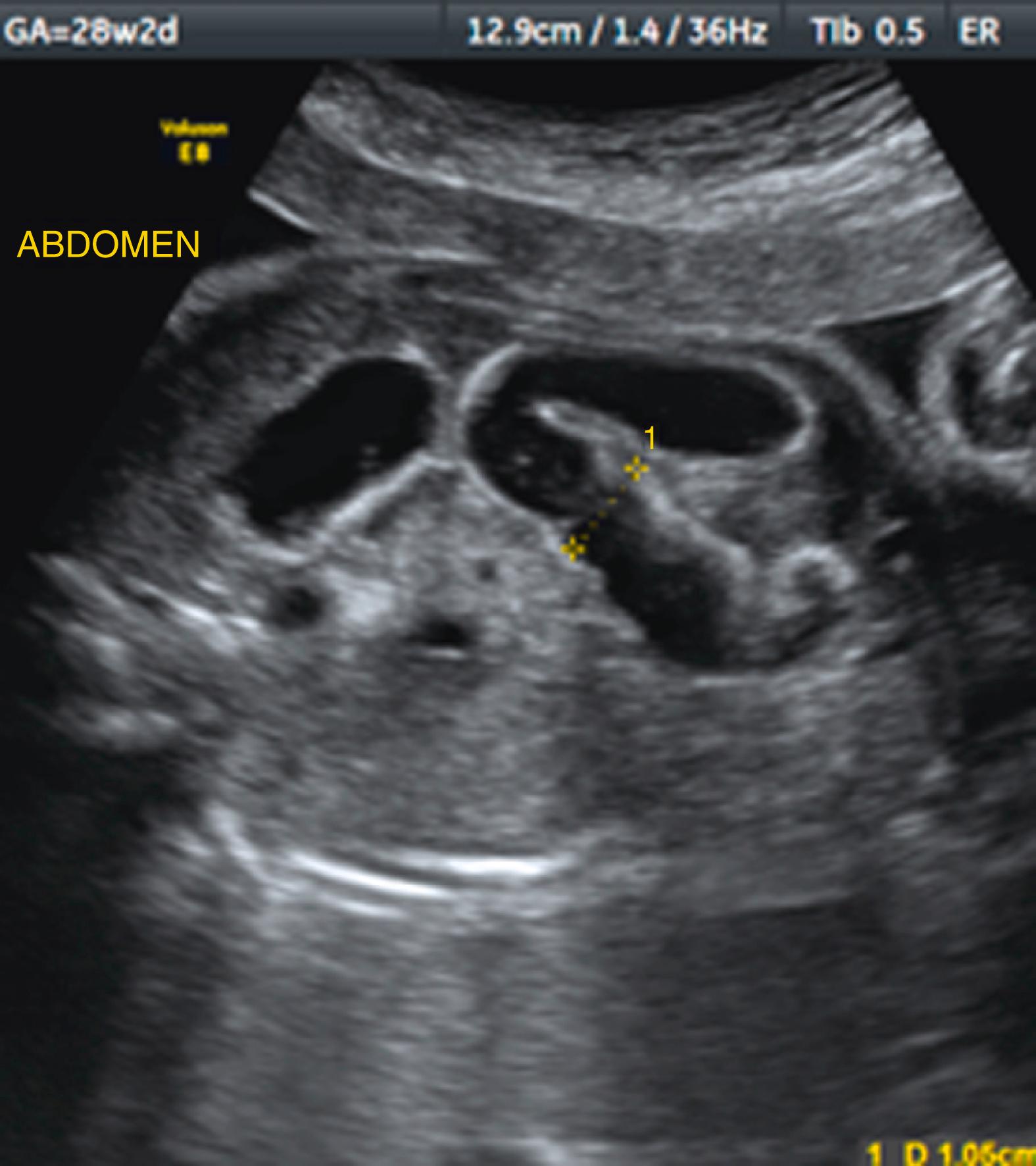
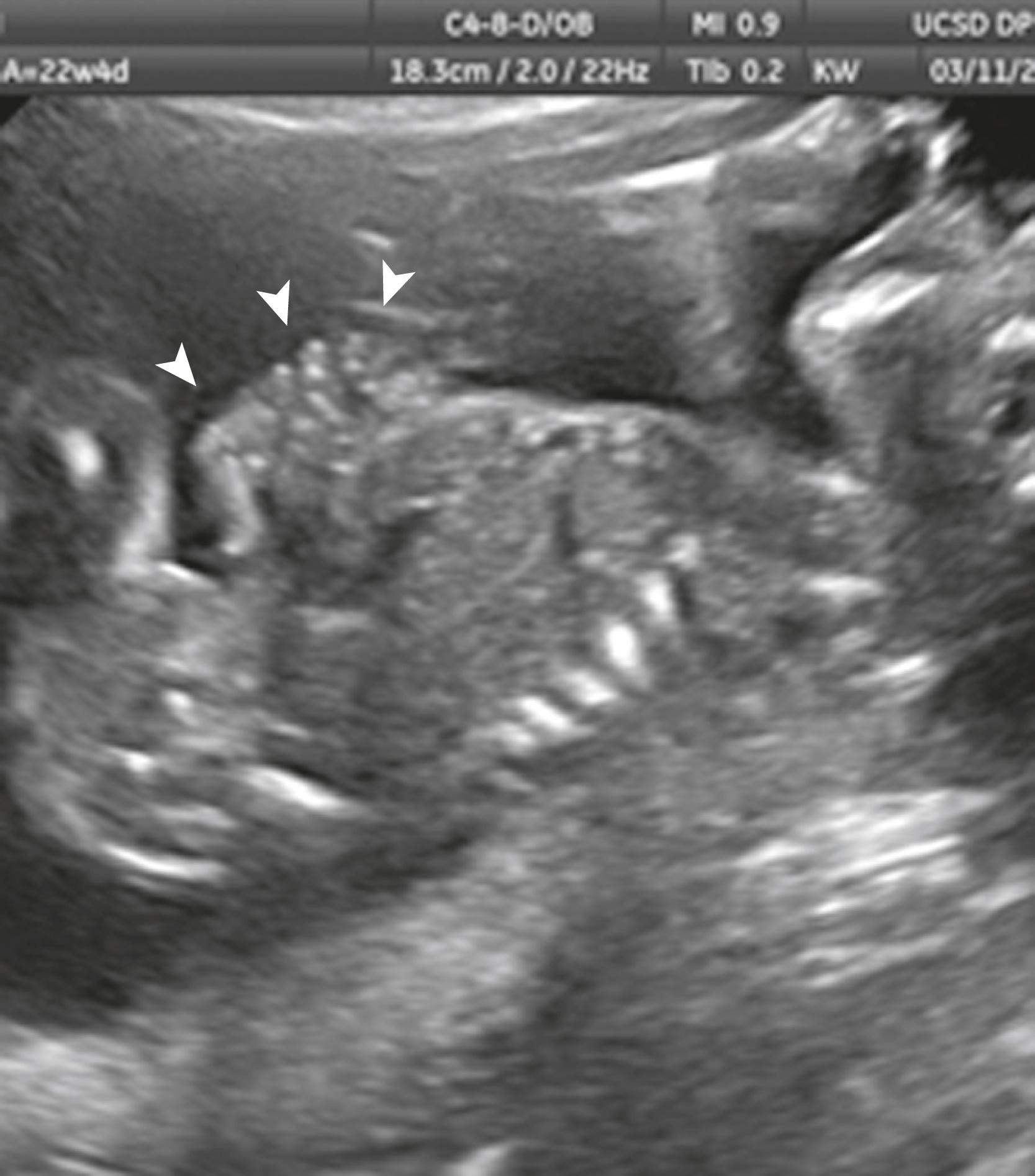
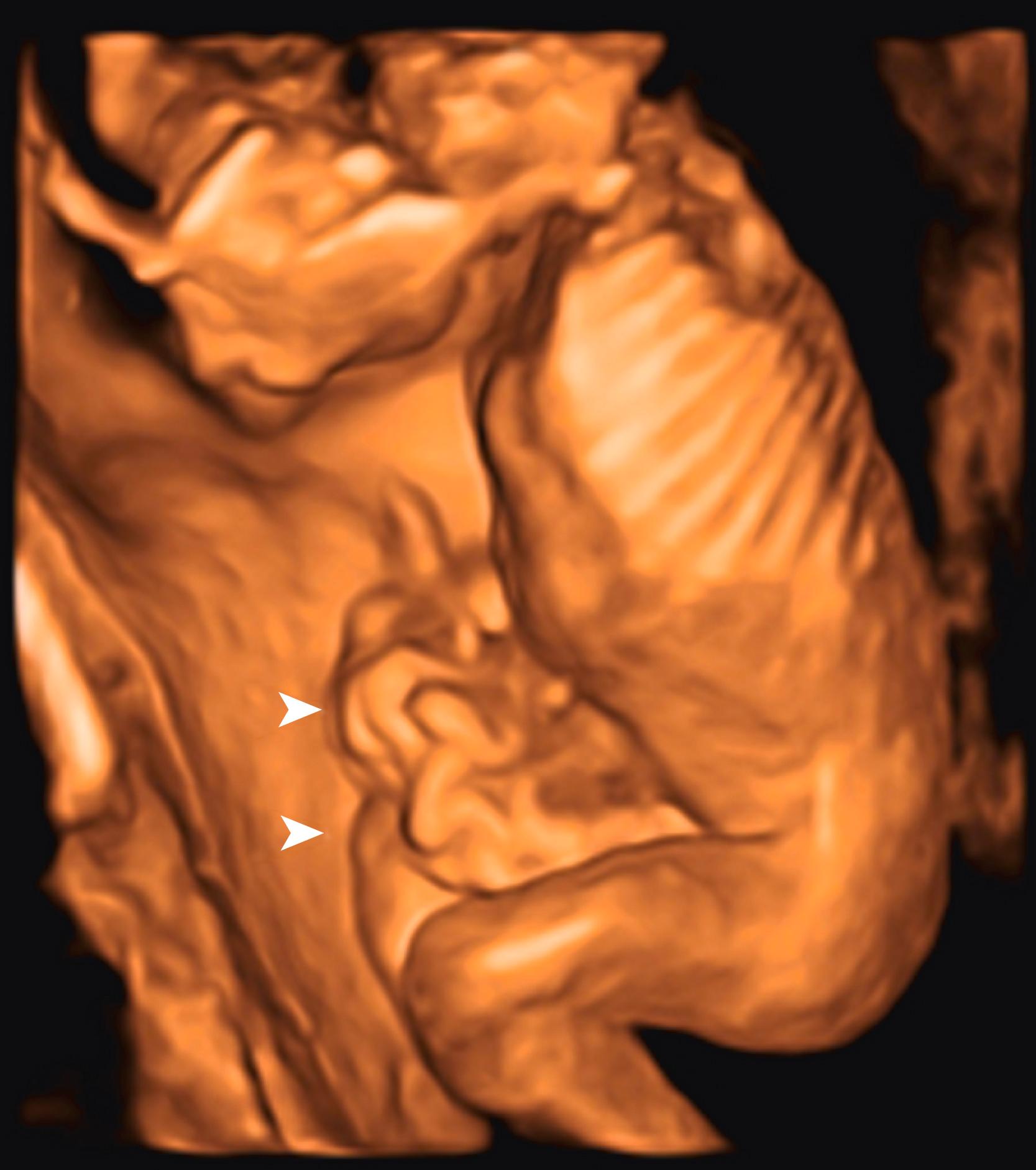
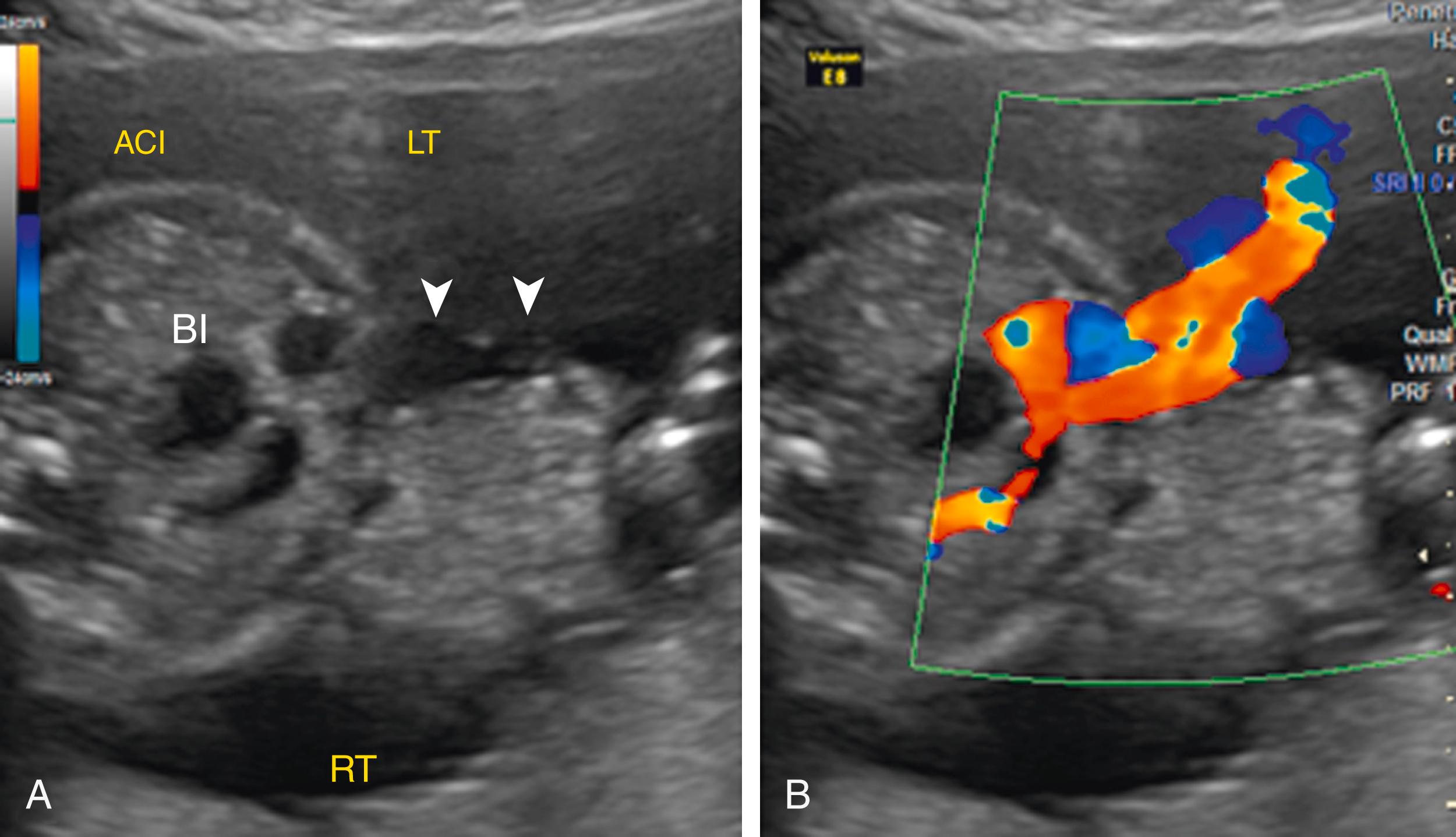
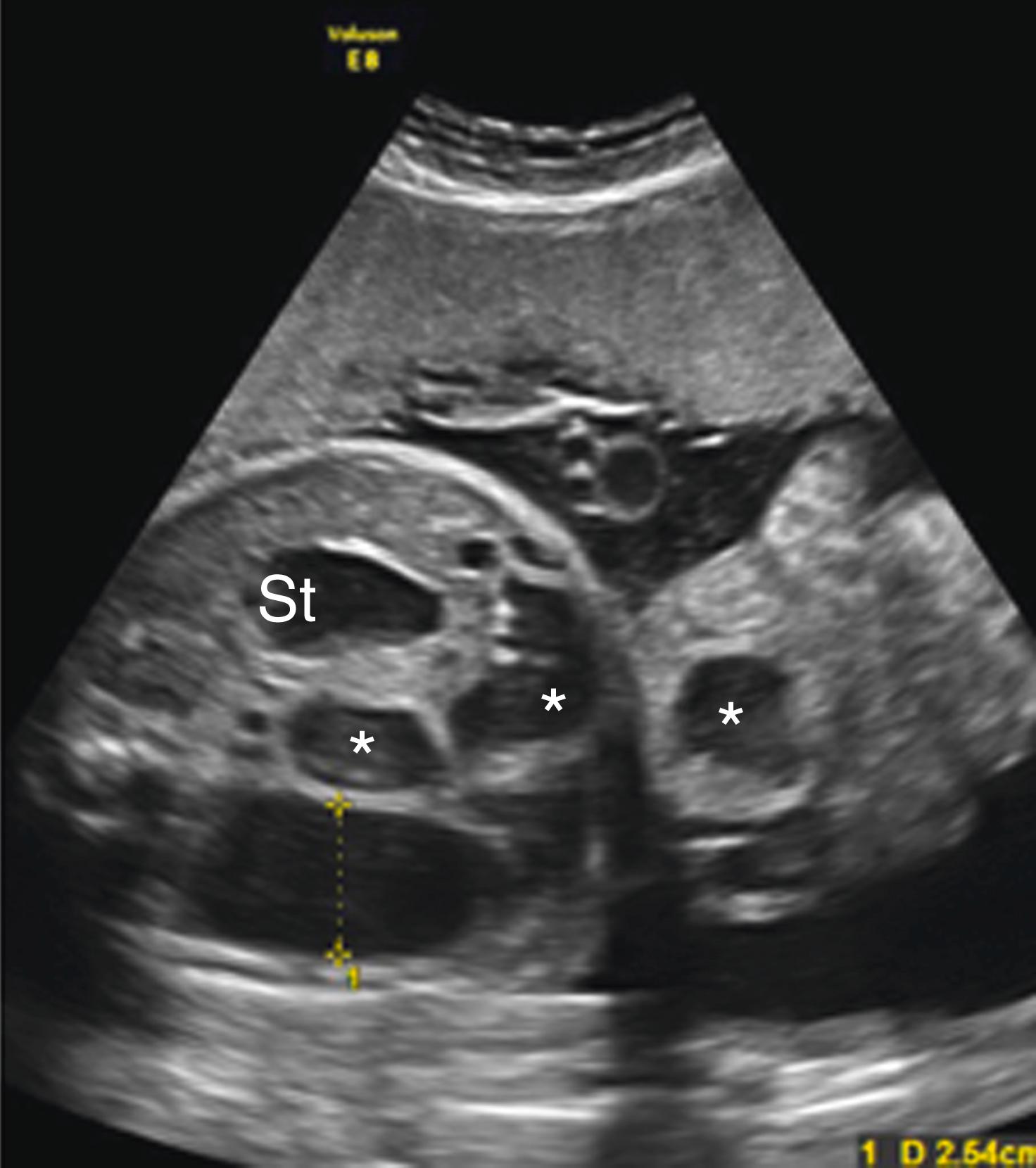
Ascites appears as an echolucent fluid accumulation within the fetal abdomen, outlining the fetal liver, loops of bowel, stomach, and bladder ( Figs. 24.1–24.3 ; ![]() , , , , ).
, , , , ).
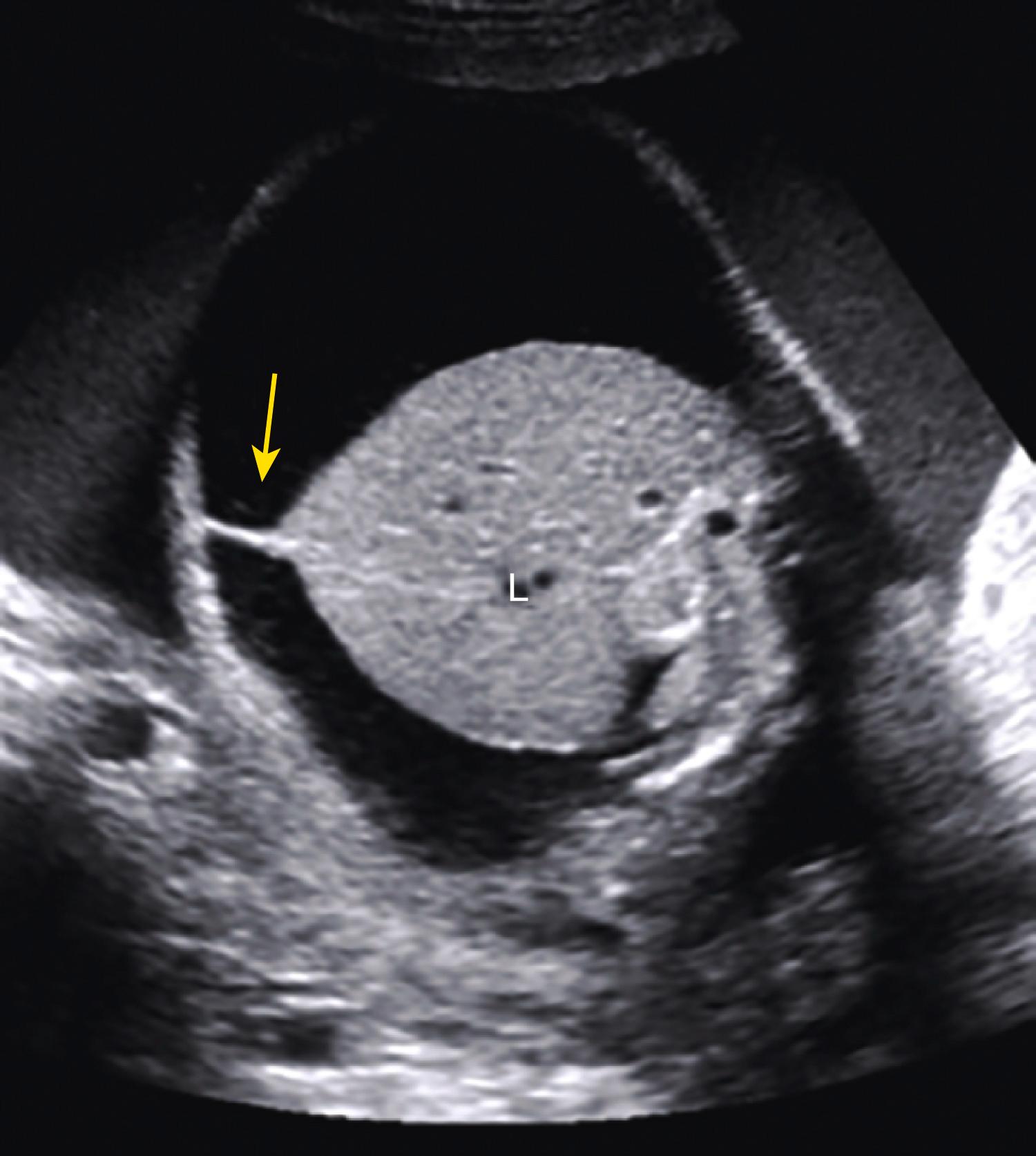
In extreme cases of ascites, the falciform ligament and/or extrahepatic portion of the umbilical vein may be outlined by fluid ( Fig. 24.4 ).
Cases of meconium peritonitis exhibit echogenic calcifications on peritoneal surfaces.
Systematic assessment of fetal anatomy , should be undertaken for other abnormalities and for evidence of hydrops fetalis, such as
Scalp and skin edema ( ![]() Video 4.6).
Video 4.6).
Pleural effusion
Pericardial effusion
Polyhydramnios
Placentomegaly (>4 cm thick)
Markedly overdistended bladder (megacystis) as seen with posterior urethral valves
Ovarian cyst
Dilated fetal bowel, particularly descending and sigmoid colon
Pseudoascites
Gastrointestinal abnormalities (e.g., meconium peritonitis) more likely to be associated with late diagnosis of fetal ascites (3% versus 30% if detected >24 weeks; P = .016)
Hydronephrosis, megacystis
Echogenic and/or dilated bowel
Hepatomegaly
Fetal growth restriction
Polyhydramnios more common (39%) than oligohydramnios (16%)
Overall prognosis depends on underlying condition and/or structural abnormalities.
Best prognosis with chylous ascites (0% mortality)
Poor prognosis if
Ascites diagnosed at <24 weeks (32% mortality versus 0% mortality if detected >24 weeks; P = .003)
Associated fetal hydrops (approximately 75% mortality)
Worst prognosis with metabolic storage disease (100% mortality)
30%–75% of cases of isolated fetal ascites resolve spontaneously. , ,
Detailed ultrasound examinations to detect associated fetal anomalies.
Doppler assessment of umbilical and middle cerebral artery flow.
Fetal echocardiogram to confirm normal anatomy.
Maternal blood testing including
Blood type and antibody screen
Kleihauer-Betke testing to detect fetal hemoglobin in maternal circulation (fetomaternal hemorrhage)
Rapid plasmin reagin (RPR)
TORCH ( t oxoplasmosis, o ther agents, r ubella, c ytomegalovirus, and h erpes simplex), Listeria, and parvovirus antibody titers
Genetic disease carrier screening
Amniocentesis with microarray testing or noninvasive prenatal testing (NIPT) to assess
Karyotype
Infection (polymerase chain reaction [PCR] for cytomegalovirus [CMV] and parvovirus)
Genetic or metabolic disease
Cordocentesis to assess hemoglobin, Coombs test, and infection.
Consider diagnostic paracentesis for protein concentration, triglyceride, cultures, and lymphocyte count to determine transudate versus exudate; low protein concentration, negative cultures, and lymphocyte count >90% suggest fetal chylous ascites.
Serial ultrasound examinations every 1–2 weeks to monitor fetal growth, amniotic fluid, worsening ascites, and progression to generalized hydrops fetalis.
Begin fetal nonstress test and/or biophysical profile testing at viability.
Delivery in tertiary care facility is recommended.
Consider therapeutic fetal paracentesis prior to delivery.
Prevents abdominal dystocia and facilitates vaginal delivery
Decreases mechanical compression of lungs
Facilitates neonatal resuscitative efforts
Cesarean delivery should be reserved for obstetric indications.
Neonatal therapeutic paracentesis should be considered if ascites causing respiratory compromise.
Continue diagnostic evaluation to reveal underlying etiology of ascites.
Abdominal ultrasound
Paracentesis with cell count, culture, and fluid analysis
Chylous ascites typically will have
High cell count (predominantly lymphocytes)
Total protein 2.5–7.0 g/dL
Serum to ascites albumin gradient (SAAG) <1.1 g/dL
High triglyceride (>200 g/dL), low-cholesterol
Low glucose (<100 mg/dL)
Consider lymphangiography
Total parental nutrition (TPN) and/or medium chain triglyceride-based formula (e.g., monogen feeds) reduce chyle volume. ,
Periodic therapeutic paracentesis may be warranted to improve respiratory function.
Surgical management if meconium peritonitis or malrotation suspected.
Ascites may be the first finding in hydrops fetalis and should prompt a systematic fetal assessment to rule out this condition.
The patient should be thoroughly evaluated to rule out fetal anemia, infection, and other etiologies of hydrops fetalis.
Continued surveillance is important to rule out evolving hydrops fetalis; however, many cases of isolated fetal ascites resolve spontaneously.
Intestinal atresia represents complete obstruction of the bowel lumen; stenosis is a narrowing of the lumen of the fetal bowel. Both can produce a distended proximal gastrointestinal tract.
Intestinal atresias occur in approximately 1–3:10,000 live births.
Duodenal atresia prevalence varies from 1:5000 to 1:10,000.
Jejunoileal atresia occurs in 1:3000.
Colonic atresia is seen in 1:2000 to 1:5000.
Anal atresia is present in 1:5000.
Up to one-third have multiple atretic sites.
The cause of intestinal atresias varies by location of the obstruction.
Duodenal atresia —lack of revacuolization during the solid core stage of embryonic intestinal development
Jejunoileal and colonic atresias —vascular accident, intestinal volvulus, intussusception, internal hernia, or strangulation as in the case of tight abdominal wall defect with gastroschisis or omphalocele
Bowel loops >7 mm are suggestive of obstruction; normal colon and rectal diameter progressively enlarges with advancing gestational age ( Table 24.1 ).
| Descending Colon Diameter (mm) | Rectal Diameter (mm) | |||
|---|---|---|---|---|
| Gestational Age, Weeks | Mean | 95% CI | Mean | 95% CI |
| 19-20 | 3.52 | 0.79–6.26 | 3.64 | 1.45–5.82 |
| 21 | 3.59 | 0.86–6.32 | 3.79 | 1.61–5.97 |
| 22 | 3.69 | 0.96–6.41 | 3.95 | 1.78–6.13 |
| 23 | 3.82 | 1.09–6.54 | 4.14 | 1.97–6.31 |
| 24 | 3.98 | 1.26–6.70 | 4.34 | 2.17–6.52 |
| 25 | 4.18 | 1.46–6.90 | 4.57 | 2.40–6.74 |
| 26 | 4.43 | 1.70–7.15 | 4.82 | 2.64–6.99 |
| 27 | 4.71 | 1.99–7.43 | 5.08 | 2.91–7.26 |
| 28 | 5.04 | 2.32–7.76 | 5.38 | 3.20–7.55 |
| 29 | 5.42 | 2.69–8.14 | 5.69 | 3.52–7.87 |
| 30 | 5.84 | 3.12–8.57 | 6.04 | 3.86–8.21 |
| 31 | 6.32 | 3.60–9.05 | 6.41 | 4.23–8.58 |
| 32 | 6.86 | 4.13–9.58 | 6.80 | 4.63–8.98 |
| 33 | 7.45 | 4.72–10.17 | 7.23 | 5.05–9.40 |
| 34 | 8.10 | 5.37–10.82 | 7.68 | 5.51–9.85 |
| 35 | 8.81 | 6.09–11.53 | 8.17 | 5.99–10.34 |
| 36 | 9.59 | 6.87–12.31 | 8.68 | 6.51–10.85 |
| 37 | 10.44 | 7.71–13.16 | 9.23 | 7.16–11.40 |
| 38 | 11.35 | 8.63–14.08 | 9.81 | 7.64–11.98 |
| 39 | 12.34 | 9.61–15.07 | 10.43 | 8.25–12.61 |
| 40 | 13.40 | 10.66–16.15 | 11.08 | 8.89–13.26 |
Dilated bowel and/or colon may not be evident until the third trimester.
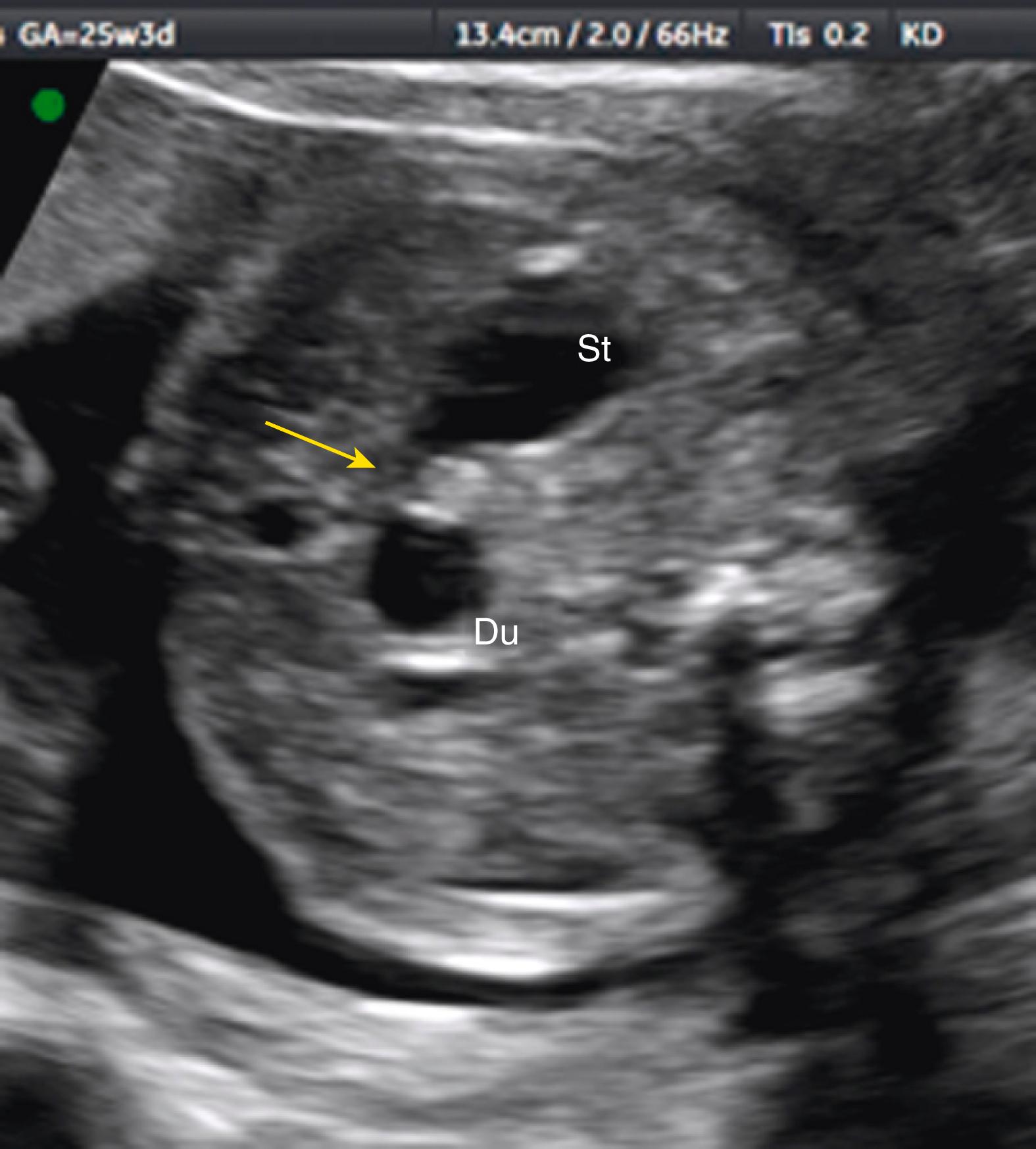
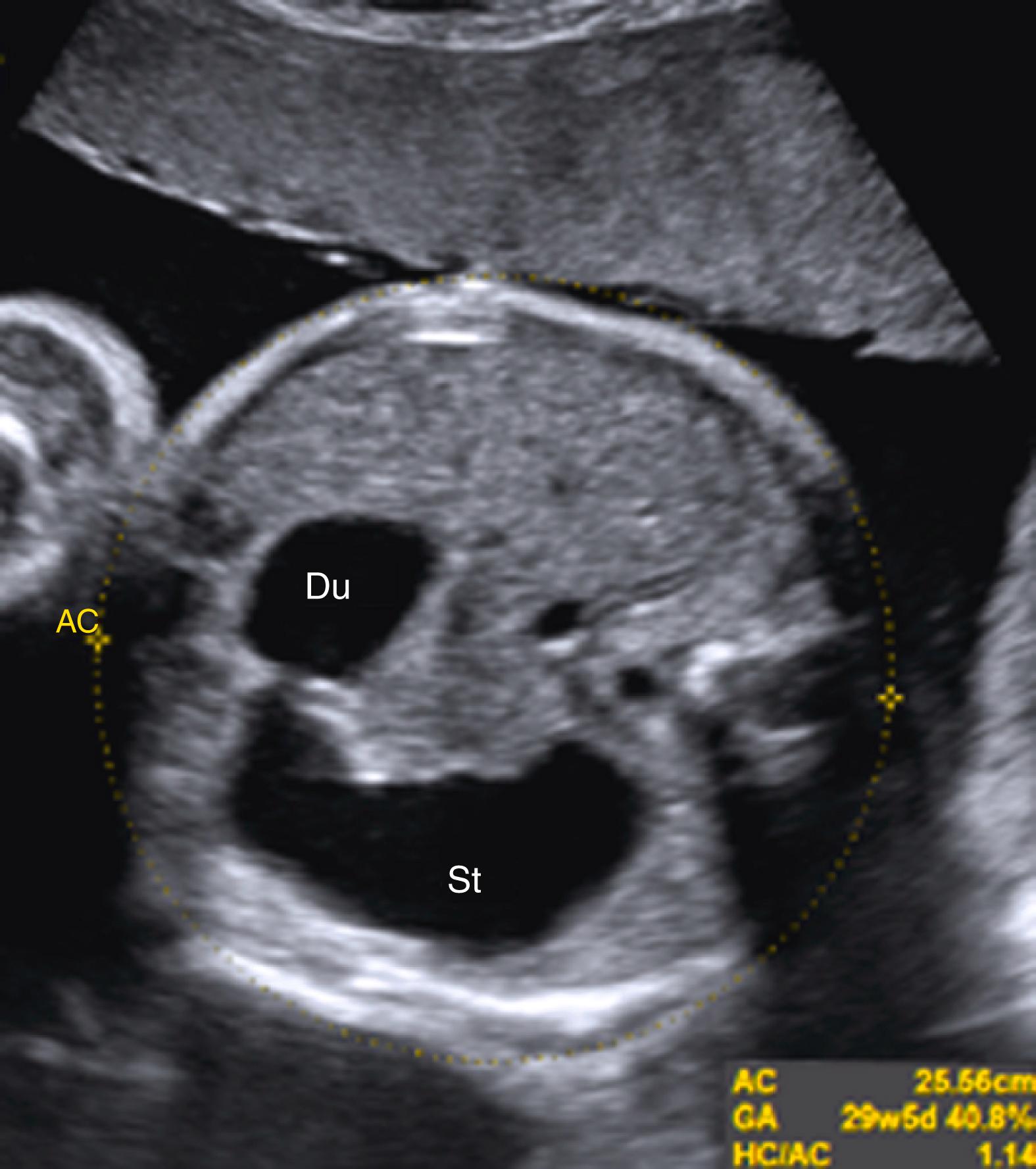
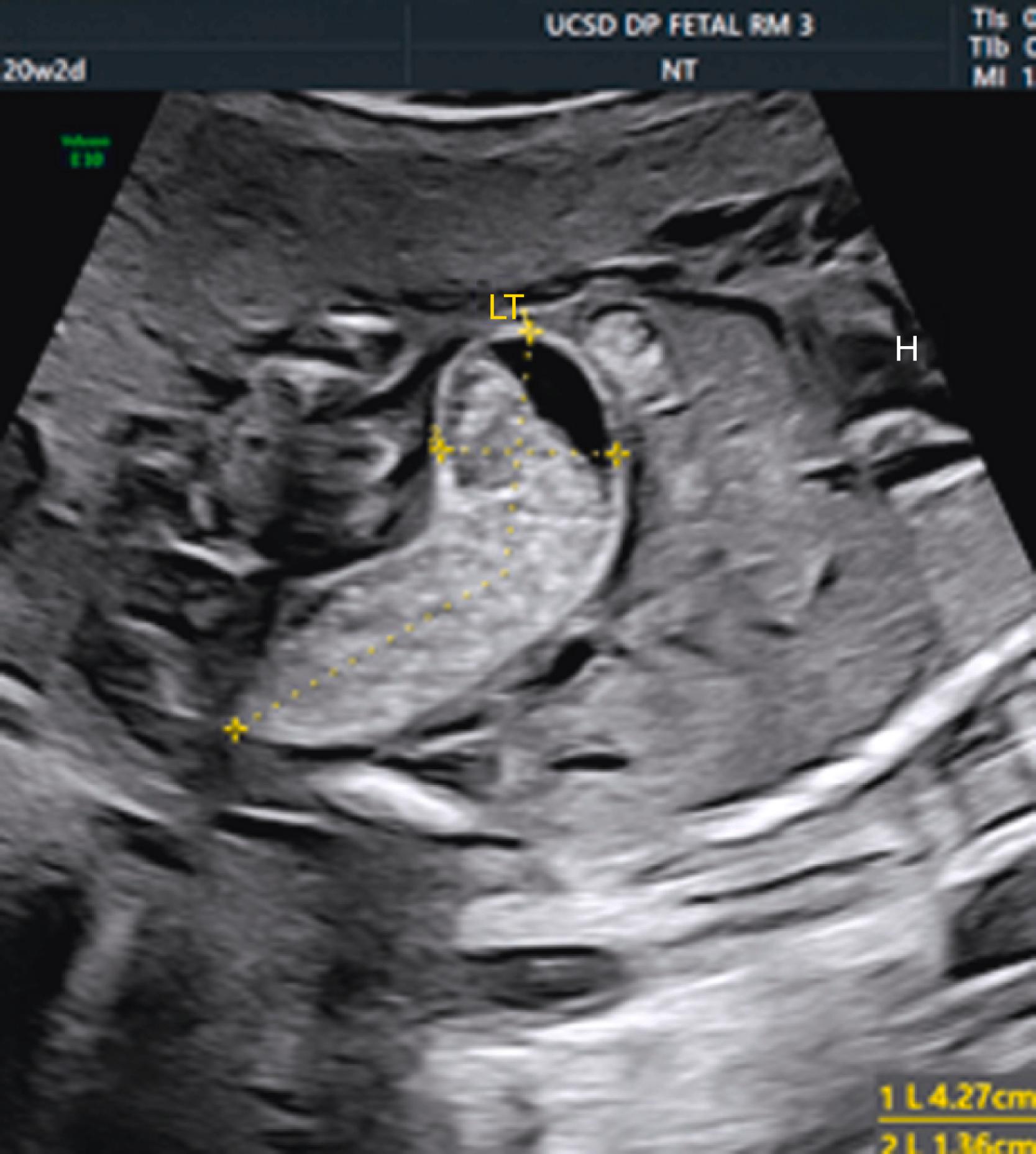
Duodenal atresia produces classic echolucent “double bubble.”
Represents two fluid-filled structures: the stomach and dilated proximal fluid-filled duodenum ( Fig. 24.7 ; ![]() )
)
A thin echolucent connection between these two echolucent structures, most commonly visible after the late second trimester, confirms the diagnosis of duodenal atresia ( Fig. 24.8 ; ![]() , ).
, ).
Polyhydramnios is typical with duodenal atresia and is particularly marked in the third trimester.
Distal intestinal lesions are difficult to diagnose prenatally; many cases of jejunoileal atresia may remain undetected before delivery.
Detection rate of nonduodenal small bowel atresia is approximately 50% (95% confidence interval [CI], 38.0%–63.2%), with only 25% of ileal atresia identified in utero (95% CI, 4.0%–58.0%).
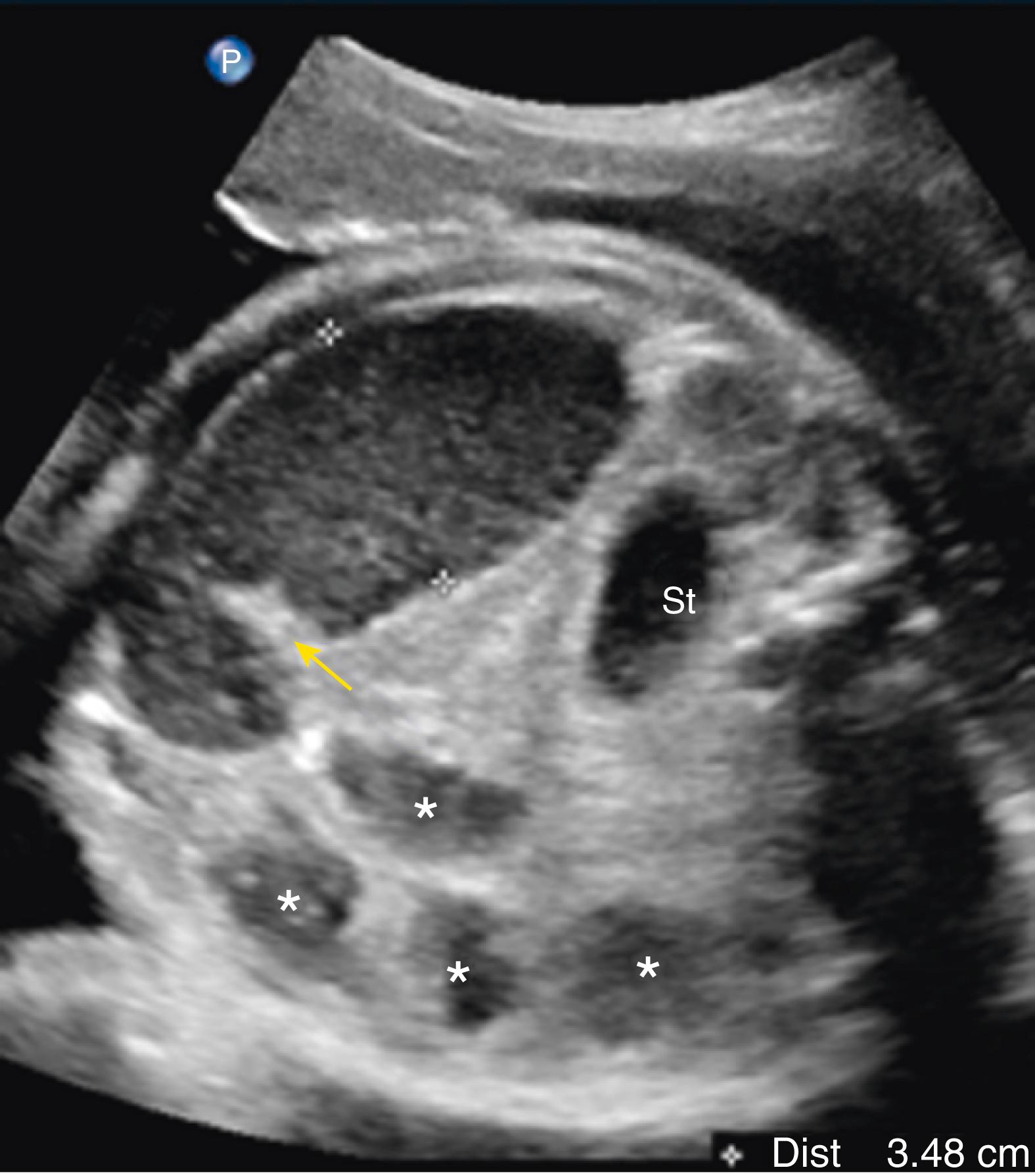
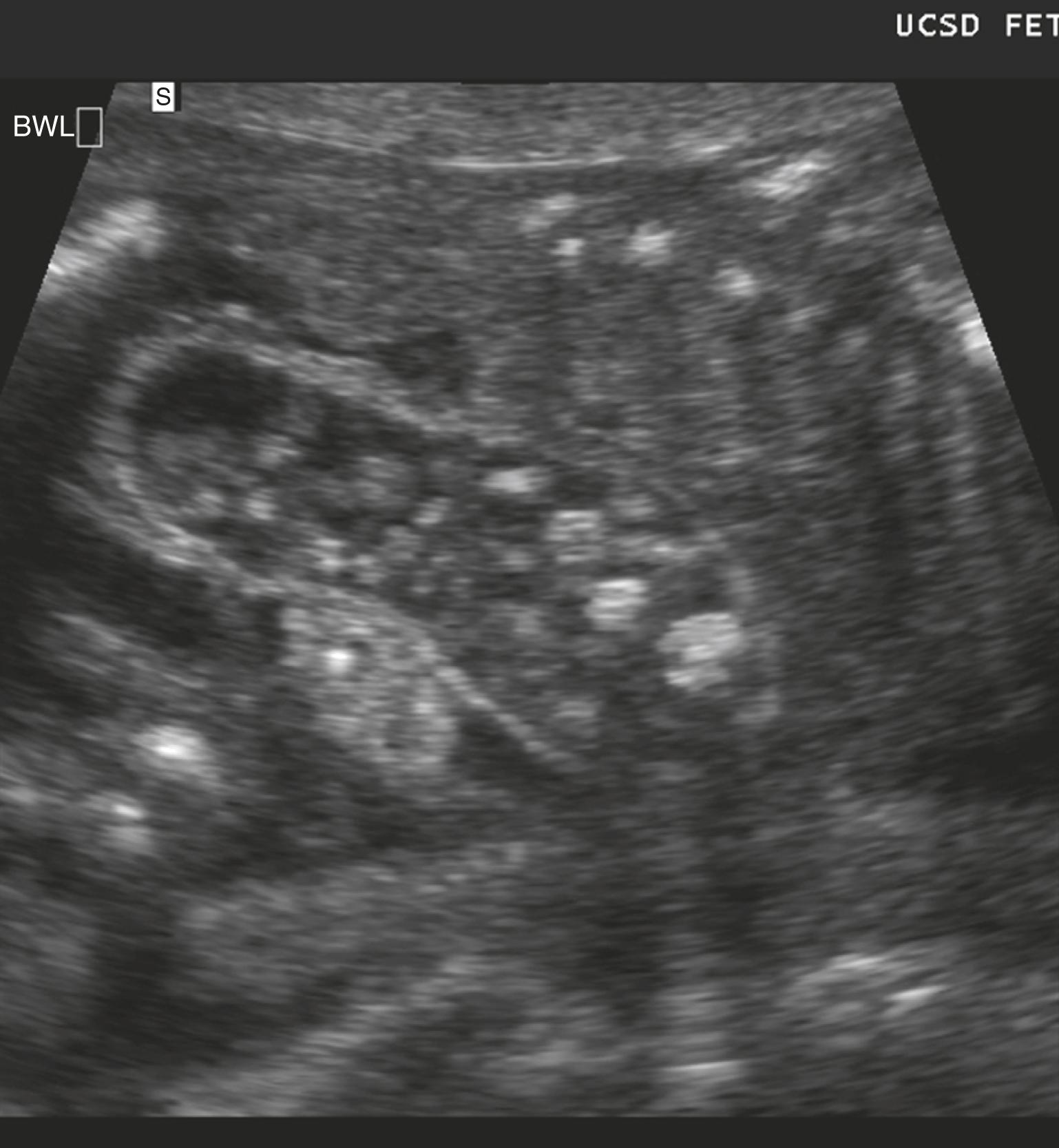
Jejunoileal atresia produces multiple dilated loops of bowel with increased proximal peristalsis.
Multiple dilated bowel loops likely indicate jejunal obstruction ( Fig. 24.9 ), whereas smaller multiple dilated loops of bowel may indicate more distal ileal obstruction ( Fig. 24.10 ; ![]() , ).
, ).
Colon obstruction may be challenging to diagnose prenatally. ,
Isoechoic meconium fills the dilated colon, making echolucency less apparent.
Colonic atresia primarily occurs proximal to splenic flexure with distal microcolon, which is not visible on prenatal ultrasound.
Late in pregnancy, the colon can become markedly dilated ( Figs. 24.11–24.13 ).
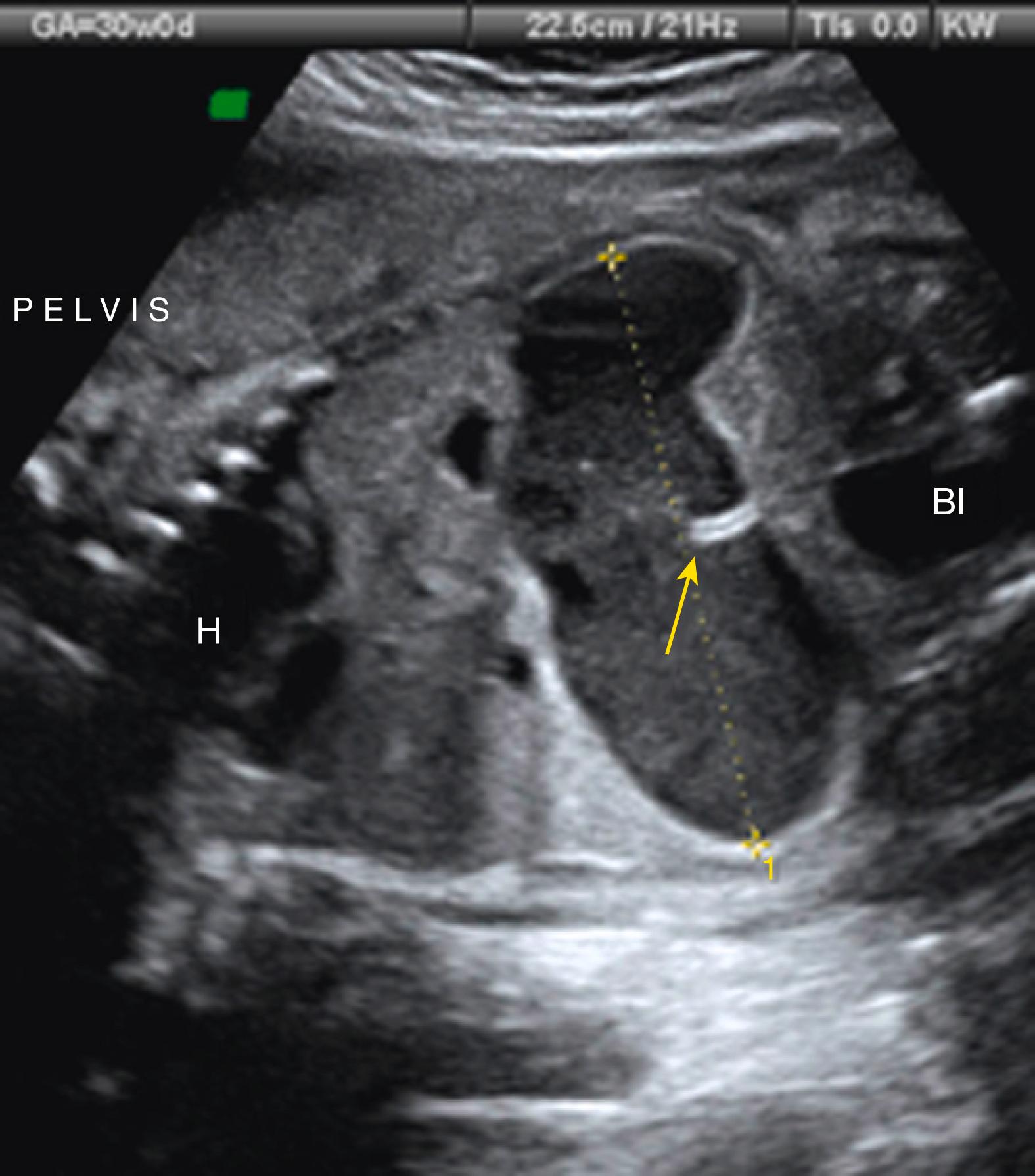
A U- or V-shaped segment of dilated echolucent bowel is suggestive of anorectal atresia ( Fig. 24.14 ).
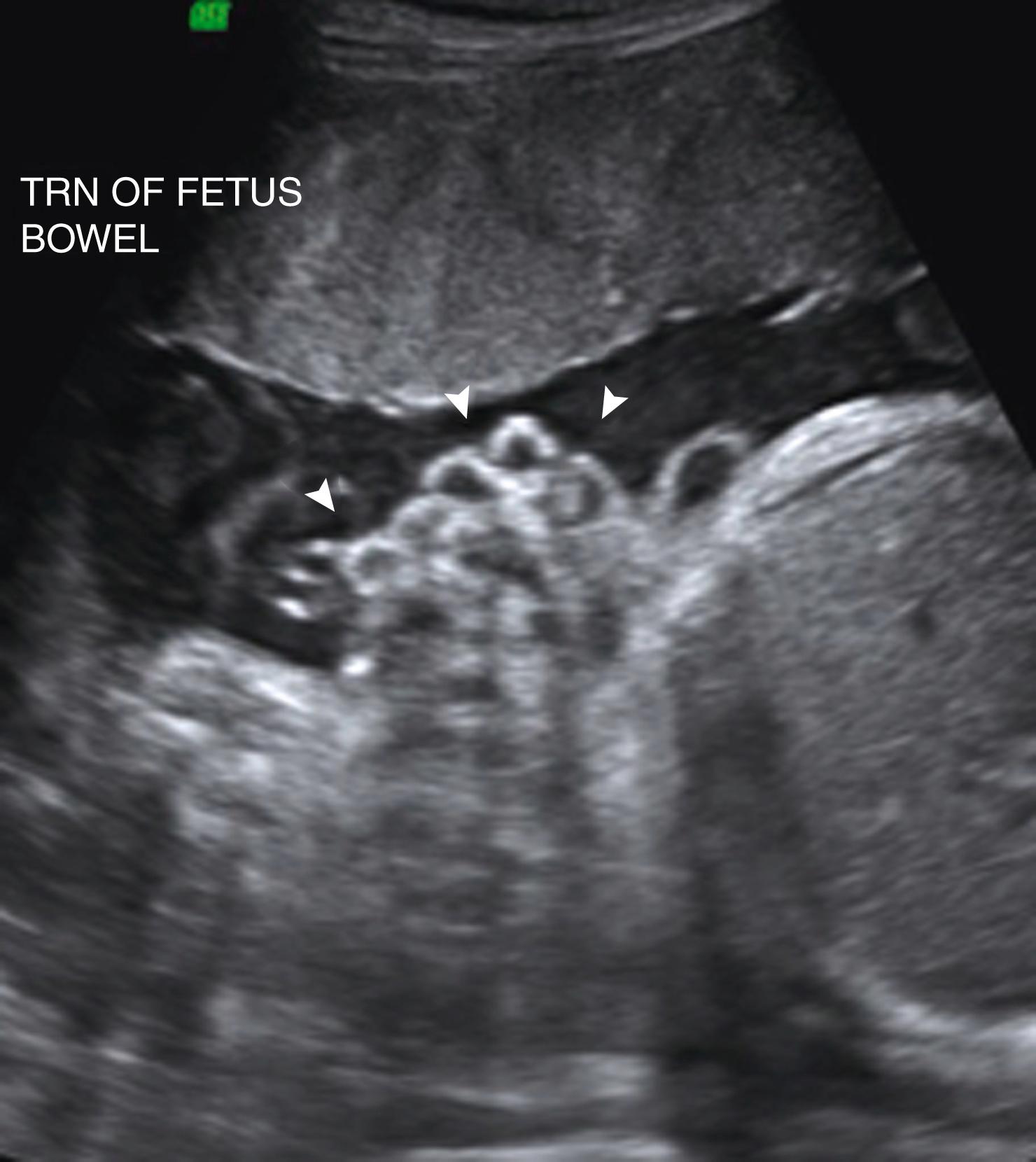
Imperforate anus will result in a dilated rectum with echogenic meconium contents ( Fig. 24.15 ; ![]() ).
).
Dilated colon containing echogenic material may represent a cloacal anomaly or rectourinary fistula ( Fig. 24.16 ).
Careful sonographic anatomic survey is needed to detect other anomalies.
Mildly dilated, normal colon
Markedly distended normal fetal stomach or gallbladder
External constriction by annular pancreas or Ladd bands encircling duodenum
Meconium ileus, particularly associated with cystic fibrosis
Hirschsprung disease (aganglionosis primarily affecting distal colon and rectum)
Intestinal malrotation
Congenital chloride diarrhea
Hydroureter
Hydrocolpos
Umbilical vein varix
Other abdominal cystic masses that can mimic distended bowel include
Choledochal cyst
Ovarian cyst
Duplication cyst
Mesenteric cyst
Renal cyst
Splenic cyst
Urachal cyst
Nearly half of intestinal atresia cases have an associated abnormality.
Trisomy 21 may be present in 30% of duodenal atresia cases.
Congenital heart defects (e.g., atrial and ventricular septal defects).
Gastrointestinal malformations
Malrotation
Meconium ileus and peritonitis
Volvulus
Annular pancreas (seen in 20%–30% of duodenal atresia cases)
Esophageal atresia
Anorectal atresia
Genitourinary malformations.
Ventral wall defects.
Skeletal defects, primarily with anal atresia (VATER syndrome [ v ertebral defects, imperforate a nus, t racheo e sophageal fistula, r enal defects], VACTERL association [ v ertebral abnormality, a nal atresia, c ardiac defect, t racheo e sophageal fistula, r enal agenesis, and radial l imb abnormality], OEIS complex [ o mphalocele, e xstrophy of the bladder, i mperforate anus, s pinal deformities], caudal regression).
Overall prognosis depends on level of lesion and other associated anomalies, particularly aneuploidy.
Live birth rate is lower in duodenal atresia compared with ileojejunal atresia, owing to higher rate of associated congenital anomalies.
Survival data have shown improvement over time from approximately 60% in the 1960s and 1970s to 90%–100% in the 1990s and 2000s.
Jejunoileal atresia has consistently lower long-term survival (60%–90%), secondary to short bowel syndrome.
Long-term total parenteral nutrition (TPN) may lead to cholestasis and subsequent liver damage.
Small bowel transplantation may be necessary; 5-year patient survival rate is 55%; graft survival rate is 45%.
Amniocentesis with microarray or noninvasive prenatal testing (NIPT) to assess karyotype and cystic fibrosis status.
Fetal echocardiogram.
Consider MRI to determine level of obstruction. ,
Monitor fetal growth, amniotic fluid, worsening bowel distention, and progression to bowel perforation with meconium peritonitis.
Fetal nonstress and/or biophysical profile testing in third trimester.
Pediatric surgery consultation.
Delivery in tertiary care facility is recommended.
Therapeutic amnioreduction may reduce risk of preterm labor and prolapsed umbilical cord or abruption with rupture of membranes.
Cesarean delivery should be reserved for obstetric indications.
Orogastric or nasogastric decompression to prevent aspiration
IV fluid hydration
Confirm diagnosis of intestinal atresia
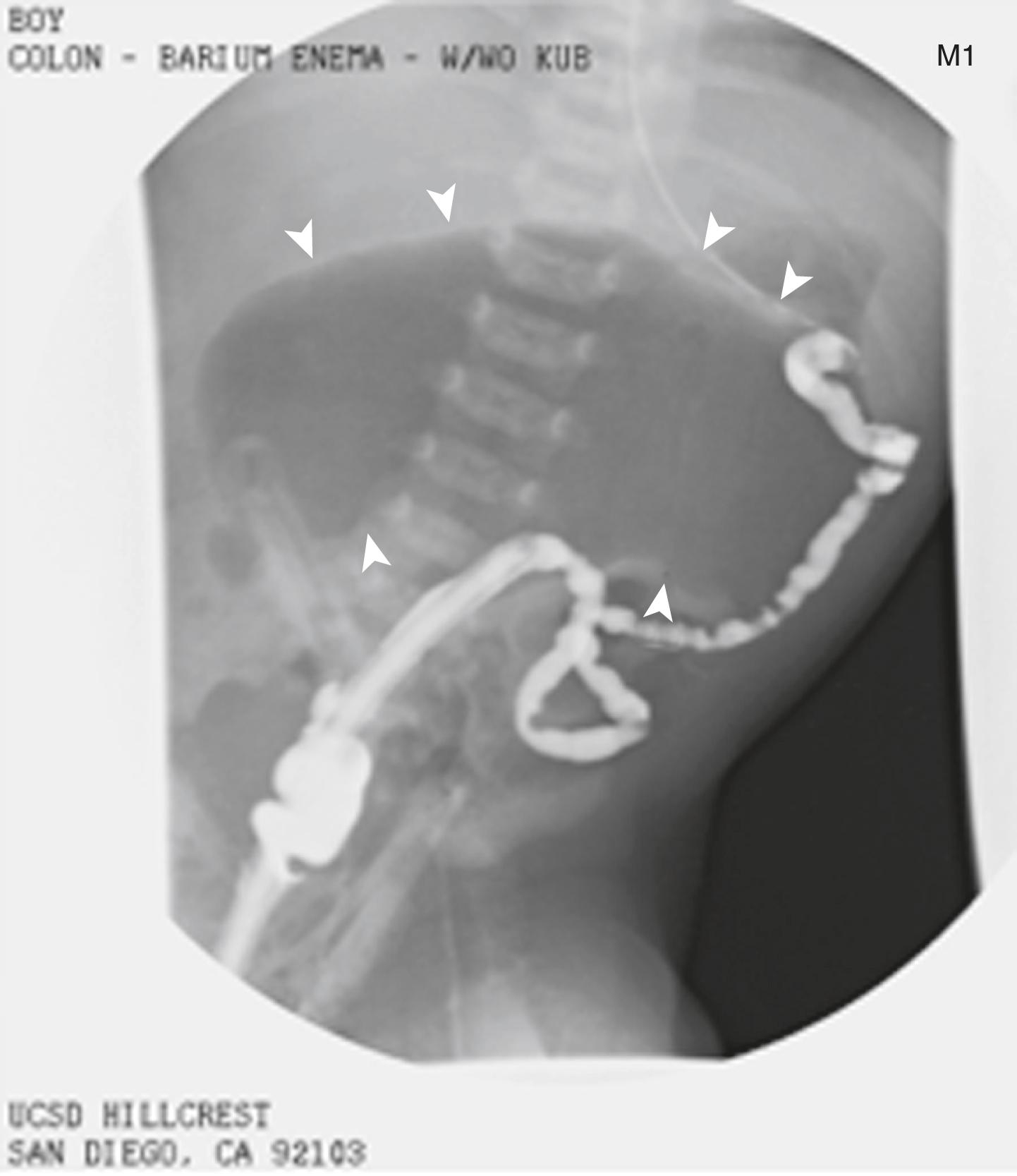
Plain abdominal radiograph
Upper gastrointestinal contrast-enhanced radiograph
Barium enema
Surgical management depends on site of lesion and associated conditions; most are treated with excision of abnormal segment and primary anastomosis :
Duodenal atresia is treated with direct duodenojejunostomy or duodenoduodenostomy.
Jejunoileal atresia is managed with tapering enteroplasty or intestinal plication.
Colonic atresia is repaired with primary anastomosis.
Multiple intestinal atresias are managed with multiple staged anastomoses and enteroplasty; approximately 25% require >100 days on TPN.
Dilated loops of fetal bowel may indicate obstruction distal to dilated segment of bowel or colon.
Proximal small bowel (duodenal) atresia is more easily identified on second-trimester and third-trimester fetal imaging than more distal (jejunal or colonic) atresia.
Marked polyhydramnios is typical in third trimester with proximal small bowel atresia and may result in preterm labor, premature rupture of membranes, and prolapsed umbilical cord.
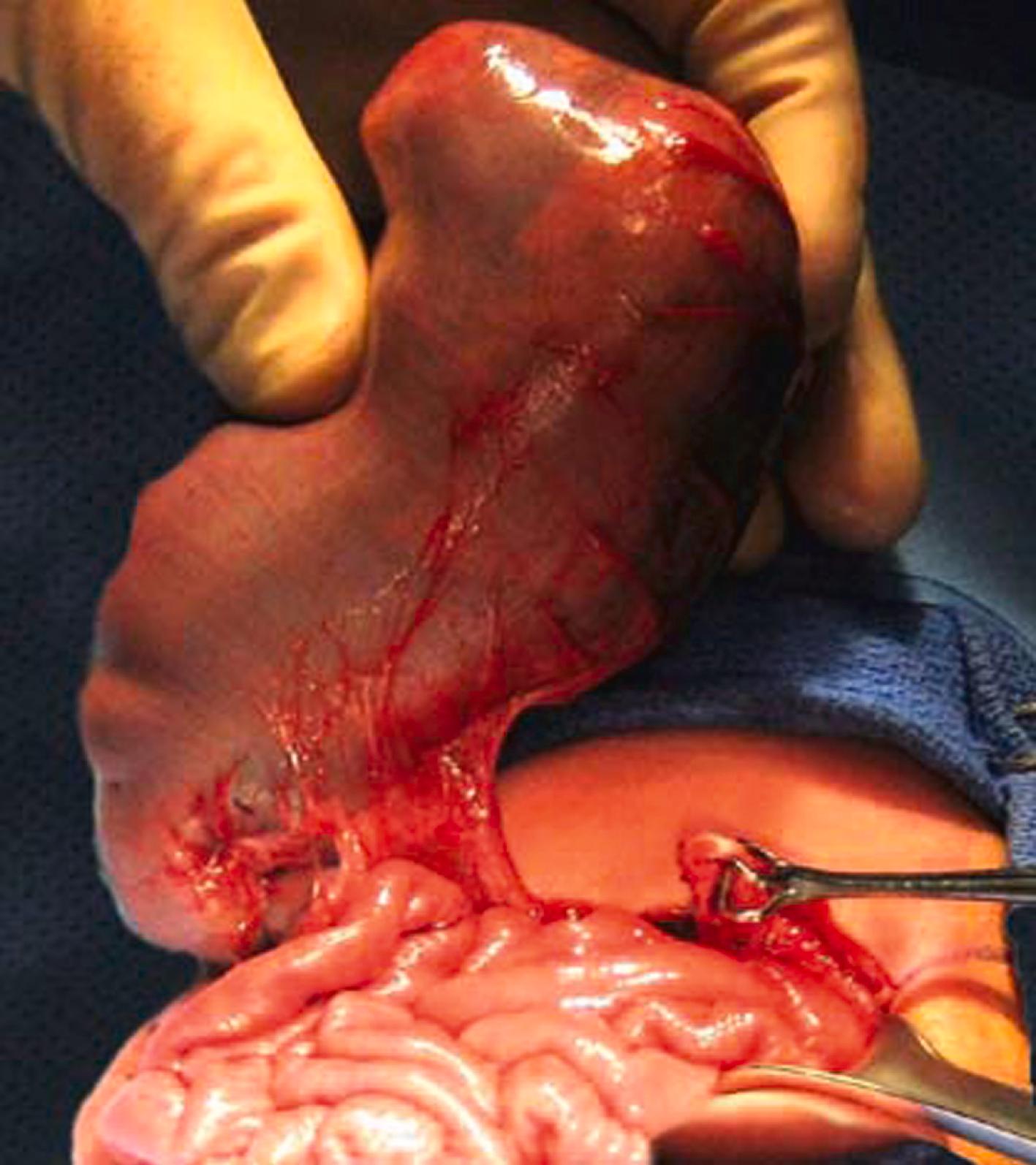
Gastroschisis is a full-thickness paraumbilical defect through which bowel herniates. In contradistinction to omphalocele, the bowels are not enveloped by a membrane.
The incidence of gastroschisis worldwide is approximately 1–5:10,000 live births. Although the incidence in US increased threefold between 1997 and 2008 (2.9–6.4:10,000), it decreased to 3.3:10,000 by 2018 ( P < .01).
Risk factors for developing gastroschisis include young maternal age, smoking, vasoactive drug use, and low socioeconomic status.
Women younger than 25 years old have a two to three times higher risk of gastroschisis, with prevalence of gastroschisis in women younger than age 20 increasing from 8:10,000 to 18:10,000 since the mid-1990s.
Smoking increases risk of gastroschisis (odds ratio [OR] = 3.4; 95% CI, 1.1–10.5).
Illicit drug use (relative risk [RR] 2.14; 95% CI, 1.48–3.07) and alcohol consumption (RR 1.40; 95% CI, 1.13–1.70) increase the risk of gastroschisis.
Gastroschisis is increased approximately 1.5 times in areas where opioid prescription rates are high (5.1:10,000 live births; CI, 4.9–5.3) compared to low opioid prescription areas (3.2:10,000 live births; CI, 3.1–3.4).
Black women have a lower risk of gastroschisis (RR = 0.49; 95% CI, 0.38–0.63).
The embryogenesis of gastroschisis is unclear. There are five main proposed theories leading to gut herniation and clinical presentation of gastroschisis:
Embryonic (somatopleural) mesenchyme fails to differentiate; thus abdominal wall mesoderm fails to form.
Amnion rupture at base of the umbilical ring during physiologic gut herniation in late embryonic period.
Right umbilical vein prematurely involutes.
Right vitelline (omphalomesenteric) artery disrupted.
Abnormal body wall folding.
Multifactorial etiology of gastroschisis includes identified gene variants ( NOS3, NPPA, ADD1, ICAM1, ICAM4, ICAM5, and MTHFR ) associated with environmental exposures.
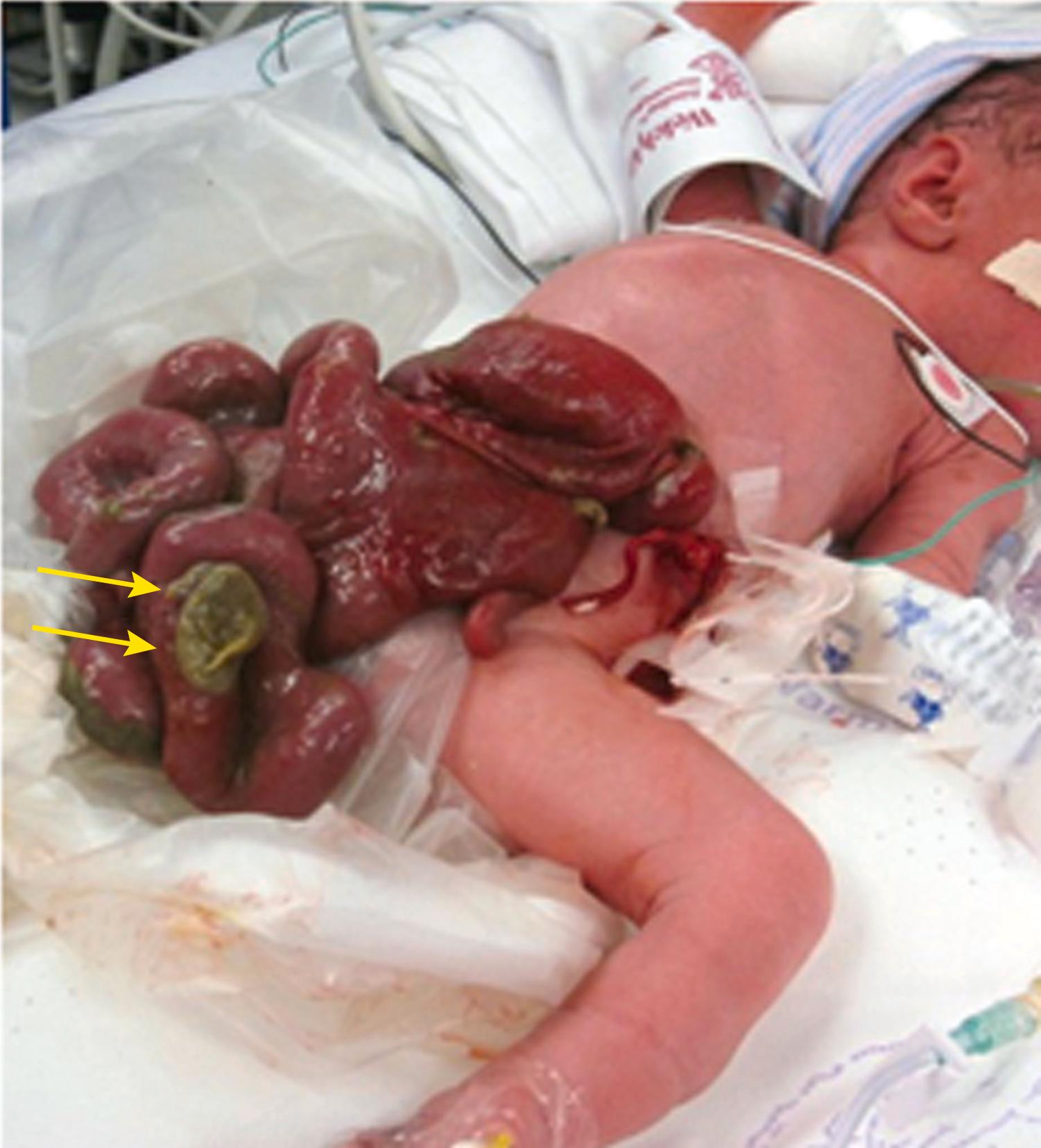
Multiple loops of bowel are seen floating freely in amniotic fluid, with a typical “cauliflower” appearance ( Figs. 24.17 and 24.18 ; ![]() , , ).
, , ).
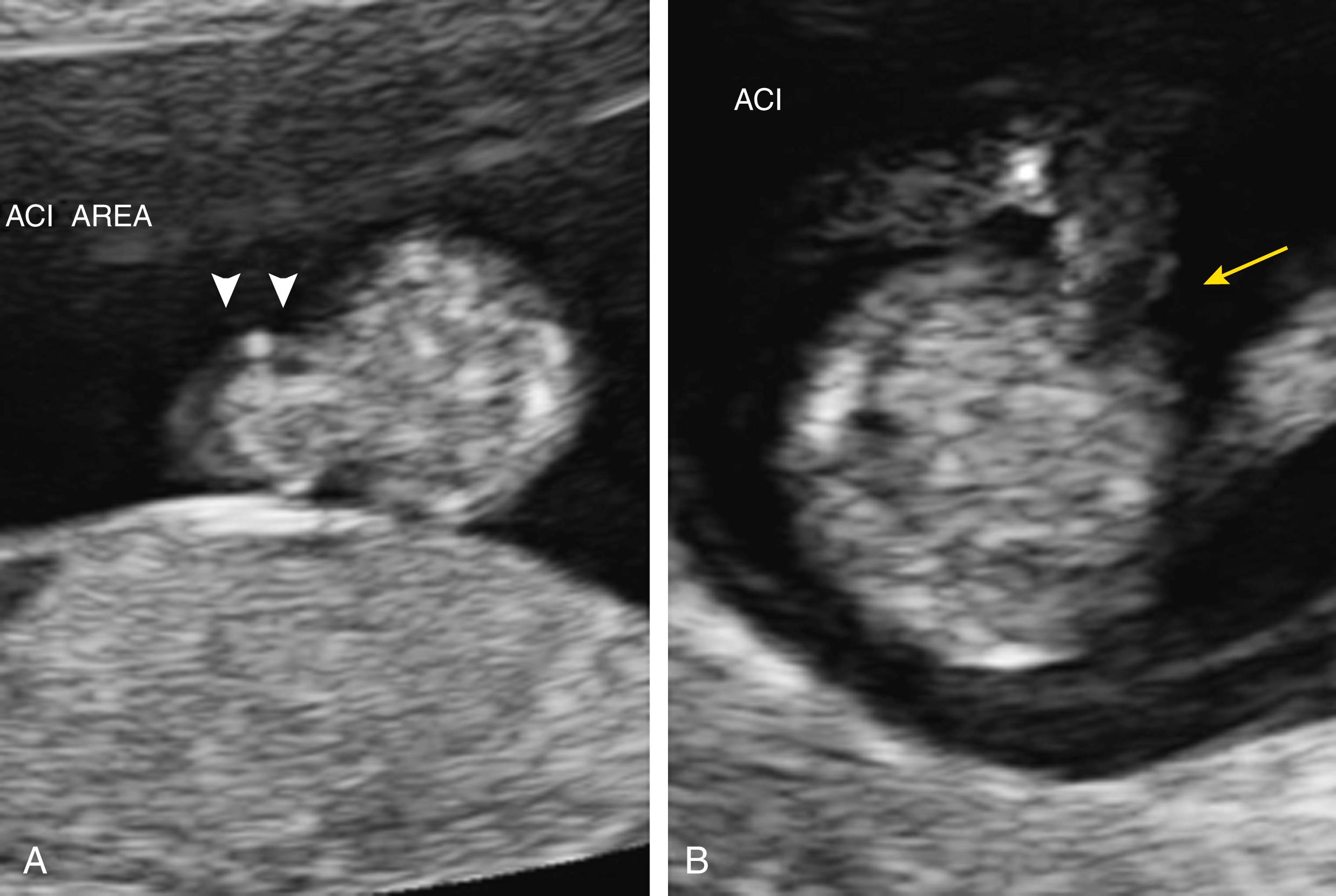
Abdominal wall defect is typically located to the right of the abdominal cord insertion ( Fig. 24.19 ; ![]() , ); left-sided defects are much less common.
, ); left-sided defects are much less common.
Intraabdominal and extraabdominal dilated loops of bowel may be seen late in pregnancy ( Figs. 24.20 and 24.21 ).
Complex gastroschisis if intraabdominal bowel dilation and/or liver or bladder herniation.
Maternal serum alpha fetoprotein (AFP) is elevated with gastroschisis; with widespread use or prenatal screening, 95% of gastroschisis cases are diagnosed prenatally.
Excluding omphalocele is important, as 40% of omphalocele cases are associated with aneuploidy; isolated gastroschisis is not typically associated with aneuploidy, making invasive prenatal testing unwarranted.
Normal physiologic gut herniation ( Fig. 24.22 )
Occurs between 6th and 10th week of embryonic development.
Loop of intestines herniates, rotates around the superior mesenteric artery, and returns to abdominal cavity.
Gut herniation persisting beyond 12 weeks is likely gastroschisis.
Ruptured omphalocele (umbilical cord located medially within the herniated intestinal mass, as opposed to intestines being lateral to the umbilical cord insertion as seen with gastroschisis).
Limb–body wall complex (larger size abdominal mass, often adherent to placenta and/or with short umbilical cord).
Pentalogy of Cantrell.
Umbilical cord cysts.
Urachal cysts.
Loops of normal umbilical cord.
Bladder or cloacal exstrophy.
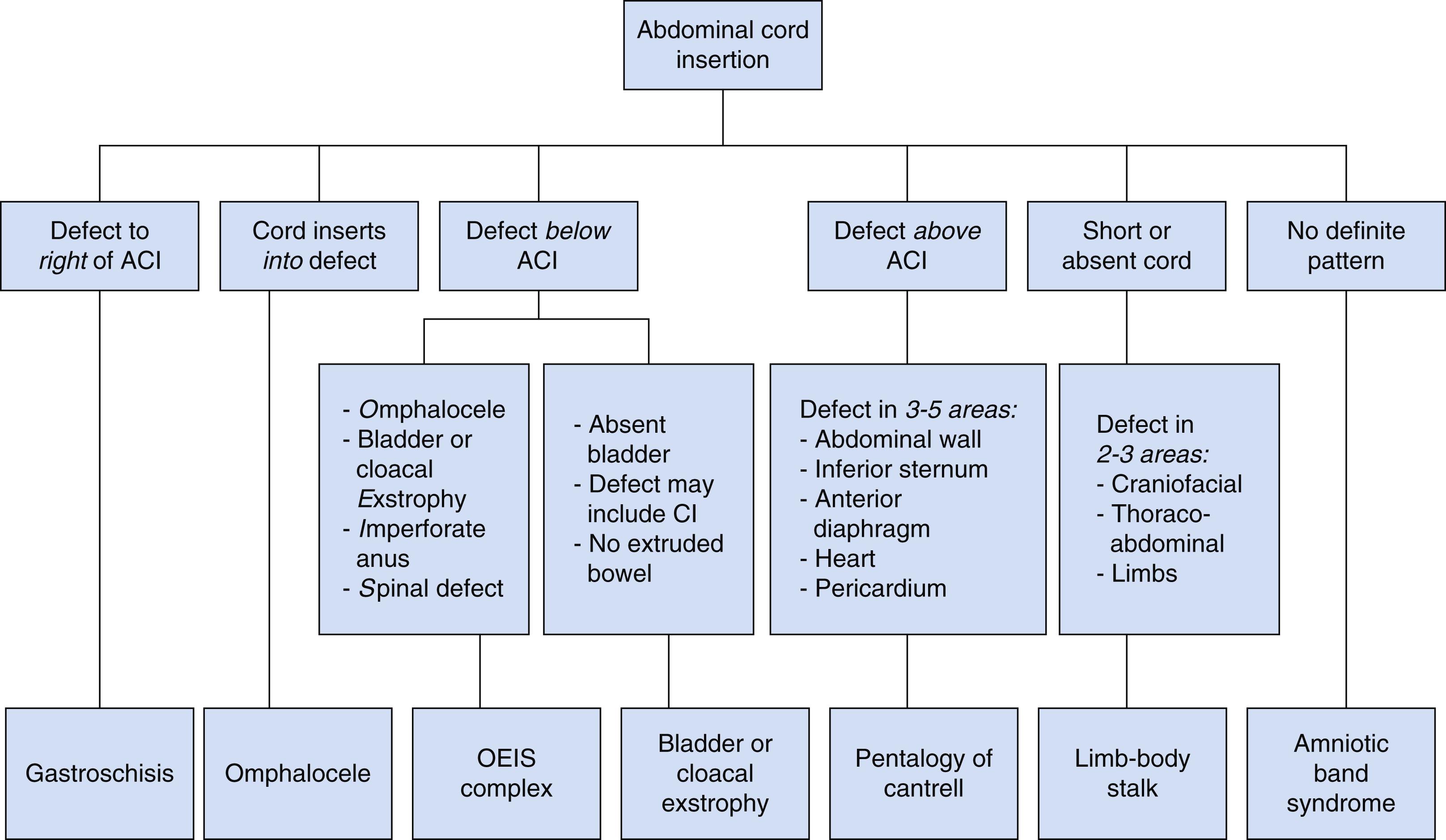
See Fig. 24.23 for diagnostic algorithm for diagnosing suspected fetal abdominal wall defects.
Approximately 85% of gastroschisis cases occur in isolation.
Other associated gastrointestinal malformations (complex gastroschisis), including bowel atresia, malrotation, volvulus with dilated loops of bowel, and perforation.
Vessels are constricted at level of abdominal defect.
Torsion of the mesentery leads to bowel ischemia and necrosis.
Nongastrointestinal abnormalities, including arthrogryposis, cardiac defects, and genitourinary anomalies (e.g., bladder herniation), are infrequent (<5%).
Oligohydramnios is common; however, polyhydramnios may accompany gastroschisis with concomitant bowel atresia or poor bowel function.
Fetal growth restriction (FGR) is often present.
In one study of over 4000 live births with gastroschisis, the median birth weight was 2410 ± 300 g, delivering at a median 36 weeks’ gestational age.
FGR may be overestimated using standard Hadlock formulas for estimating fetal weight, as herniated abdominal contents result in an undersized abdominal circumference (AC).
Siemer (biparietal diameter [BPD] and femur length) or Shepard (modified AC and BPD) formulas may be more accurate for calculating estimated fetal weight in fetuses with gastroschisis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here