Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Disease and injury frequently result in pain and hyperalgesia. These abnormal sensory events arise in part from the action of inflammatory mediators on the peripheral terminals of nociceptive neurons. In this chapter we begin by reviewing the different ways in which such mediators bring about the activation or sensitization of nociceptive terminals. We then consider the biological effects and potential importance of different inflammatory mediators. The list of mediators has steadily been increasing and includes not only traditionally recognized molecules such as arachidonic acid metabolites and bradykinin but also other small molecules such as adenosine triphosphate and nitric oxide. Additionally, evidence has accumulated for an important role of a series of inflammatory cytokines and chemokines, such as tumor necrosis factor-α and interleukin-1β, and growth factors, particularly nerve growth factor, which are all capable of changing the response properties of pain-signaling neurons. They achieve this in a variety of ways, including activation or sensitization of nociceptive terminals, as well as regulation of gene expression by nociceptors. Immune cells are an important source of inflammatory mediators, cytokines, and some growth factors. Recently, it has become clear that they modulate pain processing not just by release of mediators into peripherally damaged or diseased tissue but also by release of the same mediators into the central nervous system.
A long-standing interest for pain scientists has been the identification of chemical mediators released into injured or diseased tissues that are responsible for the abnormal pain states associated with these disorders. For some time, attention was focused on a small number of molecules such as prostaglandins and bradykinin. These factors were known to be produced as a result of tissue damage or inflammation and were thought to be responsible for activation and sensitization of peripheral pain-signaling sensory neurons; that is, they were seen as the principal peripheral pain mediators. During the past decade or so, evidence has emerged for many novel pain mediators. The old ones have not disappeared, although their roles have been redefined in some cases. Prostaglandin E 2 (PGE 2 ), for instance, is now recognized as playing a prominent role in central nervous system (CNS) as well as peripheral tissues. The newly identified mediators include a variety of factors produced and released from non-neuronal cells, often immune and glial cells. There is now a rapidly expanding evidence base that these are important mediators of persistent pain states and can act at a number of loci.
This chapter focuses on the cellular characteristics of nociceptive afferent neurons, their ion channels, and their signal transduction pathways and discusses the ways in which inflammatory mediators impinge on these basic properties. In particular, we first review the cellular mechanisms of activation and sensitization of nociceptors. Then we discuss the roles and actions of particular immune cells and specific pain mediators, starting with a group of small molecules often rapidly released into damaged tissue. We conclude with a review of the actions of another group of peripheral pain mediators and modulators: the pro-inflammatory cytokines, some chemokines, and some neurotrophic factors, which in addition to their traditionally recognized roles, are all capable of changing the response properties of pain-signaling neurons. The topic of neuro-immune interactions within the CNS is considered in Chapter 4 .
A large number of endogenously generated factors produce pain when injected into peripheral tissue. Many of these substances can also sensitize nociceptors. That is, they reduce the threshold for activation of nociceptors by one or more stimulus modalities and/or increase the responsiveness of nociceptors to suprathreshold stimulation. This process of sensitization is recognized as being of critical importance in many chronic pain states; it is precisely this aberrant excitability of nociceptors that causes a large part of the sensory abnormality. Some features of the sensitization process are described in Chapter 1 . Here we first review the cellular mechanisms by which sensitization occurs.
Sensory nerves express a variety of receptors for inflammatory mediators. Different classes of nociceptors express distinct patterns of receptors. The receptors fall into three main classes: G protein–coupled receptors (GPCRs), ligand-gated ion channels, and the cytokine receptors or receptor tyrosine kinases ( Fig. 3-1 ).
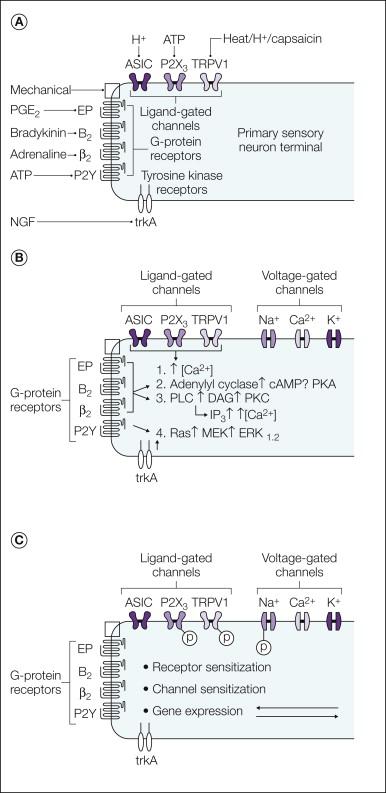
Many mediators produced during inflammation, such as bradykinin, serotonin, prostaglandins, and chemokines, act via GPCRs. These receptors elicit a specific biochemical response that depends on the type of G protein that is activated. Activation of G s stimulates adenylate cyclase to raise the level of cyclic adenosine monophosphate (cAMP) and activate protein kinase A (PKA) in the neuron, whereas G i inhibits the activity of adenylate cyclase to lower cAMP levels. Although many cAMP effects are mediated by PKA, other mechanisms may be operative. For example, cAMP can activate Epac (exchange protein directly activated by cAMP), a guanine nucleotide exchange factor, which leads to activation of the ϵ isoform of protein kinase C (PKC-ϵ). Stimulation of G q/11 activates phospholipases, notably phospholipase C (PLC), which generates inositol triphosphate (IP 3 ) and diacylglycerol (DAG) from the membrane lipid precursor phosphatidylinositol 4,5-bisphosphate (PIP 2 ). G q activation can also stimulate PLA 2 , which cleaves membrane phospholipids at the sn-2 position to produce the prostaglandin precursor arachidonic acid. G-protein control of cellular function can also involve direct action of βγ subunits on ion channels and enzymes, such as PLC (see ).
Some inflammatory mediators act by directly gating the ion channels expressed by sensory neurons. Notable examples in this class are adenosine triphosphate (ATP; acting via P2X channels), protons (acting via acid-sensing ion channels [ASICs] and transient receptor potential vanilloid 1 [TRPV1]), and the lipid activators of TRPV1. All these ion channels are cation selective and are permeable to either sodium ions or both monovalent and divalent cations. In all cases the ion flow evoked by channel opening depolarizes the sensory neurons and leads to neuronal firing.
The third general type of receptor includes cytokine receptors activated by mediators such as interleukin-1 (IL-1) or tumor necrosis factor-α (TNF-α) and the receptor tyrosine kinases for neurotrophic factors, such as the receptors for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell line–derived neurotrophic factor (GDNF), and artemin. Both classes of receptors have monomers derived from a single transmembrane segment with a large extracellular ligand-binding domain. The cytosolic domain of receptor tyrosine kinases contains an intrinsic protein tyrosine kinase catalytic site, whereas the cytosolic domain of cytokine receptors is generally associated with a separate protein kinase that is recruited to the complex either directly or via adapter proteins. The functional receptors are either dimers or trimers, which either exist normally or are formed by cross-linking of adjacent monomers by the ligand. In either case, ligand binding activates kinase pathways that affect gene transcription and can also elicit acute effects on neuronal function.
In addition to receptor-mediated signaling, cells also signal via nitric oxide (NO). NO is an important intercellular mediator and is produced by many cells that have close physical association with neurons both in the periphery and within the spinal cord. NO is formed from l -arginine following activation of the enzyme nitric oxide synthase (NOS) by calcium and other co-factors, including calmodulin. Three forms of NOS have been identified: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS), each with a distinct physiological role. nNOS and eNOS are both Ca 2+ /calmodulin dependent and are present in both the spinal cord and brain, whereas iNOS is functionally Ca 2+ independent and normally expressed in macrophages, inflammatory cells, and glia (for review see ). NO diffuses to its site of action, where it stimulates guanylate cyclase to produce cyclic guanosine monophosphate (cGMP). In turn, cGMP modifies intracellular processes, including activation of protein kinases, ion channels, and phosphodiesterases. NO can also act in other ways, for example, by activating cyclooxygenase (COX) enzymes and by S -nitrosylation of proteins ( ).
Sensory nerves are activated and sensitized by inflammatory mediators in several ways (see Fig. 3-1 B). Some mediators directly activate cation channels and thus depolarize neurons toward the voltage for initiation of an action potential. Other receptors activate intracellular pathways and influence neuronal sensitivity and excitability indirectly. These mechanisms include GPCR-mediated production of the second-messenger molecules NO, COX, and lipoxygenase products of arachidonic acid. Phosphorylation or dephosphorylation of membrane proteins often regulates the transduction and transmission of sensory signals ( Fig. 3-1 C), and this can occur via PKA-, PKC-, mitogen-activated protein kinase (MAPK)-, or phosphatidylinositol-3′-kinase/Akt-mediated phosphorylation or by dephosphorylation via protein phosphatases such as calcineurin. In addition to phosphorylation, some of the mediators that act on nociceptors can stimulate biochemical processes such as methylation and lipid modification of proteins, and these pathways may be important in nociceptive neurons.
In general, the effect of sensitization is to increase the probability that a given stimulus (ligand or voltage) will activate the target receptor or ion channel or increase the probability that the neuron will be excited. Protein phosphorylation is a well-known mechanism for controlling the activity of ion channels. For example, activity of the heat-sensitive ion channel TRPV1 is modified by both PKC- and PKA-mediated phosphorylation ( ), and the level of membrane expression is regulated by src-mediated phosphorylation ( ). Control of transduction channel activity can also be regulated by hydrolysis of PIP 2 and removal of the tonic inhibition caused by PIP 2 binding to the ion channel (see, e.g., ). Ion channels that control the excitability and firing frequency of sensory neurons are also substrates for regulation by PIP 2 ( ) and phosphorylation ( ).
Neuronal sensitization can occur through changes in the level of protein expression, either by transcriptional control altering the production of proteins or by changing the trafficking such that an altered amount of the protein is functionally expressed. Transcriptional control is an important long-term mechanism underlying the effects of neurotrophin receptor activation. In some cases, sensitization has been associated with the de novo expression of molecules important for nociception in neurons that do not normally express the protein ( ).
There is a considerable body of evidence that kinins contribute to the pathophysiological processes accompanying both acute and chronic inflammation. Bradykinin and the related peptide kallidin (Lys 0 -bradykinin) are formed from kininogen precursor proteins following the activation of plasma or tissue kallikrein enzymes during inflammation, tissue damage, or anoxia. The activity of these kinins is terminated by several degradative enzymes. Kininase I liberates the biologically active metabolites des-Arg 9 -bradykinin and des-Arg 10 -kallidin, whereas kininase II and endopeptidases form inactive metabolites ( ). The biologically active kinins activate two distinct types of G protein–linked receptors. Bradykinin and kallidin act preferentially at the B 2 receptor, whereas des-Arg 9 -bradykinin and des-Arg 10 -kallidin act with much higher affinity at the B 1 receptor than at the B 2 receptor.
B 2 receptors are expressed constitutively on a wide range of cell types, including nociceptive sensory nerves, and administration of bradykinin evokes pain and sensitizes polymodal nociceptors (see ). Bradykinin acts directly on sensory nerves and can also act indirectly by evoking the release of other inflammatory mediators from non-neuronal cells. There is good pharmacological evidence that the acute and some of the long-term effects of bradykinin are mediated via the B 2 receptor. For example, peptide and non-peptide B 2 receptor antagonists have analgesic and anti-hyperalgesic actions in animal models of inflammatory pain ( ; ; ; ; ; ), as well as in some neuropathic pain models ( ). Interestingly, thermal hypersensitivity is still evoked by complete Freund’s adjuvant (CFA)-induced inflammation in mice lacking the B 2 receptor ( ), but carrageenan-evoked thermal hypersensitivity is reduced ( ).
In contrast to B 2 receptors, B 1 receptors are not normally expressed at significant levels in normal tissue, except in some vascular beds, but their expression is induced by tissue injury and infection. This up-regulation of B 1 receptors requires de novo protein synthesis ( , DeBlois et al 1991), and there is evidence that the induction is stimulated by the release of cytokines such as IL-1β and TNF-α from immunocompetent cells in the damaged tissue ( ). Some effects of B 1 agonists are mediated via non-neuronal cells, where activation of the B 1 receptor evokes the release of PGE 2 and PGI 2 , NO, and various cytokines ( ). There is also immunocytochemical and autoradiographic evidence that the B 1 receptor is expressed in a subset of sensory neurons ( ) and that the level of expression is increased during inflammation ( ). The mechanisms regulating expression of the B 1 receptor in sensory neurons are not well understood but are likely to involve cytokines, as found in other cell types, and neurotrophins. Functional expression of sensory neuron B 1 receptors is up-regulated by exposure to the neurotrophins GDNF and neurturin. Under such conditions, B 1 receptor activation evokes sustained enhancement of the heat-gated current mediated by TRPV1 ( ).
There is good pharmacological evidence that B 1 receptors have an important role in the hyperalgesia associated with persistent inflammation. Although B 1 agonists do not normally affect nociceptive thresholds in animals, they evoke hyperalgesia following inflammation ( ). Furthermore, peptide B 1 antagonists such as des-Arg 10 -HOE140 and des-Arg 8 Leu 9 -bradykinin ( ), as well as non-peptide B 1 antagonists ( ), inhibit thermal or mechanical hyperalgesia in models of joint, paw, or tail inflammation. These data are consistent with the finding that mice lacking the B 1 receptor show reduced thermal ( ) and mechanical ( ) hyperalgesia after CFA treatment.
The relative importance of the changes in subtypes of bradykinin receptors is variable and depends on the inflammatory condition, with evidence of a shift toward a dominant role of B 1 receptors in chronic conditions in which B 1 receptor expression is up-regulated (see, e.g., ). Although many studies have focused on the peripheral role of kinin receptors, there is also evidence from studies involving selective antagonists and knockout mice that B 1 and B 2 receptors expressed in the spinal cord influence spinal processing of nociceptive signals in inflammatory conditions ( ; ).
B 1 and B 2 receptors couple through G q α to stimulate PLC, which results in phosphoinositide hydrolysis, DAG production, and mobilization of intracellular Ca 2+ from intracellular stores. They can also act through G i α to inhibit adenylate cyclase and stimulate the MAPK pathways ( ). A significant body of evidence supports the idea that bradykinin activates sensory neurons via a DAG–PKC pathway. Bradykinin causes the translocation of a specific PKC isoform, PKC-ϵ, from the cytoplasm to the plasma membrane of dorsal root ganglion (DRG) neurons ( ), and the excitatory effects of bradykinin are inhibited by the PKC inhibitor staurosporine ( ), which also attenuates the responses of skin afferents ( ). Furthermore, the bradykinin responses of many, but not all, neurons are reduced or abolished when PKC activity is down-regulated by prolonged exposure to phorbol esters ( ).
PKC activators depolarize sensory neurons by opening a cation-permeable ion channel ( ), and several pieces of information indicate that bradykinin exerts its effects, in part, by sensitizing or opening the heat-sensitive TRPV1 ion channel. Bradykinin activates ion channels in DRG neurons with properties similar to those of TRPV1 channels ( ); this agonistic effect requires the presence of PKC-ϵ and is blocked by PKC inhibitors ( ). Bradykinin also increases the capsaicin sensitivity of TRPV1 and reduces the temperature threshold for activation from approximately 42°C toward or below normal body temperature via a PKC mechanism ( ).
Activation of TRPV1 cannot explain all the excitatory effects of bradykinin inasmuch as activation of vagal and visceral afferents by bradykinin is retained in TRPV1 knockout mice ( ) and bradykinin can stimulate DRG neurons from TRPV1 −/− mice ( ). Bradykinin can also act via PLC to activate TRPA1 ( ), and bradykinin-evoked responses were significantly attenuated in sensory neurons from both TRPV1 and TRPA1 knockout mice ( ). One possibility is that TRPV1 and TRPA1 act in concert. In this scenario ( ), activation of PLC evokes TRPV1 gating and calcium influx. Because TRPA1 is often co-expressed with TRPV1 and because TRPA1 can be activated by increases in the intracellular calcium concentration ( , Zuborg et al 2007), a small calcium influx through TRPV1 may activate TRPA1.
Failure to inhibit bradykinin responses in all sensory neurons with staurosporine or prolonged exposure to phorbol esters ( ) suggests that excitation can be mediated by a PKC-independent mechanism. Other evidence points to different phospholipase-linked mechanisms resulting in activation of TRPV1. One proposal is that binding of PIP 2 to TRPV1 inhibits channel activity ( ) and its hydrolysis by B 2 receptor–mediated activation of PLC potentiates channel opening by removing this tonic inhibition ( ). However, the inhibitory influence of PIP 2 on TRPV1 has been challenged, and there is evidence that PIP 2 binding potentiates rather than inhibits TRPV1 ( ). Phosphoinositide binding may have both inhibitory and potentiating effects on TRPV1, depending on the level of stimulation ( ).
B 2 receptor activation also stimulates the 12-lipoxygenase pathway and leads to the production of endogenous TRPV1 agonists (e.g., 12-hydroperoxyarachidonate [HPETE] and leukotriene B 4 [LTB 4 ]. Bradykinin-evoked activation of TRPV1-like currents, neuronal firing, and behavioral responses are blocked by lipoxygenase inhibitors, consistent with a contribution of this pathway ( ). Other data point to a role of COX products since the COX inhibitor flurbiprofen inhibits the heat sensitization induced by bradykinin in a skin–nerve preparation ( ).
Two other ionic mechanisms have recently been proposed for bradykinin-evoked activation of DRG neurons. Depolarization resulting from inhibition of M-type potassium currents and activation of a calcium-activated chloride current, encoded by TMEM16A, have been proposed as important PLC-linked mechanisms for the excitatory actions of bradykinin ( ).
The enzymatic breakdown of arachidonic acid yields a variety of bioactive lipid molecules that have diverse physiological roles, including important actions in inflammation and pain. These molecules are not stored but are synthesized de novo from membrane lipids. The first step is release of arachidonic acid from phospholipids by the action of PLA 2 enzymes. Arachidonic acid is then metabolized to prostaglandins via the COX enzymes; to leukotrienes, 5-HPETE, and 5-hydroxyeicosatetraenoic acid (HETE) via 5-lipoxygenase; to 12-HPETE and 12-HETE via 12-lipoxygenase; to lipoxins via 15-lipoxygenase; and to epoxyeicosatetraenoic acids via the action of cytochrome P450.
Non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit COX enzymes, are the most widely used and effective drugs for the clinical treatment of inflammatory pain and hyperalgesia. NSAIDs have no obvious effect on normal pain thresholds but attenuate the abnormal pain responses in inflammatory conditions. Two COX enzymes, COX-1 and COX-2, are responsible for the first steps in prostaglandin synthesis. These enzymes have two catalytic enzymatic activities: a COX activity responsible for the production of PGG 2 from arachidonic acid and a peroxidase activity that reduces PGG 2 to form PGH 2 , the first steps in prostanoid biosynthesis.
In general, COX-1 is considered to have a “housekeeping” role in almost all tissues mediating physiological responses. In contrast, COX-2 is not constitutively expressed (except in the kidney, vas deferens, and importantly, the brain) but is induced in inflammatory conditions. In the periphery, COX-2 expression is induced in cells involved in inflammation (macrophages, monocytes, and synoviocytes) and is primarily responsible for synthesis of the prostaglandins involved in acute and chronic inflammatory states. COX-2 expression is induced in peripheral tissues in animal models of arthritis, and up-regulated expression is seen in human rheumatoid arthritic joints, although relatively little expression has been noted in human osteoarthritic joints. Both COX-1 and COX-2 are expressed constitutively in DRG neurons and in the spinal cord. Normally, COX-1 is expressed in small and medium-sized DRG neurons and in neurons and astrocytes in the spinal cord. Enzyme expression in both neuronal and non-neuronal cells in the spinal cord is up-regulated after peripheral inflammation and nerve injury (see ), and intraspinal release of PGE 2 is enhanced during peripheral inflammation ( ).
The important roles of spinal cord COX enzymes are not discussed in detail here but are covered in Chapter 28 . The available information indicates that COX inhibition at both peripheral and central sites can contribute to the anti-hyperalgesic effects, with the predominant clinical effect being mediated centrally. Certainly, prostaglandins produced in the periphery after tissue injury can sensitize peripheral nerves and induce hyperalgesia in animal models of inflammation, thus suggesting that a component of hyperalgesia could be due to a peripheral action. However, the finding that intrathecal administration of COX-2–selective inhibitors suppresses experimentally induced inflammatory hyperalgesia also argues for a central site of action ( ). The observations that COX-2 inhibitors have clinical efficacy similar to that of non-selective NSAIDs and that COX-2 inhibitors exert a rapid effect after surgery also argue that they act in these conditions at central sites where COX-2 is constitutively expressed.
PGH 2 is metabolized by different prostaglandin synthetases to a range of prostaglandins. Prostaglandins such as PGE 2 , PGD 2 , and PGI 2 are produced during inflammation and act with some specificity on different prostanoid receptors, termed EP, DP, and IP, respectively. Each of the prostanoid receptors has distinct coupling to G proteins, and the pattern of coupling determines the biochemical consequence of receptor activation. Four major types of EP receptors (EP 1–4 ) have been described, and splice variants of the EP 3 subclass have also been identified, which probably explains the multiplicity of transduction pathways that have been associated with this receptor. In situ hybridization studies have shown the presence of mRNA for IP, EP 1 , EP 3 , and EP 4 receptors in DRG neurons. About half the neurons express EP 3 receptor mRNA; 40%, IP mRNA; 30%, EP 1 mRNA; and 20%, EP 4 mRNA, with some degree of co-expression ( ). Of these, EP 1 , EP 4 , IP, and some splice variants of EP 3 receptors (EP 3B and EP 3C ) couple positively via G s to stimulate adenylate cyclase and raise cAMP levels.
A major peripheral effect of PGE 2 and PGI 2 is to sensitize afferent neurons to noxious chemical, thermal, and mechanical stimuli (see, for example, ). In contrast, PGD 2 shows little or no such activity ( ). The importance of these receptor subtypes in the periphery is confirmed by the findings that EP 3 −/− and IP −/− mice show reduced hyperalgesia after lipopolysaccharide (LPS) administration ( ). In contrast, intrathecal administration of PGE 2 induced normal mechanical allodynia in wild-type and EP 3 −/− mice but not in EP 1 −/− mice, thus illustrating that the EP 1 receptor plays a role in prostaglandin-induced spinal sensitization ( ).
The potential role of lipoxygenase products in inflammatory pain is less clear, and although the levels are increased in inflammatory conditions, evidence of a direct role in nociception is lacking. The major effect of these lipids is to recruit immune cells and alter microvascular permeability. Intradermal injection of LTB 4 or 8R,15S-diHETE decreases mechanical and thermal thresholds in rats ( ; ; ) and humans ( ), and LTB 4 sensitizes dental afferents ( ). The sensitizing actions of LTB 4 require the presence of polymorphonuclear (PMN) leukocytes and are thus likely to be indirect ( ). 8R,15S-diHETE reduces the thermal and mechanical thresholds of C fibers ( ) and excites some C-fiber neuromas ( ). A role of LTB 4 in experimental antigen (ovalbumin)-induced mechanical hyperalgesia has been shown by using the LTB 4 antagonist CP10596 ( ). More recently, the cysteinyl-leukotriene receptor CysLT2 was found to be expressed in about 40% of rat DRG neurons, preferentially in small-diameter neurons. Intraplantar administration of the CysLT2 agonist LTC 4 strongly enhanced the nocifensive response evoked by the P2X 3 agonist αβ-me-ATP but was without effect on thermal sensitivity, thus suggesting a lack of effect on TRPV1 channels ( ).
One probable action for some lipoxygenase products is to activate TRPV1 channels inasmuch as 12S-HPETE, 15S-HPETE, 5S-HETE, and LTB 4 all open TRPV1 channels in DRG neurons ( ). The behavioral effects of 8R,15S-diHETE noted earlier are unlikely to be due to such an action since this lipid shows very weak agonist effects on TRPV1.
In addition to the leukotrienes, lipoxygenases can also convert eicosapentaenoic and docosahexaenoic acids into active signaling molecules. Formation of some of these metabolites requires the sequential action of COX-2 or cytochrome P450 followed by lipoxygenase-mediated oxidation ( ). The resulting molecules have been named resolvins because of the roles that they are thought to play in the resolution phase of inflammation, and they have attracted interest for their analgesic potential ( ). The resolvins RvE1 and RvD1 potently reduce thermal and mechanical hypersensitivity in inflammatory pain models. Resolvins produce these effects by stimulating G i/o -coupled GPCRs located both on DRG neurons and in the spinal cord, thereby effectively inhibiting the activity of the sensory neuron ion channels TRPA1 and TRPV1, as well as C-fiber evoked long-term potentiation in the spinal cord ( ).
Linoleic acid is converted into several hydroxyl and carbonyl derivatives (9-HODE, 13-HODE, 9-oxoODE, and 13-oxoODE) by both lipoxygenase pathways and non-enzymatic lipid peroxidation reactions. In experimental situations, formation of these mediators is increased by depolarization of the spinal cord with a high-K + solution ( ). Extended exposure to heat also significantly increases the tissue concentration of these oxidized linoleic acid metabolites in mouse skin biopsy samples. Application of 9-HODE to cultured trigeminal neurons stimulates TRPV1, and administration in vivo evokes nocifensive behavior and thermal hypersensitivity, which is absent in Trpv1 − / − mice, thereby demonstrating that TRPV1 mediates the nociceptive effect of 9-HODE (Patwardhan et al 2010). Thus, oxidized linoleic acid metabolites, such as the endocannabinoid anandamide and several lipoxygenase products formed from arachidonic acid, can act as direct TRPV1 agonists ( ).
During conditions characterized by oxidative stress, such as inflammation or reperfusion after ischemia, a range of lipid peroxidation products are formed in reactions between free radicals and membrane lipids. Many of the lipids formed are well-known reactive, electrophilic molecules that bind covalently to proteins such as hydroxynenonal, cyclopentenone prostaglandins, isoprostanes, and related species. The covalent modification of redox-sensitive transcription factors initiates specific signaling cascades that may act to modify or protect against oxidative conditions, but the electrophilic lipids also stimulate nociceptive sensory neurons directly by activating TRPA1 ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here