Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nociceptors are a specialized class of primary afferents that respond to intense, noxious stimuli. Unmyelinated nociceptors signal the burning pain from intense heat stimuli applied to the glabrous skin of the hand, as well as the pain from sustained pressure. Myelinated nociceptors signal the sharp pain from heat stimuli applied to hairy skin and from sharp mechanical stimuli. Both myelinated and unmyelinated nociceptors signal pain from chemical stimuli. Following a cutaneous injury, enhanced pain in response to cutaneous stimuli, called hyperalgesia , develops at the site of injury (primary hyperalgesia) and in the surrounding uninjured skin (secondary hyperalgesia). Tissue injury leads to enhanced responsiveness of nociceptors, called sensitization , which accounts for primary hyperalgesia. This sensitization is due to the local release of inflammatory mediators. Secondary hyperalgesia is due to sensitization of neurons in the central nervous system. When nerves are severed, spontaneous activity and ectopic mechanical, thermal, and chemical sensitivity develop in the injured nociceptors. The properties of nearby, uninjured nociceptors are also changed. In both injured and uninjured nociceptors, responsiveness to adrenergic agents can develop, which may account for involvement of the sympathetic nervous system in certain forms of neuropathic pain.
One of the vital functions of the nervous system is to provide information about the occurrence or threat of injury. The sensation of pain, by its inherent aversive nature, contributes to this function. In this chapter we consider the peripheral neural apparatus that responds to noxious (injurious or potentially injurious) stimuli and thus provides a signal to alert the organism to potential injury. Investigators have studied cutaneous sensibility by recording from single nerve fibers in different species, including humans. Stimuli are applied to the receptive field (i.e., area of the tissue responsive to the applied stimulus) of single fibers, and the characteristics of the neural response are noted. We concentrate on the skin for three reasons. First, sensory receptors in the skin have been more thoroughly studied than receptors in any other tissue. Second, the opportunity to perform correlative psychophysical studies in animals and humans allows powerful inferences to be made regarding function. Third, cutaneous pain sensation is of great clinical significance. Diseases such as post-herpetic neuralgia and others associated with small-fiber neuropathies have profound effects on cutaneous sensory function and often lead to severe pain.
Highly specialized sensory fibers, alone or in concert with other specialized fibers, provide information to the central nervous system (CNS) not only about the environment but also about the state of the organism itself. In the case of the sensory capacity of the skin, cutaneous stimuli may evoke a sense of cooling, warmth, or touch. Accordingly, certain sensory fibers are selectively sensitive to these stimuli. Warm fibers, which are predominately unmyelinated, are exquisitely sensitive to gentle warming of their punctate receptive fields. These fibers have been shown to exclusively signal the quality and intensity of the warmth sensation ( ). Similarly, a subpopulation of the thinly myelinated, Aδ fibers respond selectively to gentle cooling stimuli and encode the sense of cooling ( ). For the sense of touch, different classes of mechanoreceptive afferent fibers are exquisitely sensitive to deformations of the skin. These low-threshold mechanoreceptors encode such features as texture and shape.
A relatively high threshold for an adequate stimulus distinguishes the remaining class of cutaneous receptors. Because these receptors respond preferentially to noxious stimuli, they are termed nociceptors ( ). Among the many varieties of sensory receptors, nociceptors are distinctive in that they typically respond to the multiple energy forms that produce injury (thermal, mechanical, and chemical stimuli) and provide information to the CNS regarding the location and intensity of noxious stimuli. Nociceptors may be subclassified with respect to four criteria: (1) unmyelinated C-fiber afferents (conduction velocity <2 m/sec) versus myelinated A-fiber afferents (conduction velocity >2 m/sec), (2) modalities of stimulation that evoke a response, (3) response characteristics, and (4) distinctive chemical markers (e.g., receptors expressed on the membrane). We first consider the properties of cutaneous nociceptors and then review how their function is thought to relate to the sensation of pain.
Tissue damage results in a cascade of events that lead to enhanced pain in response to natural stimuli, termed hyperalgesia . A corresponding increase in the responsiveness of nociceptors, called sensitization , occurs. The characteristics of hyperalgesia and its neurophysiological counterpart sensitization are discussed in a later section. Finally, we consider how nociceptors may play a role in accounting for the often severe pain that accompanies nervous system injury and disease.
Nature might have designed nociceptors such that each had the capacity to respond to the full complement of stimulus energy forms that pose potential risks to the organism (thermal, mechanical, and chemical). What nature has adopted instead is a mixed strategy whereby many nociceptors respond to multiple stimulus modalities (polymodal) and others have more specialized response properties. These specialized response properties probably at least in part account for different aspects of nociceptive sensory function (e.g., burning, aching, pricking, prickle, itch). As delineated later, nociceptors have distal effector functions as well, and specialization may also play a role here. The end result is that nociceptors have a complex biology and heterogeneous properties.
The receptive field of a nociceptor is often first localized by use of mechanical stimuli. Various other stimulus modalities are then applied to this receptive field. In most early studies of nociceptors, only heat and mechanical stimuli were used to study nociceptors. Therefore, the nomenclature of CMH and AMH is often used to refer to C-fiber mechano-heat–sensitive nociceptors and A-fiber mechano-heat–sensitive nociceptors, respectively. If a fiber responds to heat and mechanical stimuli, the fiber will in most cases respond to chemical stimuli as well ( ). Thus, CMHs and AMHs may also be referred to as polymodal nociceptors.
The issue of whether a given nociceptor responds to a particular stimulus modality is perilous because the presumed lack of response to a given modality may in fact represent failure to apply the stimulus with sufficient intensity. The problem with the application of high-intensity stimuli is that the stimulus may alter the properties of the nociceptor in an enduring manner. A selection bias occurs: nociceptors with lower thresholds are more likely to be studied. The easiest way to find a nociceptor for electrophysiological study is to apply squeezing (mechanical) stimuli to the skin and thus identify the receptive field. This selection process identifies what are termed mechanically sensitive afferents (MSAs). In time it has become apparent that selection bias from this approach has led to oversight of an important class of nociceptors: mechanically insensitive afferents (MIAs). Because these fibers by definition have high mechanical thresholds (or are unresponsive to mechanical stimuli), finding the mechanical receptive field of these fibers is difficult. An alternative technique described by has been to apply electrical stimuli to the skin to identify the putative receptive field. With this technique it turns out that about half of the Aδ-fiber nociceptors and 30% of the C-fiber nociceptors are MIAs, with MIAs being defined as afferents that have very high mechanical thresholds (>6 bar = 600 kPa = 60 g/mm 2 ) or are unresponsive to mechanical stimuli ( ). MIAs have also been reported in the knee joint ( ), viscera ( ), and cornea ( ). As will be seen, this MIA–MSA distinction is of significance with regard to distinguishing nociceptor types. From the perspective of nomenclature, it is well to emphasize that MIAs are not defined as fibers that have no response to mechanical stimuli but rather as fibers that have a very high threshold (or no sensitivity at all) such that demonstration of a response to mechanical stimuli in electrophysiological studies is difficult.
CMHs are commonly encountered cutaneous afferents, and activity of sufficient magnitude in these fibers is thought to evoke a burning pain sensation. The size of the receptive field appears to scale with the size of the animal. Typical values for monkey are between 15 and 20 mm 2 ( ), and for human they are near 100 mm 2 ( ). There are often discrete areas of mechanical sensitivity (hot spots) within the receptive field, but in many fibers the areas of mechanical responsiveness tend to fuse over the region of the receptive field. Most CMHs respond to chemical stimuli (though not as well as A-fiber nociceptors; ) and can therefore be considered polymodal.
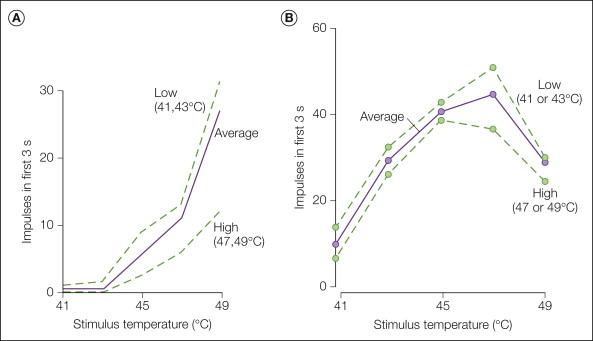
Responses to heat stimuli have been studied in considerable detail. The response of a typical CMH to a random sequence of heat stimuli ranging from 41–49°C is shown in Figure 1-1 A. It can be seen that the response increases monotonically with stimulus intensity over this temperature range, which encompasses the pain threshold in humans. One ion channel involved in the transduction of heat at nerve terminals is thought to be the neuronal transient receptor potential ion channel V1 (TRPV1); activity in this channel increases with increasing temperature ( ). A detailed description of the neuronal ion channels involved in stimulus transduction is presented in Chapter 2 (for review see ). Signal transduction molecules on keratinocytes may also play a role in heat transduction by inducing the release of adenosine triphosphate (ATP), which activates purinergic receptors (P2X 3 and P2Y 2 ) on the free nerve endings (see Fig. 1-4 ).
Two types of heat response are observed following a stepped heat stimulus. Quick C (QC) fibers exhibit their peak discharge during the rising phase of the heat stimulus, whereas slow C (SC) fibers exhibit their peak discharge during the plateau phase ( Fig. 1-2 B). The heat thresholds ( Fig. 1-2 C) and mechanical thresholds of QC fibers are significantly lower than those of SC fibers, thus suggesting that they may be located more superficially in the epidermis. QC fibers respond more vigorously to pruritic stimuli than do SC fibers, which suggests that they may be important in itch sensations ( ).
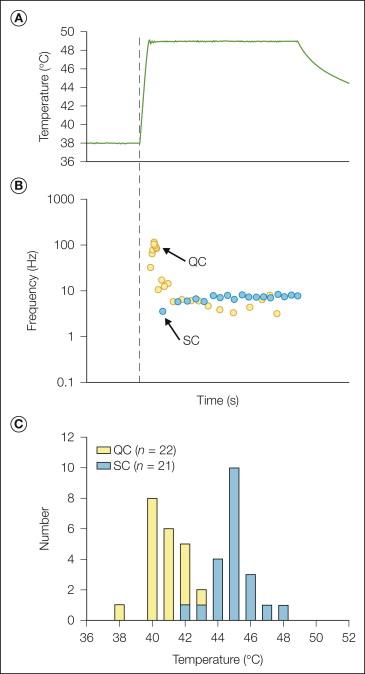
Thermal modeling studies combined with electrophysiological analysis have indicated that (1) the heat threshold of CMHs depends on the temperature at the depth of the receptor and not the rate of increase in temperature, (2) transduction of heat stimuli (conversion of heat energy to action potentials) occurs at different skin depths for different CMHs ( ), and (3) suprathreshold responses of CMHs vary directly with the rate of increase in temperature ( ; ). The depth of the heat-responsive terminals of CMHs varies quite widely (ranging from 20–570 μm; ). When a stepped temperature stimulus is applied to the skin, the temperature increases in the subsurface levels more slowly because of thermal inertia. The disparity in the surface temperature and the temperature at the level of the receptor varies directly with depth and indirectly with time. Given that the depth of CMH terminals varies widely, true heat thresholds are obtained when the rate of increase in temperature is very gradual or when the duration of the stimulus is very long. Although the literature reflects a wide range of heat thresholds for CMHs, when tested with these types of heat stimuli, the heat threshold of the majority of CMHs is in a remarkably narrow range of 39–41°C ( ).
The response of CMHs is also strongly influenced by the stimulus history. Both fatigue and sensitization are observed. One example of fatigue is the observation that the response to the second of two identical heat stimuli is substantially less than the response to the first stimulus. This fatigue is dependent on the time between stimuli, with full recovery taking longer than 10 minutes. A similar reduction in the intensity of pain after repeated heat stimuli is observed in human subjects ( ). Fatigue is also apparent in Figure 1-1 A, where the response to a given stimulus varied inversely with the intensity of the preceding stimulus. A decrease in the response to heat is also observed following mechanical stimuli applied to the receptive field or electrical stimuli applied to the nerve trunk ( ). This suggests that fatigue in response to a given stimulus modality can be induced by heterologous stimulation, that is, by excitation with a stimulus of a different modality. Interestingly, recovery from cross-modal or heterologous fatigue is faster than recovery from fatigue induced by a stimulus of the same modality. Presumably, this is because these heterologous stimuli do not activate and therefore do not fatigue the stimulus transduction apparatus in the same way. Alternatively, fatigue may arise from independent effects on spike initiation (from antidromic stimulation) and transduction (from natural stimulation at the receptive field). Fatigue in response to heat stimuli is also seen in vitro when small (and presumably nociceptive) dorsal root ganglion (DRG) cells are repetitively tested with heat stimuli ( ). The enhanced response, or sensitization, that may occur in CMHs after tissue injury is described below in the section on hyperalgesia.
Responses to mechanical stimuli are covered in more detail later. Suffice it here to indicate that CMHs usually display a slowly adapting response to mechanical stimuli of a given force. As noted later, MSA CMHs have a graded response to punctate stimuli, but their stimulus–response functions become saturated at levels substantially below the threshold for pain.
C-fiber MIAs are heterogeneous with regard to responses to chemical and heat stimuli, and some respond only to mechanical stimuli (but of course with a very high mechanical threshold). The sensitivity to mechanical stimuli has no obvious correlation to the heat threshold ( ). In contrast to CMH afferents, some C-fiber MIAs in humans are vigorously excited when challenged with histamine or capsaicin. In addition, the activity observed in these C-fiber MIAs parallels the duration of the perception of itch (histamine) or burning pain (capsaicin) ( ). C-fiber MIAs may therefore act as chemosensors. In addition to pronounced chemosensitivity, these fibers have some other interesting properties that could account for pain in response to tonic pressure stimuli or the neurogenic flare response (see below).
Low-threshold C-fiber mechanoreceptors that do not respond to heat have been described in the cat ( ) and rabbit ( ). In primates, including humans, these fibers have been found in proximal areas of the body ( ) and the hairy skin on the forearm ( ). These afferents are strongly activated by innocuous mechanical stimuli moved slowly across the receptive field, but they also respond to pinprick stimuli. The neuronal activity in these fibers is not critical for the perception of touch and, according to one imaging study, leads to the activation of the insular but not the sensory cortex (Olausson et al 2003). Low-threshold C-fiber mechanoreceptors are thought to mediate the sensation of “pleasant” touch and may therefore play an important role in “affiliative” behavior ( ).
Some mechano-insensitive C fibers are reported to be activated by non-noxious and noxious cold and hot stimuli. It has been hypothesized that activity in these afferents may mediate the “hot–burning” sensations caused by such stimuli. These afferents may also be involved in mediating psychophysical phenomena such as “paradoxical heat” or the thermal grill illusion ( ).
C-fiber afferents differ not only in their receptive features but also in their conductive properties. In fact, their conductive and receptive properties appear to correlate. When unmyelinated C-fiber afferents are activated repetitively by electrical stimuli, their conduction latency increases gradually (i.e., the conduction velocity of the afferent decreases). In addition, with increasing stimulation frequency, the amount of this activity-dependent slowing increases. Slowing in C-fiber MIAs is greater than in C-fiber MSAs ( ), and mechanosensitive nociceptive afferents show more pronounced slowing than do cold-sensitive C fibers, low-threshold C fibers, or sympathetic efferent C fibers ( ). This difference in slowing properties indicates that the ion channels responsible for conduction may be different and suggests that the ion channels responsible for spike initiation at the receptive terminal may also differ between C-fiber classes.
A-fiber nociceptors are thought to evoke pricking pain, sharpness, and perhaps aching pain. As a general rule, A-fiber nociceptors do what C-fiber nociceptors do, but do it more robustly. They respond at higher discharge frequencies, and the discriminable information supplied to the CNS is greater (e.g., ).
Two types of A-fiber nociceptors are apparent ( ). A summary of their properties is presented in Table 1-1 . Type I fibers are typically responsive to heat, mechanical, and chemical stimuli and may therefore be referred to as AMHs or polymodal nociceptors. Because the heat thresholds are high with short-duration stimuli (typically >53°C), the responsiveness of these fibers to heat has in some studies been overlooked. Consequently, these fibers have been called high-threshold mechanoreceptors (HTMs) by many investigators (e.g., ). Heat sensitivity in type I fibers is most likely mediated by the vanilloid receptor–like protein 1 (VRL1, renamed TRPV2) since it has a similar high threshold for activation by heat and is expressed in neurons with small myelinated axons ( ). When heat thresholds are determined with long-duration temperature stimuli, however, thresholds are in the mid-40–50°C range ( ). Type I AMHs are seen in hairy and glabrous skin ( ) and have also been described in the cat and rabbit ( ). The mean conduction velocity of type I AMHs in the monkey is 25 m/sec and extends as high as 55 m/sec. Thus, by conduction velocity criteria, type I AMHs fall into a category between that of Aδ and Aβ fibers. Nearly all type I AMHs are MSAs. Their receptive field size is similar to that of CMHs, but the presence of “hot spots” in response to mechanical stimuli is much more obvious.
| CHARACTERISTIC | TYPE I | TYPE II |
|---|---|---|
| Heat threshold to short stimuli | High | Low |
| Heat threshold to long stimuli | Low | Low |
| Response to intense heat | Slowly increasing | Adapting |
| Response latency to intense heat | Long | Short |
| Peak latency to intense heat | Late | Early |
| Mechanical threshold | Most are MSAs | Most are MIAs |
| Conduction velocity | Aδ and Aβ fibers | Aδ fibers |
| Sensitization to heat injury | Yes | No |
| Location | Hairy and glabrous skin | Hairy skin |
Type II A-fiber nociceptors were encountered only infrequently in early studies. It turns out that this is because the thresholds to mechanical stimuli place the majority of these fibers in the MIA category. Many have no demonstrable response to mechanical stimuli. When an unbiased electrical search stimulus is used, however, the prevalence of type I and type II A-fiber nociceptors in the hairy skin of the primate is similar. They do not occur in the glabrous skin of the hand (where type I AMHs are prevalent). Their mean conduction velocity, 15 m/sec, is also lower than that of type I AMHs. Their responses to heat resemble those observed in CMHs, and they may also be mediated by the vanilloid receptor 1 (VR1 or TRPV1). Responses to endogenous inflammatory/algesic mediators resemble those seen with type I A-fiber nociceptors ( ).
Examples of the differing responses of the two types of A-fiber nociceptors to a heat stimulus are shown in Figure 1-3 . Type I fibers exhibit a distinctive, gradually increasing response to heat. They sensitize to burn and chemical injury and probably play a role in the development of hyperalgesia. Type II fibers respond to heat in similar fashion to CMHs: early peak frequency and a slowly adapting response ( ). As noted later, type II A-fiber nociceptors are thought to signal first pain sensation in response to heat and may also contribute to pain caused by the application of capsaicin to the skin ( ).
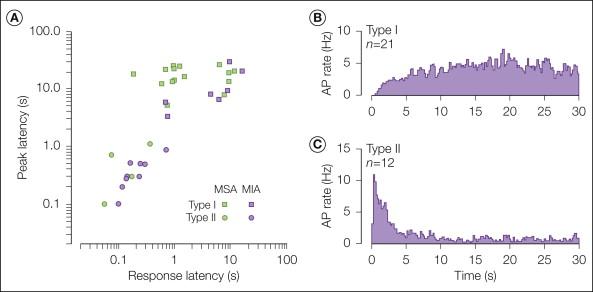
The conduction velocity of small myelinated Aδ fibers is, by definition, faster than that of unmyelinated C fibers. However, the terminal cutaneous branches of nociceptive Aδ fibers may conduct at a velocity characteristic of unmyelinated fibers (i.e., <2 m/sec) ( ). In addition, these unmyelinated terminals may branch off the main axon several centimeters proximal to their cutaneous receptive field.
The anatomical and biochemical features of nociceptive afferents have been studied extensively to correlate these features with their receptive properties. A wide range of cell markers have been used to classify nociceptive afferents and to study their peripheral and central projections. These markers include molecules expressed on the cell surface (e.g., receptors, glycoconjugates), molecules stored and released from nociceptive afferents (e.g., peptides), and enzymes. Expression of receptors for neurotrophic factors is of interest since these factors may regulate the sensitivity of nociceptive afferents in physiological and pathological states such as inflammation and neuropathy. The size of neuronal populations expressing or co-expressing different markers varies between species ( ) and changes with the developmental stage ( ). Inflammation of the innervated tissue or a peripheral nerve lesion can cause substantial changes in the expression of these molecules. With the ongoing discovery of new marker molecules and the refinement of histological techniques, classification of nociceptive afferents is undergoing constant change and revision. Despite these “challenges,” however, classification of nociceptive afferents based on the expression of biochemical markers is instructive inasmuch as certain different neuronal populations are distinguishable across species. Sophisticated genetic manipulations have allowed the peripheral and central projections of defined neuronal populations to be studied in great detail. In addition, ablation experiments have been used to study the role of defined neuronal populations in animal behavior.
The cell bodies of nociceptive somatic and visceral afferents are located in DRGs. Slowly conducting Aδ and C fibers, including nociceptors, have small cell bodies ( ). Some of these are labeled with an antibody directed against a neurofilament protein (NF200) and are therefore thought to correspond to the somata of small myelinated Aδ afferents.
Small DRG cells are subdivided into peptidergic neurons (i.e., neurons containing peptides such as substance P [SP], calcitonin gene–related peptide [CGRP], and somatostatin [SST]) and “non-peptidergic” neurons. In the rat, about 40% of DRG cells, 50% of C fibers, and 20% of Aδ fibers are classified as peptidergic ( ). Non-peptidergic, nociceptive neurons contain fluoride-resistant acid phosphatase (FRAP) ( ), and their somata and axons bind the plant isolectin B4 (IB4) from Griffonia simplicifolia ( ). It is common practice to classify neurons as “peptidergic” or “non-peptidergic” based on their binding of IB4. However, considerable co-localization of SP or CGRP and IB4 or FRAP has been reported in rats but less so in mice ( ). In vivo intracellular recordings combined with immunohistochemistry have shown that cells containing SP or CGRP or cells binding IB4 are nociceptive and that non-nociceptive cells do not label with these markers ( ; ).
A group of mas-related genes (Mrgs) have been discovered that are selectively expressed in small DRG neurons and encode G protein–coupled receptors (GPCRs) ( ). Independently, sensory neuron–specific GPCRs (so-called sensory neuron–specific receptors [SNSRs]) in which the encoding genes were identical to some of the previously described Mrgs were identified shortly thereafter ( ). For some Mrgs (MrgA–C) identified in mice, no ortholog genes exist in human or non-human primates, but closely related Mrgs (so-called MrgXs) have been identified. For other Mrgs (MrgD–G), however, ortholog genes exist in humans. Mrgs are expressed mainly in non-peptidergic, IB4-positive neurons, with some Mrgs being expressed in distinct IB4 subpopulations. In in vitro recordings, MrgD + DRG cells showed characteristics typical of nociceptors (e.g., broad action potentials, expression of tetrodotoxin [TTX]-resistant sodium channels) ( ). Receptors encoded by Mrgs respond to a variety of ligands, including β-alanine, cortistatin, peptides derived from different opioid precursors, and different RFamide peptides ( ), and they probably modulate excitability and sensitivity in this class of nociceptive afferents.
Expression of some markers appears to be related to the peripheral target tissue innervated by the neuron. Thus, almost all visceral afferents are peptidergic, but only about half the afferents projecting to the skin are (e.g., ) and only a small percentage of afferents projecting to muscle are labeled with IB4 ( ). MrgD-positive fibers exclusively innervate the skin, and they terminate in more superficial skin layers than do their peptidergic counterparts ( Fig. 1-4 ) ( ). Peptidergic and non-peptidergic afferents project to distinct dorsal horn laminae, with peptidergic fibers projecting mainly to lamina I and lamina II outer and IB4-binding afferents projecting preferentially to lamina II inner (e.g., ; but see also ).
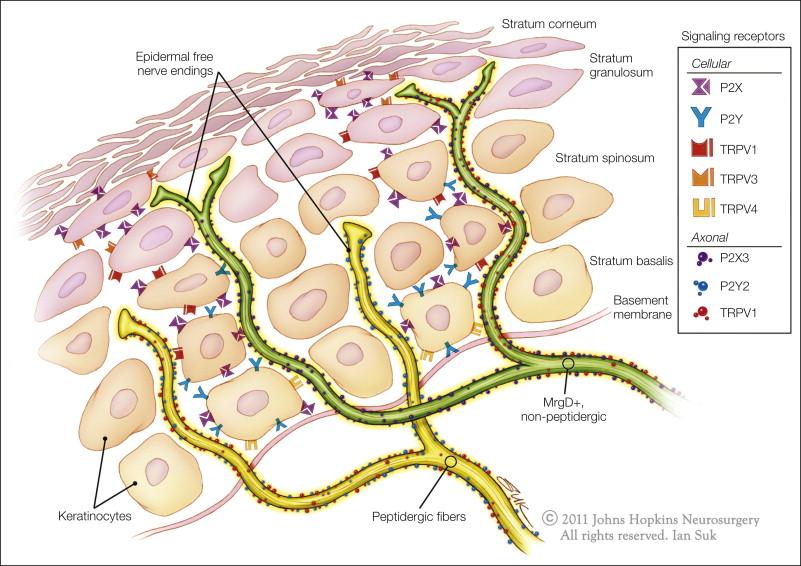
Although all nociceptive neurons depend on nerve growth factor (NGF) during early development, in the adult only peptidergic neurons express its receptor TrkA (tropomyosin-related kinase A) ( ). In contrast, most IB4-positive DRG cells do not express TrkA ( , but see also ) but express one of the glial-derived neurotrophic factor (GDNF) family receptors (GDNFRα1–4) together with receptor tyrosine kinase Ret ( ).
Peptidergic and non-peptidergic neurons express different receptors involved in signal transduction, and they may therefore display different sensitivity to a given stimulus. Thus the P2X 3 receptor, which mediates nociceptor excitation by ATP, is primarily expressed in IB4-positive neurons ( ). In contrast, TRPV1, which mediates responses to heat, capsaicin, and protons, is expressed in only a minority of IB4-positive cells in mice ( ). In rats, however, this segregation is less obvious since about half of both IB4-positive and -negative cells express TRPV1 ( ; ; ). Species differences also exist in the co-expression of different Mrgs and their co-expression with other markers of nociceptive neurons ( ).
Activation of one fiber by action potential activity in another is referred to as coupling . Coupling of action potential activity occurs between C fibers in the normal peripheral nerve of the monkey ( ). Coupling frequently involves conventional CMHs. Coupling is eliminated by injecting small amounts of local anesthetic at the receptive field of the CMH, thus indicating that the site of coupling is near the receptor. Collision studies indicate that the coupling is bidirectional. Sympathetic fibers do not appear to be involved in this coupling as demonstrated by experiments in which the sympathetic chain is stimulated or ablated ( ). The role of coupling is unknown but it may relate to the flare response or other efferent functions of nociceptors (see below). Coupling between peripheral nerve fibers is also one of the pathological changes associated with nerve injury (e.g., ). In this case, coupling occurs at the site of axotomy.
Immunostaining for protein gene product (PGP) 9.5, a carboxy-terminal ubiquitin hydrolase, has proved particularly sensitive in identifying small-diameter afferents in the skin ( ). Vertical sections reveal that epidermal axons emerge from the superficial dermal nerve plexuses running beneath the epidermis. Schwann cells encase the axons at the dermal level, but as the axons rise into the epidermis between keratinocytes, the Schwann cell encasements are lost ( ). Both clear round and large dense-core vesicles are noted at the epidermal penetration site. The vesicles are similar morphologically to vesicles present in other cells involved in hormone and neurotransmitter secretion. It is presumed that these vesicles secrete their contents into tissues on activation (see the efferent role of nociceptors below). Some of these fibers appear to innervate Langerhans cells. In small-fiber neuropathies in which patients have pain and deficits in cutaneous pain sensibility, these axonal terminals stained by PGP 9.5 are markedly decreased ( ).
As illustrated in Figure 1-4 , free nerve endings can be traced far into the epidermal layer. These free nerve endings are probably sensory and serve the sensations of pain, temperature, and itch. The parent axons of these unmyelinated terminals are probably both myelinated and unmyelinated. Some of these free nerve endings are peptidergic and contain SP or CGRP ( ). Others are non-peptidergic and reach into the superficial layers of the epidermis.
We now examine the evidence that CMHs signal pain. In glabrous skin of the hand, two types of fibers, CMHs (not AMHs) and warm fibers, respond to short-duration heat stimuli (≤5 seconds) at temperatures near the pain threshold in humans (i.e., around 45°C). It is of interest, therefore, to compare how warm fibers and CMHs encode information about noxious heat stimuli. Warm fibers respond vigorously to gentle warming of the skin and are thought to signal the sensation of warmth ( ). An example of the response of a warm fiber to stimuli in the noxious heat range is shown in Figure 1-1 B. The response of warm fibers is not monotonic over this temperature range. In the example shown in Figure 1-1 B, the total response evoked at 49°C was less than that at 45°C. Psychophysical studies in humans demonstrate that pain increases monotonically with stimulus intensities between 40 and 50°C. Because the responses of CMHs increase monotonically over this temperature range ( Fig. 1-1 A) and the responses of warm fibers do not ( Fig. 1-1 B), it follows that CMHs probably signal the sensation of heat pain to the glabrous skin of the hand ( ).
Other evidence in support of a role for CMHs in pain sensation includes the following: (1) human judgments of pain in response to stimuli over the range of 41–49°C correlate well with the activity of CMH nociceptors over this range ( Fig. 1-5 , ); (2) selective A-fiber ischemic blocks or C-fiber (local anesthetic) blocks indicate that C-fiber function is necessary for perception of thermal pain near the pain threshold ( ); (3) the stimulus interaction effects observed in psychophysical experiments ( ) are also observed in recordings from CMHs ( Fig. 1-1 A); (4) the latency to pain sensation on glabrous skin following stepped changes in temperature is long and consistent with input from CMHs ( ); and (5) in patients with congenital insensitivity to pain, microscopic examination of peripheral nerves indicates an absence of C fibers ( ).
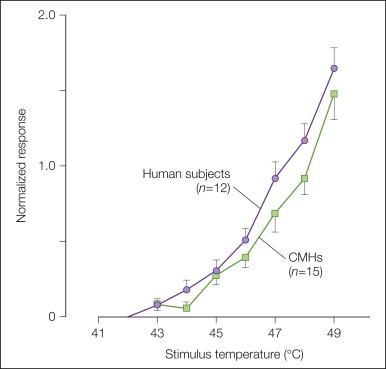
Microneurography has been used to record from nociceptive afferents in awake humans and allows correlations between the discharge of afferents and the reported sensations of the subject. The technique involves percutaneous insertion of a microelectrode into fascicles of nerves such as the superficial radial nerve at the wrist. These studies have demonstrated that the properties of nociceptors in humans and monkeys are similar. In some experiments the microelectrode is also used to stimulate an identified, single nerve fiber in awake human subjects to evoke specific sensations. Some, however, argue that the size of the stimulating electrode is too large to stimulate individual units ( ). Given this reservation, the following evidence from microneurographic studies in humans points to the capacity of CMH activity to evoke pain: (1) intraneural electrical stimulation of presumed single identified CMHs in humans elicits pain ( ), (2) the heat threshold for activation of CMHs recorded in awake humans is just below the pain threshold ( ), and (3) a linear relationship exists between responses of CMHs recorded in awake humans and ratings of pain over the temperature range 39–51°C ( ).
We noted above that the heat threshold of CMHs is dependent on temperature at the level of the receptor and is independent of the rate of change in temperature. At the same time when threshold temperature is measured at the surface of skin, CMHs have a lower threshold when the rate of increase in temperature is slow. As discussed earlier, the reason for this relates to thermal inertia.
Human pain thresholds are sometimes measured as the temperature that corresponds to the first report of pain as skin temperature is increased linearly (Marstock technique). Investigators have noted that faster rates of change in temperature lead to lower estimates of the heat pain threshold ( ). This is the opposite of the situation with the surface temperature threshold of CMHs but fits with the finding that suprathreshold responses of CMHs vary directly with the rate of increase in temperature. Thus it is unlikely that the threshold responses of CMHs are responsible for the heat pain thresholds. Rather, it appears that nociceptors must reach a certain discharge frequency (about 0.5 impulses/sec) for pain to be perceived ( ).
As shown in Figure 1-6 , a long-duration heat stimulus applied to the glabrous skin of the hand in human subjects evokes substantial pain for the duration of the stimulus. CMHs exhibit a prominent discharge during the early phase of the stimulus, but this response adapts within seconds to a low level. In contrast, type I AMHs are initially unresponsive but then discharge vigorously. Therefore, type I AMHs probably contribute to the pain during a sustained, high-intensity heat stimulus ( ).
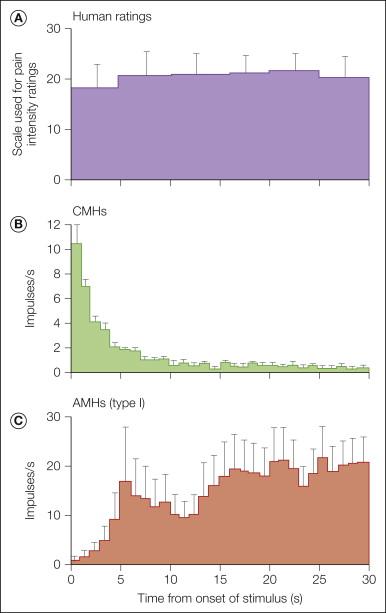
In hairy skin, stepped heat stimuli evoke a double pain sensation ( ). The first perception is a sharp pricking sensation, and the second sensation is a burning feeling that occurs after a momentary lull during which little if anything is felt. Myelinated afferent fibers must signal the first pain since the latency of response to the first pain is too small to be carried by C fibers ( ). Type II A-fiber nociceptors (see Fig. 1-3 ) are ideally suited to signal this first pain sensation: (1) the thermal threshold is near the threshold temperature for the first pain ( ), (2) the receptor utilization time (time between onset of the stimulus and activation of the receptor) is short ( ), and (3) the burst of activity at the onset of the heat stimulus is consistent with the perception of a momentary pricking sensation. The absence of a first pain sensation to heat stimuli applied to the glabrous skin of the human hand correlates with the failure to find type II A-fiber nociceptors on the glabrous skin of the hand in the monkey.
The preceding discussion indicates that nociceptors may signal pain in response to heat stimuli. However, two caveats are in order: (1) This does not mean that activity in nociceptors always signals pain. It is clear that low-level discharge rates in nociceptors do not always lead to sensation (e.g., ). Central mechanisms, including attentional and emotional states, quite obviously play a crucial role in whether and how much nociceptor activity leads to the perception of pain. (2) It is probable that receptors other than nociceptors signal pain in certain circumstances. For example, the pain in response to light touch that occurs after certain nerve injuries or with tissue injury appears to be signaled by activity in low-threshold mechanoreceptors (see below).
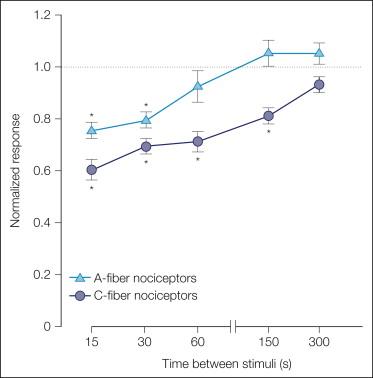
A-fiber and C-fiber MSAs respond well to punctate mechanical stimuli. When a controlled-force stimulus is applied to the receptive field, the response is greatest at the onset of the stimulus and then slowly adapts. Like heat, repeated presentations of a mechanical stimulus lead to pronounced fatigue. A-fiber nociceptors recover faster from fatigue than do C-fiber nociceptors ( Fig. 1-7 ).
Much has been learned about the features of a mechanical stimulus that determine the response of nociceptors to mechanical stimuli. The discharge of nociceptors increases with increased force and pressure, but these functions vary depending on probe size: the smaller the probe, the greater the response ( ). For cylindrical probes of different diameter, the discharges are comparable if the intensity of the stimulus is calculated according to force per length of the perimeter of the cylindrical probe. This suggests that the stress/strain maximum that occurs at the edge of the cylindrical stimulus is the critical parameter for excitation of nociceptor terminals.
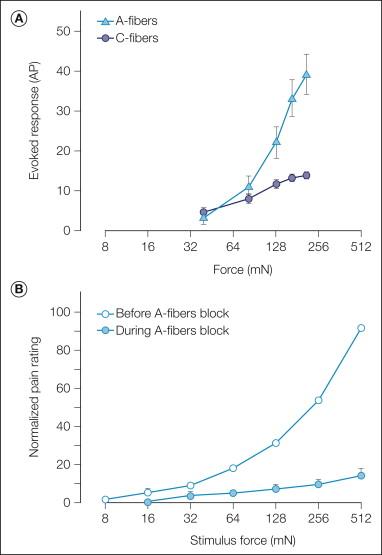
For a given probe size, the response of A-fiber nociceptors increases monotonically with force, whereas the response of C-fiber nociceptors becomes saturated at higher force levels ( Fig. 1-8 A; ). In general, the discharge in A fibers is greater than that in C fibers.
The area of the receptive field that responds to mechanical stimuli also responds to heat stimuli ( ). However, the transducer elements that account for mechanosensitivity are probably different from those responsible for heat. For example, the heat response of nociceptors is readily sensitized by a heat injury, whereas the mechanical response is not (see below).
A-fiber nociceptors appear to be responsible for the sharp pain reported in response to punctate mechanical stimuli: (1) the reaction time to perception of pain is short, (2) the stimulus–response function of A-fiber nociceptors ( Fig. 1-8 A) is comparable to the pain ratings of human subjects ( Fig. 1-8 B) over a similar force range, and (3) the pain in response to sharp probes is dramatically reduced during selective blockade of A-fiber function ( Fig. 1-8 B; ).
Pretreatment of the skin with capsaicin abolishes heat pain sensitivity but does not greatly affect mechanical pain ( ). This suggests that the A-fibers involved in sharp pain are capsaicin insensitive; they could be type I AMHs or HTMs.
When long-duration mechanical stimuli are applied to human subjects, the pain increases throughout the stimulus ( ). However, the response of MSAs to long-duration suprathreshold stimuli adapts with time. Although C-fiber MIAs are, by definition, normally insensitive to mechanical stimuli, they develop a response to prolonged mechanical stimulation ( ). In addition, the pain associated with a tonic stimulus persists through selective A-fiber blockade ( ). Thus it appears that C-fiber MIAs signal the pain associated with tonic pressure.
Cold pain differs from heat pain in a number of important factors: (1) the cold pain threshold (≈14°C on hairy skin; ) is much farther from resting skin temperature (33°C) than the heat pain threshold (about 45°C), (2) the slope of the stimulus–response function is much steeper for heat pain than for cold pain ( ), and (3) the lag in response between stimulus onset and pain report suggests that cold pain is subserved by deeper receptors whereas heat pain seems to be subserved by superficial receptors. demonstrated that cold pain could be evoked by cold stimuli applied within the veins of human subjects. A local anesthetic applied within the vein, but not in the overlying skin, abolished cold pain sensibility. It is therefore possible that cold pain is served, at least in part, by vascular receptors.
Just as the sensation of warmth is served by a specific set of primary afferents (predominantly C fibers), the sense of cooling is served by a specific set of primary afferents (i.e., cold fibers). Cold fibers are predominantly of the A type. They exhibit ongoing activity at room temperature, and their response increases markedly with gentle cooling. Stimuli that induce cold pain are not encoded well by these cold fibers. Although the majority of nociceptors have some response to ice stimuli applied to the skin, showed that all A-fiber nociceptors respond to cold stimuli below 0°C. C-fiber nociceptors may play a role in signaling cold pain sensation as well ( ). A non-selective cation channel has been identified (called ANKTM1 or transient receptor potential ankyrin 1 [TRPA1]) that has an activation threshold (17.5°C) comparable to the cold pain threshold ( ). This channel is found in a subset of nociceptive sensory neurons that are responsive to intense heat and capsaicin. However, the role of TRPA1 in mediating noxious cold is still debated.
Many chemical agents produce pain when applied to the skin. In many cases the pain from these agents probably results from tissue injury and is therefore indirect. (Chemical mediators associated with inflammation are described later.) One exception that has received a lot of attention is capsaicin. Intradermal injection of capsaicin produces intense burning pain that lasts for several minutes. When capsaicin is injected into the receptive field of C-fiber MSAs, the response is weak (relative to the heat response) and of short duration ( ). In contrast, A-fiber and C-fiber MIAs exhibit a long-lasting, vigorous response to capsaicin ( ), thus suggesting that these fibers are responsible for the pain induced by capsaicin. The pungent effects of capsaicin appear to be mediated by the TRPV1 receptor expressed on nociceptive fibers. This receptor appears to be activated by heat and protons (acid) as well.
Another chemical of interest is histamine, which produces a long-lasting itch when applied to the skin. Injection of histamine into the receptive field of C-fiber MSAs leads to a lasting response ( ). Iontophoresis of histamine into the receptive field of a subpopulation of C-fiber MIAs also produces a vigorous, long-lasting response ( ), which suggests that both CMHs and C-fiber MIAs may play a role in histamine-induced itch. Histamine probably activates nociceptors via the H 1 receptor located on peripheral terminals.
Because cowhage spicules produce an intense itch that is not blocked by topical antihistamines ( ), and they provide a useful tool to investigate the chronic itch in patients that is resistant to antihistamine treatment. In about half of normal subjects, cowhage-induced itch is greatly attenuated during selective blockade of myelinated fibers. Although C-fiber MIAs do not respond to cowhage, QC fibers and A-fiber nociceptors respond vigorously to cowhage ( ). The active ingredient in cowhage is the cysteine protease mucunain, which activates nociceptive terminals via protease-activated receptor 2 (PAR-2) and PAR-4 ( ).
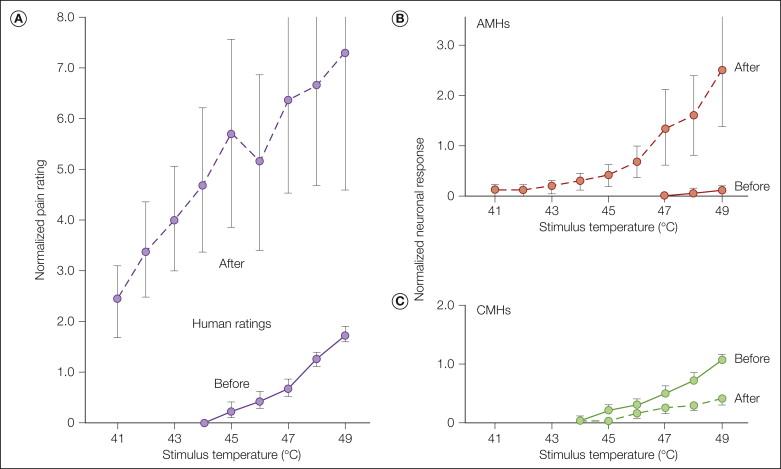
To understand the peripheral neural mechanisms of pain induced by noxious stimuli is to understand only one aspect of pain sensibility. There is, in fact, a dynamic plasticity that relates stimulus intensity and sensation. Of great biological importance in this regard is the phenomenon of hyperalgesia. Hyperalgesia is defined as a leftward shift of the stimulus–response function that relates the magnitude of pain to stimulus intensity. An example of this is seen in Figure 1-9 A, which shows human judgments of pain induced by heat stimuli before and after a burn. It is evident that the threshold for pain is lowered and pain in response to suprathreshold stimuli is enhanced.
Hyperalgesia is a consistent feature of somatic and visceral tissue injury and inflammation. Pharyngitis is associated with hyperalgesia in pharyngeal tissues such that merely swallowing induces pain. Micturition in the presence of a urinary tract infection is painful, again reflecting the presence of hyperalgesia. In arthritis, slight motion of the joint results in pain. A sunburn leads to pain with light touch and gentle heating.
The peripheral neural mechanisms of hyperalgesia have been studied in various tissues, including the joints, cornea, testicle, gastrointestinal tract, and bladder. Much of the theoretical work on hyperalgesia, however, has evolved from studies of the skin, and it is this work that will receive attention here.
Hyperalgesia occurs not only at the site of injury but also in the surrounding uninjured area. Hyperalgesia at the site of injury is termed primary hyperalgesia , whereas hyperalgesia in the uninjured skin surrounding the injury is termed secondary hyperalgesia ( ). Hyperalgesia exemplifies the functional plasticity of the nervous system. As we will see, the neural mechanisms for primary and secondary hyperalgesia differ.
In discussing hyperalgesia, it is useful to consider the following variables: (1) energy form of the injury, (2) type of tissue involved, (3) energy form of the test stimulus, and (4) location of the testing relative to the area injured. These variables interact in complex ways. For example, it will be shown that nociceptors will become sensitized to mechanical stimuli (the energy form of the test stimulus), but only after certain forms of injury (i.e., injection of inflammatory mediators).
An experimental design frequently used for study of the neural mechanisms of hyperalgesia is to characterize the response properties of a given fiber, then apply a manipulation that under usual circumstances would produce hyperalgesia, and finally assess whether this manipulation has altered the response properties of the fiber in question. Cutaneous hyperalgesia has been studied after thermal injury (burn or freeze lesions), after local administration of chemicals (e.g., capsaicin, mustard oil, or menthol), after a mechanical injury to the skin (e.g., incision, crushing), and after exposure to ultraviolet radiation. The main features of the hyperalgesia that develops after these various injuries are quite similar.
As shown in Figure 1-10 , the relative locations of the injury site, the test site, and the receptive field of the sensory neuron being studied dictate whether the experiment provides information regarding the mechanisms of primary or secondary hyperalgesia ( ). These three variables may interact in any of six ways. As shown in Figure 1-10 , when the injury and the test site coincide ( Fig. 1-10 A and B), the study has provided a basis by which to consider the mechanism of primary hyperalgesia, whereas when the test site and the injury site diverge ( Fig. 1-10C– F), the study has provided a basis by which to account for secondary hyperalgesia.
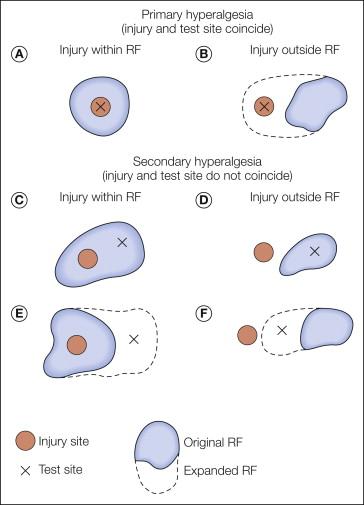
When the paradigms shown in Figure 1-10 A and B are used, it is found that under certain circumstances, nociceptors exhibit an increased response to the test stimulus. Thus, peripheral neural mechanisms are likely to account for at least some aspects of primary hyperalgesia. In contrast, primary afferent nociceptors do not develop an enhanced response to the test stimulus when the paradigms shown in Figure 1-10C– F are investigated. By default, therefore, the mechanism for secondary hyperalgesia must reside within the CNS.
We first consider the situation in which a burn injury is applied to the skin and the test stimulus is heat applied to the location of the burn injury. When a burn is applied to the glabrous skin of the hand, marked hyperalgesia to heat develops as shown in Figure 1-9 A ( ). The hyperalgesia is manifested as a leftward shift of the stimulus–response function that relates the magnitude of pain to stimulus intensity. For example, the 41°C stimulus was not painful before the burn but after the injury was as painful as the 49°C stimulus before the injury.
Substantial evidence favors the concept that the primary hyperalgesia to heat stimuli that develops at the site of a burn injury is mediated by sensitization of nociceptors ( ). Sensitization is defined as a leftward shift of the stimulus–response function that relates the magnitude of the neural response to stimulus intensity. Sensitization is characterized by a decrease in threshold, an augmented response to suprathreshold stimuli, and ongoing spontaneous activity. These properties correspond to the properties of hyperalgesia ( Table 1-2 ).
| HYPERALGESIA (SUBJECT RESPONSE) | SENSITIZATION (FIBER RESPONSE) |
|---|---|
| Decreased pain threshold | Decreased threshold for response |
| Increased pain in response to suprathreshold stimuli | Increased response to suprathreshold stimuli |
| Spontaneous pain | Spontaneous activity |
To explain the hyperalgesia that occurs with a burn on the glabrous skin of the hand, a correlative analysis of subjective ratings of pain in humans with responses of nociceptors (CMHs and type I AMHs) in anesthetized monkeys was performed ( ). Test heat stimuli were applied to the glabrous skin of the hand before and after a 53°C, 30-second burn. The burn led to prominent hyperalgesia in the human subjects ( Fig. 1-9 A). The CMHs showed a decreased response following the burn ( Fig. 1-9 C), whereas the type I AMHs were markedly sensitized ( Fig. 1-9 B). Thus, it is likely that for thermal injuries on the glabrous skin of the hand, AMHs, not CMHs, code for the heat hyperalgesia.
Sensitization is not a uniform property of nociceptors. Tissue type and the nature of the injury are important variables. For example, CMHs that innervate hairy skin become sensitized, whereas as described above, CMHs that innervate the glabrous skin of the hand do not become sensitized to a burn injury ( ). Thus, CMHs appear to play a role in accounting for hyperalgesia to heat stimuli on hairy skin ( ). These data support the conclusion that the hyperalgesia to heat stimuli that occurs at the site of an injury is due to sensitization of primary afferent nociceptors.
Distinguishing hyperalgesia to mechanical stimuli in the primary and secondary zones may be incorrect in some respects since the mechanism for hyperalgesia in the two zones may have some common elements. The mechanisms discussed in this section, however, will be limited to those applicable to the primary zone.
Different forms of mechanical hyperalgesia have been characterized. One form is evident when the skin is gently stroked with a cotton swab and is referred to as “stroking hyperalgesia,” “dynamic hyperalgesia,” or “allodynia.” The second form of hyperalgesia is evident when punctate stimuli, such as von Frey probes, are applied and, accordingly, has been termed “punctate hyperalgesia.” Hyperalgesia to tonic stimulation with a blunt probe, called “pressure hyperalgesia,” and impact hyperalgesia to shooting small bullets against the skin at a controlled velocity have also been described in the primary hyperalgesic zone ( ). As discussed in the later section on secondary hyperalgesia, the mechanism for these different forms of mechanical hyperalgesia is probably different. Stroking hyperalgesia is thought to be signaled by low-threshold mechanoreceptors, whereas punctate hyperalgesia is mediated at least in part by nociceptors. Pressure hyperalgesia and impact hyperalgesia are probably mediated by sensitized C fibers. Another form of mechanical hyperalgesia termed “progressive tactile hypersensitivity,” which may contribute to the allodynia associated with inflammation, has been described ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here