Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Over the last decades, cardiovascular monitoring techniques used in the intensive care unit (ICU) and operating room (OR) have progressively evolved from invasive toward less invasive approaches. In the 1990s, the pulmonary artery catheter (PAC) was at its apogee, as it was the only method to assess and monitor hemodynamics at the bedside. The PAC has provided clinicians with measurements of cardiac output (CO), pulmonary artery pressure (PAP), cardiac filling pressures, and mixed venous blood gases. Modified versions of PAC provided continuous monitoring of CO and of mixed venous oxygen saturation (SvO 2 ). However, a progressive decline of use of the PAC has been observed since 1995. In addition to invasiveness, there were multiple reasons for such a decline: (1) evidence of insufficient physician knowledge in measuring and interpretating the PAC data , ; (2) a report from a nonrandomized outcome study showing increased mortality associated with PAC vs. no PAC ; (3) findings from randomized controlled trials showing no outcome benefits of using PAC in ICU patients , ; (4) development of bedside echocardiography in the ICU that allows better assessment of cardiac function; (5) emergence of transpulmonary thermodilution (TPTD), esophageal Doppler (ED), and minimally invasive or noninvasive pulse wave analysis (PWA) CO monitors. The latter monitoring systems, which provide real-time and continuous CO and fluid responsiveness variables, belong to the class of functional hemodynamic monitoring devices. Use of such devices in goal-directed therapy strategies in the perioperative period of high-risk surgical patients has been reported to improve outcome in comparison to standard management using central venous pressure (CVP) and mean arterial pressure (MAP). During recent years, the importance of predicting fluid responsiveness has also been emphasized in the ICU setting. This can be explained by (1) the demonstration that approximately half of ICU patients are fluid nonresponders and (2) the fact that fluid overload is associated with increased mortality of ICU patients. Numerous studies have documented the superiority of dynamic over static variables to predict fluid responsiveness. Accordingly, many intensivists and anesthesiologists prioritize the use of devices that provide dynamic variables such as pulse pressure variation (PPV) and stroke volume variation (SVV) or that facilitate tests such as passive leg raising (PLR) , or end-expiratory occlusion (EEO). Thus it is easy to understand why in this context, the place of the PAC, which cannot provide adequate assessment of fluid responsiveness, has decreased over time.
In this chapter, we review the most used hemodynamic monitoring devices in the ICU and the OR settings, ranging from the invasive to less invasive ones. We emphasize the clinical relevance of the hemodynamic information they provide and the advantages and limitations of their use, knowing that they generally gain in safety what they lose in precision. We also discuss the place of hemodynamic monitoring in patients with shock and in patients in the OR setting.
The most commonly used PAC model is a fluid-filled catheter of 7 or 7.5F external diameter and 80-cm length that connects to an electronic pressure transducer. The distal lumen, ending at the tip of the catheter, enables blood sampling and pressure measurement at the level of the pulmonary artery. A few millimeters before its termination, a latex balloon surrounds the catheter. Temporary balloon inflation totally occludes the pulmonary artery branch (10–15 mm diameter) into which the catheter has been placed and allows the measurement of the pulmonary occlusion artery pressure (PAOP). A proximal lumen at the level of the right atrium allows measuring the right atrial (central venous) pressure. A thermistor located close to the tip of the catheter continuously senses the changes in blood temperature induced by thermal injection via the proximal lumen. This allows calculating thermodilution CO according to the Stewart-Hamilton principle.
Based on this minimal configuration, alternative PAC models can be equipped with:
An ultra-fast thermistor providing calculation of right ventricular (RV) ejection fraction and RV end-diastolic volume
A thermal filament for continuous CO measurement
A fiberoptic probe for reflectance photometry and continuous assessment of SvO 2
The PAC provides the physician with hemodynamic variables (right atrial pressure, PAP, PAOP, and CO) and with tissue perfusion variables such as SvO 2 and the mixed venous carbon dioxide pressure (PvCO 2 ). Simultaneous acquisition of arterial blood gases allows calculation of oxygen consumption (VO 2 ) and oxygen delivery (DO 2 ), using the time-honored Fick principle.
The PAOP is obtained after inflation of the distal balloon with 1.5 mL of air. This temporarily occludes a branch of the pulmonary artery of around 13 mm diameter and thus interrupts flow through the column of blood linking the balloon with a pulmonary vein of similar diameter. The PAOP reflects the pressure in a large pulmonary vein and thus approximates both the left atrial pressure (LAP) and left ventricular (LV) end-diastolic pressures (LVEDP).
It is recommended to measure PAOP at the end of expiration, a time when intrathoracic pressure is closest to its value at atmospheric pressure. Positive end-expiratory pressure (PEEP) or auto-PEEP may lead to overestimation of PAOP at end expiration as a measure of LV filling pressure (LVFP). Obtaining transmural PAOP, a better marker of LVFP, requires subtraction from the end-expiratory PAOP a value that estimates the transmitted PEEP or auto-PEEP into the thorax. This value can be obtained by the product of PEEP (or auto-PEEP) by the ratio of the difference between the PAOP values at end expiration, and at end inspiration over the driving pressure (difference between plateau pressure and PEEP).
Even if it reflects LAP, PAOP overestimates LVEDP in cases of mitral stenosis or insufficiency and underestimates LVEDP in the case of severe aortic insufficiency or reduced LV compliance.
Although the main interest of the transmural PAOP is to estimate the LVFP, the intramural PAOP is also used to reflect the pulmonary capillary pressure (Pcp). However, because PAOP reflects the pressure in a large pulmonary vein, it underestimates Pcp, particularly when CO is high and when pulmonary venous resistance is elevated, as during acute respiratory distress syndrome (ARDS). , The Pcp could be estimated from the PAP trace decay during the seconds after the distal balloon inflation, after extrapolation back toward time zero of the slow component of the pressure decay. For adequate precision, this calculation requires a computerized mathematical method. Because Pcp is not measured in routine practice, PAOP provides only a rough estimate of the Pcp needed to differentiate between hydrostatic and increased permeability pulmonary edema. However, authentic increased permeability pulmonary edema also can be associated with elevated PAOP.
The CO can be measured using the thermodilution principle. Two methods of measurement are currently used:
The intermittent thermodilution method requires the injection of a cold saline bolus through the proximal lumen of the catheter. The decrease in blood temperature is recorded by the distal thermistor, and CO is calculated from the Stewart-Hamilton equation. At least three measurements must be averaged for a reliable estimation of CO. Severe tricuspid regurgitation leads to underestimation of CO.
The “continuous” thermodilution method is based on automatic heating of blood by means of a proximal thermal filament (located about 15 cm from the tip of the PAC). The external monitor activates the heating filament for 1–4 seconds in a pseudorandom sequence. The resulting series of heat signals from the distal thermistor are analyzed stochastically to determine a single thermodilution curve. This technique has the advantage of continuously displaying CO and avoiding repeated manipulations of the lines and bolus injections. However, it does not enable real-time CO monitoring, because the average of successive CO measurements is delayed as compared with the intermittent technique. This limits the ability to detect rapid hemodynamic changes induced by therapy or to track hemodynamic instability during high-risk surgery.
Because pulmonary arterial blood blends the mix from all venous territories of the body, measuring SvO 2 with the PAC enables assessment of global tissue oxygenation.
Two techniques are currently available. The first one requires sampling of the pulmonary artery blood through the distal tip of the PAC. The second technique uses PAC models, which enable a continuous “in vivo” monitoring of SvO 2 through fiberoptic spectrophotometry. The latter method has the advantage of avoiding repeated blood samplings and provides real-time monitoring of SvO 2 .
Physiologically, SvO 2 depends on arterial oxygen saturation (SaO 2 ), VO 2 , CO, and hemoglobin concentration (Hb), according to the formula derived from the Fick equation applied to oxygen: SvO 2 = SaO 2 − [VO 2 / (CO × Hb × 13.4)]. Because CO, Hb, and SaO 2 are the key determinants of DO 2 , SvO 2 is an integrative variable, which is considered a marker of the global balance between actual VO 2 and DO 2 . SvO 2 values range from 65% to 77% in healthy subjects.
Useful interpretation of SvO 2 presents several difficulties. First, a low value of SaO 2 results in a decreased SvO 2 , which is no longer considered a marker of the VO 2 /DO 2 balance. Second, a normal or high SvO 2 value can be observed during certain shock states (e.g., sepsis) because of impaired oxygen extraction capability. This difficulty emphasizes that when SaO 2 is normal, SvO 2 is a marker of the balance between DO 2 and VO 2 but does not faithfully reflect oxygen demand. Third, for constant VO 2 , Hb, and SaO 2 , the relation between SvO 2 and CO is hyperbolic. Thus although changes in SvO 2 parallel changes in CO in low CO states, marked changes in CO do not significantly alter SvO 2 in hyperdynamic ones. Fourth, in shock states characterized by DO 2 /VO 2 dependency, changes in CO result in parallel changes in VO 2 such that SvO 2 will not change if DO 2 is below its critical value. Fifth, SvO 2 is the flow-weighted average of the venous saturation values from all organs of the body. Organs having high blood flow and low oxygen extraction, such as the kidneys, have a greater influence on SvO 2 than organs with low blood flow and high oxygen extraction, such as the myocardium. In sepsis, interpretation of SvO 2 is further complicated by the local maldistribution of blood flow. Nevertheless, in any shock state, monitoring SvO 2 is helpful because a low value (<65%) should prompt clinicians to increase DO 2 in order to improve tissue oxygenation. On the other hand, a high value of SvO 2 suggests that attempts to increase DO 2 have little chance to improve tissue oxygenation significantly or ultimately benefit outcome.
The veno-arterial carbon dioxide tension (PCO 2 ) difference (ΔPCO 2 ) is the difference between PvCO 2 and PCO 2 in arterial blood (PaCO 2 ). Its normal value ranges from 2 to 5 mm Hg.
The Fick equation applied to carbon dioxide (CO 2 ) indicates that the CO 2 excretion (equivalent to CO 2 production [VCO 2 ] in a steady state) equals the product of CO by the difference between the CO 2 content in the mixed venous blood and in the arterial blood. The normal relationship between CO 2 content and PCO 2 is almost linear over the usual physiologic range of the CO 2 contents. Thus by substituting PCO 2 for CO 2 content, ΔPCO 2 = k × VCO 2 / CO, where k is a constant. Accordingly, ΔPCO 2 would be linearly related to VCO 2 and inversely related to CO.
The ΔPCO 2 is considered a marker of the adequacy of venous blood flow to remove the total CO 2 produced by the peripheral tissues. An increased ΔPCO 2 suggests that the CO is not high enough with respect to the global metabolic conditions. A high ΔPCO 2 should prompt clinicians to consider measures aimed at increasing CO so as to reduce tissue hypoxia. Conversely, a normal ΔPCO 2 suggests that increasing CO is not a priority.
The complications associated with the use of a PAC are relatively rare and can be related to (1) PAC insertion (e.g., arterial puncture, bleeding, pneumothorax, ventricular extrasystoles), (2) PAC maintenance (e.g., bacterial colonization, infectious endocarditis, venous thromboembolism), (3) balloon inflation (e.g., rupture of a pulmonary artery branch, false aneurysm of the pulmonary artery), and (4) PAC withdrawal (e.g., transient ventricular arrhythmias, knotting of the catheter). Some of these complications relate to the poor experience of the user and to the PAC length of stay. The PAC should be carefully removed as soon as no longer needed, and its use should not be prolonged longer than 3 or 4 days.
Two commercialized devices integrate the TPTD and the PWA methods: the PiCCO (Pulsion, Germany) and the VolumeView (Edwards LifeSciences, USA). They require the insertion of a standard central catheter in the superior vena cava and a specific thermistor-tipped arterial catheter, which is usually inserted in the femoral artery. The arterial catheter can also be inserted in the radial, brachial, and axillary arteries if site-specific catheters are used. In practice, a bolus of cold saline (15 mL) is injected through the venous catheter. The thermistor at the tip of the arterial catheter detects the change in blood temperature induced by the cold bolus injection. In addition to providing TPTD-derived variables, cold bolus injections allow calibrating the PWA-based CO, which is derived from the analysis of the ABP waveform.
Similar to the intermittent PAC thermodilution, the area under the TPTD is inversely related to CO according to the Stewart-Hamilton principle. Because the thermistor is located in a femoral artery and not in a pulmonary artery, the TPTD curve has a longer appearance time, a less negative peak value, and a longer return time to baseline temperature compared with the PAC thermodilution curve. Nevertheless, there is a good agreement between CO values measured by the two methods in humans. Although the PAC thermodilution should theoretically provide more accurate CO measurements (less indicator loss between the injection and the sampling sites) than TPTD, the latter has the advantage of being less influenced by respiration and severe tricuspid regurgitation. Cold saline (<8°C) injection should be preferred to room-temperature saline injection to avoid multiplying the risks of errors of measurements and to avoid overestimating CO. The result of three cold bolus injections performed at random should be averaged to obtain an acceptable precision. The least significant change is around 12%, which is comparable with that of the PAC.
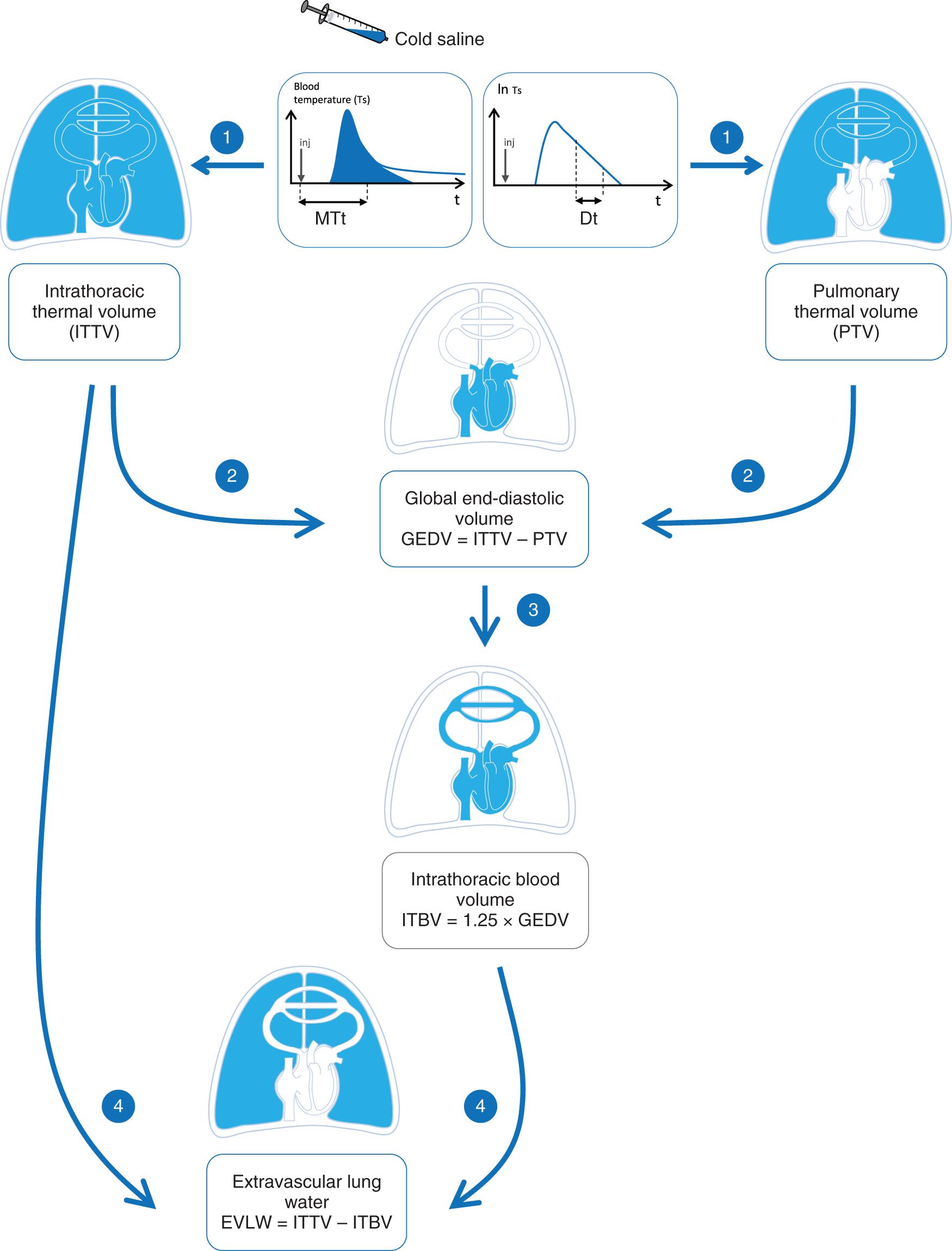
Through a combined analysis of the thermodilution curve and of its natural logarithmic transformation, TPTD can estimate several volumes of fluid inside the thorax ( Fig. 32.1 ). In the PiCCO device, the global end-diastolic volume (GEDV) equals the product of CO by the difference between mean transit time and downslope time. In the VolumeView device, the calculation of GEDV takes into account the ratio of the maximal ascending and descending slopes of the TPTD curve. The GEDV represents the sum of the maximal volume of the four cardiac chambers and thus represents a volumetric measure of global preload. As such, it can be helpful for identifying the mechanism of shock and following the effects of therapies, but like every static preload marker, it cannot be used to assess preload responsiveness.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here