Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
It is not entirely clear how a chapter on pregnancy fits into a book titled Anesthesia and Coexisting Disease . Pregnancy is not a disease, but a normal physiologic condition, usually associated with and dependent on relatively good health. For most so-called coexisting diseases in this book, the clinical situation being analyzed and discussed is that of a patient needing surgery, usually or often unrelated to the disease under discussion, where the disease process affects anesthesia and perioperative management. In contrast, pregnant women predominantly receive care from anesthesiologists for procedures and events related to the pregnancy and undergo procedures specific to pregnancy (e.g., labor analgesia and cesarean delivery). Anesthesia for these pregnancy-related events is extensively discussed in books and chapters specifically about obstetric anesthesia and cannot possibly be treated properly in a single chapter in this book. Many coexisting medical conditions and comorbidities require only minor adjustments to obstetric anesthesia care or require the same assessments and alterations that the anesthesiologist would consider for the nonpregnant patient. In this chapter we will begin with the assumption that the reader is familiar with routine obstetric anesthesia procedures and care, then we will focus on anesthesia care of the pregnant woman in unusual clinical situations when the woman’s other medical conditions add significant complexity to obstetric anesthesia care rather than on pregnancy complications or fetal issues. These areas include the care of the pregnant woman with hypertensive diseases of pregnancy (gestational hypertension and preeclampsia/eclampsia), significant cardiovascular disease, and obesity, the most common coexisting condition in pregnancy. Two areas where pregnancy (and the postpartum period) does function more as a coexisting disease are the pregnant woman who requires surgery during pregnancy but unrelated to the pregnancy itself and the breastfeeding woman who needs an anesthetic. These two areas will be included in our discussion.
Any anesthesiologist caring for the pregnant woman in any clinical scenario must understand the physiologic changes of pregnancy that impact anesthesia care. Most changes in the cardiovascular system are caused by the hormonal changes of early pregnancy, then later too by growth and anatomic changes of late pregnancy. Higher progesterone levels result in increased production of nitric oxide and prostacyclin, which together with a decreased response to norepinephrine and angiotensin result in relative vasodilation. Increased concentrations of relaxin lead to renal artery dilation and, through reduction in aortic stiffness, dilation of the proximal aorta. Systemic vascular resistance decreases in early pregnancy with a decrease of ∼35% at 20 weeks, presumably as a result of alteration in receptor number and function as well as vascular smooth muscle changes induced by progesterone. Systemic vascular resistance slowly rises later but remains ∼20% lower at term than the prepregnancy level. Despite lower vascular tone/resistance, central venous pressure, pulmonary artery pressure, and pulmonary capillary wedge pressure remain stable throughout pregnancy due to increased vascular volume. The decrease in systemic vascular resistance during the initial weeks after conception causes a compensatory elevation of cardiac output (initially resulting from an increase in heart rate) and an increase in renin activity. Increased renin activity results in retention of sodium and, by osmotic gradient, water. About 1000 mEq of sodium will be retained by term, which results in retention of water. Plasma volume begins to rise in the fourth week of pregnancy and reaches a maximum (30–50% increase) at 28 to 34 weeks. The increase in plasma volume, combined with a 20% to 30% increase in total red blood cell mass, results in significantly elevated total blood volume, which reaches ∼100 mL/kg at term. Cardiac output rises in parallel with plasma volume, increasing by 15% at 8 weeks of gestation and reaching a maximum increase of 50% by 28 to 32 weeks. Plasma volume and cardiac output remain stable from ∼32 weeks until labor begins. In labor, cardiac output rises as a result of sympathetic stimulation (pain and stress) and autotransfusion (the displacement of blood from the contracting uterus into the circulation). Compared with prelabor output, cardiac output is increased by 20% during the first stage and 50% during the second stage of labor. Just after delivery of the placenta (the end of the third stage of labor), cardiac output is elevated 80% above prelabor levels, which corresponds to a 170% increase relative to the prepregnancy level. Cardiac output falls to the prelabor level in 24 to 48 hours and returns to the prepregnancy level in the next 12 to 24 weeks. Twin pregnancy results in a 20% greater increase in cardiac output than singleton gestation.
| Parameter | Change From Nonpregnant Value (%) |
|---|---|
| Cardiovascular/Circulatory | |
| Intravascular fluid volume | ↑35–40 |
| Plasma volume | ↑45–50 |
| Erythrocyte volume | ↑20–25 |
| Hemoglobin/hematocrit | ↓15 |
| Cardiac output | ↑40 |
| Stroke volume | ↑20 |
| Heart rate | ↑10–15 |
| Systolic blood pressure | No change or small ↓ |
| Systemic vascular resistance | ↓15 |
| Diastolic blood pressure | ↓0–15 |
| Central venous pressure | No change |
| Femoral venous pressure | ↑15 |
| Minute ventilation | ↑50 |
| Respiratory | |
| Tidal volume | ↑40 |
| Respiratory rate | ↑10 |
| Pao 2 | ↑10 mm Hg |
| Paco 2 | ↓10 mm Hg |
| Arterial pH | No change |
| Inspiratory reserve volume | ↑5 |
| Tidal volume | ↑45 |
| Expiratory reserve volume | ↓25–30 |
| Residual volume | ↓0–10 |
| Inspiratory capacity | +15 |
| Functional residual capacity | ↓10–20 |
| Vital capacity | No change |
| Total lung capacity | ↓0–5 |
| Minute ventilation | ↑30 |
| Oxygen consumption | ↑20 |
| Renal | |
| Renal blood flow and glomerular filtration rate | ↑30–50 |
| Creatinine concentration | ↓20–50 |
Pregnancy affects echocardiographic and electrocardiographic (ECG) findings so that several findings that would be interpreted as abnormal and perhaps concerning are normal findings in pregnancy. These include an increase in left ventricular mass by 6% and right ventricular mass by 15% to 20% by term. Increases in the size and dilation of the cardiac chambers result in a mild degree of insufficiency of all valves except the aortic; it is, however, not normal to see aortic insufficiency at any stage of pregnancy. Enlargement of the heart and cephalic displacement of the diaphragm cause a horizontal shift and rotation of the heart, which results in changes in the cardiac axis on ECG. It is not abnormal to see a deep S wave in lead I and a large Q wave with negative T waves in leads III and aVF. These changes resolve after pregnancy in the normal heart.
At term the uterus can and usually does completely compress the inferior vena cava while parturients are in the supine position. Twin and singleton pregnancies cause a similar degree of obstruction. Recent magnetic resonance imaging (MRI) studies suggest that this compression is often not relieved by the usual clinical recommendation of 15-degree tilt, but rather requires 30 to 45 degrees of displacement/tilt. The importance of this compression for most pregnant women is questionable, and they (and their fetuses) show no symptoms of this compression, even during anesthesia, as alternate pathways for venous blood return have developed during the pregnancy. Compression of the inferior vena cava by the gravid uterus results in supine hypotension syndrome in a subset of pregnant women, which manifests with a short period of tachycardia followed by bradycardia and profound hypotension. It is not clear why some women have significant symptoms while many/most do not. Contrary to decades of use of the term aortocaval compression, implying aortic obstruction in addition to caval compression, most contemporary evidence suggests that the aorta is not affected by the gravid uterus.
The respiratory system is also altered by the hormonal and anatomic changes of pregnancy. Increased activity of relaxin results in relaxation of the ligaments of the rib cage, which results in displacement of the ribs to a more horizontal position. This leads to upward displacement of the diaphragm early in pregnancy even before the gravid uterus shifts the abdominal contents. The vertical dimension of the chest decreases by about 4 cm, but the diameter increases by about 5 cm, significantly increasing the volume of the lungs available for gas exchange during normal spontaneous respiration. Tidal volume increases up to 40% by term. Increased activity of progesterone, a potent respiratory stimulant, leads to an increase in tidal volume and respiratory rate so that minute ventilation is increased by 50% at term. Chronic hyperventilation driven by hormonal changes results in respiratory alkalosis with a typical Paco 2 in the range of 28 to 32 mm Hg. Secondary to the decline in Paco 2 , Pao 2 rises slightly, in the range of 104 to 108 mm Hg, during pregnancy. These changes increase the gradient between mother and fetus and improve maternal-fetal gas exchange. Clinically, the implication is that a Paco 2 in the normal range could indicate significant hypoventilation in a pregnant woman.
Decrease in the vertical size of the chest secondary to elevation of the diaphragm leads to a 25% decrease in expiratory reserve volume and a 15% decrease in residual volume, which results in a 20% decrease in functional residual capacity. A 20% increase in oxygen consumption caused by an elevated basal metabolic rate, combined with the decrease in functional residual capacity, produces more rapid desaturation during periods of apnea. In a fully preoxygenated healthy nonpregnant patient, desaturation from 100% to lower than 90% occurs in approximately 9 minutes. In a healthy patient at term, desaturation occurs in only 3 to 4 minutes, and in a morbidly obese pregnant patient desaturation can occur in a minute or less.
Edema and hyperemia of the oropharyngeal mucosa, glandular hyperactivity, and capillary engorgement secondary to elevated activity of estrogen, progesterone, and relaxin result in nasal stiffness, epistaxis, and upper airway narrowing. Therefore the rates of difficult and failed intubation in pregnant women are increased—historically quoted as ∼3.3% and 0.4%, respectively—which are more than eight times higher than in nonpregnant patients, although recent reports suggest that these numbers may overestimate the failure rate in contemporary practice (see later discussion). When providing general anesthesia to a pregnant woman for any procedure, the anesthesiologist is thus faced with a potentially difficult airway in a patient who will undergo desaturation more rapidly than a nonpregnant patient. This is one of the factors contributing to a higher mortality rate among parturient women who undergo general anesthesia than among those who undergo regional anesthesia. The increased mortality associated with general anesthesia compared to neuraxial anesthesia for cesarean delivery from difficult intubation or aspiration of gastric contents (often during or due to difficult intubation) has lessened significantly in the past few decades presumably due to improved training, protocols and awareness, and more and improved devices for airway management. Over the last decade, better preparation and the use of video laryngoscopy has improved intubation success rates in pregnant women, and the availability of supraglottic airway devices has provided a rescue pathway for failed intubation.
Normal pregnancy is a relatively hypercoagulable state. The activity of the majority of the coagulation factors (I, VII, VIII, IX, X, XII) and the levels of fibrinogen are increased, whereas the activity of physiologic anticoagulants is decreased. The latter includes a significant reduction in protein S activity and an acquired activated protein C resistance. This effect (i.e., reduction in anticoagulation activity) is doubled in in vitro fertilization (IVF) pregnancies. Deep vein thrombosis occurs in 1 per 1000 deliveries, which is 5.5 to 6 times higher than the rate in the general female population of childbearing age and reaches a maximum at 4 to 6 weeks postpartum. Platelet counts generally decrease moderately (10–20%) during gestation due to increased peripheral consumption or sequestration in a larger spleen or the placenta. Procoagulant changes during normal pregnancy are counterbalanced by significant activation of the fibrinolytic system during the postpartum period. Over the past few years protocols and national guidelines have been promulgated, increasing the indications for and use of thromboprophylaxis and anticoagulation in women during pregnancy and especially in the postpartum period. The recommendations regarding thromboprophylaxis around the time of labor and delivery or hospitalization for pregnancy-related complications should also be seriously considered in any pregnant woman undergoing nonobstetric surgery, as the surgical intervention is almost always a prothrombotic stimulus, and many of these women will be hospitalized or confined to bed for prolonged periods. Teleologically, the hypercoagulability of pregnancy has the evolutionary advantage of limiting hemorrhage at delivery, but the activated state of the coagulation system also has the somewhat counterintuitive effect of triggering disseminated intravascular coagulation due to rapid consumption of coagulation factors and platelets in response to a variety of stimuli, including bleeding/trauma itself or maternal absorption of gestational material, potentially leading to the development of a rapidly developing coagulopathic state.
Lower esophageal sphincter tone is decreased in pregnancy as a result of two factors: displacement of the stomach upward and muscle relaxation caused by the effects of progestins. Heartburn is a frequent occurrence among pregnant women. Contrary to the teachings of several generations of anesthesiologists, gastric emptying is not delayed in pregnancy as determined by multiple methods, including ultrasound examination of stomach volume and oral drug absorption studies, although it is slowed in labor.
Bile secretion is increased during pregnancy. Bile stasis is increased owing to the effect of progesterone, and together with changes in the composition of bile acids, this results in increased gallstone formation. Cholecystectomy is the second most frequent surgery during pregnancy (after appendectomy), with a reported incidence as high as 1 in 1600 pregnancies.
Pregnancy is characterized by insulin resistance caused by increased activity of hormones such as progesterone, estrogen, cortisol (2.5-fold increase at term), and placental lactogen. This insulin resistance resolves rapidly after delivery. Fasting glucose levels are lower in pregnant than in nonpregnant patients because of the high glucose utilization by the fetus.
Estrogen increases the level of thyroxin-binding globulin, which results in an elevation of total triiodothyronine (T 3 ) and thyroxine (T 4 ) levels, but levels of free T 3 and T 4 remain stable.
Increased levels of progesterone and endorphins may elevate the pain threshold. Cerebrospinal fluid volume is decreased during pregnancy, but intracranial pressure remains stable. Anesthetic requirements for both local anesthetics and general anesthetics are somewhat decreased.
Renal blood flow is increased in pregnancy, due mostly to increased cardiac output. Glomerular filtration rate increases by 50% at 12 weeks of gestation, which results in a decrease in blood urea nitrogen (BUN) and creatinine concentrations. The most important clinical implication of this is that normal BUN and creatinine levels may indicate renal dysfunction (e.g., in preeclampsia).
This section of the chapter fits most closely within the title of this book, in that pregnancy is truly the “coexisting disease” in a woman who needs surgery and anesthesia while she is pregnant. Of all pregnant women in the United States, 1% to 2% will undergo surgery unrelated to their pregnancy (∼75,000 procedures requiring anesthesia per year). The most frequent nonobstetric procedures are appendectomy, cholecystectomy, breast biopsy, and surgery required because of trauma.
Generally, elective surgery should be delayed until the patient is no longer pregnant and has returned to the nonpregnant physiologic state. Depending on how elective the procedure is, surgery is sometimes delayed until after the woman has stopped breastfeeding, but this is usually not necessary (see Anesthesia in the Breastfeeding Woman, later). Procedures that can be scheduled with some flexibility but that cannot be delayed until after delivery are best performed in the second trimester. In theory, this lessens any risk of teratogenicity with first-trimester medication administration and preterm labor, which is thought to be a greater risk in the third trimester, although both of these assumptions and recommendations have come under some scrutiny and criticism recently, and many argue that delaying indicated surgery might be the larger risk in many clinical circumstances ( Fig 32.1 ).
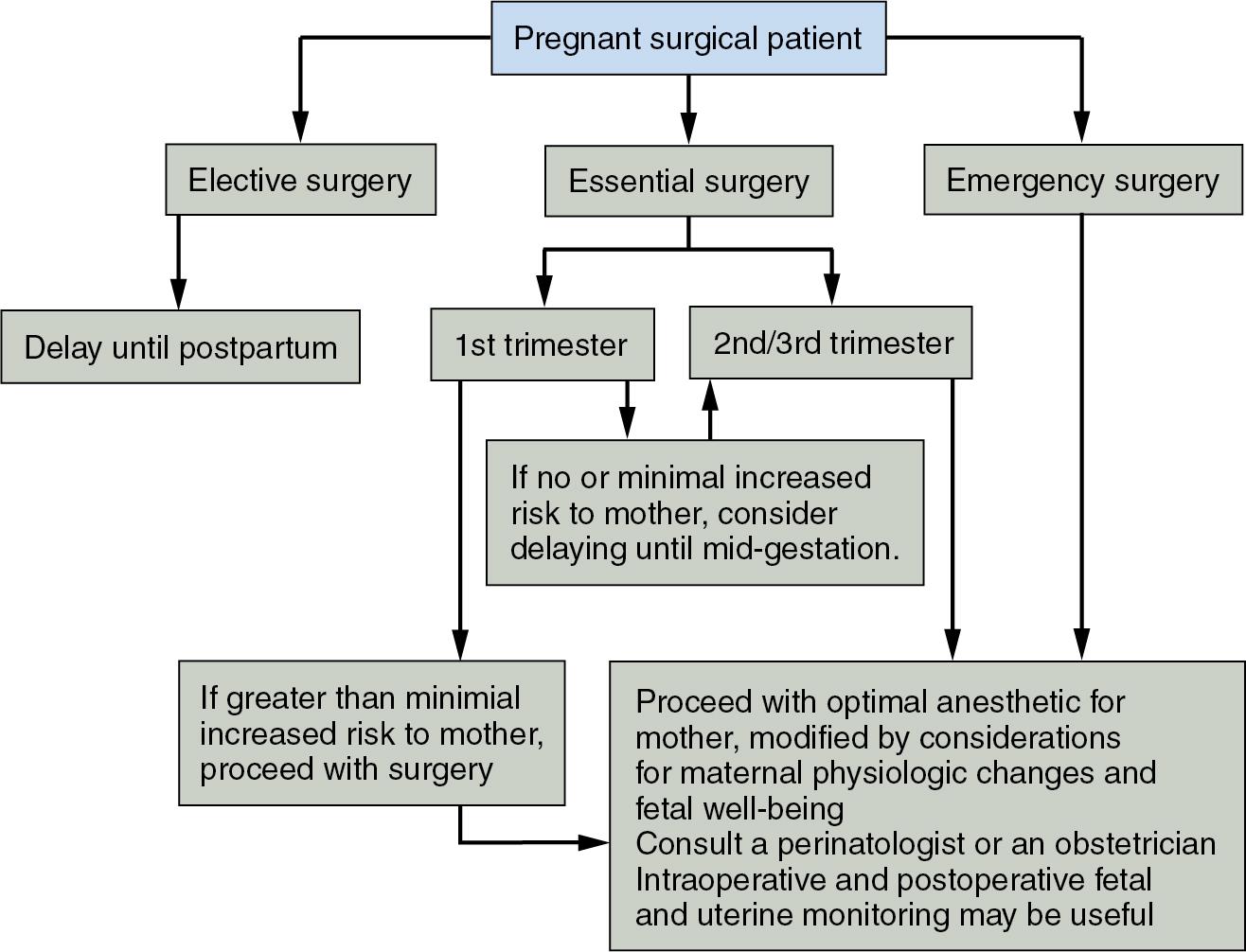
The pregnancy-specific issues involved in nonobstetric surgery during pregnancy involve taking into account pregnancy-induced anatomic, physiologic, pharmacodynamic, and pharmacokinetic alterations, minimizing fetal drug exposure when possible and monitoring for and prevention of preterm labor, a particular risk with intrabdominal surgery.
Little outcome data exist to guide anesthetic choice, but when possible most anesthesiologists (and often patients) prefer a regional anesthetic (neuraxial or peripheral nerve block) with minimal sedation, as this would appear to be the best way to minimize fetal drug exposure. While logic and reasonable caution would suggest minimizing exposure to drugs having significant effects on the human brain, no commonly used anesthetic agents have been shown to cause teratogenicity or other significant effect on the fetus. Most anesthetic decisions should be based on the usual anesthetic and surgical considerations as well as maternal status and safety, not on the presence of the fetus. For the most commonly performed operative procedures during pregnancy (appendectomy and cholecystectomy), general anesthesia is required, particularly since these operations are now almost always performed laparoscopically. A decade ago there was significant controversy about whether laparoscopic surgery was safe during pregnancy. There were concerns with issues of the effect of intraperitoneal pressure and potentially increased Pa CO2 , but clinical experience and some animal studies have mostly put these concerns to rest, and laparoscopic appendectomies and cholecystectomies have become the standard approach, even for pregnant women.
Airway management during surgery depends on gestational age. By late second trimester, most anesthesiologists would opt for rapid-sequence induction and intubation to reduce the risk of aspiration, as is standard for cesarean delivery under general anesthesia. Earlier in gestation, before ∼20 weeks, controversy exists as to the level and nature of this risk, and the need for rapid-sequence induction or intubation will depend on the specific nature of the patient and operative procedure.
Teratogenicity may occur at any stage of gestation. However, most organogenesis occurs in the first trimester. Although many commonly used anesthetics are teratogenic at high dosages in animals, no studies or data support teratogenic effects of anesthetic or sedative medications in humans at the dosages and time periods used for anesthesia care. There was literature in the 1960s suggesting a link between chronic benzodiazepine and other sedative use and fetal/neonatal cleft lip and palate, but even this issue of chronic exposure has been mostly refuted. There is some evidence of a link between maternal high-dose diazepam treatment and intrauterine growth restriction, but single or limited doses of benzodiazepines are almost certainly safe when used in the usual manner for perioperative anxiety. That being said, it is common to attempt to avoid these medications in pregnancy due to concerns about effects on the fetal brain and the history of controversy about their use.
Nitrous oxide has been suggested to be teratogenic in animals when administered for prolonged periods (1–2 days); the concern is biologically plausible given its effect on DNA synthesis through inhibition of methionine synthetase. As with other purported effects of anesthetics, however, it has been difficult to document clinical effects in humans. Teratogenesis has been seen in animals only under conditions (long, repeated exposure) that are not likely to be reproduced in clinical care or relevant to single exposure perioperatively. Nitrous oxide is of course widely used for labor analgesia, so fetal exposure near and at term is common without generating too much concern from either clinicians or patients, although actual data on fetal/neonatal safety are surprisingly limited. Recent studies suggest that volatile anesthetics stimulate neuronal apoptosis in rats, but it is not obvious whether these data can be extrapolated to humans. Widespread neuronal apoptosis is associated with memory and learning deficits in laboratory animals, but again this has not been shown in humans.
Propofol and ketamine are all safe intravenous (IV) induction agents. Induction doses for these medications are unchanged in pregnancy. The choice of induction agent is usually based on provider preference and the clinical status of the patient (e.g., presence of dehydration, valvular heart disease, dysrhythmia, hypertension, or preeclampsia). None of these agents has been clearly shown to be teratogenic or have adverse effects on human brain development. Thiopental is no longer available in the United States but is also an accepted induction agent during pregnancy.
In the past decade or so there has been increasing concern in the pediatric anesthesia arena about the possible negative effects of anesthetics on behavior and learning in children, especially upon repeat or long exposure. These concerns could also be relevant to in utero exposure. While studies in young animals show a variety of behavioral and cellular neurotoxic effects of a variety of drugs and of anesthetic exposure in general, the results from human clinical studies and reviews of large databases of pediatric anesthetics have not generally shown much effect. A detailed discussion of this complex issue that is under intense investigation around the world is beyond the scope of this chapter, but the available evidence would not support any recommendation to withhold anesthesia from a pregnant woman who needs it on the basis of concern about drug effects on the fetus.
Pregnant patients are more sensitive to the action of nondepolarizing muscle relaxants but may have increased clearance of both these medications, so dosing of these medications may need to be more frequent and based on neuromuscular monitoring. Succinylcholine dose is unchanged in pregnancy; its volume of distribution is increased, but systemic pseudocholinesterase activity is decreased, resulting in increased variability in duration of action, but this is unlikely to be of any clinical significance. Muscle relaxant reversal in pregnancy involves a few unique issues. The use of neostigmine is unproblematic, but the vagolytic medication given to prevent bradycardia and other effects requires some thought. Glycopyrrolate does not cross the placenta, but neostigmine, although also a quaternary amine, does appear to cause bradycardia in some fetuses; thus atropine, which does cross the placenta, is the recommended vagolytic adjuvant in pregnancy. Suggamadex has not been approved for use in pregnancy, as very few drugs have been, but with suggamadex there is reason to consider avoiding it due to its known effect of binding steroid hormones, including progestins, which could have unknown effects on pregnancy. However, no deleterious effects or decreases in progesterone levels were reported after high doses in early pregnancy in rats.
After almost any surgical procedure during pregnancy, fetal heart rate should be monitored in the recovery room, intermittently for previable fetuses and continuously for the viable fetus. Uterine activity should also be monitored because contractions are most likely to occur proximate to the procedure and as the tocolytic effect of volatile anesthetics wears off. The use of tocolytic drugs (e.g., magnesium, indomethacin) has not been studied perioperatively, but they are commonly used. Opioids can be used as needed to control postoperative pain, as a short course of opioids is unlikely to have any detrimental effect on the fetus, but consideration should be given to peripheral nerve blocks or epidural analgesia when possible. Nonsteroidal antiinflammatory drugs (NSAIDs) are often avoided due to concerns about an increased miscarriage rate and premature closure of the ductus arteriosus seen in some studies of chronic use, although short-term perioperative use is probably safe particularly before the third trimester.
The statement of the American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice titled “Nonobstetric Surgery During Pregnancy” recommends that an obstetric consult be obtained before surgery and that use of fetal monitoring be individualized. The only absolute recommendation in our institution’s policy on surgery during pregnancy is that every pregnant woman undergoing surgery must have an obstetrician on staff who is aware of her condition and able to consult as to the decisions regarding issues of fetal and labor monitoring, timing of surgery, and other issues that may arise perioperatively, including availability for possible emergent cesarean delivery. In consultation with the surgeon and anesthesiologist, the obstetrician can also make decisions about administration of corticosteroids to increase fetal lung maturity if preterm labor seems likely and the fetus is viable, and assess venous thromboembolism risk and recommend thromboprophylaxis, which is often indicated in pregnant women who are hospitalized.
Cardiac surgery requiring cardiopulmonary bypass (CPB) is rarely needed in pregnant women and when possible should be delayed until after pregnancy as maternal and fetal mortality after CPB are 3% to 13% and 16% to 38%, respectively. If surgery and CPB are unavoidable (aortic dissection, mechanical valve thrombus, severe valvular lesion with maternal decompensation, etc.), then the parameters shown to improve fetal outcomes are shorter CPB and aortic cross-clamp times, normothermia, higher pump flows (>2.5 L/min/m 2 ), perfusion pressure greater than 70 mm Hg, and maintaining the hematocrit above 28%. If cardiac surgery is necessary, concomitant cesarean delivery (if fetus is viable) or staged CPB may be the preferable strategy. Fetal heart monitoring may be useful intraoperatively as fetal arrhythmias or bradycardia can be treated by improving maternal hemodynamics. If cardiac surgery is performed in pregnancy, the second trimester has the best maternal and fetal outcomes. Continuous fetal monitoring and uterine contraction monitoring for 12 to 24 hours postoperatively is recommended as uterine contractions are the most important predictor for fetal demise, and contractions can occur on rewarming.
The issue concerning anesthesia for a breastfeeding woman, and what to tell her to do after the anesthetic, has been controversial for years. The concern is mostly that anesthetic drugs, when passed into breast milk and consumed by the breastfeeding infant, could be toxic or at least cause sedation or behavioral problems. Recommendations that breastfeeding women who receive anesthesia care express milk and discard it (“pump and dump”), for some period of time, often 24 hours, have been common over the past decades. With increasing understanding of the magnitude of drug transfer (very low in most cases) and with improved pharmacokinetic profiles of many anesthetics, combined with the further establishment of the benefits of breastfeeding, recommendations have changed significantly in the past few years. A strong consensus appears to have been reached that there is less of an issue than there used to appear to be, and almost all women are now advised the resume breastfeeding after surgery and anesthesia as soon as they are awake and able to breastfeed. A recent formal statement from the American Society of Anesthesiologists (ASA) and a detailed set of guidelines and recommendations from the Association of Anaesthetists of Great Britain and Ireland (AAGBI) both state that pump and dump is unnecessary.
The AAGBI document contains some of the most detailed and evidenced-based guidelines. It makes the following points: (1) Breastfeeding is of proven benefit to women and infants, (2) most drugs are transferred in very low quantities into breastmilk, (3) most anesthetic drugs leave the woman’s system or decrease in concentration very quickly (e.g., propofol, volatile anesthetic agents), (4) many recommendations have erred on the side of caution with little evidence that the advice to discard breast milk for 12 or 24 hours is based on science or clinical experience, and (5) as the infant will be ingesting any of the drugs of concern orally, and oral availability of most anesthetic/analgesic/sedative medications is low, there is a built-in element of safety ( Table 32.2 ).
| Proven benefits of breastfeeding to women and infants |
| Most drugs transferred in very low quantities to breast milk |
| Most modern anesthetic drugs leave the woman’s system or decrease in concentration very quickly (e.g., propofol, volatile anesthetic agents) |
| Infant consumes drugs orally; oral availability low |
| Limit doses of long-acting opioids and sedatives (benzodiazepines) when possible |
| Drugs with possible issues: tramadol, codeine, analgesic dose aspirin, sugammadex |
There are a few drugs that are controversial and might and usually can be avoided in breastfeeding women. Tramadol and codeine are both metabolized by cytochrome CYP2D6, and this metabolism is greatly affected by the specific genetic variant present; the US Food and Drug Administration (FDA) advises against their use in breastfeeding women, although similar agencies in other countries have not taken this position. As there are many other analgesics to choose from, it would seem that avoidance of these two should not be difficult. Benzodiazepines and other long-acting sedatives should be used in limited doses, but the usual perioperative use of these medications should be not be problematic. When necessary for postoperative analgesia, opioids can and should be used, but at the lowest effective doses, and the infant should be monitored for any signs of any sedation. Sugammadex is a large molecule probably not found in any significant concentration in breast milk, but it does interfere with pharmacologic contraception so could be avoided in women relying on that form of birth control.
Because this is a chapter about pregnancy as a “coexisting disease” when anesthesia care is required, we will not go into detail on general strategies of standard obstetric anesthesia management of labor pain or operative delivery in healthy pregnant women, a subject more properly accessed in books and review articles devoted exclusively to obstetric anesthesia and analgesia. We will focus on obstetric anesthesia in women with some of the more common coexisting diseases that occur or exist during pregnancy and that make anesthesia or analgesia care more complex for the anesthesiologist.
Ideally all patients, and especially patients with significant comorbidities, should be assessed by the anesthesiology team on admission to labor and delivery. The anesthesiology team should be aware of complex pregnant patients as early as possible (i.e., preferably before admission), but at a minimum a communications pathway and system should be established between the obstetric team and the covering anesthesia team to guarantee that the anesthesiology staff is informed when a patient is admitted and a complicated delivery is anticipated or when patient characteristics indicate increased anesthetic difficulties or risk ( Table 32.3 ). Medical comorbidities, including cardiac disease and obesity, now account for a large percentage of maternal morbidity and mortality. Pulmonary aspiration and failed intubation previously accounted for three-fourths of all maternal deaths related to anesthesia care, but significant advances in anesthetic care over the past 20 to 30 years, including the development of videolaryngoscopy, supraglottic airways, and the adoption of airway algorithms and (probably) better training of anesthesiologists, have led to a decline in the rate of aspiration and associated morbidity. In fact, there was only one case of aspiration associated with labor and delivery in the ASA’s Closed Claims Project database between 2005 and 2013. Researchers also noted that no cases of death due to aspiration were reported in the United Kingdom between 2000 and 2005, compared to 1.5 cases per 1000 during the 1940s. For patients with significant medical complications (e.g., pulmonary hypertension, severe left-sided obstructive lesions, severe cardiomyopathy, respiratory failure), consideration of transfer to a tertiary or quaternary medical center with relevant experience and facilities (e.g., maternal intensive care, cardiac surgery, extracorporeal membrane oxygenation [ECMO]) should be strongly considered.
| Morbid obesity (BMI >40) |
| Facial and neck edema |
| Extremely short stature |
| Airway issues |
| History of anesthesia complications/problems (e.g., malignant hyperthermia susceptibility, history of difficult intubation) |
| Significant scoliosis or history of major spine surgery |
| Significant cardiac, respiratory, or neurologic disease |
| Coagulopathy/thrombocytopenia (pathophysiologic or pharmacologic) |
| Severe allergy/anaphylaxis history |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here