Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When it comes to anesthetic care, children are not merely small adults. Many considerations come into play beyond underlying medical conditions: the child’s age, developmental stage (physically and psychologically), physiology, social circumstances, and family factors all play a significant role.
The topic of anesthesia-induced developmental neurotoxicity has garnered widespread attention in recent years. Mounting evidence from animal studies over the past 2 decades has consistently shown increased and accelerated neuroapoptosis upon exposure to virtually every known anesthetic agent. It is unclear whether the observed acute neuroapoptosis leads to permanent brain cell loss and how or if the degree of immediate neuronal injury correlates with subsequent neurocognitive sequelae. While inconclusive, results from studies to date generally suggest that repeated or prolonged anesthetic exposure at a young age (<3 years) may be associated with subsequent behavioral and learning difficulties. The data led to a black box warning on anesthetic agents in 2018. Recent results from a large international, multicenter, randomized controlled trial suggests a single exposure of short duration appears to have little consequence. Further high-quality evidence is needed before changes in practice can be justified. At present, no change in current practice is recommended for lifesaving and/or truly emergent or urgent procedures requiring anesthesia. However, anesthesiologists may consider delaying elective surgeries until after age 3 years when possible.
Anxiety is a normal and expected response to anticipation of surgery and anesthesia. In fact, anxiety is sometimes the only indication for sedation or anesthesia for nonpainful diagnostic procedures. An appreciation of circumstances and patient factors that contribute to preanesthetic anxiety is important in designing an approach that minimizes apprehension and potential psychological trauma.
Reasons for perianesthetic anxiety vary by age group. Separation anxiety becomes prominent around 8 to 10 months of age while fears of body disfigurement, loss of control, and death arise in older children. An understanding of the different categories of fears is essential in selecting the age-appropriate approach. Strategies for mitigating perianesthetic anxiety include but are not limited to medical play, distraction with toys/electronic devices, make-believe, storytelling, and pharmacologic agents.
Other psychological or behavioral concerns may influence perioperative management. Children affected by autism spectrum disorder (ASD) may struggle in the perioperative environment due to sensory sensitivities. Coordination with the child’s parent to determine the best course of action to mitigate anxiety and provide the least threatening experience possible for the child is essential: Each child with ASD has unique needs, and a single approach will not suffice for all. Other psychiatric concerns such as stress-related disorders (e.g., posttraumatic stress disorder), mood disorders, and disruptive behavior disorders (e.g., oppositional defiant disorder) may be present and need to be considered in order to individualize care.
Neonates and infants are vulnerable to perioperative hypothermia. Body heat is lost more rapidly in this age group than in older children or adults, owing to the ratio of large body surface area to body weight/volume, minimal subcutaneous fat, and a decreased ability to produce heat. Neonates are not able to shiver; they generate heat via nonshivering thermogenesis mediated by brown fat metabolism.
It is very difficult to reestablish normothermia once hypothermia ensues in newborns and infants. Prevention of hypothermia is essential. Strategies include minimizing exposure of skin (swaddling, hats, etc.), room warming, heat lamps, forced-air blankets, fluid warming, humidifiers in the anesthesia circuit, and minimizing fresh gas flows. Potential complications associated with intraoperative hypothermia include surgical wound infections, negative nitrogen balance, delayed wound healing, delayed postoperative anesthetic recovery, impaired coagulation, and prolonged hospitalization.
The airway of a term newborn differs in several ways from that of an adult. Newborns have a proportionally larger head and tongue, a larynx that is situated higher in the neck, a short and mobile epiglottis, and vocal cords whose anterior commissure is slanted inferiorly. Airway obstruction occurs more readily owing to the larger tongue size relative to the oral cavity. Infants are obligate nose breathers due to this anatomy: choanal atresia causes significant difficulties with breathing and feeding. The cricoid cartilage (as opposed to the vocal cords in adults) is the narrowest portion of the larynx in pediatric patients. As in adults, angulation of the right mainstem bronchus favors right endobronchial intubation if the tracheal tube is inserted beyond the carina. The importance of the smaller absolute dimensions of the upper and lower airways in newborns and infants cannot be overemphasized: One millimeter of airway edema in a 4-mm infant airway reduces cross-sectional area by 75% and increases resistance to airflow by a factor of 16. The relative size of the head and tongue decreases as the child ages, and the position of the larynx in the neck moves to the lower position as seen in adults during early childhood.
Significant physiologic differences exist between young children and adults. Oxygen consumption (VO 2 ) is much greater on a per kilogram basis in children compared to adults, owing to the difference in the ratio of surface area to volume. In addition, the high compliance of lung parenchyma and chest wall in newborns and infants predisposes to alveolar collapse, with resultant V/Q mismatching and hypoxemia.
Pulmonary vascular resistance (PVR) gradually decreases over the first several months of life, but the pulmonary vasculature remains reactive; PVR can increase dramatically under conditions of acidosis, hypoxemia, and hypercarbia. The foramen ovale and ductus arteriosus can reopen under these circumstances, with reversion to fetal circulatory patterns resulting in significantly decreased pulmonary blood flow and profound hypoxemia. Anatomic closure of the foramen ovale occurs between 3 months and 1 year of age, although 20% to 30% of adults have a probe-patent foramen ovale. Functional closure of the ductus arteriosus normally occurs 10 to 15 hours after birth, with anatomic closure taking place in 4 to 6 weeks. Ductus arteriosus constriction occurs in response to increased arterial oxygenation that develops after birth. Nevertheless, the ductus arteriosus may reopen during periods of arterial hypoxemia.
Heart rate is the main determinant of cardiac output and systemic blood pressure in neonates and young infants. Due to a relative decrease in contractile elements, contractility of the neonatal myocardium is decreased compared to that in older children and adults. Stroke volume is relatively fixed due to a paucity of elastic elements. The Frank-Starling mechanism is not operational under most circumstances. As such, increases in cardiac output in the newborn are dependent on increases in heart rate for the most part.
Total body water content and extracellular fluid (ECF) volume are increased proportionately in neonates. The ECF volume is equivalent to approximately 40% of body weight in neonates, compared with 20% in adults. By 18 to 24 months of age, the proportion of ECF volume relative to body weight is similar to that in adults. In addition to fluid replacement, newborns and young infants may also require glucose supplementation. Maintenance glucose requirement for newborns is 6 to 8 mg/kg/min. Term newborns are capable of maintaining normoglycemia for up to 10 hours with no exogenous glucose administration. Careful monitoring, with likely need for glucose replacement, is necessary for neonates born to diabetic mothers.
Perioperative fluid administration for pediatric patients can be divided into several components:
Replacement of fluid deficits from fasting
Maintenance fluid requirement
Replacement of blood loss
Replacement of evaporative losses
Fluid maintenance and replacement of deficits are based on the Holliday-Segar formula for caloric expenditure of children of different sizes. Caloric expenditure based on weight and water requirement is approximately 1 mL/kcal expended per day. This is the basis for the 4:2:1 rule ( Table 31.1 ). Blood loss is generally replaced 3:1 for each milliliter of blood loss with isotonic crystalloid, and evaporative loss replenishments are guided by estimations based on type of procedure and associated area of surgical exposure ( Table 31.2 ).
| Weight | Caloric Expenditure | Water Requirement | Fluid Maintenance a |
| 0–10 kg | 100 kcal/kg/day | 100 mL/kg/day | 4 mL/kg/h (for first 10 kg) |
| 10–20 kg | 50 kcal/kg/day | 50 mL/kg/day | 2 mL/kg/h (for second 10 kg) |
| ≥20 kg | 20 kcal/kg/day | 20 mL/kg/day | 1 mL/kg/h (for each additional kg above 20 kg) |
a Fluid maintenance rates are additive. For example, a 25-kg child requires 4 mL/kg/h for the first 10 kg (40 mL/h) plus 2 mL/kg/h for the second 10 kg (20 mL/h) plus 1 mL/kg/h for each additional kg above 20 kg (5 mL/h), totaling 65 mL/h (40 + 20 + 5) as the hourly fluid maintenance rate.
| NORMAL SALINE OR LACTATED RINGER SOLUTION (ML/KG/H) | |||
| Procedure | Maintenance | Replacement | Total |
| Minor surgery (e.g., herniorrhaphy) | 4 | 2 | 6 |
| Moderate surgery (e.g., pyloromyotomy) | 4 | 4 | 8 |
| Extensive surgery (e.g., bowel resection) | 4 | 6 | 10 |
The glomerular filtration rate is greatly decreased in term newborns but increases nearly fourfold by 3 to 5 weeks. Newborns are obligate sodium losers and cannot concentrate urine as effectively as adults. Therefore adequate exogenous sodium and water must be provided during the perioperative period. Conversely, newborns excrete volume loads more slowly than adults and are more susceptible to fluid overload. Decreased renal function can also delay excretion of drugs dependent on renal clearance for elimination.
At term, the liver has significant glycogen stores that can be converted to glucose for use by the neonate. The newborn’s glycogen stores, on a per kilogram basis, are at least equal to the stores in most adults. Hepatic capacity for biotransformation and metabolism of drugs, however, is diminished until several months of age.
The hematologic system undergoes significant changes after birth. In fetal life the lower P 50 of fetal hemoglobin (Hb) allows the fetus to extract O 2 from maternal Hb. In the first 2 months of life, as fetal Hb is replaced by adult Hb, P 50 increases from 19 mm Hg to 22 mm Hg and then eventually to the typical adult level of 26 mm Hg. In addition to the change in Hb type (fetal to adult), Hb concentration changes as well. Physiologic anemia occurs between 2 and 3 months of age. In view of the decreased cardiovascular reserve of neonates and the leftward shift of the oxyhemoglobin dissociation curve, it may be useful to maintain the neonate’s hematocrit (Hct) closer to 40% than 30%, as is often accepted for older children. Typical blood cell values are delineated in Table 31.3 .
| Age | Hemoglobin (g/dL) | Hematocrit (%) | Leukocytes (1000/mm 3 ) |
| Cord blood | 14–20 | 45–65 | 9–30 |
| Newborn | 13–20 | 42–66 | 5–20 |
| 3 months | 10–14 | 31–41 | 6–18 |
| 6 months to 12 years | 11–15 | 33–42 | 6–15 |
| Young adult male | 14–18 | 42–52 | 5–10 |
| Young adult female | 12–16 | 37–47 | 5–10 |
There is no need for routine preoperative Hb determination in healthy children for surgeries not expected to produce significant blood loss. Preoperative Hb measurement may be prudent in symptomatic children ahead of major surgery or ahead of lesser procedures necessary within the window of physiologic anemia. Based on estimated blood volume ( Table 31.4 ), calculation of the maximal allowable blood loss is useful to guide transfusion therapy ( Table 31.5 ).
| Age Group | Estimated Blood Volume (mL/kg) |
| Premature neonate | 90–100 |
| Term neonate | 80–90 |
| Infants | 75–80 |
| Children >1 year | 70–75 |
| A 3-kg term neonate is scheduled for intraabdominal surgery. The preoperative Hct is 50%. What is the maximum allowable blood loss (MABL) to maintain the Hct at 40%? |
| MABL− EBV × [(Hct high − Hct low )/Hct average ] |
| EBV = 3 kg × 85 mL/kg = 255 mL |
| Hct high − Hct low = 50% − 40% = 10% |
| Hct average = (50% + 40%)/2 = 45% |
| MABL = 255 mL × [(50% − 40%)/45%] = 56.1 mL |
a These calculations are only guidelines and do not consider the potential impact of fluid infusion therapy on the measured Hct.
Pharmacologic responses to drugs may differ in pediatric patients and adults. They manifest as differences in anesthetic requirements, response to muscle relaxants, and pharmacokinetics.
Full-term neonates require lower concentrations of volatile anesthetics than infants aged 1 to 6 months. Furthermore, the minimum alveolar concentration (MAC) in preterm neonates decreases with decreasing gestational age. MAC steadily increases until age 2 to 3 months, but after 3 months the MAC steadily declines with age, although there are slight increases at puberty. Sevoflurane is unique among the currently used volatile anesthetics. The MAC of sevoflurane in neonates and infants remains constant.
Despite immature neuromuscular junctions, greater total body water content, and immature muscle composition in young infants, the dosage of nondepolarizing neuromuscular blocking agents is not changed on a per kilogram basis compared to adults. Duration of action of these drugs may be prolonged; the use of train-of-four monitoring is critical to determine depth of blockade. Antagonism of neuromuscular blockade is generally unaffected in infants, but requirements for anticholinergics may be decreased owing to longer clearance times than in adults. Sugammadex is becoming frequently used in pediatric patients; however, careful consideration of associated risks of bradycardia, anaphylaxis, recurarization, and interference with contraception is warranted and may require additional patient counseling. Neonates and infants require more succinylcholine on a per kilogram basis than do older children to produce similar degrees of neuromuscular blockade; this is due to the increased ECF and larger volume of distribution characteristic of this age group.
Pharmacokinetics differ in neonates and infants compared with adults. For example, uptake of inhaled anesthetics is more rapid in infants than in older children or adults because of the infant’s high alveolar ventilation relative to functional residual capacity. More rapid uptake may unmask negative inotropic effects of volatile anesthetics, resulting in an increased incidence of hypotension in neonates and infants upon inhalational induction of anesthesia.
An immature blood-brain barrier and decreased ability to metabolize drugs could increase the sensitivity of neonates to the effects of hypnotics. As a result, neonates might require lower doses of intravenous (IV) induction agents. On the other hand, older children and adolescents generally require a higher dose of IV induction agents compared to adults (up to 3 mg/kg of propofol in children and teenagers compared to 1.5–2 mg/kg for adults).
Decreased hepatic and renal clearance of drugs, which is characteristic of neonates, can produce prolonged drug effects. Clearance rates increase to adult levels by age 5 to 6 months, and during early childhood may even exceed adult rates. Protein binding of many drugs is decreased in infants, which could result in high circulating concentrations of unbound and pharmacologically active drugs.
The majority of children tolerate general anesthesia without incident. However, cardiac arrests do occasionally occur. Respiratory events are by far the most common cause of pediatric cardiac arrest. Many arrests also result from either the critical health condition of the patient (especially complex congenital heart disease) or surgically related complications. The incidence of anesthesia-related cardiac arrest reported in infants is 15:10,000, with a range of 9.2 to 19:10,000. Overall, children experience anesthesia-related cardiac arrest at a rate of 3.3:10,000 anesthetics. The incidence of anesthesia-related cardiac arrest reported for all pediatric age groups is 1.8:10,000.
More than 50% of arrests occur among infants. Patients with congenital heart disease are at significantly higher risk of perioperative cardiac arrest while undergoing noncardiac procedures. High American Society of Anesthesiologists (ASA) physical status and emergency status have been shown to be independent negative predictors of survival from perioperative cardiac arrest. Accidental IV injection of local anesthetic and local anesthetic systemic toxicity are also common causes.
Management of a perioperative cardiac arrest depends on its cause. Initial management is guided by the same principles used for any pediatric cardiac arrest. Certification in pediatric advanced life support (PALS) is recommended for anesthesiologists regularly caring for infants and children. The reader is referred to the latest PALS algorithm published by the American Heart Association ( https://cpr.heart.org/en/resuscitation-science/cpr-and-ecc-guidelines/algorithms ). An underlying respiratory cause of cardiac arrest should always be sought. The overall outcome for children following anesthesia-related cardiac arrest is much better than for in-hospital nonanesthesia-related arrests with respect to survival and development of new neurologic deficits.
As defined by the Committee on Fetus and Newborn of the American Academy of Pediatrics, preterm newborns are classified based on gestational age rather than birth weight as in the past. Preterm morbidity also correlates better with gestational age than with birth weight. A preterm newborn is one born before 37 weeks of gestation. Table 31.6 illustrates the traditional classification of preterm newborns by weight and the related approximate gestational age. The term ELGAN (extremely low-gestational-age newborn) refers to a preterm newborn delivered before 28 weeks of gestation regardless of birth weight. ELGANs have immaturity of all organ systems and represent the most vulnerable of all pediatric patients, with the highest morbidity and mortality. Age terminology for preterm neonates and infants is defined in Table 31.7 .
| Weight-Based Category a | Birth Weight (g) | Estimated Gestational Age (weeks) |
| LBW | <2500 | 31–35 |
| VLBW | 1000 to <1500 | 26–30 |
| ELBW | <1000 | <26 |
a ELBW, Extremely low birth weight; LBW, low birth weight; VLBW, very low birth weight. VLBW and ELBW newborns are considered micropremies.
| Term | Definition |
| Gestational age (GA) | First day of LMP to birth in weeks |
| Chronologic age (CA) | Time since birth in weeks or months |
| Postmenstrual age | GA + CA in weeks or months |
| Corrected postconceptual age | CA − (40 − GA) in weeks or months |
Newborns are classified as small, appropriate, or large for gestational age based on normal values established for weight at various gestational stages.
Lack of surfactant leads to development of neonatal respiratory distress syndrome (RDS). The incidence is inversely proportional to the gestational age and birth weight. Sufficient surfactant is present in most fetuses by 35 weeks of gestation; however, 5% of newborns diagnosed with RDS are born at term.
RDS is usually apparent within minutes of birth; it is evidenced by tachypnea, prominent grunting, intercostal and subcostal retractions, and nasal flaring. Grunting reflects the newborn’s effort to mitigate alveolar collapse. Cyanosis and dyspnea progressively worsen. If untreated, apnea and irregular respirations, signs of impending respiratory failure, develop. The clinical course, chest radiograph, and blood gas analysis help establish the clinical diagnosis of RDS.
Surfactant is administered to preterm newborns either immediately in the delivery room or later as a rescue treatment. It increases lung compliance and stabilizes the alveoli at end exhalation. Surfactant administration decreases the need for high concentrations of inspired oxygen, ventilatory support, and high ventilatory pressures. Unfortunately it has not decreased the incidence of subsequent chronic lung disease or bronchopulmonary dysplasia (BPD). Current evidence also supports nasal continuous positive airway pressure (NCPAP) as the optimal treatment for newborns with RDS. It is proven to reduce the risk of death and BPD. Other strategies such as noninvasive positive pressure ventilation and high-flow nasal cannula are useful in special situations. Caffeine is also a mainstay of RDS treatment.
During anesthesia the arterial oxygen saturation should be maintained near its preoperative levels. An arterial catheter (ideally in a preductal artery) is useful to monitor oxygenation, avoid hyperoxia, and prevent respiratory and metabolic acidosis during the intraoperative and postoperative periods. Pneumothorax from barotrauma is an ever-present danger and should be considered if there is sudden cardiorespiratory decompensation. Maintaining the Hct near 40% helps optimize systemic oxygen delivery. Excessive hydration should be avoided; fluid resuscitation using smaller total volumes of colloids such as 5% albumin (10–20 mL/kg increments) should be considered over crystalloids.
BPD is a form of chronic lung disease of infancy. As mentioned, the incidence of chronic lung disease in ex–preterm newborns has not decreased despite widespread use of surfactant in the treatment of RDS.
BPD is a clinical diagnosis defined as oxygen dependence at 36 weeks of postconceptual age (PCA) or oxygen requirement (to maintain Pao 2 >50 mm Hg) beyond 28 days of life in infants with birth weights under 1500 g. Pulmonary dysfunction in patients with BPD is most pronounced during the first year of life. Infants with mild BPD may eventually become asymptomatic, but airway hyperreactivity frequently persists into adulthood.
Maintenance of adequate oxygenation (Pao 2 >55 mm Hg and Spo 2 >94%) is necessary to prevent or treat cor pulmonale and to promote growth of lung tissue and remodeling of the pulmonary vascular bed. Reactive airway bronchoconstriction is treated with bronchodilating agents. Diuretic administration is often needed to treat interstitial fluid retention and pulmonary edema to improve gas exchange.
Preoperative assessment of the child with BPD should focus on any recent respiratory decompensation and need for intervention. Ongoing drug therapy (bronchodilators, diuretics) as well as baseline oxygen saturations provide valuable clues to the severity of BPD. In children with a history of mechanical ventilation, an endotracheal tube (ETT) one to a half-size smaller than that predicted for age should be used because subglottic stenosis may be present. Tracheomalacia and bronchomalacia may also present as sequelae of past prolonged intubation. Airway hyperreactivity is likely; thus a deep plane of anesthesia, often including neuromuscular blockade, must be established prior to airway instrumentation. Indeed, children with active or prior BPD can be assumed to have lifelong airway hyperreactivity and should be treated similarly to those with asthma. Oftentimes increased peak inspiratory pressures (PIPs) are required, reflecting decreased pulmonary compliance. Adequate oxygen should be delivered to maintain a Pao 2 of 50 to 70 mm Hg. Patients with metabolic alkalosis from furosemide therapy may exhibit a compensatory retention of CO 2 . Fluid should be administered judiciously to avoid pulmonary edema.
Laryngomalacia is a congenital or acquired condition of excessive flaccidity of the laryngeal structures, especially the epiglottis and arytenoids. It can result from lack of normal neural control of laryngeal muscles or from pressure on the laryngeal cartilage, leading to inadequate laryngeal rigidity and structural collapse with normal respiratory efforts. Laryngomalacia accounts for more than 70% of persistent stridor in neonates and young infants.
Bronchomalacia is seen in infants who have had a prolonged course in the neonatal intensive care unit (NICU). Risk factors include long periods of mechanical ventilation, poor nutrition, intercurrent infections, and other impediments to normal growth and development. The cartilage of the major airways is weakened; when affected infants bear down, these airways can collapse partially or completely. Infants with bronchomalacia generally also have a component of BPD. These two conditions together can lead to significant respiratory difficulties. Even a mild viral respiratory infection may worsen the situation sufficiently to require hospitalization.
Retinopathy of prematurity (ROP) is a retinal disorder of pathologic vasculogenesis affecting preterm infants. It is a leading cause of childhood blindness and considerable visual morbidity worldwide. The risk of retinopathy is inversely related to birth weight and gestational age, occurring in up to 70% of premature infants weighing less than 1000 g at birth.
Approximately 80% to 90% of mild cases of ROP undergo spontaneous regression with little or no residual visual disability. However, infants with ROP have an increased risk of developing visual and retinal problems later in life, including myopia, amblyopia, strabismus, glaucoma, retinal tear, and retinal detachment.
Laser photocoagulation of the peripheral retina is the mainstay of ROP treatment. Central vision is preserved at the expense of variable peripheral visual field loss. Scleral cryotherapy and lens-sparing vitrectomy have also been used with success.
When dealing with an infant with ROP there are challenges of limiting hyperoxia while avoiding hypoxemia. Currently there are no established guidelines for specific intraoperative goals of oxygen saturation for preterm infants presenting for surgery.
The optimal intraoperative oxygen saturation for these patients has yet to be determined, so it remains prudent to limit oxygen supplementation for preterm infants with or without ROP, especially in those less than 32 weeks of PCA. Supplemental oxygen should be used judiciously based on the patient’s clinical needs. Many advocate for maintaining a stable intermediate range of oxygenation (89–94%).
Apnea of prematurity (AOP) is a result of the immaturity of the respiratory control centers in the newborn brainstem. The severity of AOP is inversely proportional to the gestational age of the newborn at birth.
Affected newborns exhibit both primary (central) apnea, in which there is simply a lack of effort to breathe in the absence of any obstruction, and obstructive apnea. Mixed episodes of central and obstructive apnea are also seen. The CO 2 response of infants with AOP is decreased compared to infants without AOP. Diagnosis of AOP is made on clinical grounds, and the criteria are somewhat variable. The diagnosis is made if an infant exhibits apnea longer than 15 to 20 seconds, apnea associated with heart rates below 80 to 100 beats per minute, or apnea associated with significant decreases in oxygen saturation.
Treatment of AOP is begun once other causes of apnea (e.g., infection, central nervous system [CNS] disorder) have been eliminated. Some cases of AOP are associated with anemia and resolve with treatment. Other nonpharmacologic treatments include nasal CPAP and, in very severe cases, mechanical ventilation. Methylxanthines are the mainstay of drug therapy for AOP. These central stimulants increase the sensitivity of the respiratory centers to CO 2 . Various forms of methylxanthines are used, with caffeine being the most common.
Postanesthetic apnea has many similarities with AOP. Preterm newborns who are at risk for AOP based on their corrected PCA are also at increased risk for developing postanesthetic apnea. The incidence is inversely related to PCA. Regional anesthesia without the addition of systemic sedatives and opioids may decrease the risk for postanesthetic apnea in at-risk infants. Regardless, it is recommended to keep formerly premature infants whose PCA is less than 50 to 60 weeks for overnight observation after anesthesia. Term infants over 44 weeks PCA may qualify for outpatient surgery.
Hypoglycemia is the most common metabolic problem occurring in newborns and young infants, with many different causes ( Table 31.8 ). Inadequate glycogen stores and immature gluconeogenesis are important risk factors. The incidence of symptomatic hypoglycemia is highest in those born small for gestational age (SGA).
| Maternal Factors |
| Intrapartum administration of glucose |
| Drug treatment |
|
| Maternal diabetes/gestational diabetes |
| Neonatal Factors |
| Depleted glycogen stores |
|
| Increased glucose utilization (metabolic demands) |
|
| Limited glycogen stores |
|
| Hyperinsulinism/endocrine disorders |
|
| Decreased glycogenolysis/gluconeogenesis/utilization of alternate fuels |
|
Serum glucose levels are rarely below 35 to 40 mg/dL in the first 24 hours of life or below 45 mg/dL thereafter. CNS or systemic signs of hypoglycemia such as agitation, seizures, apnea, lethargy, or mottling and pallor will usually be observed when serum glucose concentrations fall below 30 to 40 mg/dL in term infants during the first 72 hours and less than 40 mg/dL thereafter.
Infants with symptoms other than seizures should receive an IV bolus of 2 mL/kg (200 mg/kg) of 10% dextrose. If the infant is experiencing convulsions, an IV bolus of 4 mL/kg of 10% dextrose is indicated. Following bolus administration, a 10% dextrose infusion should be continued at 8 mg/kg/min and titrated to maintain the serum glucose above 40 to 50 mg/dL.
Those at particular risk of hypocalcemia are neonates born prematurely or with low birth weight, particularly neonates with intrauterine growth retardation, neonates of insulin-dependent diabetic mothers, and neonates with birth asphyxia associated with prolonged and difficult deliveries. Late neonatal hypocalcemia occurring 5 to 10 days after birth is usually due to ingestion of cow’s milk, which contains high levels of phosphorous; it is not seen in breast-fed infants as human breast milk has a lower phosphate content.
The clinical manifestations of hypocalcemia include irritability, seizures, and lethargy; hypocalcemic tetany is very uncommon. Under anesthesia, hypocalcemia will manifest as hypotension and depressed cardiac performance. Treatment with IV calcium should be considered in newborns presenting with hypotension without an obvious cause. It is important to evaluate both total and ionized calcium.
Management of hypocalcemia involves correction of hypocalcemia as well as hypomagnesemia and any other metabolic or acid-base abnormalities. Intravenous calcium dosage is based on the amount of elemental calcium administered. The starting dose is 10 to 20 mg/kg of elemental calcium. Calcium gluconate 10% provides 9 mg/mL of elemental calcium, and calcium chloride provides 27.2 mg/mL of elemental calcium. These doses have been shown to increase ionized calcium, blood pressure, and cardiac contractility.
Bradycardia and even asystole have been reported with rapid IV administration of calcium. As such, IV calcium should be given over 5 to 10 minutes with electrocardiographic (ECG) monitoring. If calcium is given via an umbilical venous line, the tip should be confirmed to be in the inferior vena cava and not too near the right atrium; administration of calcium too close to the heart can result in dysrhythmias.
Congenital diaphragmatic hernia (CDH) is a defect in the diaphragm that is associated with a variable amount of intraabdominal organ extrusion into the thoracic cavity. It has an incidence between 1:2500 and 1:3000 live births. Concomitant anomalies are seen in approximately 50% of CDH cases. Some associated congenital syndromes include Beckwith-Wiedemann, CHARGE ( c oloboma, h eart defects, a tresia of the choanae, r etardation [intellectual disability], g enital anomalies, and e ar anomalies), and trisomies 21 and 18.
Prenatal diagnosis of CDH has increased from approximately 10% in 1985 to nearly 60% in present day. The most common findings include displacement of the heart and gastrointestinal segments into the thorax. Most newborns present in the first few hours of life with respiratory distress (from mild dyspnea to cyanosis) and apparent dextrocardia in left-sided lesions. Typical physical findings include a scaphoid abdomen and a barrel-shaped chest with decreased breath sounds, distant or rightward displaced heart sounds, and bowel sounds in the chest. A chest radiograph typically shows a bowel gas pattern in the chest and a mediastinal shift.
Pulmonary parenchymal and vascular hypoplasia increase PVR. This causes right-to-left shunting of blood through the ductus arteriosus, with persistence of fetal circulatory patterns. Persistent pulmonary hypertension (HTN) of the newborn ensues, and if uncorrected, permanent pulmonary HTN follows.
Care of a newborn with a severe CDH starts immediately in the delivery room. Prompt endotracheal intubation and placement of a naso/orogastric tube for decompression of the stomach are recommended for most cases to minimize lung and even heart compression. Once considered a surgical emergency, the current approach aims at medical stabilization before surgical repair.
Specific goals of preoperative medical management include achievement of a preductal oxygen saturation of at least 90% and correction of metabolic acidosis. Crystalloid fluid and blood products are administered to maintain intravascular volume and red blood cell mass. Adequate sedation is administered in an effort to minimize increases in PVR. Mechanical ventilation should be accomplished with the lowest settings possible (goal, PIP <25 cm H 2 O), allowing for moderate permissive hypercarbia in an effort to minimize ventilator-induced lung injury. Surgery should be delayed until PVR has decreased and ventilation can be maintained with low PIPs and reasonable supplemental oxygen requirement. If pulmonary HTN persists or recurs, trials of inhaled nitric oxide and high-frequency oscillatory ventilation are initiated; extracorporeal membrane oxygenation (ECMO) may also be considered. In the case of severe pulmonary hypoplasia, an ex-utero intrapartum treatment (EXIT) procedure may be planned.
Endotracheal intubation should be carried out with avoidance of gastric distention. Avoiding positive pressure mask ventilation, the patient should undergo a rapid-sequence induction of anesthesia followed by tracheal intubation. “Awake” intubation should only be considered when no alternative is possible; it results in a sudden increase in PVR with agitation and ingestion of air with crying, thereby increasing risks of right-to-left shunting and lung compression, respectively. In addition to routine monitors, two pulse oximeters (pre- and postductal locations) are useful to monitor the degree of shunting. A preductal arterial cannulation (right radial) is recommended for monitoring systemic blood pressure, acid-base status, and other blood analyses. Venous access should be avoided in the lower extremities in case venous return is impaired from compression of the inferior vena cava following reduction of the hernia.
Anesthesia can be maintained with intravenous agents (e.g., opioids, ketamine, dexmedetomidine, benzodiazepines), a nondepolarizing muscle relaxant, and (if tolerated) low concentrations of inhaled anesthetics. Nitrous oxide should be avoided. In the case of EXIT procedure, extensive planning and coordination with all involved parties (surgical team, obstetric team, pediatric and obstetric anesthesia teams as well as the operating room [OR] nursing/technical staff and NICU team) is necessary to ensure safety of both mother and newborn.
Repair of CDH via thoracoscopic approach is becoming more common, but open repair is still the most common. Newborns with CDH have, almost by definition, pulmonary dysfunction that limits the patient’s ability to tolerate thoracoscopy and associated CO 2 insufflation and one-lung ventilation. The primary advantages of thoracoscopic repair are smaller surgical incisions, less postoperative pain, and decreased risk of long-term thoracic and rib deformities. However, thoracoscopic repairs tend to be longer in duration and are very challenging for the anesthesiologist, since compromise of cardiorespiratory functions may be even more significant than that seen with open repairs. The two most obvious challenges are lung compression and significant hypercarbia from CO 2 insufflation, further worsening ventilation with resultant respiratory acidosis that increases PVR.
In the open surgical technique, reduction of the diaphragmatic hernia is accomplished through either a left subcostal abdominal incision or a thoracotomy incision. Depending upon the size of the defect, prosthetic material may be used to close the diaphragm. Throughout the intraoperative course, airway pressures should be monitored and maintained below 25 to 30 cm H 2 O to minimize the risk of barotrauma and pneumothorax. After reduction of the herniated contents, an attempt to inflate the hypoplastic lung is not recommended; it is unlikely to expand, and excessive positive airway pressures may damage the contralateral lung. In addition to lung hypoplasia, these neonates are likely to have an underdeveloped abdominal cavity. Hernia reduction can cause increased intraabdominal pressure, with cephalad displacement of the diaphragm, decreased functional residual capacity, and compression of the inferior vena cava. To prevent excessively tight abdominal surgical closures in infants with large defects, it is often necessary to create a ventral hernia (which can be repaired later) and close the skin or place a silastic pouch.
Postoperative management of neonates with CDH presents significant challenges. The long-term outcome of these patients is ultimately determined by the degree of pulmonary hypoplasia. There is no effective treatment for pulmonary hypoplasia.
Esophageal atresia (EA) is the most frequent congenital anomaly of the esophagus, with an approximate incidence of 1 in 4000 neonates ( Fig. 31.1 ). More than 90% have an associated tracheoesophageal fistula (TEF). The most common form of EA/TEF (type C) represents 90% of all cases and presents as a blind upper esophageal pouch and a distal esophagus that connects to the trachea via a fistula tract, typically on the posterior aspect near the carina.
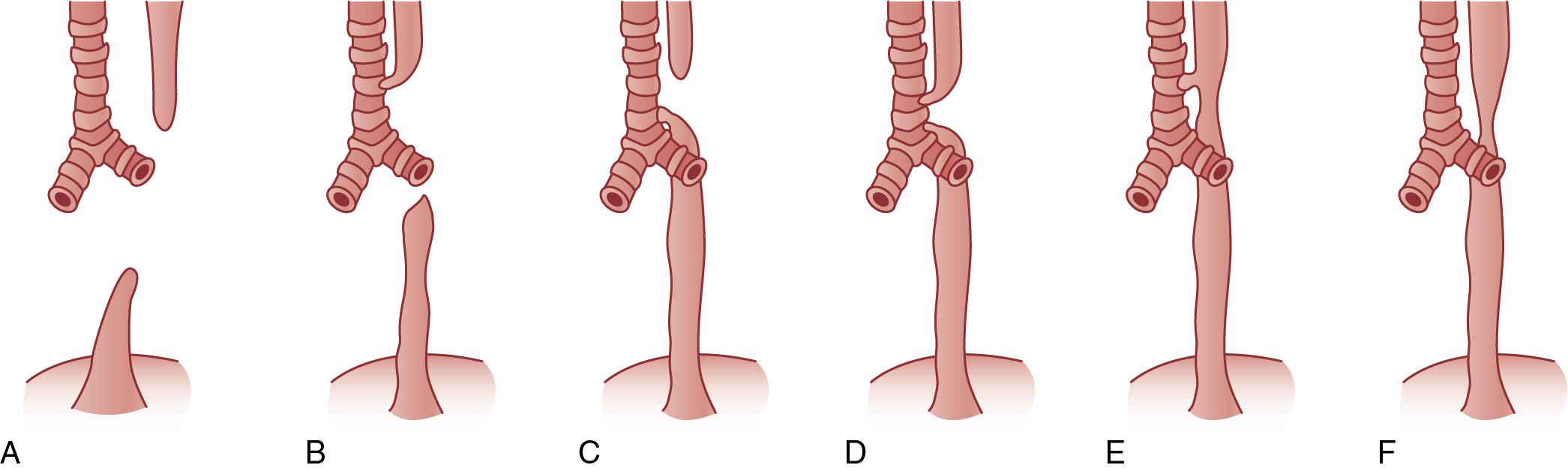
More than 25% of infants with EA have other congenital anomalies, most often with the VATER ( v ertebral defects, imperforate a nus, t racheo e sophageal fistula, and r enal dysplasia) or VACTERL (VATER with c ardiac and l imb anomalies) associations. TEF and EA are also commonly seen in some chromosomal abnormalities such as trisomies 13, 18, and 21. Approximately 20% of neonates with EA have major congenital heart defects, and 30% to 40% are born preterm.
EA should be suspected if maternal polyhydramnios is present. It is usually diagnosed soon after birth when an oral catheter cannot be passed into the stomach or when the neonate exhibits cyanosis, coughing, and choking during oral feedings. Plain radiographs of the chest and abdomen will reveal coiling of a nasogastric tube in the esophageal pouch and possibly an air-filled stomach in the presence of a coexisting TEF. In contrast, pure EA may present as an airless scaphoid abdomen. Infants with an isolated TEF without EA may elude diagnosis until later in life when they may present with recurrent pneumonias and refractory bronchospasm.
Initial therapeutic measures include maintaining a patent airway and preventing aspiration of secretions. The infant is fed nothing by mouth, given IV fluids, and placed in a head-up position to minimize regurgitation of gastric secretions through the fistula. Continuous suctioning of the proximal esophageal segment prevents aspiration of oropharyngeal secretions. Endotracheal intubation is avoided if possible because of the potential to worsen distention of the stomach, which can lead to gastric rupture. One-lung ventilation may be necessary until the stomach can be decompressed.
Primary repair without initial gastrostomy is routine. Repair of a TEF is urgent. A thorough evaluation for associated anomalies, particularly congenital heart disease, should be undertaken preoperatively. If the newborn is too unstable for a complete primary repair, a staged approach with an initial gastrostomy under local anesthesia may be all the neonate can safely tolerate.
Minimally sedated fiberoptic intubation with preservation of spontaneous respiration allows for optimal positioning of the ETT while minimizing the risk of ventilatory impairment associated with gastric distention due to positive pressure ventilation and passage of gases through the fistula. Awake intubation is not recommended unless there is no alternative. Induction of anesthesia can also be done via the inhalational or IV route. Regardless, spontaneous respiration should be maintained as much as possible, with positive pressure ventilatory maneuvers kept to the lowest possible inspiratory pressures. Use of muscle relaxant should be avoided or at least delayed until decompressive gastrostomy is accomplished. Proper placement of the ETT is critical; it should be above the carina but below the TEF. The ETT must be above the carina because the right lung is compressed during thoracotomy. The ETT may also be subject to obstruction with secretions during surgical manipulation or displacement into the fistula; great vigilance must be exercised to ensure adequate ventilation and delivery of volatile anesthetics. Low-dose volatile anesthetics in conjunction with air/O 2 /opiate are usually well tolerated if the neonate is adequately hydrated. In addition to routine monitors, an arterial catheter is useful for blood gas monitoring. Ligation of the TEF and primary esophageal anastomosis is usually performed via a right thoracotomy. Thoracoscopic approach is also becoming more common.
Omphalocele and gastroschisis are defects of the anterior abdominal wall that permit external herniation of the abdominal viscera. They are the most common congenital abdominal wall defects, with important differences between them ( Table 31.9 ).
| Omphalocele | Gastroschisis | |
| Gender distribution | Male > female | Male = female |
| Preterm birth | 30% | 60% |
| Location | Within umbilical cord | Periumbilical cord (right) |
| Sac | Present | Absent |
| Associated anomalies | >50% (cardiovascular 20%) | Rare |
| Surgical intervention | Not urgent | Urgent |
| Prognostic factors | Associated anomalies | Condition of bowel |
Omphalocele manifests as external herniation of abdominal viscera through the base of the umbilical cord. By definition the defect is larger than 4 cm. A defect smaller than 4 cm is termed an umbilical hernia . The abdominal contents are contained within a sac formed by the peritoneal membrane internally and the amniotic membrane externally, without overlying skin. Most cases involve only intestinal herniation, with herniation of liver and intestine occurring half as frequently. Over 50% of cases are associated with other congenital structural or chromosomal anomalies. Approximately 30% of neonates with omphaloceles are born preterm. Cardiac defects and prematurity are the major causes of mortality.
Gastroschisis manifests as external herniation of abdominal viscera through a small (usually <5 cm) defect in the anterior abdominal wall. In most cases the defect occurs laterally, just to the right of the normally inserted umbilical cord. Unlike an omphalocele, a hernia sac is absent, and the exposed viscera is in direct contact with amniotic fluid.
In most cases, only intestines are herniated. Gastroschisis is rarely associated with other congenital anomalies except for intestinal atresia, which occurs in 10% of cases.
Gastroschisis requires urgent surgical intervention to limit evaporative and thermal losses. Upon delivery, the exposed viscera is at once covered in warm saline-soaked gauze, with careful positioning of the newborn to prevent kinking of the mesentery. Options for surgical management include traditional primary closure, staged closure with prefabricated silos, and more recently sutureless or plastic closure repair, where the umbilical cord itself is used to cover the abdominal wall defect, which is then allowed to close with epithelialization and granulation over time. The introduction of these sutureless techniques can often avoid intubation and anesthesia in some patients (smaller, uncomplicated defects).
Decompressing the stomach with an oro/nasogastric tube decreases risks of regurgitation, aspiration pneumonia, and further bowel distention. Broad-spectrum antibiotic prophylaxis is initiated along with fluid resuscitation to replace evaporative losses (150–300 mL/kg/day). As there is considerable protein loss and third-space fluid translocation, protein-containing solutions (5% albumin) should constitute approximately 25% of the replacement fluids. A urinary catheter should be placed to monitor a goal of 1 to 2 mL/kg/hr of urine output.
Initial medical management of omphalocele is similar to that of gastroschisis. Surgical intervention is essential but not urgent; the hernia sac provides some protection against evaporative and thermal losses. The high incidence of coexisting congenital anomalies warrants a thorough preoperative evaluation of the major organ systems, especially the heart. Although primary closure is desirable, it is not possible in most cases, owing to the large defect size and the unacceptable increase in intraabdominal pressure with one-stage reduction. Abdominal compartment syndrome can ensue with respiratory compromise, decreased venous return, poor organ perfusion, anuria, profound acidosis, and bowel necrosis. If primary closure is deemed not feasible, the viscera should be covered with a prosthetic silo and then slowly reduced over a period of days to weeks.
Important aspects of anesthetic management for omphalocele and gastroschisis closure include preservation of normothermia and fluid resuscitation. Intubation is best achieved with a rapid-sequence induction. The ETT should allow for ventilation with PIP greater than 20 cm H 2 O. Primary closure may require higher PIPs, at least in the initial postoperative period. Repair of a large defect will require maximal muscle relaxation intraoperatively and during the initial postoperative period. Nitrous oxide is avoided because of its potential to hinder hernia reduction from potential bowel distention. Given the underdeveloped abdominal cavity, tight surgical abdominal closure can result in compression of the inferior vena cava and decreased diaphragmatic excursion. Monitoring airway pressures is helpful for detecting changes in pulmonary compliance during abdominal closure. Primary closure is not recommended if inspiratory pressures are above 25 to 30 cm H 2 O or if intravesical or intragastric pressures are above 20 cm H 2 O. Changes in ventilatory parameters and oxygen requirement can help the surgeon decide on treatment strategy. High ventilatory pressures and excessive Fio 2 are indications for postponing immediate abdominal closure.
Evidence of unacceptably high intraabdominal pressure requires removal of fascial sutures and closure of only the skin or addition of a prosthesis such as a silo, which consists of a silastic or Teflon mesh that is sutured to the fascia of the defect. After the silo is in place, the herniated viscera is gradually returned to the peritoneal cavity over successive days and can often be done at the bedside without anesthesia. Final closure is typically done in the OR.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here