Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuromuscular blockade is used extensively in pediatric anesthesia and intensive care settings to optimize conditions for endotracheal intubation, ventilation, and surgical and diagnostic procedures. A thorough understanding of the maturational changes in neuromuscular anatomy and physiology, along with an understanding of age-related responses to neuromuscular blocking agents (NMBAs), is fundamental to the safe and effective use of these agents in children.
Fundamentally, the mechanisms of neuromuscular transmission are the same in children and adults ( Fig. 13.1 ) ( ; ; ; ). Acetylcholine synthesized by the nerve cell is stored in discrete vesicles at the terminal of a motor neuron. When an electrical impulse reaches the presynaptic nerve terminal, calcium influx causes stored acetylcholine to be released into the synaptic cleft between the nerve and the surface of the skeletal muscle. Acetylcholine molecules cross the cleft to bind to nicotinic acetylcholine receptors embedded in the postsynaptic muscle membrane, causing a conformational change in the receptor that briefly opens an ion channel, allowing sodium and calcium ions to enter and potassium to exit the postsynaptic muscle cell. Net entry of ions depolarizes the muscle membrane, opening voltage-gated sodium channels that further depolarize the muscle cell to a point above the threshold to generate an action potential. Propagation of the action potential along the muscle fiber ultimately results in muscle contraction.
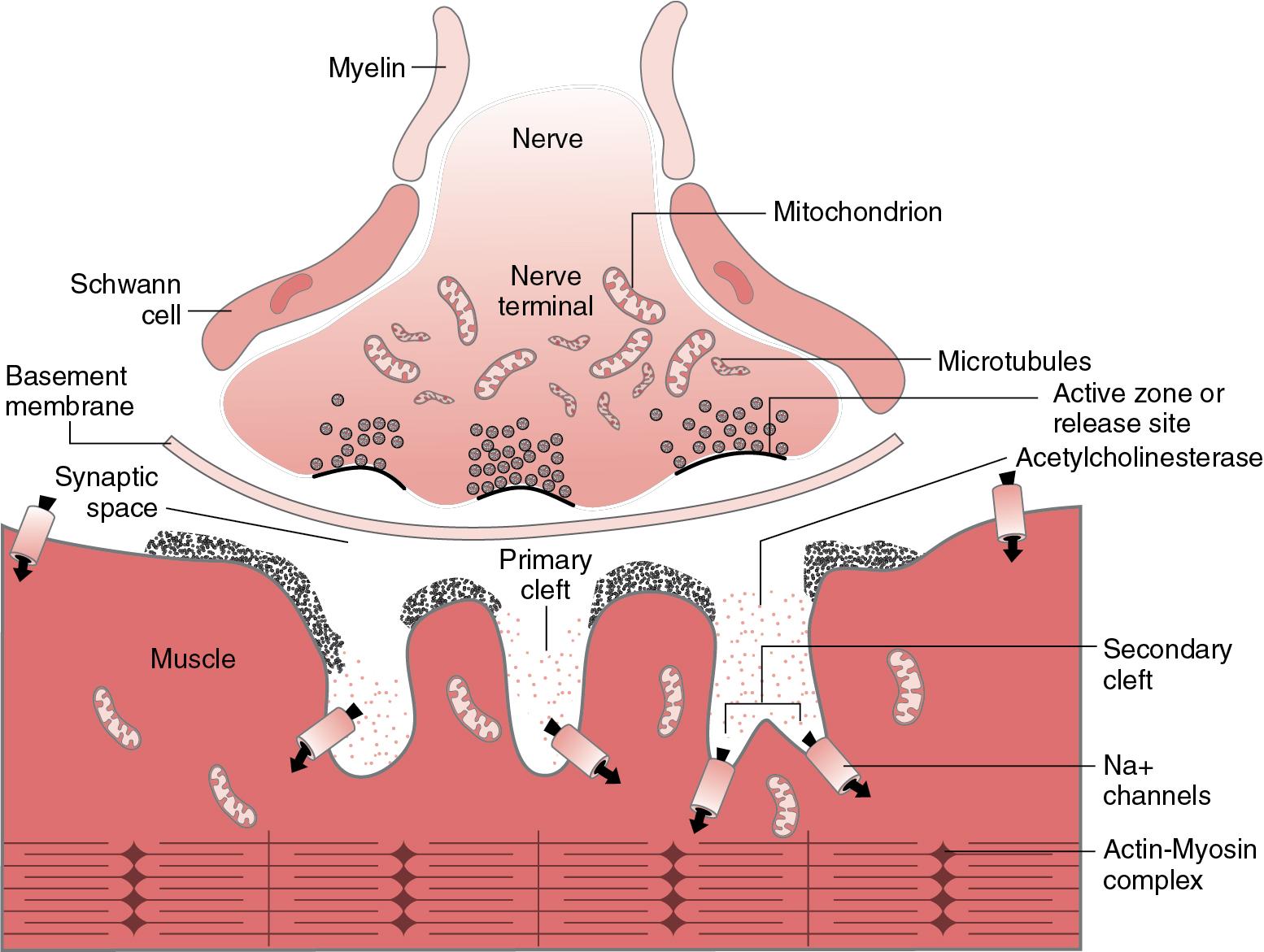
Acetylcholinesterase enzyme present in the synaptic cleft rapidly breaks down acetylcholine, terminating its action in neuromuscular signal transmission. The metabolic byproducts are then recycled into new acetylcholine molecules in the nerve terminal. The large amount of acetylcholine released, and the extensive number of receptors available relative to the number needed to initiate an action potential at the motor end plate, results in a large margin for safety. Failure of transmission of signal is rare in the normal state ( ; ).
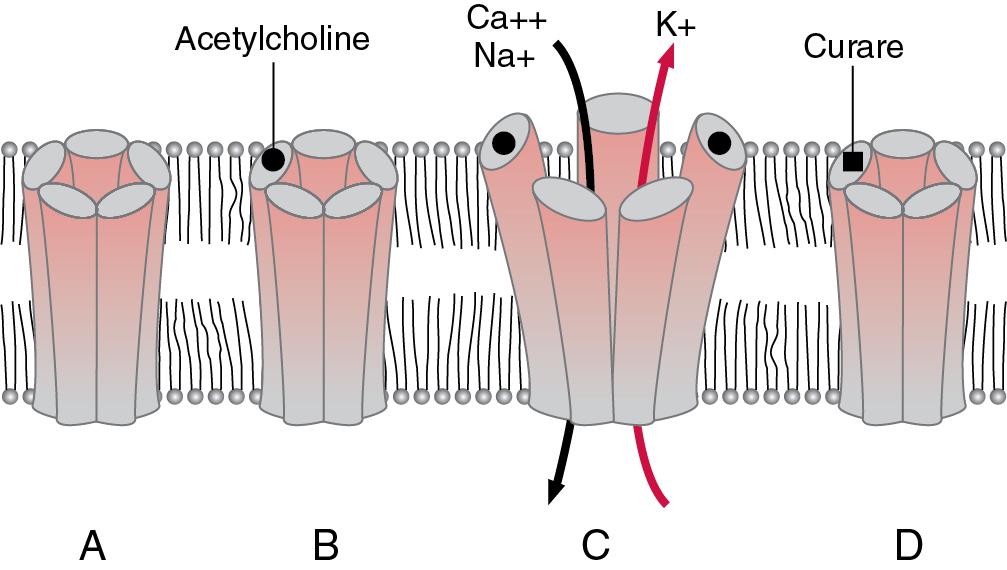
Nicotinic acetylcholine receptors normally exist in high concentration in discrete areas along the surface of the postsynaptic muscle cell at the neuromuscular junction. They also exist in smaller amounts on the presynaptic (neuronal) terminal where they play a role in modulation of acetylcholine release ( ). A representation of the mature nicotinic acetylcholine receptor at the neuromuscular junction and its function is shown in Fig. 13.2 . It is comprised of five subunits (two alpha, one beta, one delta, and one epsilon), clustered around an ion channel. For acetylcholine to activate nicotinic receptors at the motor end plate of the neuromuscular junction, two acetylcholine molecules must simultaneously bind to the receptor, one at each of the alpha subunits. These subunits are not functionally identical, as they show a different affinity for some nondepolarizing neuromuscular blocking drugs ( ). Blockade of just one alpha subunit by a competitive antagonist prevents ion channel opening.
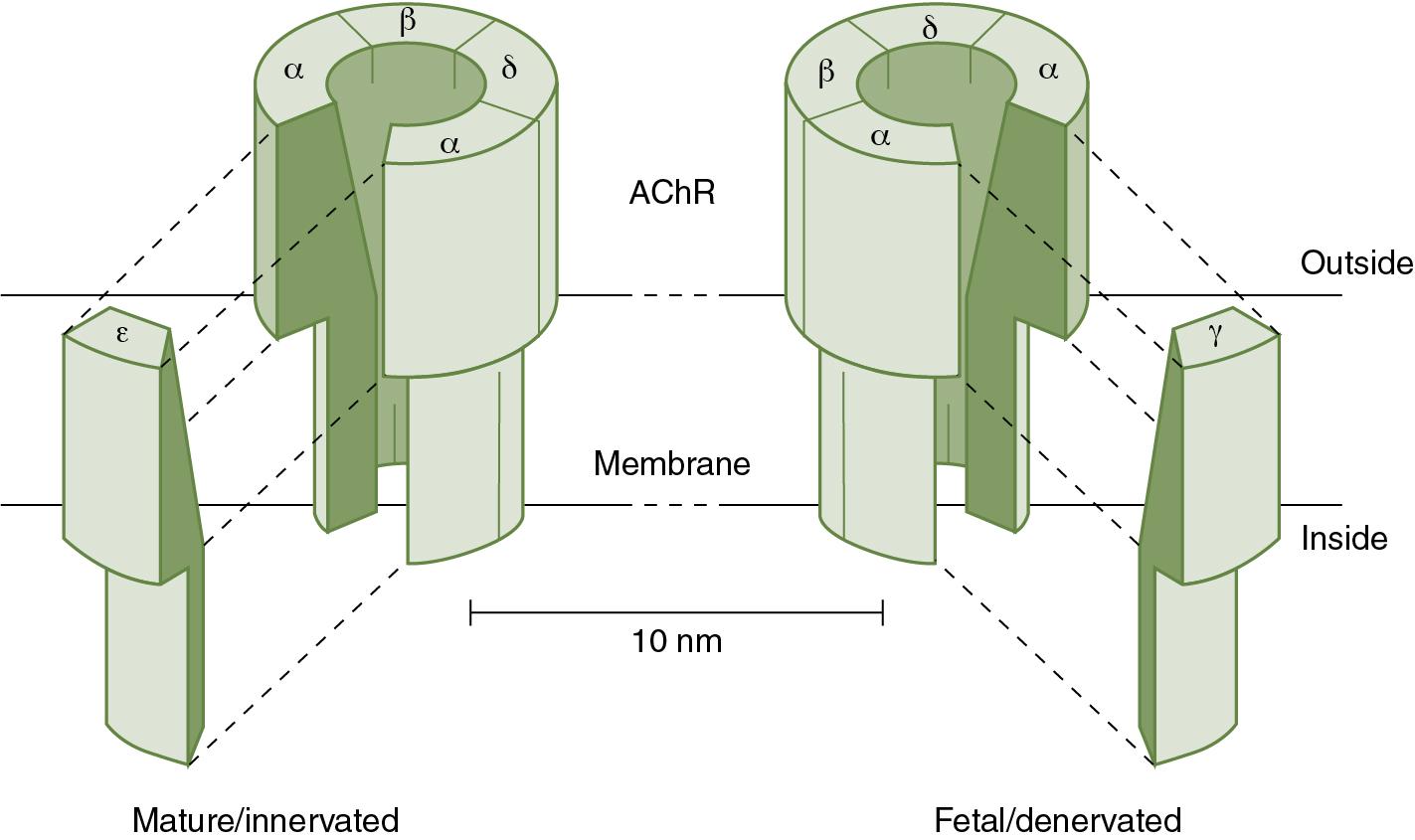
A structurally different subtype of the nicotinic acetylcholine receptor exists at the neuromuscular junction during development. In this so-called fetal (or immature) receptor, a gamma subunit exists in place of the epsilon subunit ( Fig. 13.3 ). This fetal receptor type differs functionally in that it has a longer ion channel opening time (2- to 10-fold) when activated compared with the mature receptor ( ). The half-life of the immature receptor is also just 24 hours compared with around 2 weeks in the mature form ( ; ). Animal studies demonstrate that neonates may have a mixture of mature and fetal acetylcholine receptors at the neuromuscular junction, and the presence of these receptors may impact neuromuscular transmission. The characteristic differences between mature and fetal acetylcholine receptors are shown in Table 13.1 .
| Mature Receptors | Fetal Receptors * |
|---|---|
| ε subunit Localized to end-plate region Metabolically stable (half-life = 2 wk) Larger single-channel conductance Shorter mean open time Agonists depolarize less easily Competitive agents block more easily |
γ subunit Junctional and extrajunctional sites Metabolically unstable (half-life = 24 hr) Smaller single-channel conductance Twofold to 10-fold longer mean open time Agonists depolarize more easily Competitive agents block less easily † |
* Fetal receptors are more sensitive to pancuronium, vecuronium, mivacurium, and rocuronium but not to d-tubocurarine or gallamine.
† Immature junctional receptors have the same characteristics as upregulated extrajunctional receptors.
During fetal and early postnatal development, the density of acetylcholine receptors at the motor end plate becomes more pronounced as muscle fibers increase in size. Neuromuscular transmission becomes more efficient and reliable as mature acetylcholine receptors at the neuromuscular junction replace the fetal forms of the receptor ( ; ). This change in receptor subtype is promoted primarily through increased nerve activity resulting in muscle stimulation, leading to downregulation of fetal acetylcholine receptor synthesis. Animal studies demonstrate that the transition from fetal to mature acetylcholine receptor may occur more quickly in some muscle groups (diaphragm, tibialis anterior) compared with others (extraocular muscles, soleus), perhaps because of differences in muscle fiber type ( ). The timing of receptor type transition is less well-characterized in humans, although one small study showed that no fetal acetylcholine receptors were present beyond 31 weeks gestation in intercostal muscles ( ). Regardless, without the receptor transition from fetal to mature, muscle development is severely impaired ( ).
Beyond changes in receptor subtype, neuromuscular transmission may be immature in the first few months of life for several other reasons ( ; ; ). First, animal studies suggest that a smaller amount of acetylcholine is released from the neuronal presynaptic terminal in neonates, reducing the margin of safety ( ; ). Secondly, both human and animal studies demonstrate that structural maturation of the neuromuscular junction is incomplete at birth and continues to mature postnatally ( ; ; ). The function of the neuromuscular junction becomes increasingly more efficient during early infancy, but maturation may not be fully complete until 1 to 2 years of age in humans ( ; ). Therefore synaptic transmission may be relatively limited with decreasing age, and this inefficiency may alter the clinical response to administration of neuromuscular blocking agents in less predictable ways, especially in the youngest children ( ; ; ; ).
In addition to maturational changes at the neuromuscular junction, changes also occur in the makeup of skeletal muscle during postnatal development. Skeletal muscle has two major types of muscle fiber, fatigue-resistant type 1 (slow-twitch) muscle fibers and type II fibers (fast-twitch), that are more easily fatigued. Some peripheral muscles progressively convert to a greater proportion of fast-twitch fibers postnatally, whereas muscle fibers of the diaphragm increase their percentage of slow-twitch muscle over the first months of life, making the diaphragm more fatigue-resistant ( ; ; ). There is evidence from animals that fast- and slow-twitch muscles may respond differently to certain neuromuscular blocking agents ( ).
Nerve activity is known to suppress messenger RNA expression that leads to the synthesis of fetal acetylcholine receptors, and this mechanism is thought to be responsible for the downregulation and replacement of the fetal type of acetylcholine receptor early in development. The genes that synthesize the fetal acetylcholine receptor still exist in mature functioning muscle though. In denervation states later in life (e.g., spinal cord injury, direct muscle trauma, burn injury, muscle disuse atrophy, or with the prolonged use of muscle relaxants), upregulation of fetal acetylcholine receptors may occur, and these receptors may proliferate at sites on the muscle membrane away from the neuromuscular junction (so-called extrajunctional receptors), even beyond the neonatal period. Up- and downregulation of extrajunctional acetylcholine receptors may therefore influence dosing and potential side effects of neuromuscular blocking drugs in these conditions. For example, proliferation of extrajunctional receptors is associated with resistance to nondepolarizing neuromuscular blockade and may also be associated with hyperkalemia following exposure to succinylcholine (as discussed later) ( ).
Fundamentally, monitoring of neuromuscular function is essential to the safe use of neuromuscular blocking agents in children ( ; ; ; ; ). This should include supramaximal electrical stimulation (20% to 25% more than necessary for a maximal response) of an isolated nerve with measurement of the evoked muscle response. This is typically accomplished with a peripheral nerve stimulator with either qualitative (estimation of response by tactile or visual means) or quantitative (measurement of response with numeric display) assessment. Neuromuscular monitoring aids in the determination of dosing of neuromuscular blockers, helps determine the appropriate doses of reversal agents, and allows a determination of the adequacy of reversal from the effects of drugs acting at the neuromuscular junction.
Basic definitions used to describe various aspects of neuromuscular monitoring in this chapter are shown in Box 13.1 , and the pharmacodynamic definitions are shown in Box 13.2 . Although several types of monitoring modalities are available, the most commonly used methods in children clinically, and in the study of muscle relaxants in children, are train of four (TOF) monitoring, tetanic stimulation (including posttetanic count [PTC]) and single-twitch height ( Fig. 13.4 ). Double burst stimulation is used to facilitate assessment of residual neuromuscular blockade, especially when TOF ratio is between 0.3 and 0.7. Although double burst suppression has been shown to be more sensitive than TOF monitoring in pediatric patients, its use in children is not widespread ( ).
A single, square-wave supramaximal stimulus applied for 0.2 to 0.3 milliseconds at regular intervals
A baseline single-twitch response is measured prior to administration of neuromuscular blockade
Frequency is commonly one stimulus/second (1.0 Hz) or one every 10 seconds (0.1 Hz)
Does not distinguish between depolarizing and nondepolarizing block
Four supramaximal stimuli applied at 2 Hz (one every 0.5 seconds)
Train-of-four count (TOFC) is the number of individual responses detected on TOF measurement (range 0–4)
TOF fade is defined as decrement in the response of T4 to T1
TOF ratio (ratio of height of T4/T1) is used to evaluate the degree of nondepolarizing block
TOF ratio <1.0 occurs with nondepolarizing block (amplitude of responses are progressively diminished)
TOF ratio = 1.0 in depolarizing block (amplitude of responses are equally diminished)
TOF ratio >0.9 is considered full recovery from neuromuscular blockade
High-frequency stimulation (typically 50 Hz) for 5 seconds
Painful in awake children
Response may diminish (fade) over the 5 seconds in infants without blockade or in the setting of nondepolarizing block (see Fig. 13.6 )
Recovery for several minutes should occur prior to subsequent measurements to avoid potentiation of response
Tetanic stimulation followed by a series of single stimuli at 1 Hz
The number of measured responses defines the PTC
Can be used when TOF reveals no response (profound block or early stages of recovery) to assess degree of block
Higher PTC indicates lesser degree of blockade
May be used to predict timing of subsequent return of TOF response
Recovery for several minutes should be allowed prior to subsequent measurements to avoid potentiation of response
The dose of neuromuscular blocker required to produce 95% depression of baseline single-twitch response
A measure of potency
Two to three times the ED 95 is the usual recommended dose of neuromuscular blocking agent in order to speed the onset of blockade and to optimize relaxation for endotracheal intubation
The dose of neuromuscular blocker required to produce 50% depression of baseline single-twitch response
The time from administration of neuromuscular blocker until return of 25% of baseline single-twitch response
Defines the clinical duration
Four twitches would usually be seen on TOF testing at this time
Redosing of neuromuscular blocker may be appropriate at this time (20% to 25% of initial dose)
Earliest time that reversal of block with anticholinesterase agents should be administered
The time from administration of neuromuscular blocker until return of 95% of baseline response
Defines the time at which full recovery from blockade has occurred
The calculated difference in time from the time of recovery to 25% (T 25 ) and the time to recovery to 75% (T 75 ) of baseline response
Defines the rate of recovery from neuromuscular blockade
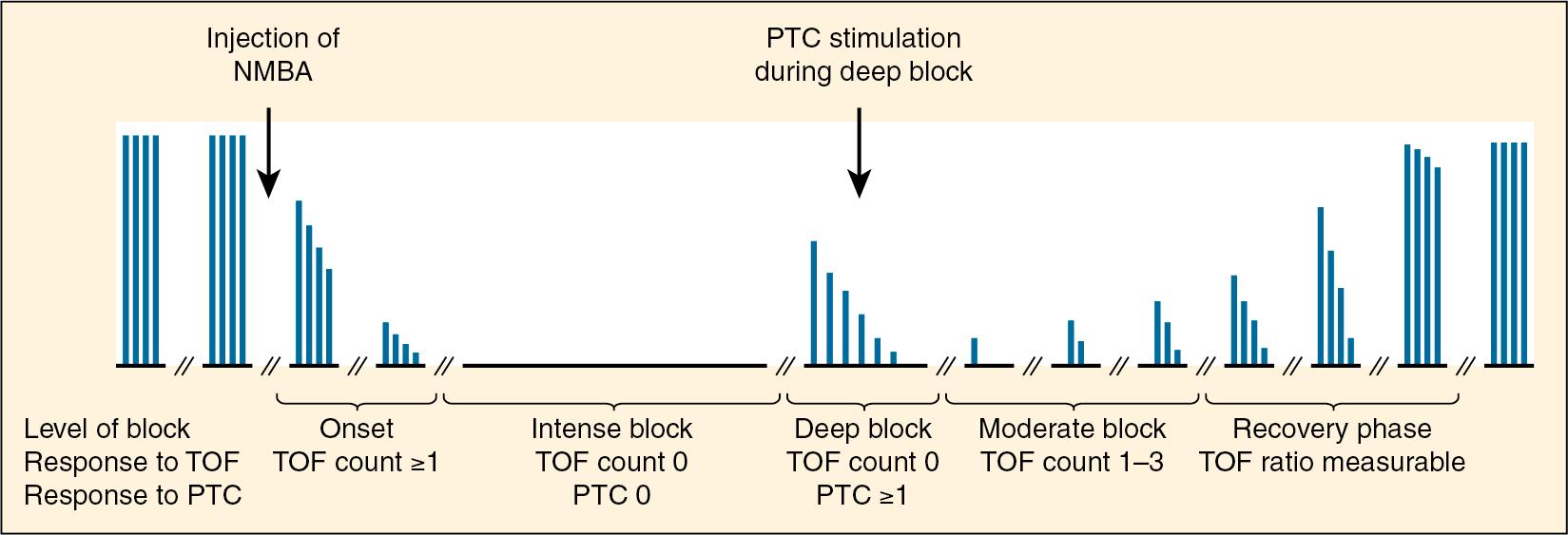
In monitoring neuromuscular blockade, it is important to recognize that 75% of acetylcholine receptors at the neuromuscular junction need to be occupied by an antagonist before any response may be seen on peripheral nerve stimulation. Ninety-five percent of receptors must be occupied in order to completely abolish the twitch response ( ). Neonates may demonstrate blockade at lower receptor occupancy ( )
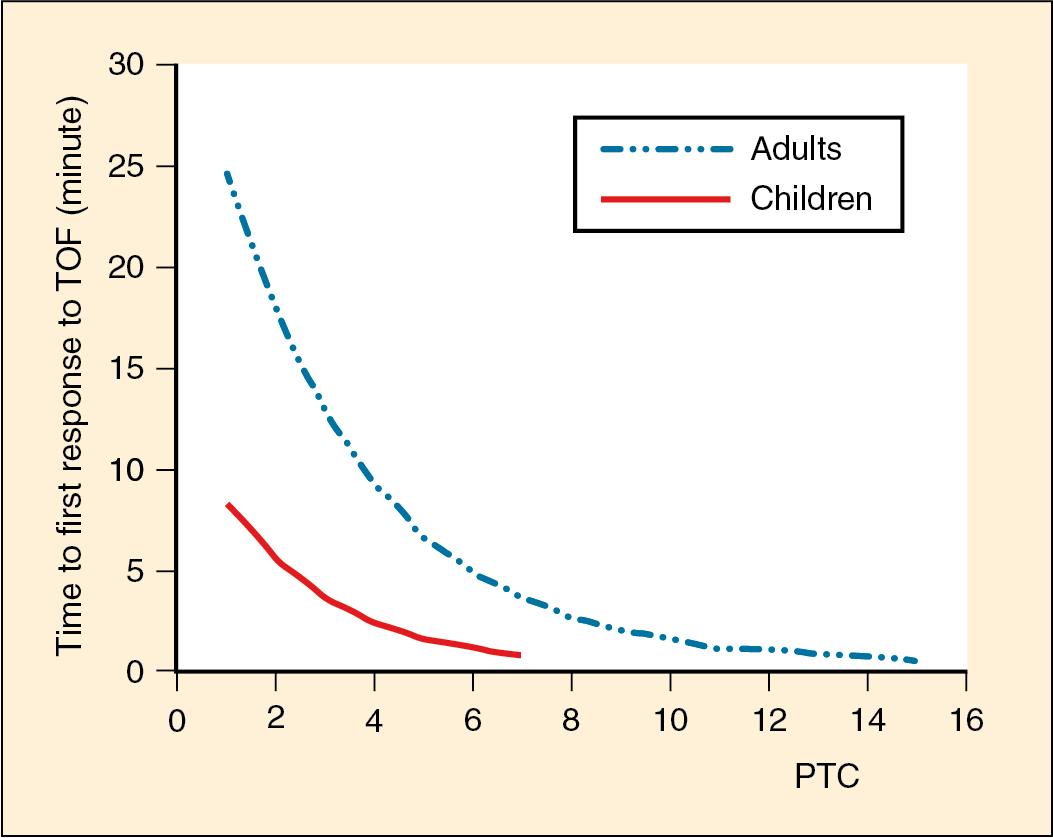
Tetanic stimulation may temporarily increase the availability of acetylcholine at the neuromuscular junction and lead to immediate potentiation of response to subsequent stimulation in the setting of competitive blockade. In the setting of deep block, the PTC is a measure of that response and allows an assessment of the degree of blockade when no response to TOF is initially demonstrated ( ; ; ). Measurement of PTC may therefore predict the time that return of TOF will occur. Fig. 13.5 demonstrates this phenomenon and also shows that children (ages 2 to 5 years) recover more quickly from blockade with rocuronium compared with adults. For similar reasons, posttetanic potentiation (PTP) may also improve TOF response when twitches are present prior to tetany but a decremental response to TOF fade is seen. Potentiation of response may be diminished in infants less than 2 months old ( ).
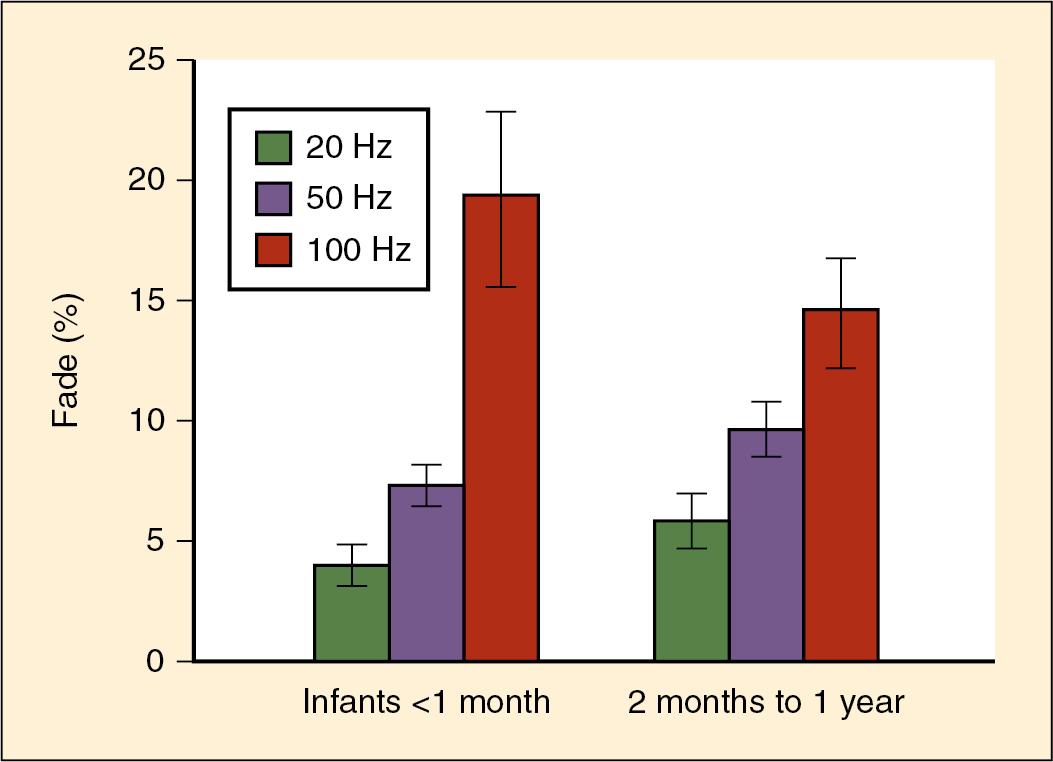
Infants (especially neonates) may show fade to tetanic stimulation (>50 Hz) even without exposure to neuromuscular blocking agents, and this response may be more pronounced in the premature infant, where posttetanic exhaustion may last up to 15 minutes. The degree of fade to tetanic stimulation increases with increasing duration and higher frequency of stimulation ( Fig. 13.6 ). The amplitude of response to stimulation is also worsened by administration of nitrous oxide ( ; ; ). Goudsouzian studied the force of contraction of the thumb in 37 infants and children anesthetized with halothane (no muscle relaxant given) and showed that the TOF ratio was lower in infants less than 2 months of age compared with older infants and children. These results suggest that in infants with limited reserve capacity for neuromuscular transmission and susceptibility to fatigue, muscle function may be further impaired perioperatively with exposure to anesthetics ( ).
As in adults, measurement of the evoked response of the adductor pollicis muscle upon stimulation of the ulnar nerve is the most common site for peripheral nerve function monitoring in infants and children. Although other sites may be used for monitoring neuromuscular function, different monitoring locations may demonstrate unique responses to neuromuscular blockade. As such, a thorough understanding of these differential responses is key to appropriate and effective use of neuromuscular monitoring in children.
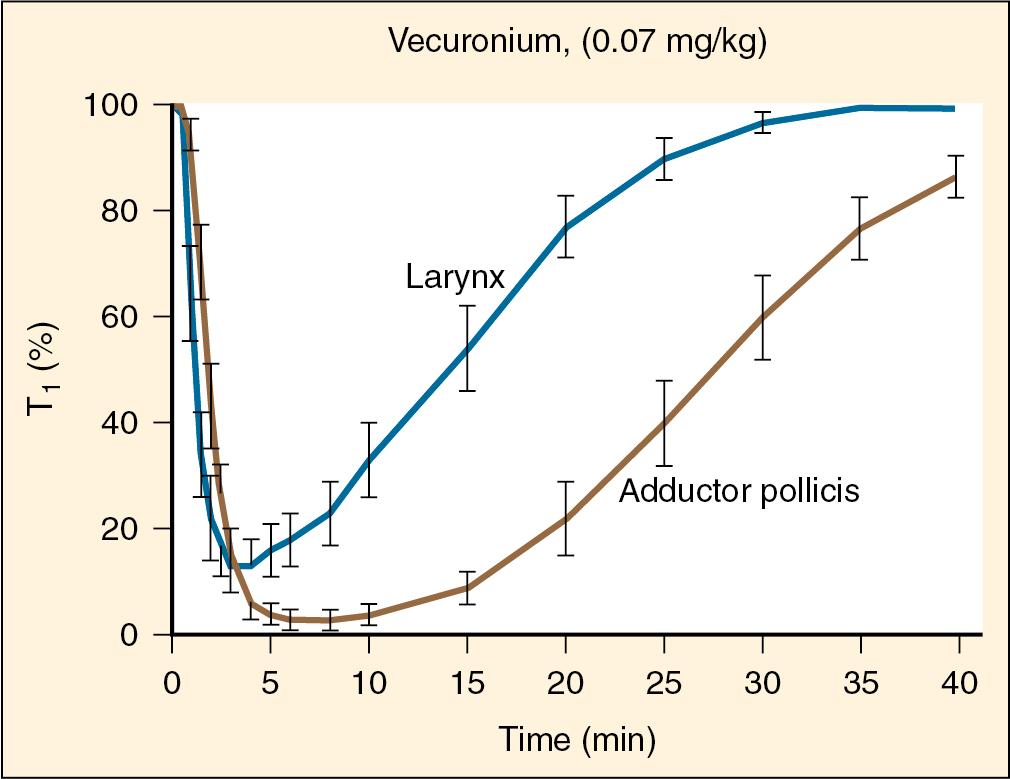
The diaphragm and laryngeal muscles generally show a faster onset of neuromuscular blockade compared with adductor pollicis. Higher relative blood flow to these central muscles and the resulting more rapid equilibration between plasma and muscle may account for this phenomenon ( ; ; ; ) Because the laryngeal muscles are blocked more quickly, intubation conditions may be favorable prior to complete loss of response measured at the adductor pollicis ( ).
Although onset is faster, the diaphragm and laryngeal muscles demonstrate a relative resistance to blockade, with recovery occurring more quickly in those muscles ( Fig. 13.7 ). In adults, 90% blockade of adductor pollicis is associated with only 24% blockade of diaphragm function ( ). Infants and children show a similar diaphragm resistance pattern. Although infants (especially neonates) may be overall more sensitive to neuromuscular blockade for certain nondepolarizing NMBAs (e.g., pancuronium and other aminosteroid agents), this sensitivity appears to occur at both the diaphragm and adductor pollicis equally. For example, as children age, the relative ratio of blockade at the two muscles (58% to 75% greater diaphragm resistance compared with adductor pollicis) does not significantly change with age from early infancy up to 10 years ( ).
Facial nerve monitoring (measuring orbicularis oculi [controls eyelid] or corrugator supercilii [controls eyebrow] muscle response) may be more practical during surgical procedures when the upper extremities are unavailable because of patient positioning. However, like the diaphragm, these muscles may be relatively resistant to neuromuscular blockade such that monitoring the response to facial nerve stimulation may overestimate the return of muscle function at adductor pollicis ( ). In fact, using the facial nerve has been associated with greater postoperative residual neuromuscular blockade in adults ( ). It should also be noted that unlike the diaphragm, muscles involved in the support of the upper airway (e.g., geniohyoid) are at least as sensitive to neuromuscular blockade as adductor pollicis ( ). Pharyngeal function parallels adductor pollicis function such that a TOF ratio of <0.9 increases the risk of aspiration in adult volunteers ( ). For a variety of reasons then, facial nerve monitoring may be useful to detect laryngeal muscle function to predict intubating conditions in children, but it is an inferior substitute for adductor pollicis for assessment of return of adequate neuromuscular function ( ). As such, it is preferable to use the adductor pollicis when the extremity is available at the completion of surgery ( ; ; ).
Surveys indicate that many clinicians do not routinely monitor neuromuscular blockade in adults ( ; ; ). In lieu of monitoring, clinical signs such as head lift, hand grip strength, or measurement of respiratory tidal volume may be used to judge the adequacy of neuromuscular function. However, several studies in adults indicate that these parameters may not correlate consistently with adequate return of neuromuscular function ( ; ; ). In children, respiratory parameters such as tidal volume or minute ventilation are also inadequate surrogates for return of adequate muscle strength and have been shown to poorly correlate with return of neuromuscular function following blockade ( ). Even when standard doses of reversal agents are administered, use of clinical signs alone to assess for adequacy of recovery has been shown to be associated with TOF ratio of just 0.5 in children at the time of extubation ( ). Fundamentally, then, neuromuscular monitoring is essential to the safe use of muscle relaxants in children.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here