Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Concentrations of minerals and electrolytes in extracellular fluid (ECF) are maintained nearly constant, despite large day-to-day variations in the dietary intake of salt and water. Such homeostasis is governed primarily by the kidneys through an array of intricate processes that may be influenced by intrarenal and extrarenal vasoactive substances and hormones. Although the basic tenets governing nephron function and homeostasis of body fluid composition have changed little over the past decade, major advances stemming from genetic research have greatly elucidated the structure and function of many renal tubular electrolyte transporters during both health and disease. A major objective of this chapter is to enhance the understanding of electrolyte (and fluid) pathophysiology based on newer information.
The kidneys are retroperitoneal paired organs located on each side of the vertebral column. A normal adult kidney measures 11 to 12 cm in length, 5 to 7.5 cm in width, and 2.5 to 3 cm in thickness. In the adult male, it weighs 125 to 170 g, and in the adult female, it weighs 115 to 155 g. Beneath its fibrous capsule lies the cortex, which contains the glomeruli, the convoluted proximal tubules, the distal tubules, and the early portions of the collecting tubules. The remainder of the tissue, the medulla, contains the pars recta, the loop of Henle, and the middle and distal portions of the collecting duct. The inner medulla borders the renal pelvis, where urine is received from the collecting ducts. The ducts and loops are arranged into cone-shaped bundles called pyramids, which have tips that project into the renal pelvis and form papillae. The pelvis drains into the ureters, which in the adult human descend a distance of 28 to 34 cm to open into the fundus of the bladder. The walls of the pelvis and ureters contain smooth muscles that contract in a peristaltic manner to propel urine to the bladder.
Despite accounting for only 0.5% of body weight, the kidneys receive about 25% of the cardiac output, with a blood flow of approximately 4 mL/min per gram of kidney tissue. Renal blood flow (RBF) in women is slightly lower than it is in men, even when normalized for body surface area, averaging 592 ± 153 mL/min per 1.73 m 2 and 654 ± 163 mL/min per 1.73 m 2 , respectively ( ). In children between the ages of 6 months and 1 year, normalized RBF is half that of adults but increases progressively to reach adult levels at about 3 years of age ( ). After the age of 30 years, RBF decreases progressively; by the age of 90 years, it is approximately half of the value present at 20 years ( ). This generous supply provides not only for the basal metabolic needs of the kidneys but also for the high demands of ultrafiltration.
The basic arterial supply of the kidneys is a single renal artery that divides into large anterior and posterior branches and subsequently into segmental or interlobar arteries. The latter form the arcuate and interlobular arteries. These blood vessels are end-arteries and therefore predisposed to tissue infarction in the presence of emboli. The arcuate arteries are short, large-caliber vessels that supply blood to the afferent arterioles of the glomeruli at a mean pressure of 45 mm Hg, which is higher than that found in most capillary beds. This high hydraulic pressure and large endothelial pore size lead to enhanced glomerular filtration ( ).
Glomerular capillaries have many anastomoses but recombine to form the efferent arteriole. The latter subdivide into an extensive peritubular capillary network. This arrangement allows solute and water to move between the tubular lumen and the blood. These networks rejoin to form the venous channels, through which blood exits the kidneys.
Ninety percent of RBF goes to the cortex, which accounts for 75% of the renal weight, whereas the medulla and the rest of the kidneys receive 25% of the RBF. Although cortical blood flow is 5 to 6 mL/g per minute, outer medullary blood flow decreases to 1.3 to 2.3 mL/g per minute, and the flow to the papilla is as low as 0.22 to 0.42 mL/g per minute ( ). The unevenness in the distribution of RBF between the cortex and the medulla is necessary to develop and maintain the medullary gradient of osmotically active solutes that drive the countercurrent exchange/multiplier, which is essential for the elaboration of concentrated urine. Outer medullary blood flow may preferentially supply the loop of Henle, thereby accounting for the striking influence of loop diuretics in that region. Furthermore, papillary blood flow is far greater than the metabolic needs of the renal parenchyma and is well adapted to the countercurrent concentrating mechanism characteristic of this region.
RBF remains almost constant over a range of systolic blood pressures from 80 to 180 mm Hg, a phenomenon known as autoregulation. Consequently, glomerular filtration is also constant over this range of pressures as a result of adaptations in the renal vascular resistance ( ; ). Because the changes in resistance that accompany graded reductions in renal perfusion pressure occur in both denervated and isolated perfused kidneys, autoregulation appears not to depend on extrinsic neural or hormonal factors ( ). According to the “myogenic hypothesis” first proposed by , the stimulus for vascular smooth muscle contraction in response to increasing intraluminal pressure is either the transmural pressure itself or the increase in the tension of the vascular wall. An increase in perfusion pressure, which initially distends the vascular wall, is followed by a contraction of the resistance vessels and a return of blood flow to basal levels.
There are only a few studies of autoregulation of RBF in developing animals. Aortic constriction in adult animals reduces renal perfusion by 30% but has minimal effects on RBF and glomerular filtration rate, compared with the significant changes observed in 4- to 5-week-old rats ( ). Furthermore, it has been demonstrated that autoregulation of RBF in young rats occurs at renal perfusion pressures between 70 and 100 mm Hg, compared with pressures of 100 to 130 mm Hg in adult rats ( ). A similar increase in the pressure set point for autoregulation has been found in dogs ( ). It appears that autoregulation of RBF occurs in the very young and is sufficient to maintain blood flow constant over a wide range of perfusion pressures that are physiologically adequate for the age. No such human studies are available.
Several substances have been proposed to participate in the autoregulation of RBF, including vasoconstrictor and vasodilator prostaglandins, kinins, adenosine, vasopressin, the renin-angiotensin-aldosterone system, endothelin, and endopeptidases ( ; ; ; ). Nitric oxide (NO), previously known as endothelium-derived relaxing factor (EDRF), has also been shown to play an important role in regulating renal vascular tone through its vasodilatory action. Bradykinin, thrombin, histamine, serotonin, and acetylcholine act on endothelial receptors to activate phospholipase C, which in turn results in the formation of inositol triphosphate and diacylglycerol, resulting in the release of intracellular calcium ( ; ). This in turn stimulates the synthesis of NO from l -arginine. Other factors that stimulate the formation of NO include hypoxia, calcium ionophores, and mechanical stimuli to the endothelium. NO increases RBF by decreasing efferent arteriolar vascular resistance, whereas glomerular filtration remains unchanged ( ).
Because in mature kidneys, autoregulation is lost at arterial pressures less than 80 mm Hg, the lower physiologic pressures prevailing in the newborn period may be expected to limit this important control mechanism. There is evidence both to support and to refute this conclusion ( ; ).
The glomerulus is a specialized capillary cluster arranged in loops that functions as a filtering unit. The capillary walls may be viewed as a basement membrane lined by a single layer of cells on either side. In contact with blood are endothelial cells, which contain many fenestrations; podocytes, with their foot processes, line the other side of the basement membrane.
The route by which water and other solutes are filtered from the blood is not fully understood, but it appears that plasma ultrafiltrate traverses the large fenestrations of the glomerular capillary endothelium and penetrates the basement membrane and the slit pores located between the podocyte foot processes. Filtration of large molecules is greatly influenced by the size and charge of the specific molecule, as well as by the integrity and charge of the glomerular basement membrane. Abnormalities in various structural proteins of the slit-pore diaphragm such as nephrin, podocin, and α-actinin may be responsible for several proteinuric disorders ( ). In general, the endothelium and the lamina rara interna of the glomerular basement membrane slow the filtration of circulating polyanions such as albumin ( ). The lamina rara externa and the slit pores slow the filtration of cationic macromolecules such as lactoperoxidase ( ). Neutral polymers such as ferritin are not filtered because of their large molecular size and shape ( ). Molecules with a radius of 4.2 nm or more are excluded from the glomerular filtrate. In practical terms, red cells, white cells, platelets, and most proteins are restricted to the circulation.
Among the main functions performed by the kidneys is the process of glomerular filtration. The glomerulus is primarily responsible for the filtration of plasma. The glomerular filtration rate (GFR) is the product of the filtration rate in a single nephron and the number of such nephrons, which range from 0.7 to 1.4 million in each kidney ( ). Clearance, which is defined as the volume of plasma cleared of a substance within a given time, provides only an estimate or approximation of GFR.
Although tubular reabsorption and tubular secretion may influence the blood level of numerous medications and endogenously produced substances such as urea, creatinine, and uric acid, the degree of elimination of such substances depends largely on GFR. Hence, in individuals with renal impairment, estimation or measurement of GFR is crucial in determining the dosage adjustment and choice of medications needed to achieve effectiveness while avoiding toxicity. GFR is also a major factor that affects electrolyte composition and volume of body fluids, as well as acid-base homeostasis.
Glomerular filtration is driven by hydrostatic pressure, which forces water and small solutes across the filtration barrier. In healthy individuals, changes in hydrostatic pressure rarely affect single-nephron GFR because autoregulatory mechanisms sustain or maintain a constant glomerular capillary pressure over a large range of systemic blood pressure ( ). Hydrostatic pressure is opposed by the oncotic pressure produced by plasma proteins and the hydrostatic pressure within Bowman’s capsule. Mathematically, this relation can be expressed by the equation:
SNGFR is the single-nephron glomerular filtration rate; K f is the glomerular ultrafiltration coefficient; P and p are the average hydraulic and osmotic pressure differences, respectively; and P uf is the net ultrafiltration pressure. As plasma water is filtered, the proteins within the capillaries become more concentrated, so oncotic pressure increases at the distal end of the glomerular capillary loop, and the rate of filtration ceases at the efferent capillary ( ). Under normal conditions, about 20% of the plasma water that enters the glomerular capillary bed is filtered; this quantity is referred to as the filtration fraction.
RBF has the greatest influence on GFR. Renal parenchymal disorders interfere with autoregulation of RBF, such that GFR may decrease, even with low-normal mean arterial blood pressure (MABP). Still more pronounced changes in GFR may occur with hypotension or hypertension, which may accelerate ischemic or hypertensive injury. Clearance of a molecule may serve as an indicator of GFR only if the assayed molecule is biologically inert and freely permeable across the glomerular capillary, if it remains unchanged after filtration, and if it is neither reabsorbed nor secreted by the tubule. The exogenous-filtration marker inulin (a fructose polymer) has all of these attributes and is the ideal standard for measuring GFR. However, inulin-clearance measurement is rarely used clinically because it is an expensive and cumbersome method. Instead, measurement of an endogenous small molecule such as serum creatinine (molecular weight, 0.113 kDa), which is derived from muscle metabolism at a relatively constant rate and is freely filtered at the glomerulus, is a practical, rapid, and inexpensive means for estimating GFR, thereby aiding clinical decisions. Thus in the steady state, creatinine production and urinary creatinine excretion are equal even when GFR is reduced.
Serum-creatinine concentrations vary by age and gender. In 1-year-old girls, values are 0.35 ± 0.05 mg/dL (mean ± SD) and rise gradually to 0.7 ± 0.02 mg/dL (mean ± SD) by 17 years of age; boys have corresponding mean values that are 0.05 mg/dL higher until 15 years of age and 0.1 mg/dL higher subsequently ( ). Expected creatinine-excretion rates in 24-hour urine collections are often used to validate such collections. Values range from 8 to 14 mg/kg per day in neonates and in infants younger than 1 year of age, with an increase to about 22 ± 7 mg/kg per day (mean ± SD) in preadolescent children of either gender ( ). Subsequently, creatinine excretion in boys is 27 ± 3.4 mg/kg per day.
In healthy children with proportional height and weight, GFR can be estimated by creatinine clearance (CrCl) as calculated by updated Schwartz’s “bedside” formula, which does not rely on measurement of urinary creatinine or timed urine collections:
Height is in centimeters, S cr is the serum-creatinine concentration in mg/dL, and k is a constant proportion to muscle mass (0.41). Normal CrCl ranges from 90 to 143 mL/min per 1.73 m 2 , with a mean of 120 mL/min per 1.73 m 2 ( ).
Although more cumbersome, calculation of CrCl based on values obtained in 12- or 24-hour urine collections provides a better estimate of GFR. Once the completeness of such collections is validated based on expected creatinine excretion, CrCl is calculated using the formula:
where U is the urinary concentration of creatinine in mg/dL, V is the total urine volume in mL, min is the time of collection in minutes, and P cr is the serum concentration of creatinine in mg/dL. To standardize the clearance of children of different sizes, the calculated result is multiplied by 1.73 m 2 (surface area of a standard man in meters squared) and divided by the body surface area of the child in meters squared.
In children with impaired renal function, GFR estimates based on creatinine methods may grossly overestimate the true GFR, because tubular and gastrointestinal secretion of creatinine increases disproportionately. Hence, serum creatinine concentrations are less reflective of filtration at the glomerulus. For example, Schwartz’s formulas overestimate GFR by 10% ± 3% when GFR is greater than 50 mL/min per 1.73 m 2 but by 90% ± 15% when GFR is less than 50 mL/min per 1.73 m 2 . Other limitations of creatinine-based GFR determinations stem from variations of analytical assays, reference values ranging from 0.1 to 0.6 mg/dL in children younger than 9 years of age, diurnal variation in serum creatinine levels resulting from high intake of cooked meat or intense exercise, influence of body mass index, and inaccurate urine collections—all of which make comparisons of GFR difficult over time, especially in growing children ( ). Using cimetidine to block tubular secretion of creatinine before measuring CrCl in urine collections may improve such measurements ( ).
Measurement of cystatin-C, a 13-kDa serine proteinase produced at a constant rate by all nucleated cells, is purported to be a superior endogenous marker of filtration, because cystatin-C is less susceptible to variation than is plasma creatinine. A meta-analysis compared the correlation between GFR measured by inulin clearance, radiolabeled methods, nonlabeled iothalamate or iohexol, and either plasma creatinine or cystatin-C concentrations measured nephelometrically ( ). The correlation between GFR and cystatin-C was significantly higher compared with plasma creatinine (0.846 versus 0.742, P < 0.001). Thus cystatin-C measurements are becoming increasingly popular in clinical practice, and reference ranges have been generated in children up to 16 years of age ( Table 6.1 ) ( ; ; ; ).
| 90% CONFIDENCE LIMITS | P * | ||||
|---|---|---|---|---|---|
| Age Group | n | Reference Interval | Lower Limit | Upper Limit | |
| Preterm infants | 58 | 1.34–2.57 | 1.07–1.42 | 2.47–2.86 | 0 |
| Full-term infants | 50 | 1.36–2.23 | 1.24–1.44 | 2.03–2.32 | 0 |
| 9 days–1 year | 65 | 0.75–1.87 | 0.71–0.86 | 1.78–1.91 | 0 |
| 2–3 year | 72 | 0.68–1.60 | 0.65–0.79 | 1.39–1.67 | 0.011 |
| 4–16 year | 162 | 0.51–1.31 | 0.48–0.68 | 1.26–1.35 | — |
* Statistical significance versus the oldest group (including Bonferroni’s correction factor 5).
Studies in renal transplant donors and in individuals with various renal disorders have shown that plasma-creatinine concentration changes minimally as GFR falls to about 50 mL/min per 1.73 m 2 ( Fig. 6.1 ) ( ). This compensation is largely the result of hypertrophy and hyperfiltration of the remaining nephrons. When more than 50% of the nephrons cease to function and “renal reserve” is outstripped, serum creatinine may rise rapidly in a parabolic fashion (see Fig. 6.1 ). Thus when a more accurate clinical assessment of GFR is desirable for research purposes, radiolabeled methods with an identity exceeding 97% give a better approximation of GFR relative to inulin clearance and may be more useful in aiding clinical decisions. In multicenter investigations conducted in the United States using a uniform method for GFR measurement, 125 I-iothalamate is often used because this isotope has low radiation exposure and long isotope half-life and can be assayed at a central laboratory ( ). Otherwise, 99m Tc-diethylenetriaminepenta-acetic acid (Tc-DTPA) is commonly used to estimate GFR for routine clinical purposes. In other countries, 51 Cr-ethylenediaminetetra-acetic acid (Cr-EDTA), which delivers a greater radiation dosage, is also popular, as are nonlabeled iothalamate and iohexol methods.

Although GFR may fluctuate, the kidneys retain the ability to regulate the rate of solute and water excretion according to changes in intake. This regulation is achieved by changes in tubular reabsorption rates—a phenomenon known as glomerular-tubular balance ( ). The result is preservation of ECF volume and chemical composition. Glomerular-tubular balance can be disturbed by several factors, including volume expansion, loop diuretics, and inappropriate secretion of antidiuretic hormone (ADH).
The proximal tubule is the site of reabsorption of large quantities of solute and filtered fluid ( Fig. 6.2 ). Many transporters subserving tubular electrolyte transport have been characterized at the genetic level, and various pathologic disorders have been elucidated ( ). Under physiologic conditions, the proximal convoluted tubule isotonically reabsorbs 50% to 60% of the glomerular filtrate ( ). The initial portion of the proximal convoluted tubule reabsorbs most of the filtered glucose, amino acids, and bicarbonate. Glucose and amino acids are absorbed actively, whereby they are transported against their electrochemical gradient, coupled to sodium (Na + ). Active Na + transport at the peritubular membrane provides the driving force that ultimately is responsible for other transport processes. The system is driven by Na + , K + , (activated) adenosine triphosphatase (Na + /K + -ATPase), or the Na + “pump,” which requires the presence of K + in the peritubular fluid and is inhibited by ouabain. Micropuncture studies show that around 50% to 70% of the filtered Na + is reabsorbed in this segment, mostly by a process of active cotransport.

The major fraction of filtered bicarbonate (HCO 3 − ) is absorbed early in the proximal convoluted tubule. Hydrogen (H + ) gains access to luminal fluid via an Na + /H + electroneutral exchange mechanism and forms carbonic acid. The latter is dehydrated to H 2 O and CO 2 under the influence of carbonic anhydrase. CO 2 diffuses into the cell, and HCO 3 − is reformed and ultimately absorbed into the bloodstream. In general, the concentration of HCO 3 − is maintained at 26 mmol/L, which is slightly below the renal threshold of approximately 28 mmol/L ( ).
The renal clearance of glucose is exceedingly low, even after complete maturation of glomerular filtration. The amount filtered increases linearly as plasma glucose increases. Initially, all filtered glucose is reabsorbed until the renal threshold has been exceeded (at around 180 mg/dL), at which point filtered glucose appears in the urine. However, maximal tubular glucose (T mG ) reabsorption is attained at a filtrate glucose concentration of about 350 mg/mL ( ). The reabsorption of glucose in the proximal tubule occurs via a carrier-mediated, Na + /glucose cotransport process across the apical membrane, followed by passive facilitated diffusion and active Na + extrusion across the basolateral membrane.
Apart from Na + , other solutes reabsorbed in the proximal tubule include K + , Ca 2+ , P 2− , Mg 2 + , and amino acids. These are discussed in detail in other sections of this chapter.
The loop of Henle makes the formation of concentrated urine possible and contributes to the formation of dilute urine ( ). This dual function is achieved through the unique membrane properties of the loop, the postglomerular capillaries, and the hypertonicity of the interstitium. The proximity of the descending and ascending portions of the loop allows it to function as a countercurrent multiplier, whereas the capillaries serve as countercurrent exchangers (see Fig. 6.2 ). The descending loop of Henle abstracts water from tubular fluid, increasing the intraluminal concentrations of NaCl and other solutes. However, the intraluminal osmolality remains in equilibrium with the interstitium, where 50% of the osmolality results from urea. In the thin ascending limb of the loop of Henle, there is passive efflux of NaCl and urea into the interstitium. The thick ascending limb of the loop of Henle, by being impermeable to water, contributes to the formation of dilute urine.
The final creation of hypotonic or hypertonic urine depends on the distal tubules and collecting ducts and their interaction with ADH. In the distal convoluted tubule, Na + reabsorption occurs against a steep gradient, largely under the influence of aldosterone. K + is secreted by the distal tubule in association with Na + reabsorption and H + secretion. Moreover, this segment of the nephron acidifies the urine and is the only site of new bicarbonate formation. At the end of the collecting duct, about 1% of the filtered water and about 0.5% of the filtered Na + appear in the final urine.
ADH plays a pivotal role in water homeostasis by acting on the most distal portion of the nephron. ADH is a cyclic octapeptide that, along with its carrier protein, neurophysin, is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus ( ). The prohormone migrates along the nerve axons to the posterior pituitary gland, where it is stored as arginine vasopressin. It is released through exocytosis ( ).
Several variables affect ADH secretion. Physiologically, the most important factor is plasma osmolality. A very small rise in plasma osmolality is sufficient to trigger a response from the sensitive osmoreceptors located in and around the hypothalamic nuclei, leading to ADH secretion. Conversely, plasma ADH concentrations are less than 1 pg/mL at a physiologic plasma osmolality of less than 280 mOsm/kg water. The antidiuretic activity of ADH is maximal at plasma osmolality of greater than 295 mOsm/kg water, when plasma ADH exceeds 5 pg/mL ( ). Once plasma osmolality exceeds this limit—thus surpassing the capacity of the ADH system to affect maximal fluid retention—the organism depends on thirst to defend against dehydration. Intracerebral synthesis of angiotensin II largely mediates this thirst response and the oropharyngeal reflex. Atrial natriuretic peptide (ANP) opposes the release of ADH and of angiotensin II. In summary, plasma osmolality and Na + are maintained within a narrow range. The upper limit of this range is determined by the sensitivity of the thirst mechanism located in the hypothalamus, whereas its lower range is affected by ADH release.
Nonosmolar factors also influence ADH secretion and may be the key stimuli of ADH secretion in pathologic disorders, leading to hypovolemia and hypotension. These changes are mediated by low-pressure (located in the left atrium) and high-pressure (located in the carotid sinus) baroreceptors. Experimental studies suggest that this nonosmotic pathway of ADH release is less sensitive than the osmotic pathway and is triggered by a 5% to 10% fall in blood volume, whereas a 1% to 2% increase in ECF osmolality can trigger ADH release.
Euvolemic states of ADH excess can lead to hyponatremia with an elevated fractional excretion of urate (>10%), hypouricemia (serum uric acid level <4 mg/dL), increased urinary sodium excretion (urine sodium >30 mEq/L), and increased fraction excretion of sodium >0.5%) ( ; ). These conditions result in hyponatremia. Conversely, inhibitors of ADH release or primary or acquired nephropathies may result in the inability to respond to ADH or to conserve water, and these inhibitors are often accompanied by polyuria with urine osmolality (U osm ) of less than 150 mOsm/kg, dehydration, and hypernatremia.
ADH has a major effect on the medullary thick ascending limb and thereby influences the countercurrent multiplier mechanism and urinary concentration. More directly, ADH binds to V 2 receptors in the basolateral membrane of the collecting duct, causing the activation of adenylate cyclase and the formation of cyclic 3′,5′-adenosine monophosphate (cAMP) ( ; ). This results in the insertion of aquaporin-2 water channels in apical membranes and in the activation of apical Na + channels, which causes water conservation ( ). These effects are counterbalanced by prostaglandin E 2 (PGE 2 ) and the calcium-sensing receptor in cells of the medullary thick ascending limb that mediate saluresis and diuresis.
Polyuric syndromes can be separated on the basis of urine osmolality and generally consist of water diuresis, solute diuresis, or a mixed water-solute diuresis with typical U osm of less than 150 mOsm/kg, 300 to 500 mOsm/kg and 150 to 300 mOsm/kg, respectively ( ). The etiology of polyuria may be facilitated by obtaining a urinalysis; a measurement of urine pH; and measurements of electrolytes, creatinine, osmolality, glucose, urea nitrogen, and bicarbonate, preferably in a timed urine collection together with the corresponding serum values. Such assessment may serve to prevent dehydration, acid-base disturbances, hypokalemia, or hypernatremia, which often accompany such polyuric disorders ( Table 6.2 ) ( ). Proper correction of acute hypernatremia is needed to prevent brain demyelination. Normal saline infusion may be the agent of choice in polyuric conditions associated with solute diuresis, whereas ADH and electrolyte-free fluid administration may be appropriate in cases of “pure” water diuresis. The recommended rate of correction of hypernatremia is about 10 mEq/L per 24 hours, amounting to a fall in plasma osmolality of about 20 mOsm/kg H 2 O per day ( ).
| Abbreviation (Term) | Formula * |
|---|---|
| PS (principal solute) | — |
| PS% (percent principal solute) | (100)(PS) (U osm )(TV) |
| C osm(NE) (nonelectrolyte osmolal clearance) | C osm – C osm(E) |
| Ch 2 o (free water clearance) | V – C osm |
| Ch 2 o(e) (electrolyte–free water clearance) | V – C osm(E) |
| U TS (urine total solute) | (U osm ) (TV) |
| U E (urine electrolyte solute) | (2) (U [Na] + U [K] ) (TV) † |
| C osm (osmolal clearance) | (U osm )V O osm |
| one1C osm(E) (electrolyte osmolal clearance) | (U [Na] + U [K] )V P [Na] |
| U NE (urine nonelectrolyte solute) | U TS – U E |
| UAG (urinary anion gap) | U [Na] + U [K] – U [Cl] |
* P [Na] , Plasma sodium concentration; P osm , plasma osmolality; TV, total 24-hr urinary volume; U [Cl] , urine chloride concentration; U [K] , urine potassium concentration; U [Na] , urine sodium concentration; U osm , urine osmolality; and V, urine volume/unit time.
† U E calculations assume that the corresponding anions are monovalent.
The renin-angiotensin-aldosterone axis plays a key role in control of vascular tone, Na + and K + homeostasis, and, ultimately, circulatory volume and cardiovascular and renal function. Renin is an enzyme with a molecular weight of 40 kDa that is synthesized and stored in the juxtaglomerular apparatus surrounding the afferent arterioles of the glomeruli ( ). The primary stimuli for renal renin release are reductions in renal-perfusion pressure, Na + restriction, and Na + loss as detected by the specialized macula densa cells located in the distal tubule. Mechanical (stretch of the afferent glomerular arterioles), neural (sympathetic nervous system), and hormonal (PGE 2 and prostacyclin) stimuli act in an integrated fashion to regulate the rate of renin secretion ( Fig. 6.3 ).
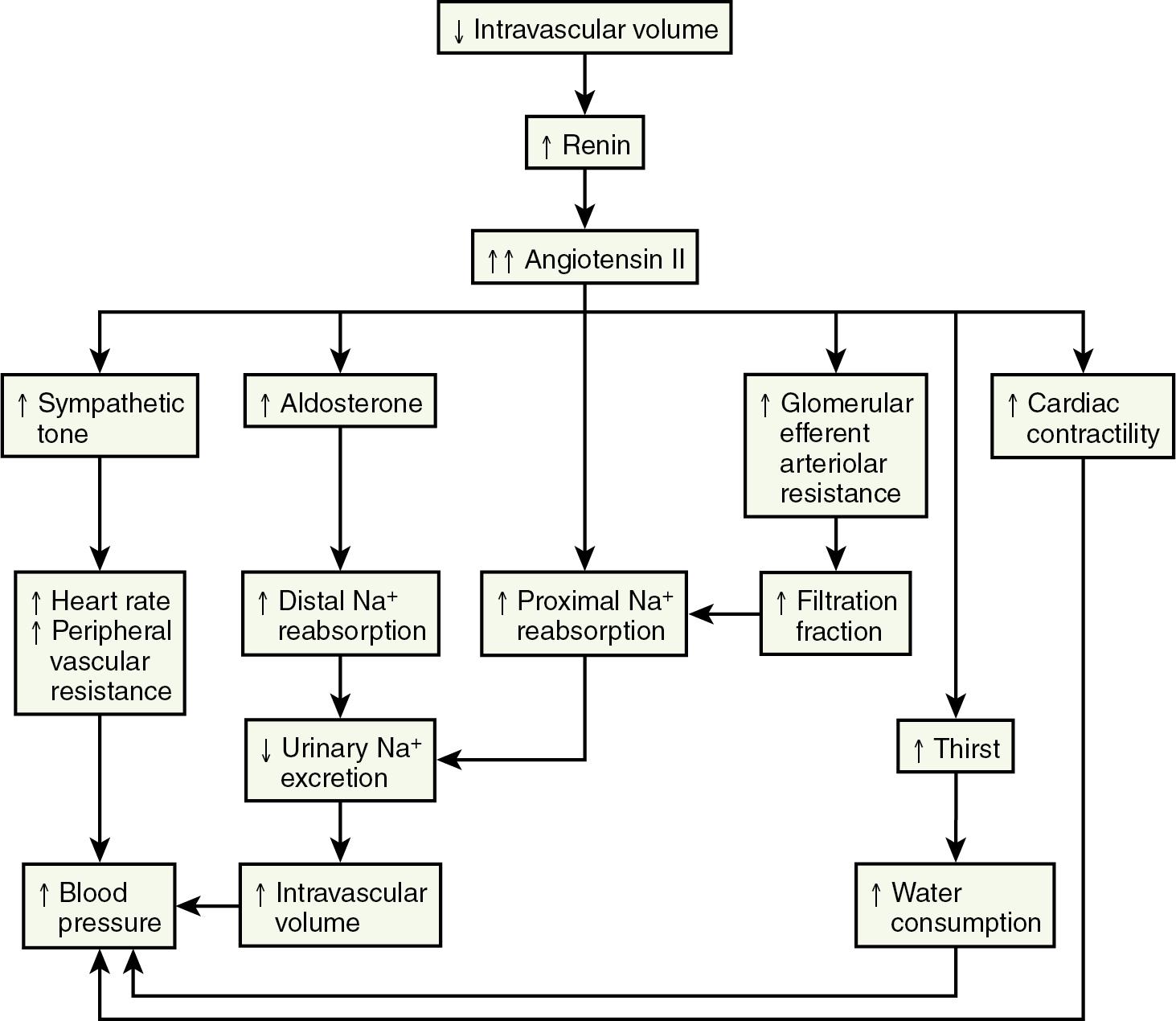
Once released into the circulation, renin cleaves the leucine-valine bond of angiotensinogen, forming angiotensin I. Angiotensin-converting enzyme that is present in the lungs, kidneys, large caliber vessels, and other tissues cleaves the carboxyl terminal (histidine-leucine dipeptide) from angiotensin I to form the biologically active angiotensin II ( ).
Angiotensin II has numerous important hemodynamic functions that are mediated largely by binding to angiotensin-II T1 receptors in endothelial cells, tubular epithelial cells, and smooth muscle ( Box 6.1 ) ( ). It plays a key role in regulating blood volume and long-term blood pressure through stimulation of several tubular transporters of Na + that are mainly located in the proximal tubule and through its effects in enhancing aldosterone secretion and Na + reabsorption in the distal tubule. As a potent direct smooth-muscle vasoconstrictor and as an enhancer of ADH and sympathetic nervous system activity, angiotensin II also participates in short-term blood pressure regulation in disorders associated with volume depletion or circulatory depression. Research has uncovered multiple nonhemodynamic functions that are primarily mediated by binding to T1 receptors of angiotensin II, which are particularly important in the pathophysiology of progressive renal injury ( ).
AT 1 receptor stimulation
Vasoconstriction (preferentially coronary, renal, cerebral)
Sodium retention (angiotensin, aldosterone production)
Water retention (vasopressin release)
Renin suppression (negative feedback)
Myocyte and smooth muscle cell hypertrophy
Stimulation of vascular and myocardial fibrosis
Inotropic/contractile (cardiomyocytes)
Chronotropic/arrhythmogenic (cardiomyocytes)
Stimulation of plasminogen activator inhibitor-1
Stimulation of superoxide formation
Activation of sympathetic nervous system
Increased endothelin secretion
AT 2 receptor stimulation
Antiproliferation/inhibition of cell growth
Cell differentiation
Tissue repair
Apoptosis
Possible vasodilation
Kidney and urinary tract development
A rise in plasma aldosterone concentration stimulates urinary K + secretion, thus allowing maintenance of K + balance. Aldosterone also increases the excretion of ammonium (NH 4+ ) and magnesium (Mg 2+ ) and increases the absorption of Na + in the distal tubule, both by increasing the permeability of the apical membrane and by increasing the activity of Na + , K + -adenosine triphosphatase (ATPase) ( ). The net effect is to generate more negative potential in the lumen, a driving force for increased K + secretion. In addition, aldosterone enhances reabsorption of sodium in the cortical collecting duct through activation of the epithelial sodium-specific channel (ENaC) ( ). In performing these functions, aldosterone plays a key role in regulating fluid and electrolyte balance. Long-term aldosterone administration to healthy volunteers increases the ECF volume. Clinical edema does not occur, however, because after several days, the kidneys “escape” from the Na + -retaining effect while maintaining the K + -secretory effect ( ).
ANP is secreted by atrial monocytes in response to local stretching of the atrial wall in cases of hypervolemia (e.g., congestive heart failure or renal failure) and ultimately results in the reduction of intravascular volume and systemic blood pressure ( ). In the kidneys, ANP acts in the medullary collecting duct to inhibit sodium reabsorption during ECF expansion. ANP induces hyperfiltration, natriuresis, and suppression of renin release, and it inhibits receptor-mediated aldosterone biosynthesis ( ). In the cardiovascular system, it diminishes cardiac output and stroke volume and reduces peripheral vascular resistance. Some of these effects are mediated through the influence of ANP on vagal and sympathetic nerve activity.
The internal environment of the body consists of fluids contained within compartments. Water accounts for 50% to 80% of the human body by weight. The variation in water content depends on tissue type: Adipose tissue contains only 10% water, whereas muscle contains 75% water. Total body water (TBW) decreases with age, mainly as a result of loss of water in ECF. For clinical purposes, TBW is estimated at 60% of body weight in infants older than age 6 months, as well as in children and adolescents. This value is very inaccurate for low-birth-weight premature infants, in whom TBW constitutes as much as 80% of total body weight ( ; ). In term infants younger than 6 months of age, TBW may be approximated as 75% of total body weight ( ). Newer formulas that consider the height (cm) and weight (kg) but not the degree of adiposity or the child’s surface area have improved the estimation of TBW, particularly in healthy children between 3 months and 13 years of age ( ; ). TBW can be determined as follows:
Intracellular fluid (ICF) represents about two-thirds of TBW, which is equivalent to 30% to 40% of total body weight. However, the proportion of ECF is much greater than that of ICF in preterm infants and reaches 60% of TBW at term. The membranes retaining this fluid allow the passive diffusion of water, whereas active transport mechanisms maintain an internal solute milieu different from that found outside the cells. K + , P 2− , and Mg 2+ are intracellular ions, and Na + and Cl − are predominantly extracellular.
ECF accounts for about one-third of TBW and is made up of two compartments: plasma and interstitial fluid. Plasma water represents 4% to 5% of body weight and 10% of TBW. It is the milieu in which blood cells, platelets, and proteins are suspended. Blood volume is usually estimated as a changing proportion with respect to body weight. When expressed as milliliters per kilogram of body weight, it decreases with age from 80 mL/kg at birth to 60 mL/kg in adulthood.
Interstitial fluid accounts for 16% of body weight and has a solute composition almost identical to that of intravascular fluid, except for a lower protein concentration. In general, the bulk distribution of ions and fluids between these two compartments is determined by the Donnan effect and Starling forces.
The transcellular fluid compartment (1% to 3% of body weight) is a specialized subdivision of the ECF compartment. Separated from blood by endothelium and epithelium, it represents fluid collections such as cerebrospinal fluid, aqueous and vitreous humors of the eyes, synovial fluid, pleural fluid, and peritoneal fluid.
Although all nephrons of the mature kidneys are formed by 36 weeks’ gestation during healthy intrauterine life, hyperplasia continues until the sixth postnatal month; thereafter, cell hypertrophy is responsible for increases in renal size. Growth in the size of the kidney tends to be directly proportional to increase in height ( ).
Whereas the fetal kidney receives 3% to 7% of cardiac output, RBF increases gradually after birth ( ). RBF, as measured by paraaminohippuric (PAH) acid clearance (C PAH ), correlates with gestational age. For example, C PAH is 10 mL/min per square m 2 at 28 weeks of gestation and 35 mL/min per m 2 at 35 weeks of gestation ( ). C PAH corrected for body surface area doubles by 2 weeks of age and reaches adult levels at 2 years. Furthermore, changes in RBF are associated with considerable increases in the relative RBF to the outer cortex, where most glomeruli are located ( ).
Selected renal functions measured at different ages are summarized in Table 6.3 . The GFR in the full-term newborn infant averages 40.6 ± 14.8 mL/min per 1.73 m 2 and increases to 65.8 ± 24.8 mL/min per 1.73 m 2 by the end of the second postnatal week ( ). GFR reaches adult levels after 2 years of age. Premature newborns have a lower GFR that increases more slowly than that in full-term infants. The low GFR at birth is attributed to the low systemic arterial blood pressure, high renal-vascular resistance, and low ultrafiltration pressure, together with decreased capillary surface area for filtration (see Chapter 27 , “Neonatology for Anesthesiologists”).
| Measurement | Premature Newborn | Full-Term Newborn | 1–2 Weeks | 6 Months–1 Year | 1–3 Years | Adult |
|---|---|---|---|---|---|---|
| GFR (mL/min/1.73 m 2 ) * | 14 ± 3 | 40.6 ± 14.8 | 65.8 ± 24.8 | 77 ± 14 | 96 ± 22 | Male: 125 ± 15 Female: 110 ± 15 |
| RBF (mL/min/1.73 m 2 ) | 40 ± 6 | 88 ± 4 | 220 ± 40 | 352± 73 | 540 ±118 | 620 ± 92 |
| Tm PAH (mg/min/1.73 m 2 ) | 10 ± 2 | 16 ± 5 | 38 ± 8 | 51 ± 20 | 66 ± 19 | 79 ± 12 |
| Maximal concentration ability (mOsm/kg) | 480 | 700 | 900 | 1200 | 1400 | 1400 |
| Serum creatinine (mg/dL) | 1.3 | 1.1 | 0.4 | 0.2 | 0.4 | 0.8–1.5 |
| Tm P /GFR (mg/dL) | — | 7.39 ± 0.37 | — | 5.58 ± 0.28 | 5.71 ± 0.28 | 3.55 ± 19 |
| Fractional excretion of sodium (%) | 2%–6% | <1 | <1 | <1 | <1 | <1 |
| Tm G (mg/min/1.73 m 2 ) | — | — | 71 ± 20 | — | — | 339 ± 51 |
* GFR , Glomerular filtration rate; RBF , renal blood flow; Tm PAH , tubular maximum for paraaminohippuric acid; Tm P , tubular maximum for phosphorus; Tm G , tubular maximum for reabsorption of glucose.
Despite a low GFR, full-term infants are able to conserve Na + ( ). This is explained by the existence of glomerulotubular balance, such that as GFR and the filtered load of Na + increase, so does the ability of the proximal tubule to reabsorb Na + . In contrast, preterm infants have a prolonged glomerulotubular imbalance, so GFR is high relative to tubular capacity to reabsorb Na + . The glomerulotubular imbalance is caused by structural immaturity of the proximal convoluted tubule and the incomplete development of the transport system responsible for conserving Na + . This, together with poor response of the distal tubule to mineralocorticoids in preterm infants, results in Na + wastage and susceptibility to hyponatremia.
The tubular mechanisms involved in the excretion of organic acids are poorly developed in neonates. The tubular transport of PAH, which is a weak acid, is around 16 ± 5 mg/min per 1.73 m 2 in full-term infants and about half this value in premature babies. It increases with age and reaches adult rates, ranging from 55 to 104 mg/min per 1.73 m 2 , by 12 to 18 months ( ). PAH excretion is limited by a number of factors, including low GFR, immaturity of the systems providing energy for transport, and a low number of transporter molecules. This is further accentuated by a low extraction ratio for PAH and other organic acids caused by the predominance of juxtamedullary circulation in the immature kidney, a phenomenon that allows increased shunting of blood through the vasa recta and exclusion of postglomerular blood from the proximal tubular excretory surface ( ).
The kidneys’ ability to concentrate urine is lower at birth, especially in premature infants. After water deprivation in the full-term newborn, urine concentrates to only 600 to 700 mOsm/kg, or 50% to 60% of maximum adult levels. Healthy children ranging from 6 months to 3 years of age who were given 20 mcg of desmopressin intranasally demonstrated a gradual rise in urinary concentration, starting from a mean value of 525 mOsm/kg to reach a mean maximum plateau of 825 mOsm/kg ( ). The major cause for the reduced concentration of urine in the neonate is the hypotonicity of the renal medulla ( ). Several mechanisms that contribute to interstitial hypertonicity are not well developed, including urea accumulation in the medulla, length of the loop of Henle and the collecting ducts within the medulla, and Na + reabsorption in the ascending, water-impermeable loop ( ; ; ). In addition, the collecting duct cells in immature kidneys may be less sensitive to ADH than those of mature nephrons ( ).
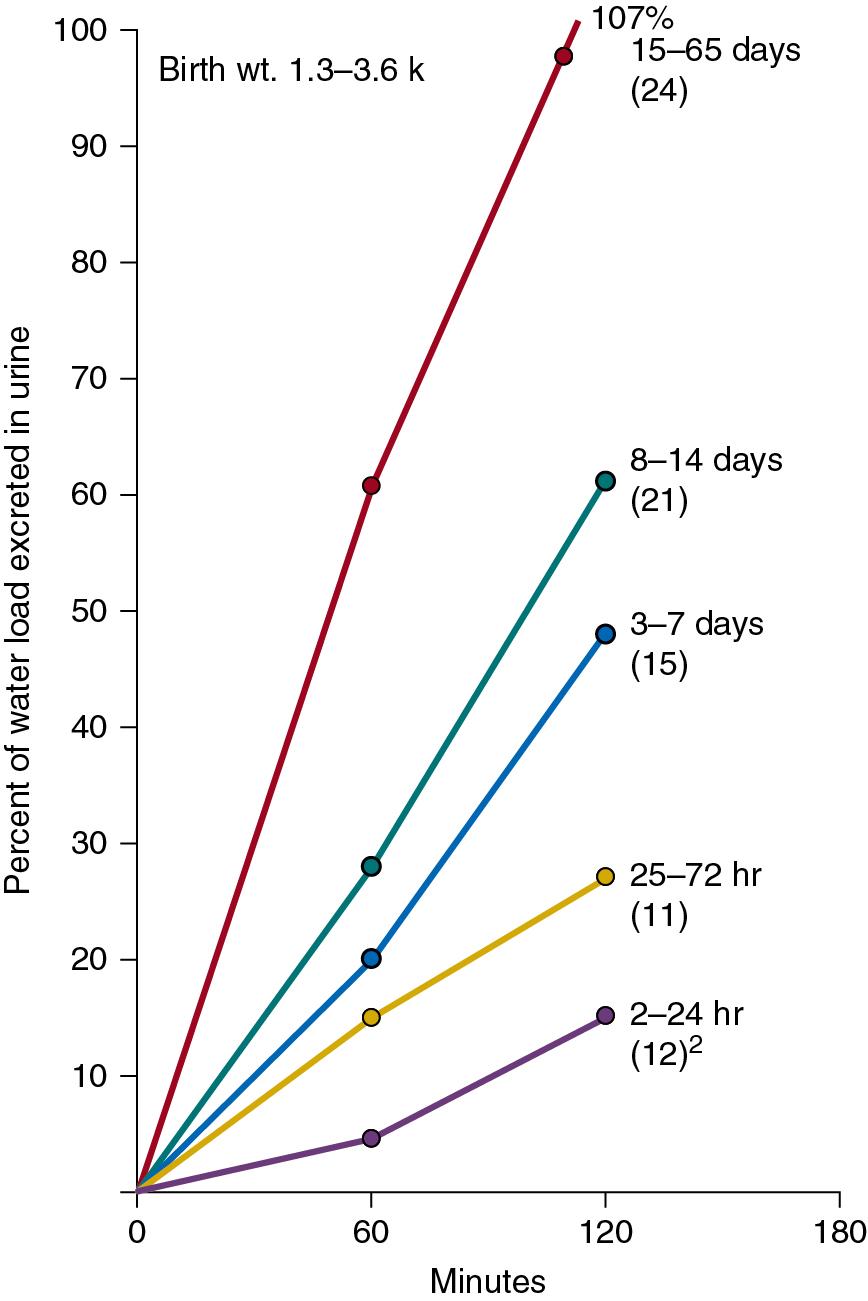
A water-loaded infant can excrete diluted urine with osmolality as low as 50 mOsm/kg. In the first 24 hours of life, however, the infant may be unable to increase water excretion to approximate water intake ( ). The diluting capacity becomes mature by 3 to 5 weeks of postnatal life ( Fig. 6.4 ).
The normal need for fluids varies markedly in low-birth-weight and full-term neonates, as well as during infancy and later childhood. This variability in fluid needs is caused by differences in the rate of caloric expenditure and growth, the ratio of evaporative surface area to body weight, the degree of renal functional maturation and reserve, and the amount of TBW at different ages. For instance, compared with older children and adults, infants have greater fluid needs because of higher rates of metabolism and growth; a surface area-to-weight ratio that is about three times greater, resulting in higher insensible fluid loss; and greater urinary excretion of solutes combined with lower tubular concentrating ability, which increases obligatory fluid loss. On the other hand, as previously noted, low-birth-weight and full-term neonates have a greater percentage of TBW compared with older children and adults ( ; ). This increase in TBW results mainly from expansion of the ECF compartment, which at birth may constitute as much as 50% of TBW. During the first 3 postnatal days, when this “extra fluid” is eliminated by the kidneys, full-term neonates require less fluid intake ( ; ; ).
The needs of low-birth-weight infants are more variable and may be markedly altered by relatively minor changes in ambient temperature or by phototherapy ( Table 6.4 ) ( ; ; ). In contrast to more mature infants, the immature skin in very low-birth-weight infants (<1500 g) allows disproportionate evaporative heat loss relative to basal metabolic rate ( ; ). This greater evaporative heat loss, together with a large body surface area, accounts for the much greater insensible fluid needs in infants with very low birth weight.
| BODY WEIGHT (G) | |||||
|---|---|---|---|---|---|
| Age (Days) | Component | 751–1000 | 1001–1250 | 1251–1500 | 1501–2000 |
| 1 | IWL † | 65 | 55 | 40 | 30 |
| Urine ‡ | 20 | 20 | 30 | 30 | |
| Stool | 0 | 0 | 0 | 0 | |
| Total | 85 | 75 | 70 | 60 | |
| 2–3 | IWL | 65 | 55 | 40 | 30 |
| Urine | 40 | 40 | 40 | 40 | |
| Stool | 0 | 0 | 0 | 5 | |
| Total | 105 | 95 | 80 | 75 | |
| 4–7 | IWL | 65 | 55 | 40 | 30 |
| Urine | 60 | 60 | 60 | 60 | |
| Stool | 5 | 5 | 5 | 5 | |
| Total | 130 | 120 | 105 | 95 | |
* Allowances for increased metabolic rate (cold stress, increased activity) are not included; these infants are in an incubator and naked.
‡ Volume required to achieve a urine osmolarity of 250 mOsm/kg of renal solute load during the first day (no sodium and protein added), 10 mOsm/kg per day on the second and third days, and 15 mOsm/kg per day on the fourth to seventh days.
Except for the first 3 postnatal days when full-term neonates require only 40 to 60 mL/kg fluid per day, in general, 100 mL of water is needed for each 100 kcal expended. Notably, an additional 15 mL of water is generated endogenously for each 100 kcal used (water of oxidation), which is also available for body functions. In preterm infants, fluid intake may be gradually increased to 150 mL/kg per day, whereas 100 to 125 mL/kg per day generally suffices for infants weighing less than 10 kg. The fluid requirement decreases to 50 mL/kg per day for those weighing 11 to 20 kg and to 20 mL/kg per day for those with body weights above 20 kg, thus the derivation of the 4, 2, 1 rule for calculating fluid maintenance. These fluid volumes are sufficient to allow excretion of dietary solute load and to replace insensible fluid loss through the skin, lungs, and intestines ( Table 6.5 ) ( ). It should be noted that energy expenditure and, therefore, fluid intake may be significantly increased with stress ( Table 6.6 ) ( ).
| H 2 O = 100–125 mL/100 kcal Expended | ||
| Components | Insensible loss (mL) | 45 |
| Sweat (mL) | 0–25 | |
| Urine (mL) | 50–75 | |
| Stool (mL) | 5–10 | |
| Food oxidation (mL) | 12 | |
| Na + = 2.5 mmol/100 kcal Expended | ||
| Components | Body growth | |
| Sweat | Variable | |
| Urine | Variable | |
| Stool | Variable | |
| K + = 2.5 mmol/100 kcal Expended | ||
| Components | As for Na + | |
| Cl − = 5 mmol/100 kcal Expended | ||
| Components | As for Na + | |
| Dextrose = 25 g/100 kcal Expended | ||
| Components | Basal metabolic rate | |
| Growth and tissue repair | ||
| Physical activity | ||
| Maintenance Solution (per liter of water) | ||
| Dextrose (g) | 50 | |
| Na + (mmol) | 25 | |
| K + (mmol) | 25 | |
| Cl − (mmol) | 50 | |
| AVERAGE HOSPITAL ENERGY REQUIREMENTS | Increases in Energy Expenditure with Stress | ||
|---|---|---|---|
| Body Weight (kg) | kcal/kg/day | ||
| 0–10 | 100 | Fever | 12% per °C |
| 10–20 | 1000 + 50/kg | Cardiac failure | >37° C |
| >20 | 1500 + 20/kg | Major surgery | 15%–25% |
| Burns | 20%–30% | ||
| Severe sepsis | Up to 100% | ||
| 40%–50% | |||
The high fractional excretion of Na + (FE Na + ) in premature infants can lead to negative Na + balance, hyponatremia, neurologic disturbances, and poor growth unless an Na + intake of 3 to 5 mmol/kg per day is given; in full-term infants and older children, 2 to 3 mmol/kg per day is sufficient ( ). Premature infants have a lower renal threshold for bicarbonate. In addition, several functional and anatomic factors combine to limit tubular excretion of weak organic acids ( ). Consequently, premature infants may need small supplements of base. Sodium bicarbonate at 1 to 2 mmol/kg per day is generally recommended for the very small premature infant. Clinically important disturbances in acid-base status are unusual in full-term neonates unless they consume excessive amounts of protein.
Because premature infants and mature neonates have a greater TBW-to-body weight ratio than older infants and children, they tolerate a greater degree of dehydration before manifesting clinical symptoms. A 10% fluid deficit in such patients may produce symptoms consistent with moderate dehydration, whereas a similar deficit in adults produces severe symptoms. However, dehydration can occur very quickly in infants because disorders such as vomiting or diarrhea very rapidly produce deficits of 50 to 100 mL/kg. Dehydration can also develop in healthy premature infants if insensible water losses are underestimated and are not adequately replaced. This situation may result from use of an open radiant warmer without appropriate plastic shields; forced convection in nonhumidified incubators; skin immaturity, resulting in greater transcutaneous evaporative fluid loss; use of phototherapy, causing insensible fluid loss; hyperthermia; or tachypnea. In older infants and children, gastrointestinal disorders are the major causes of dehydration.
The clinical signs of dehydration are a manifestation of extracellular volume depletion ( Table 6.7 ). Three factors determine the amount of extracellular volume depletion and therefore the severity of dehydration: fluid deficit, electrolyte deficit, and the speed with which dehydration occurs. The fluid deficit or antecedent deficit is the total amount of body water lost expressed as a percent decrease in body weight and can be estimated based on physical findings. The serum sodium can be normal, high, or low in children with dehydration. The severity of dehydration is affected by the electrolyte deficit, which is reflected in the serum sodium. In general, the electrolyte deficit parallels extracellular fluid losses. Therefore for the same fluid deficit, the severity of extracellular volume depletion is inversely proportional to the serum sodium. Stated differently, given the same volume loss, hyponatremic dehydration results in more severe signs than hypernatremic dehydration. Signs of volume depletion are less pronounced in patients with hypernatremia due to better preservation of the extracellular volume. This is the basis for classifying dehydration according to the serum sodium as isonatremic (130–150 mEq/L), hyponatremic (<130 mEq/L), or hypernatremic (>150 mEq/L). Isonatremic dehydration is the most commonly encountered in approximately 70% to 80% of children, followed by hyponatremic in 15% and hypernatremic in 5%. The most important factor that will determine the type of dehydration is the amount of oral intake to balance the free water losses. In most instances the amount of free water losses and free water ingested are of similar magnitude, resulting in little change in serum sodium. In infants where water intake may be decreased due to limited access or vomiting, free water losses will result in hypernatremic dehydration. In older children who may be able to satisfy their thirst or who are taking very hypotonic oral fluids, free water intake in excess of free water losses will result in hyponatremic dehydration. This is exacerbated by the stimulation of AVP secretion due to the intravascular volume depletion.
| Signs and Symptoms | Mild Dehydration | Moderate Dehydration | Severe Dehydration |
|---|---|---|---|
| Weight loss (%) | 5 | 10 | 15 |
| Fluid deficit (mL/kg) | 50 | 100 | 150 |
| VITAL SIGNS | |||
| Pulse | Normal | Increased; weak | Greatly increased; feeble |
| Blood pressure | Normal | Normal to low | Reduced and orthostatic |
| Respiration | Normal | Deep | Deep and rapid |
| GENERAL APPEARANCE | |||
| Infants | Thirsty, restless, alert | Thirsty, restless, or lethargic, but arousable | Drowsy to comatose; limp, cold, sweaty; gray color |
| Older children | Thirsty, restless, alert | Thirsty, alert, postural hypotension | Usually comatose; apprehensive, cyanotic, cold |
| Skin turgor † | Normal | Decreased | Greatly decreased |
| Anterior fontanel | Normal | Sunken | Markedly depressed |
| Eyes | Normal | Sunken | Markedly sunken |
| Mucous membranes | Moist | Dry | Very dry |
| URINE | |||
| Flow (mL/kg/hr) | <2 | <1 | <0.5 |
| Specific gravity | 1.02 | 1.020–1.030 | >1.030 |
* When hypernatremia is present, the severity of dehydration may be clinically underestimated because of the relative preservation of extracellular fluid volume (ECFV) at the expense of intracellular fluid volume (ICFV). In such states, neurologic symptoms (lethargy alternating with hyperexcitability, progressing to focal or generalized seizures) may predominate.
† With hypernatremia, the skin may have a thick, “doughy” consistency or a soft, velvety texture.
The primary goal of rehydration is to rapidly expand the intravascular volume to reestablish hemodynamic stability and tissue perfusion. If dehydration is severe, this is done by administering 20 to 40 mL/kg of a balanced salt solution (0.9% sodium chloride, plasmalyte, or lactated Ringer’s solution) rapidly and repeating the bolus fluid administration as necessary until the patient is hemodynamically stable. Further volume depletion can generally be corrected by administering 40 mL/kg of 0.9% sodium chloride over 2 to 4 hours, followed by administering the remainder of the deficit and maintenance as 0.9% sodium chloride. Caution must be taken to avoid hypotonic fluids such as 0.45% and 0.22% sodium chloride until the dehydration is corrected and AVP secretion is no longer stimulated. Hypotonic fluids may be necessary AFTER the initial phase of fluid therapy if hypernatremia is present and is associated with ongoing free water losses from high fever, voluminous diarrhea, or a renal concentrating defect such as congenital nephrogenic diabetes insipidus or renal dysplasia. Hypotonic fluids may also be required in a child with severe hyponatremic dehydration (Na <120 mEq/L) after the initial therapy with 0.9% sodium chloride. The hypotonic solution is used in order to prevent rapid correction of hyponatremia from a free water diuresis. In addition to intravenous fluid administration, oral rehydration solutions are an effective therapy for mild to moderate dehydration. The compositions of select parenteral solutions are shown in Table 6.8 .
| Fluid | M/B | Glucose (mg/dL) | Na mEq/L | Cl mEq/L | K mEq/L | Buffer mEq/L | Ca (1 mmol = 2 mEq) | Mg (1 mmol = 2 mEq) | pH | Osmolality mOsm/L mOsm/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| Human plasma | 70–110 | 135–145 | 95–105 | 3.5–5.3 | 24 | 4.4–5.2 mEq/L 2.2–2.6 mmol/L |
1.6–2.4 mEq/L 0.8–1.2 mmol/L |
7.35–7.45 | 308, 288 | |
| D 5 W | M | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 3.5–6.5 | 278 |
| D 4 0.18% NaCl | M | 4 | 31 | 31 | 0 | 0 | 0 | 0 | 4.5 | 284 |
| D 5 0.2% NaCl | M | 5 | 38 | 38 | 0 | 0 | 0 | 0 | 3.5–6.5 | 320 |
| D 5 0.45% NaCl | M | 5 | 77 | 77 | 0 | 0 | 0 | 0 | 3.5–6.5 | 405 |
| D 5 Ringer’s Lactate | M | 5 | 130 | 112 | 4 | 28 | 3 mEq/L 1.5 mmol/L |
0 | 4.0–6.5 | 530 |
| D 5 0.9% NaCl | M | 5 | 154 | 154 | 0 | 0 | 0 | 0 | 3.5–6.5 | 560 |
| D 5 Plasma-Lyte 148 | M | 5 | 140 | 98 | 5 | 50 | 0 | 3 mEq/L 1.5 mmol/L |
4.0–6.5 | 547 |
| Lactated Ringer’s | M/B | 0 | 130 | 109 | 4 | 28 | 2.7 mEq/L 1.35 mmol/L |
0 | 6–7.5 | 273 |
| Ringer’s acetate | M/B | 0 | 130 | 112 | 5 | 27 | 2 mEq/L 1 mmol/L |
2 mEq/L 1 mmol/L |
6–8 | 276 |
| Hartmann’s | M/B | 0 | 131 | 111 | 5 | 29 | 4 mEq/L 2 mmol/L |
0 | 5.0–7.0 | 278 |
| 0.9% NaCl | M/B | 0 | 154 | 154 | 0 | 0 | 0 | 0 | 4.5–7 | 310 |
| Plasma-Lyte 148 | M/B | 0 | 140 | 98 | 5 | 50 | 0 | 3 mEq/L 1.5 mmol/L |
7.4 | 294 |
| Sterofundin/Ringerfundin | M/B | 0 | 145 | 127 | 4 | 29 | 5 mEq/L 2.5 mmol/L |
2 mEq/L 1 mmol/L |
5.1–5.9 | 309 |
In most infants and children receiving parenteral solutions for brief periods, the normal fluid and electrolyte needs can be easily satisfied. The caloric needs, however, are not readily met. It is customary to provide 5% dextrose in parenteral solutions. Although this concentration provides only a fraction of the optimal number of calories (20% of total kilocalories needed by infants younger than 1 year of age), it is sufficient to prevent ketosis. In less mature neonates, higher infusion rates of 5% dextrose generally suffice to maintain blood glucose concentrations between 50 and 90 mg/dL ( ; ).
Provided that infants and children are less than 10% dehydrated and have minimal electrolyte abnormalities, a good level of consciousness, adequate bowel sounds, and absence of signs of hypovolemia, oral rehydration may be used to replace deficits and maintain fluid volume. Commercially available preparations, such as Pedialyte RS (Ross) with an Na + content of 45 mmol/L may be used. In children with diarrhea in developing countries, the World Health Organization (WHO) has recommended the use of an inexpensive and effective oral rehydration solution consisting of 90 mmol/L Na + and 111 mmol/L of glucose (total osmolarity, 311 mOsmol/L). However, glucose-based solutions with a lower osmolality may further optimize fluid and glucose-sodium coupled absorption in the small intestine ( ).
The optimal perioperative fluid volume and composition requirements in infants and children have not been adequately investigated. The formulas provided by in Table 6.9 are widely used to determine the hourly rates of intraoperative fluid volume administration, which consists of four major components:
Maintenance fluid established by based on calorie expenditure at different ages.
Estimated volume deficit incurred during preoperative fasting or gastrointestinal or other fluid deficits. One-third of such deficits may be replaced during the first hour of surgery, whereas the remaining volume may be spread over the duration of the surgery.
Severity of surgical and nonsurgical trauma. This may constitute the largest volume of fluid loss or fluid redistribution, which derives largely from the ECF compartment.
Blood losses and fluids needed to support systemic blood pressure.
| Age (yr) | Hydrating Solution During First Hour (mL/kg) | Hydrating Solution During Following Hours |
|---|---|---|
| Neonates | Maintenance fluid: 4 mL/kg/hr 5%–10% dextrose in 0.75 normal saline plus 20 mEq sodium bicarbonate/L Trauma: 6–10 mL/kg/hr for intraabdominal or 4–7 mL/kg/hr for intrathoracic surgery replaced with Ringer’s lactate |
|
| <3 | 25 | Maintenance fluid: 4 mL/kg/hr 5% dextrose in normal saline |
| 3–4 | 20 | Maintenance and trauma: basic hourly fluid 4 mL/kg 5% dextrose in normal saline + If mild trauma 2 mL/kg = 6 mL/kg/hr |
| >4 | 15 | + If moderate trauma 4 mL/kg = 8 mL/kg/hr + If maximal trauma 6 mL/kg = 10 mL/kg/hr |
* Plus blood replacement with blood or 3:1 volume replacement with crystalloid. Replace blood loss in excess of 20 mL/kg with equal volume of packed red blood cells.
A key goal of perioperative fluid management is to maintain an adequate intravascular volume without the development of hyponatremia or fluid overload ( ; ; ). Perioperative patients are at risk for developing hyponatremia because of multiple factors, including prehydration with hypotonic fluid and nausea, pain, and stress associated with surgery that may result in nonhypovolemic stimulation of ADH release during and after surgery (i.e., the inappropriate secretion of ADH) ( ; ). The limited ability of such individuals to excrete a large water load may be influenced by any preexisting edema-forming disorder, obstructive uropathy, or the use of thiazide diuretics or other drugs such as narcotics and antiemetics. However, hypotonic fluid infusion is the most important cause of acute hyponatremia developing in the intraoperative period. Acute hyponatremia results in increased water content in neurons (brain edema) without a change in solute content. This may cause subclinical symptoms such as headache, nausea, vomiting, or muscle weakness in any age group. Younger children are more susceptible to more severe hyponatremic encephalopathy because of their larger brain-to-skull ratio ( ). Unless there is a free water deficit, isotonic fluid infusion is recommended during the perioperative period. The need for potassium, calcium, chloride, and bicarbonate (or lactate or citrate, which may be converted to bicarbonate in individuals without hepatic failure) is more controversial.
The amount of dextrose commonly used is 5% (equals 5000 mg/dL or 278 mmol/L). Although this is more than 50 times more concentrated than normal plasma glucose concentration (90 to 100 mg/dL or about 5 mmol/L), the energy delivery based on the volume of fluid given to an infant weighing 10 kg amounts to 50 kcal for the first hour of surgery. Such energy supply is particularly important in preventing hypoglycemia in premature and full-term neonates, who have greater energy requirements than older children, but it may lead to hyperglycemia in 0.5% to 2% of pediatric patients. This disorder may be less common in children receiving regional anesthesia, which reduces the hyperglycemic effects of surgery. Although such transient hyperglycemia is purported to have various potential deleterious consequences, these have not been well substantiated. A review suggests that a solution of lactated Ringer’s solution with 1% dextrose is sufficient to prevent both hypoglycemia and hyperglycemia in most children, excluding premature and full-term neonates ( ). This practice, however, is not yet widely used.
There has been a growing concern that 0.9% sodium chloride has a supraphysiologic chloride concentration and may result in untoward complications such as hyperchloremic metabolic acidosis, renal vasoconstriction, delayed micturition, hyperkalemia, an increased incidence of acute kidney injury, and the need for renal-replacement therapy ( ). The benefit of balanced solutions over isotonic saline seems to be primarily in critically ill adult patients with sepsis and those with preceding acute kidney injury and previous renal replacement therapy ( ; ). It is unclear if this is applicable to children with volume depletion. Balanced solutions differ from normal saline primarily in having variable amounts of a buffering agent, such as lactate, acetate, or gluconate. Balanced solutions do not have bicarbonate as it is not stable in polyvinyl chloride bags. Balanced solutions also have variable amounts of potassium, calcium, and magnesium and have a lower sodium concentration and osmolarity in comparison to normal saline ( Table 6.8 ). A solution of 0.9% sodium chloride (Na 154 mmol/L) has a higher sodium concentration than plasma but results in normal osmolarity, whereas Plasma-Lyte (Na 140 mmol/L) and lactated Ringer’s (Na 130 mmol/L) are slightly hypotonic in relationship to plasma. Lactated Ringer’s in particular may aggravate hyponatremia and should be avoided in hyponatremic patients or those at high risk for cerebral edema. It has not been clearly established that balanced solutions are superior to 0.9% sodium chloride for fluid resuscitation or in the perioperative state, with large clinical trials yielding conflicting results. The Saline Against Lactated Ringers or Plasma-Lyte in the Emergency Department (SALT-ED) and Isotonic Solutions and Major Adverse Renal Events (SMART) trials were able to demonstrate a 1% decrease in a composite of major kidney events ( ; ). The Saline versus Plasma-Lyte 148 for ICU Fluid Therapy (SPLIT) and the Saline or Lactated Ringer’s (SOLAR) trial were unable to determine a clinically meaningful difference between the two solutions, as was a recent Cochrane review ( ; see also ; ). At the current moment, both fluids are appropriate for resuscitation and perioperative fluid management.
Guidelines for the intraoperative fluid and electrolyte management of premature and full-term neonates are largely based on available knowledge of renal physiology rather than on data obtained from clinical investigations (see Chapter 27 , “Neonatology for Anesthesiologists”). The physiology of the healthy neonate is influenced by the short tubular length and is characterized by immature reabsorption mechanisms, an activated renin-angiotensin-aldosterone system, and low circulating ADH concentrations ( ; ). Thus healthy preterm neonates weighing less than 1300 g, or of fewer than 32 weeks’ gestation, have FE Na + rates that range from 8.2% to 2.1% between 28 and 32 weeks’ gestation, with further gradual decrease to less than 1% at term; such rates may increase to 15% with stress ( ; ). The high FE Na + in preterm infants is ascribed to decreased Na + reabsorption in the proximal tubule, together with hyporesponsiveness of the distal tubule to aldosterone ( ). When combined with a negative Na + balance that results from inadequate Na + supplementation and decreased sensitivity of the collecting duct to ADH, up to one-third of such infants develop significant hyponatremia (Na + <130 mEq/L), often manifesting with neurologic disturbances during the first 6 weeks of life ( ).
Both premature and term neonates have a limited capacity to excrete K + , possibly because of distal tubular insensitivity to aldosterone. Hence, baseline reference plasma K + concentrations range from 3.9 to 5.9 mEq/L. Moreover, both preterm and term neonates are capable of producing maximally diluted urine while concentrating capacity is limited. Yet hyponatremia may develop after administration of large volumes of hypotonic fluids, because fluid excretion may be limited mainly because of low GFR. Stress may cause profound reduction in GFR in premature and term neonates through release of various extrarenal vasoactive and hormonal substances that modify the response of “immature kidneys,” thereby further disturbing fluid and electrolyte homeostasis. The higher body content of water and the higher metabolic rate, as well as a propensity to metabolic acidosis and hypocalcemia in premature newborns, are other important factors in deciding the volume and composition of intraoperative fluids.
Such considerations support the avoidance of boluses of hypotonic fluids, while keeping in mind the lower age- and size-appropriate circulatory pressures that may serve as the goal of fluid management. In the absence of the expected physiologic fluid loss, which may range from 5% to 15% of body weight during the first 3 days of postnatal life, fluid volume during this time period may be limited to 60 mL/kg per day, whereas blood pressure support may be sustained with small infusions (5 mL/kg) of 5% albumin or other blood products as needed. Beyond 3 days of life, maintenance fluid volume is gradually increased to 150 mL/kg per day. Deficits beyond the expected physiologic losses and ongoing losses and allowance for surgical trauma may be replaced by a similar fluid composition, but the volume replacement may be more gradual or less rapid than outlined for older infants and children (see Table 6.9 ). Na + bicarbonate and calcium may be supplemented, whereas K + should be limited. Also, a higher glucose concentration is generally desirable in premature infants. Prospective studies have demonstrated the safety of using isotonic fluids in neonates ( ).
The key goal of intraoperative management is to expand the circulatory volume and to maintain systemic blood pressure between the 90th and 95th percentiles for age, gender, and height percentile, so as to allow for adequate perfusion of the renal allograft ( ). Regarding fluid management, there is no difference between a cadaveric and a living related renal transplant (see Chapter 38 , “Solid Organ Transplantation”). An adult kidney may sequester up to 250 mL of blood, and in infants, nearly 50% of the cardiac output may be directed toward perfusion of the allograft. To ensure adequate perfusion of the allograft, the anesthesiologist actually needs to maximize the circulatory volume of the recipient, mainly with crystalloid or packed cytomegalovirus-safe, leukocyte-poor red blood cells if hemoglobin is below 9 g/dL, while closely monitoring the central venous pressure (CVP) and systemic MABP during vessel anastomosis. Near the completion of the vascular anastomoses, 20% mannitol (0.5 to 1.0 g/kg) and intravenous furosemide (1 mg/kg) may be given before the cross-clamps are released. Before cross-clamp release, CVP should be maintained at 8 to 12 cm H 2 O (some have suggested as much as 18 to 20 cm H 2 O), and the systolic blood pressure and MABP should be kept above 120 mm Hg and 70 mm Hg, respectively. If the MABP is inadequate to achieve good renal perfusion of the adult kidneys, a constant dopamine infusion of up to 5 mcg/kg per minute may be started. Intraoperative blood gases may be monitored frequently, because clamping of the aorta and accumulation of lactic acid can result in metabolic acidosis and vasoconstriction. The critical goal is to obtain immediate allograft function; hypotension after cross-clamp release in an infant with inadequate circulatory volume and an underperfused allograft is a potential catastrophe ( ).
Intravenous furosemide (1 to 2 mg/kg per dose), 25% salt-poor albumin (0.5 g/kg per dose), 20% mannitol (0.3 to 0.5 g/kg per dose), or isotonic solutions (10 mL/kg bolus) may be given to help promote urine output in the immediate postoperative period.
The volume and composition of intravenous fluid administered during the first 48 postoperative hours are essential to ensure continued renal function. The urine output often exceeds 5 mL/kg per hour. Thus insensible losses are quantitatively less important. Urine output is replaced on a milliliter-for-milliliter basis for approximately the first 24 hours postoperative and then can be changed to a fixed rate thereafter. In infants and children with body weight below 30 kg, the CVP should be maintained in the range of 5 to 10 cm H 2 O, and the MABP should be at greater than 70 mm Hg. One fluid solution that may be used during the first 24 to 48 hours consists of 1% dextrose, 0.45 mEq/L NaCl solution, and 10 to 20 mEq sodium bicarbonate per liter. During the first 24 to 36 hours, additional fluid boluses of 10 mL/kg of normal saline or a 5% albumin solution may be given if CVP falls below 5 cm H 2 O, with the goal of maintaining a urine output above certain arbitrary limits (5, 4, and 3 mL/kg per hour for body weight <10 kg, <20 kg, and <30 kg, respectively). It should be noted that excess fluid replacement with 0.45% NaCl has resulted in hyponatremic encephalopathy ( ). In conjunction with such fluid boluses, intravenous furosemide (1 mg/kg) is also administered because renal allografts tend to be dependent on diuretics in the early perioperative setting.
Serum electrolytes (Na + , K + , Cl − , HCO 3 − , Ca 2+ , P 2− , Mg 2+ ) may be monitored at 8- to 12-hour intervals during the first 2 postoperative days. Potassium chloride is given separately as needed when the plasma K + decreases below 3.5 mEq/L. In infants and young children, close monitoring of fluid balance and cardiovascular examination are essential to prevent electrolyte imbalance and fluid overload, which may result in severe hypertension or pulmonary edema or in reduction of intravascular volume and acute tubular necrosis (ATN). A bladder catheter inserted intraoperatively is necessary for accurate measurement of urine volume. Unless there are specific urologic indications, the catheter is removed after 4 to 5 days.
Immediate measures must be undertaken to improve postoperative oliguria. Besides the most easily correctable causes of oliguria, such as hypovolemia or a malfunctioning catheter, other potential causes include vascular bleeding or occlusion, ATN caused by prolonged cold ischemia storage, hyperacute rejection, or urinary extravasation or obstruction. In patients with oxalosis, precipitation of calcium oxalate crystals in the graft may cause acute allograft failure. Children with delayed graft function, congestive heart failure, or marked electrolyte abnormalities may require removal of fluid by hemodialysis or peritoneal dialysis. Fluid removal should be performed cautiously to avoid allograft hypoperfusion.
Hyponatremia, defined as a serum less than 135 mEq/L, is a common disorder that occurs in both the outpatient and inpatient settings ( Table 6.10 ) ( ). The body’s primary defense against developing hyponatremia is the kidney’s ability to generate a dilute urine and excrete free water. Rarely is excess ingestion of free water alone the cause of hyponatremia, as an adult with normal renal function can typically excrete over 15 L of free water per day. It is also rare to develop hyponatremia from excess urinary sodium losses in the absence of free water ingestion. For hyponatremia to develop, it typically requires a relative excess of free water in conjunction with an underlying condition that impairs the kidney’s ability to excrete free water. Renal water handling is primarily under the control of arginine vasopressin (AVP), which is produced in the hypothalamus and released from the posterior pituitary. AVP release impairs water diuresis by increasing the permeability to water in the collecting tubule. There are osmotic, hemodynamic, and nonhemodynamic stimuli for AVP release. In most cases of hyponatremia, there is a stimulus for vasopressin production that results in impaired free water excretion. The body will attempt to preserve the extracellular volume at the expense of the serum sodium, so a hemodynamic stimulus for AVP production will override an inhibitory effect of hyponatremia. There are numerous stimuli for AVP production that occur in hospitalized patients, so all hospitalized patients are at risk for hyponatremia.
| Neonates | Infants and Children |
|---|---|
| Drugs | |
| Prolonged use of diuretics in mother or infant | Diuretics (thiazides, osmotic diuretics) |
| Oxytocin for labor | Arginine vasopressin |
| Dopamine >5 mcg/kg/min | Carbamazepine |
| Prostaglandin infusion | Vincristine |
| Excessive administration of electrolyte-free solutions | Theophylline Cyclophosphamide Morphine Estrogen Barbiturates Nonsteroidal antiinflammatory agents Mannitol Hypotonic 1.5% glycine irrigant Ecstasy Selective serotonin reuptake inhibitors All conditions listed for neonates |
| Endocrine Disorders | |
| Pseudohypoaldosteronism | Hyperglycemia |
| Adrenogenital syndrome | Myxedema |
| Adrenal insufficiency problems | Glucocorticoid deficiency |
| Hypothyroidism | Decreased atrial natriuretic peptide |
| SIADH caused by asphyxia | Diabetes/ketonuria All conditions listed for neonates |
| Renal Disorders | |
| Dysplasia | Nephrotic syndrome |
| Multicystic kidneys | Acute or chronic renal failure |
| Obstructive uropathy | Medullary cystic kidneys |
| Polycystic kidney disease | Nephronophthisis |
| Renal tubular acidosis | Chronic pyelonephritis |
| Acute or chronic renal failure | Drug-induced tubulointerstitial nephritis Hypokalemic nephropathy Metabolic alkalosis Bicarbonaturia Postobstructive diuresis All conditions listed for neonates |
| Gastrointestinal Disorders | |
| Dilute formulas | Pancreatitis Cirrhosis Vomiting Diarrhea Ileus Bowel edema Protein-losing enteropathy Colonoscopy |
| Central Nervous System Disorders | |
| SIADH | |
| Cerebral salt wasting | |
| Reset osmostat | |
| Miscellaneous | |
| Negative Na + balance caused by high FE Na + in infants ≤34 weeks’ gestation | Congestive heart failure |
| Hypoalbuminemia and decreased oncotic pressure | “Third-space” from burns, peritonitis, or severe muscle injury |
| Osmotic diuresis caused by hyperalimentation and low TmG | Water intoxication (psychogenic polydipsia, dilute formulas) |
| Ketonuria | Physical and emotional stress |
| Congestive heart failure | Cystic fibrosis |
| Hydrops fetalis | Pain |
| Congenital nephrotic syndrome | Postoperative |
| Surgery | Porphyria |
| Infection | Rickettsial disease |
| Pulmonary disorders | Freshwater drowning Pseudohyponatremia in patients with hypoproteinemia, hyperglycemia, or hyperlipidemia Prolonged exercise |
Before embarking on an aggressive therapeutic regimen, it is vital to confirm that hyponatremia is in fact associated with hypoosmolality. Hyponatremia can be associated with either a normal or an elevated serum osmolality. The most common reasons for this are hyperglycemia, severe hyperproteinemia, or hyperlipidemia. Hyperglycemia results in hyperosmolality with a translocation of fluid from the intracellular space to the extracellular space, resulting in a 1.6 mEq/L decrease in the serum sodium for every 100 mg/dL elevation in the serum glucose concentration above normal. Severe hyperlipidemia, hypercholesterolemia, hyperproteinemia, or radiocontrast can cause a displacement of plasma water, which will result in a decreased sodium concentration (pseudohyponatremia) but a normal serum osmolality. Serum sodiums are currently measured by either direct- or indirect-reading ion-selective electrode potentiometry. The direct method will not result in pseudohyponatremia, because it measures the activity of sodium in the aqueous phase of serum only. The indirect method, on the other hand, can result in pseudohyponatremia because the specimen is diluted with a reagent prior to measurement. The indirect method is currently performed in approximately 60% of chemistry labs in the United States; therefore pseudohyponatremia remains an entity that clinicians need to be aware of. If hyponatremia is associated with hypoosmolality (true hyponatremia), then urinary osmolality needs to be measured in order to determine whether there is an inability to excrete free water (U osm >100 mosm/kg ).
The information that is most useful in arriving at a correct diagnosis of hyponatremia is a detailed history of fluid balance, weight changes, medications (especially diuretics), and underlying medical illnesses. Hyponatremia is usually a multifactorial disorder, and a detailed history will identify sources of salt and water losses, free water ingestion, and underlying illnesses that cause a nonosmotic stimulus for vasopressin production. Although an assessment of the patient’s volume status by physical examination and measuring the patient’s urinary electrolytes can be extremely helpful, both of these assessments can be misleading. In patients in whom hyponatremia is due to salt losses, such as diuretics, signs of volume depletion may be absent on physical examination, because the volume deficit may be nearly corrected due to oral intake of hypotonic fluids if the thirst mechanism is intact.
In general, a urinary sodium concentration less than 25 mEq/L is consistent with effective circulating volume depletion, whereas a urine sodium greater than 25 mEq/L is consistent with renal tubular dysfunction, use of diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (SIADH). However, numerous factors can affect the urine sodium, so the timing of the urinary measurements in relation to dosages of diuretics, intravenous fluid boluses, or fluid and sodium restriction helps discern the underlying cause of the hyponatremia. In some cases assessment of volume status by the measurement of a central venous pressure may be helpful.
Before SIADH can be diagnosed, diseases causing decreased effective circulating volume, renal impairment, adrenal insufficiency, and hypothyroidism must be excluded. Cortisol deficiency in particular should be ruled out, as it can be clinically indistinguishable from SIADH and can manifest in times of stress. Glucocorticoid hormones exert an inhibitory effect on AVP synthesis, which is why patients with glucocorticoid deficiency have markedly elevated AVP serum concentrations that are rapidly reversed by physiologic hydrocortisone replacement. A serum cortisol concentration in the normal range does not rule out adrenal insufficiency as the cause of hyponatremia, as the appropriate adrenal response to hyponatremia would be to increase cortisol production. An adrenocorticotropic hormone (ACTH) stimulation test should be considered if the cortisol serum concentration is not appropriately elevated in the setting of hyponatremia. Hypothyroidism can also resemble SIADH in infants.
Hospital-acquired hyponatremia is of particular concern in children because the standard of care in pediatrics has been to administer hypotonic fluids containing 0.2% to 0.45% sodium chloride as maintenance fluids ( ). The safety of this approach has never been established. Hospitalized children have numerous nonosmotic stimuli for vasopressin production that place them at risk for developing hyponatremia. There have been over 50 reported cases of neurologic morbidity and mortality during the past 10 years resulting from hospital-acquired hyponatremia in children receiving hypotonic parenteral fluids. Over half of these cases occurred in the postoperative setting in previously healthy children undergoing minor elective surgeries. Hyponatremia is especially dangerous in children with underlying CNS injury such as encephalitis, in which even mild hyponatremia (sodium >130 mEq/L) may result in cerebral edema and even herniation. In 2018, the American Academy of Pediatrics issued a clinical practice guideline on maintenance intravenous fluids, stating that the most important measures that can be taken to prevent hyponatremia are to avoid using hypotonic fluids in children who have clear risks for nonosmotic AVP secretion and to initially administer isotonic saline (0.9% sodium chloride) unless otherwise clinically indicated. These recommendations were based on over 20 prospective trials in almost 3000 patients demonstrating that isotonic fluids decrease the incidence of hyponatremia three- to sixfold in comparison with hypotonic fluids. The serum sodium should be followed in any patient receiving continuous parenteral fluid, and adjustments to the composition of intravenous fluids should be made accordingly ( ).
Clinical indications not to use 0.9% sodium chloride are primarily in disease states associated with increased free water losses such as renal concentrating defects, voluminous diarrhea, or severe burns. Furthermore, a different maintenance fluid composition and infusion rate may be required for children with neurosurgical disorders, significant congenital or acquired heart disease, hepatic or renal dysfunction, or fluid overload or edema; children undergoing or having recently received chemotherapy; or children who are <28 days old or in the NICU ( ).
A major consequence of hyponatremia is the influx of water into the intracellular space, resulting in cellular swelling. This can lead to cerebral edema and encephalopathy. The clinical manifestations of hyponatremia are primarily neurologic and related to cerebral edema from hypoosmolality. The symptoms of hyponatremic encephalopathy are quite variable among individuals, with the only consistent symptoms being headache, nausea, vomiting, emesis, and weakness. As the cerebral edema worsens, patients then develop behavioral changes and impaired response to verbal and tactile stimuli. Advanced symptoms are signs of cerebral herniation, with seizures, respiratory arrest, dilated pupils, and decorticate posturing. Hyponatremic encephalopathy accounts for one-third of the seizures encountered in the ICU setting. Not all patients have the usual progression in symptoms, and advanced symptoms can present with sudden onset ( ).
A common yet often unrecognized symptom of hyponatremic encephalopathy is neurogenic pulmonary edema ( ). Neurogenic pulmonary edema is a well-described yet underdiagnosed condition that occurs as a complication of severe CNS injury. There is typically a rapid onset of pulmonary edema following the development of cerebral edema. No cardiac etiology is found, and there is a complete and rapid resolution of respiratory symptoms following appropriate treatment of the hyponatremic encephalopathy with hypertonic saline. If not recognized early, the condition is almost universally fatal. Hyponatremic encephalopathy should be considered in any patient presenting with a noncardiogenic pulmonary edema.
The brain’s adaptation to hyponatremia initially involves a loss of cerebral blood volume and cerebral spinal fluid. This is followed by volume regulatory decrease that occurs by the extrusion of sodium, potassium, and organic osmolytes to decrease the brain osmolality. Various factors can interfere with successful brain adaptation and may play a more important role than the absolute change in serum sodium in predicting whether a patient will develop hyponatremic encephalopathy. Elevated AVP levels can increase brain water content in the absence of hyponatremia and impair brain regulatory volume mechanisms. Other factors that interfere with brain adaptation are age, hormonal factors related to gender, and hypoxemia. Hormonal factors are not a significant issue in children and are not addressed here.
Children less than 16 years of age are at increased risk for developing hyponatremic encephalopathy due to their relatively larger brain-to-intracranial volume ratio. A child’s brain reaches adult size by 6 years of age, whereas the skull does not reach adult size until 16 years of age. Consequently, children have less room available in their rigid skulls for brain expansion and are more likely than adults to develop brain herniation from hyponatremia at higher serum sodium concentrations. Children will also have a high morbidity from symptomatic hyponatremia.
Hypoxemia is a major risk factor for developing hyponatremic encephalopathy. The occurrence of a hypoxic event such as respiratory insufficiency is a major factor militating against survival without permanent brain damage in patients with hyponatremia. The combination of systemic hypoxemia and hyponatremia is more deleterious than is either factor alone. Hypoxemia impairs the brain’s ability to adapt to hyponatremia, leading to a vicious cycle of worsening hyponatremic encephalopathy. Hyponatremia leads to a decrement of both cerebral blood flow and arterial oxygen content. Patients with symptomatic hyponatremia can develop hypoxemia by at least two different mechanisms: neurogenic pulmonary edema or hypercapnic respiratory failure. Respiratory failure can occur suddenly in patients with symptomatic hyponatremia. The majority of neurologic morbidity seen in patients with hyponatremia has occurred in patients who have had a respiratory arrest as a feature of hyponatremic encephalopathy ( ).
Syndrome of inappropriate antidiuretic hormone secretion (SIADH) is one of the most common causes of hyponatremia in the hospital setting and frequently leads to severe hyponatremia (plasma Na <120 mEq/L) ( ). It is caused by elevated ADH secretion in the absence of an osmotic or hypovolemic stimulus. SIADH can occur due to a variety of illnesses, but most often it occurs due to central nervous system disorders, pulmonary disorders, and medications. The chemotherapeutic drugs vincristine and cyclophosphamide (Cytoxan) and the antiepileptic drug carbamazepine are especially common. SIADH is essentially a diagnosis of exclusion. Before SIADH can be diagnosed, diseases causing decreased effective circulating volume, renal impairment, adrenal insufficiency, and hypothyroidism must be excluded. The hallmarks of SIADH are mild volume expansion with low to normal plasma concentrations of creatinine, urea, uric acid, and potassium; impaired free water excretion with normal sodium excretion that reflects sodium intake; and hyponatremia, which is relatively unresponsive to sodium administration in the absence of fluid restriction ( Box 6.2 ). The biochemical parameters most suggestive of SIADH in adults are a spot urine sodium concentration >30 mEq/L, a fractional excretion of sodium >0.5%, fractional excretion of urea >55%, fractional excretion of urate (FEUrate) >11%, and plasma uric acid <4 mg/dL. The specificity and sensitivity of these biomarkers have not been evaluated in children.
Central nervous system disorders
Asphyxia in newborns: Intraventricular hemorrhage in neonates, mask ventilation
Encephalitis
Meningitis (viral, bacterial, tuberculous)
Cerebrovascular accident (stroke)
Postpituitary surgery
Brain tumors
Brain abscesses
Hydrocephalus
Head trauma
Guillain-Barré syndrome
Lupus cerebritis
Pulmonary disorders
Atelectasis or pneumothorax in newborns
Positive pressure ventilation
Pneumothorax
Hyaline membrane disease
Pneumonia (viral, bacterial)
Abscess
Tuberculosis
Aspergillosis
Positive-airway pressure breathing
Ligation of patent ductus arteriosus
Medications
Vincristine
Cyclophosphamide
Carbamazepine
Selected serotonin reuptake inhibitors
SIADH is usually of short duration and resolves with treatment of the underlying disorder and discontinuation of the offending medication. Fluid restriction is essential to treatment, but it is a slow method of correction and is impractical in infants, who receive most of their nutrition as liquids. All intravenous fluids should be of a tonicity of at least normal saline, and if this does not correct the plasma sodium, 3% sodium chloride may be administered as needed. If a more rapid correction of hyponatremia is needed, the addition of a loop diuretic in combination with hypertonic saline is useful.
For children with chronic asymptomatic hyponatremia from SIADH that does not respond to fluid restriction, the next step would be to increase the oral sodium intake or to give oral urea in order to increase the renal solute load, thereby inducing an osmotic diuresis. Oral urea has proven to be successful in treating chronic hyponatremia in both children and adults who did not respond to conservative measures. A once-daily dose of 15 to 30 g of urea in an adult appears to be effective and well tolerated. A lemon-flavored urea powder drink (Ure-Na by Nephcentric LLC) is now commercially available in the United States ( ).
Vasopressin 2 antagonists (vaptans) represent a relatively new class of medication for the management of SIADH. These agents are nonpeptide vasopressin V 2 receptor antagonists that selectively antagonize the antidiuretic effect of AVP and result in a urinary free water diuresis (aquaresis) without increasing loss of electrolytes. Vaptans produce an aquaresis within 1 to 2 hours of administration that abates within 12 to 24 hours. When used to treat hyponatremia, vaptans result in an approximate 5 to 7 mEq/L increase in serum sodium within the first 24 hours of administration, but the effect is highly variable. The most common side effects of vaptans are increased thirst, polyuria, and dry mouth. There are currently two FDA-approved vaptans: tolvaptan, which is available in an oral formulation, and conivaptan, which is available in an intravenous preparation. There are safety concerns with vaptans, as they have been associated with alanine aminotransferase elevation and severe hepatotoxicity with long-term use, and serious overcorrection of hyponatremia has been reported. Overcorrection is of particular concern in neurologically impaired or critically ill children with restricted access to water. These agents are inhibitors of cytochrome P450 and should not be used in conjunction with other drugs known to be metabolized by this pathway. At the present time, vaptans cannot be recommended as a first-line agent in the management of SIADH as they are expensive, are not always necessary, and present safety concerns. Vaptans do appear to be a suitable second-line agent for short-term use in patients with SIADH after conservative measures have failed ( ; ).
Hyponatremia is frequently encountered in the neurosurgical setting and has usually been attributed to SIADH. The management of patients with SIADH has generally involved fluid restriction. More recently, it has become apparent that an increasing number of neurosurgical patients with hyponatremia have a distinct clinical entity called cerebral salt wasting (CSW), a condition in which the hallmark is renal sodium loss leading to extracellular volume depletion. Management of patients with CSW involves volume expansion and salt supplementation. Because SIADH and CSW have many clinical similarities and their management is completely different, it is essential to be able to distinguish between these two conditions.
The pathogenesis of CSW is not completely understood, but it appears to be due to the release of natriuretic peptides, such as atrial natriuretic peptide, brain natriuretic peptide, and c-type natriuretic peptide. These peptides appear to lead to natriuresis via a complex mechanism involving increased GFR, inhibition of the renin angiotensin system, and inhibition of the secretion and action of AVP. This complex mechanism can lead to biochemical features that are indistinguishable from SIADH, with the patient having a low serum uric acid level and low plasma renin, aldosterone, and vasopressin levels. The key distinguishing feature between CSW and SIADH is extracellular volume depletion. Careful documentation of trends in urinary output and central venous pressure are particularly useful. An algorithm using changes in the FEUrate has recently been validated for distinguishing SIADH from CSW. Although both SIADH and CSW are associated with hypouricemia and an elevated FEUrate of >11%, the FEUrate normalized following the correction of hyponatremia in SIADH, whereas the FEUrate remained persistently elevated following the correction of serum sodium in CSW ( ).
From a practical standpoint, the administration of normal saline should be an adequate prophylaxis against developing clinically significant hyponatremia (<130 mEq/L) in SIADH. If clinically significant hyponatremia develops in a patient with a CNS disorder receiving only normal saline, then the diagnosis of CSW should be strongly considered. If there are no signs of extracellular volume depletion, then a brief period of fluid restriction could be tried. If there are signs of volume depletion or a lack of response to fluid restriction, with a further fall in the serum sodium concentration, then the patient should be managed as CSW. Patients with CSW should be volume expanded with normal saline, followed by sufficient quantities of normal saline and 3% NaCl to maintain fluid balance and a normal serum sodium. The administration of fludrocortisone may also be beneficial because aldosterone production is relatively decreased in CSW.
Despite the controversies surrounding the optimal treatment of hyponatremic encephalopathy, two aspects are generally accepted by experts in the field: Treatment should be directed based on the neurologic involvement and not the absolute serum sodium, and hypertonic saline is not indicated in the asymptomatic patient who is neurologically intact, regardless of the serum sodium. In general, correction with hypertonic saline is unnecessary and potentially harmful if there are no neurologic manifestations of hyponatremia. Symptomatic hyponatremia, on the other hand, is a medical emergency. Once signs of encephalopathy are identified, prompt treatment is required in a monitored setting before imaging studies are performed. The airway should be secured, and endotracheal intubation and mechanical ventilation may be necessary. Fluid restriction alone has no place in the treatment of symptomatic hyponatremia. If symptomatic hyponatremia is recognized and treated promptly prior to developing a hypoxic event, the neurologic outcome is good.
Patients with symptomatic hyponatremia should be treated with hypertonic saline (3%, Na = 513 mEq/L) using an infusion pump ( ). The rate of infusion should raise the plasma sodium by about 1 mEq/L per hour until (1) the patient is alert and seizure free, (2) the plasma sodium has increased by 20 mEq/L, or (3) a serum sodium of about 125 to 130 mEq/L has been achieved. If the patient is actively seizing or has impending respiratory arrest, the serum sodium can be raised by as much as 4 to 8 mEq/L in the first hour or until the seizure activity ceases. Recent studies have demonstrated that the optimal rate of correction of symptomatic hyponatremia is approximately 15 to 20 mEq in 48 hours, because patients with correction of hyponatremia in this range have a much lower mortality and an improved neurologic outcome compared with those with a correction of less than 10 mEq in 48 hours. Assuming that total body water constitutes 50% of total body weight, 1 mL/kg of 3% sodium chloride will raise the plasma sodium by about 1 mEq/L. A safe and simple approach to treating hyponatremic encephalopathy is to give a 2-mL/kg bolus with a maximum of 100 mL in repeated doses every 10 to 15 minutes until symptoms improve or until a 5- to 6-mEq/L elevation in serum sodium has been achieved. In some cases furosemide can also be used to prevent pulmonary congestion and to increase the rate of serum sodium correction ( ).
Cerebral demyelination is a rare complication that has been associated with symptomatic hyponatremia ( ). Animal data have demonstrated that correction of hyponatremia by greater than 25 mEq/L in 24 hours is associated with cerebral demyelination. This has resulted in a mistaken belief that a rapid rate of correction is likely to result in cerebral demyelination. In fact, studies have shown that the rate of correction has little to do with the development of cerebral demyelinating lesions and that lesions seen in hyponatremic patients are more closely associated with other comorbid factors or the magnitude of correction in serum sodium. In one prospective study, it was observed that hyponatremic patients who developed demyelinating lesions had been made inadvertently hypernatremic, had their plasma sodium levels corrected by greater than 25 mmol/L in 48 hours, had suffered a hypoxic event, or had severe liver disease ( ). Some data have suggested that azotemia may decrease the risk of developing cerebral demyelination ( ).
When symptomatic cerebral demyelination does follow the correction of hyponatremia, it typically follows a biphasic pattern. There is initially clinical improvement of the hyponatremic encephalopathy associated with correction of the serum sodium, which is followed by neurologic deterioration 2 to 7 days later. Cerebral demyelination can be both pontine and extrapontine. Classic features of pontine demyelination include mutism, dysarthria, spastic quadriplegia, pseudobulbar palsy, pseudocoma with a “locked-in stare,” and ataxia. The clinical features of extrapontine lesions are more varied, including behavior changes and movement disorders. Radiographic features of cerebral demyelination typically lag behind the clinical symptomatology. Cerebral demyelination is best diagnosed on MRI approximately 14 days following correction. The classic radiographic findings on MRI are symmetric lesions that are hypointense on T1-weighted images and hyperintense on T2-weighted images. Cerebral demyelination may be detected earlier on MRI with diffusion-weighted imaging.
The outcome of cerebral demyelination is not as severe as was previously believed. Cerebral demyelination has been found to be an incidental finding on neuroimaging and at autopsy in patients with chronic illnesses. In most reported cases of cerebral demyelination attributed to dysnatremia, long-term follow-up has demonstrated improvement in neurologic symptoms and regression of radiographic findings. The primary cause of brain damage in patients with hyponatremia is not cerebral demyelination but cerebral edema and herniation. Most brain damage occurs in untreated patients and is not a consequence of therapy; therefore patients with symptomatic hyponatremia need prompt therapy.
Patients with hyponatremia due to water intoxication, diarrheal dehydration, thiazide diuretics, or desmopressin (dDAVP) are at high risk for overcorrection of hyponatremia and require extreme care and monitoring. In these illnesses, once volume depletion is corrected or when the offending medication is discontinued, there will be a reversal of the urine osmolality from concentrated to dilute, resulting in a free water diuresis with a potentially rapid overcorrection of hyponatremia if saline-containing fluids are administered. This will not typically occur in SIADH, because this is saline-resistant hyponatremia. To prevent an overcorrection of hyponatremia, hypotonic fluids and possibly even dDAVP may have to be given. It has also been demonstrated that when there is an overcorrection of hyponatremia, a therapeutic relowering of the serum sodium can ameliorate the symptoms of cerebral demyelination.
Hypernatremia is defined as a serum sodium greater than 145 mEq/L. In both children and adults hypernatremia is primarily seen in the hospital setting in individuals who have restricted access to water for a variety of reasons ( ). In most instances these patients are either debilitated by an acute or chronic illness or neurologic impairment or are at the extremes of age. Hypernatremia is a particularly common problem in the intensive care unit because most patients are either intubated or moribund and have restricted access to fluids. Additional contributing factors for hypernatremia in the intensive care setting are excess sodium administration, renal concentrating defects, gastrointestinal fluid losses, and dialysis-related complications. A serum sodium greater than 145 mmol/L should always be considered abnormal and evaluated thoroughly to prevent the development of significant hypernatremia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here