Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Knee dislocations and knee instabilities are characterized by anterior, posterior, lateral, and medial directions. Combinations of the main components cause rotational instability.
The anatomic areas of insertion are of paramount importance for both the reinsertion of a torn ligament and reconstruction.
First-line management of knee dislocations is focused on vascular injuries, proper repositioning, and external fixation.
Early refixation/reconstruction leads up to more favorable results. For the posterolateral corner, augmentation is associated with less residual instability.
Many different types of implants and sutures exist. Refixation/reconstruction should start with the posterior cruciate ligament (PCL) at 70 degrees of knee flexion, followed by posterolateral/posteromedial corners at 30 degrees of flexion, and then finished with the anterior cruciate ligament (ACL) in close to full extension.
In delayed reconstruction techniques, the knee may present subluxated, and a thorough arthrolysis may be necessary before reconstruction. An external fixator with a hinge may be useful in these challenging cases.
In a meta-analysis, early suture of both the ACL and PCL was found to lead up to better results than reconstruction or conservative treatment. The results of Schenck type IV injuries are less favorable than those of Schenck type II or III injuries. Vascular injuries are present in up to 25% of the cases with a posterior ligament component, and in 20% of the cases with vascular injury, limb amputation has been reported.
A lot of confusion has been caused by the description of dimensions of all the important ligament structures about the knee. A lack of knowledge of functional anatomy has contributed to numerous fatal reconstruction techniques. Most of the debate is caused by the unique structure of each ligament insertion, that is, fanning out in a low angle radially rather than being directed perpendicular to the bony surface ( Fig. 61.1 ).

Attempts have been made to simplify the anatomic variations defining areas of isometry or near isometry. Many parts of a single ligament remain nonisometric, however, and modern ligament reconstruction therefore focuses on techniques that copy the functional behavior of the native knee. Knowledge of the insertion of ligaments and tendons still plays a pivotal role in optimizing reattachment or reconstruction of the individual structures. Diversity is immense for all ligaments, but certain functional principles have to be considered.
The most important anatomic structure for the femoral origin of the anterior cruciate ligament (ACL) is the resident's ridge. It is located along the lateral wall of the intercondylar notch and runs horizontally when the knee is flexed 120 degrees ( Fig. 61.2 ). All femoral tunnels for an ACL reconstruction should be placed inferior to this line's location.

For reconstruction of the medial patellofemoral ligament, the position of the medial epicondyle should be noted. On a sagittal radiograph, the position of a reconstruction of the medial patellofemoral ligament should be placed slightly anterior to the posterior margin of the posterior cortex of the femoral shaft ( Fig. 61.3 ).

On the tibial side, the concave shape of the medial plateau holds the medial meniscus, and the femoral condyle is able to load both the anterior and posterior horn of the meniscus during range of motion (ROM; Fig. 61.4 ).
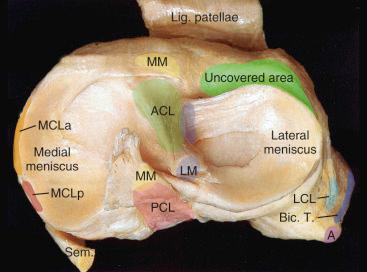
On the lateral side, the convex shape of the plateau and the lateral meniscus contribute to the greater mobility of the lateral joint. There is a combination of rolling and sliding on the lateral side that can vary substantially.
The femur rotates externally during flexion and extension around a center located in the medial femoral condyle. The mobility of the lateral compartment is shown in Fig. 61.4 . The uncovered tibial plateau enables the rotation of the tibia on the lateral side during the final 30 degrees of extension, “locking up” the joint.
Like the elbow, ankle, wrist, fingers, and jaw, the knee is categorized as a hinge joint. However, it has six degrees of freedom: translational, rotational, and varus/valgus displacements are limited by the joint facets and the adjacent cartilage, ligaments, and muscles and vary widely between individuals. The contact area of the joint facets is larger in extension than during flexion. The contact point between the femur and tibia moves from anterior to posterior, and there is more movement from anterior to posterior on the lateral than on the medial side ( Fig. 61.5 ). Interestingly, there is no “rolling back of the femur with respect to the rotational center on the medial side.”

The patella has a small contact zone on its inferior aspect in 20 degrees of flexion, and this area increases and moves superior during flexion ( Fig. 61.6 ). On the trochlea side, the contact area moves from superior to inferior as a function of increasing flexion. In 130 degrees of flexion, both the medial and lateral facets contact the intercondylar region of the medial and lateral femoral condyles (see Fig. 61.6 ). The joint facet forces are highest during extension and flexion beyond 120 degrees and are reduced during midflexion.

The origin of the ACL varies tremendously. In most knees there is a separation between an anteromedial and posterolateral bundle. The orientation of the collagen fibers of both bundles is parallel during extension and becomes twisted during flexion ( Fig. 61.7 ). The tibial footprint extends approximately 2 mm anterior to the posterior cruciate ligament (PCL) and extends far anterior underneath the transverse ligament of the menisci (see Figs. 61.1 and 61.4 ). The main stabilizing function of the ACL is to limit anterior translation and rotation of the tibia with special emphasis on 30 degrees of flexion. The intact ligaments get loaded in hyperextension and to a lesser extent in deep flexion during passive ROM ( Fig. 61.8 ). These forces increase during isolated quadriceps activation. The individual fibers react differently as a function of knee flexion and loading; most of the anterior displacing forces are transmitted by the posterolateral bundle in 20 to 30 degrees of knee flexion ( Fig. 61.9 ).



The PCL has the strongest caliber of all knee ligaments. It is divided in an anterolateral and a posteromedial (PM) bundle. The mean length is 36 mm, and the mean diameter in the middle is 13 mm. Like the ACL, both origin and insertion fan out, and the attachment sites have approximately three times the diameter of the ligament itself. The tibial attachment lies between the two articular surfaces (see Fig. 61.5 ) and starts 10 mm underneath the level of the joint surface, extending several millimeters and blending into the tibial shaft. The PCL attaches with additional slips, including a connection to blend with the posterior horn of the lateral meniscus. In cases with missing meniscofemoral ligaments, the slip from the PCL to the lateral meniscus is quite prominent. The anterolateral bundle is longer than the PM, and loading occurs predominantly in flexion. The PM bundle gets loaded in extension.
During joint development, the PCL is formed out of a dorsal plication of the synovia, which is responsible for the partially extraarticular position and highly vascular supply of the ligament ( Fig. 61.10 ). The PCL is a primary stabilizer for posterior tibial translation and a secondary stabilizer for rotational loading ( Fig. 61.11 ). Carlin and colleagues investigated the resisting forces of the PCL during posterior loading. At a 50-N posterior tibial load, the force in the PCL increased from 25 ± 11 N (30 degrees of knee flexion) to 48 ± 12 N at 90 degrees of knee flexion. At 30 degrees knee flexion, approximately 45% of the resistance to posterior tibial loading was caused by contact between the tibia and the femoral condyles; at 90 degrees of knee flexion, the entire force was transmitted by soft tissue.


The PCL is the primary stabilizer for posterior loads with between 70 and 90 degrees of knee flexion.
The anterolateral complex consists of the iliotibial band, including the Kaplan fibers, the anterolateral capsule, and a capsular structure recently named the anterolateral ligament (ALL). The anterolateral complex provides rotatory stability to the knee. The structures of the complex work synergistically as a restraint to internal tibial torque.
The anatomy of the lateral side of the knee is more heterogeneous than on the medial side. In earlier analysis, these structures were limited to the dynamic elements, such as the iliotibial tract, the biceps tendon, and the popliteus tendon. Static structures are more diverse and include the lateral collateral ligament, fabellofibular ligament, short lateral ligament, popliteofibular ligament, arcuate ligament, posterolateral capsule, posterior horn of the lateral meniscus, and the lateral coronary ligament. Mainly, some of these structures have been combined as the “arcuatum complex.” These investigators found a combination of both arcuatum and fabellofibular ligaments in 67% of all dissections. Covey named the dorsolateral structures the “popliteus complex.” Lately, the ligaments connecting the fibula and the tibia have been more thoroughly investigated, and the popliteofibular ligament was pointed out as a separate entity.
No matter which ligaments are found, for a stepwise preparation, it is useful to visualize three distinct layers:
The first layer consists of the iliotibial tract and the superficial portion of the biceps tendon. The peroneal nerve is situated on the deep side of layer I, posterior to the biceps tendon.
Layer II is formed by the retinaculum extending from the lateral aspect of the patella to two patellofemoral ligaments that extend to the lateral epicondyle and back to the fabella. Also part of layer II are the patellomeniscal ligaments, which run to the lateral aspects of the meniscus and terminate at the Gerdy tubercle.
Finally, in the deep layer III, the lateral joint capsule is found with the coronary ligament, connecting the capsule with the lateral meniscus. Posterior to the iliotibial band, the capsule includes the lateral collateral ligament (LCL) and ends at the fabellofibular ligament. A variation of structures, including the arcuatum complex and the popliteofibular ligament, is often found ( Fig. 61.12 ).

The medial stabilizing structures are the superficial and deep layer of the medial collateral ligament (MCL), the anserine foot with the flexor tendons (sartorius, semitendinosus, and gracilis), and the semimembranosus tendon, which has five communicating links: to the medial meniscus, to the deep layer of the MCL, to the posterior oblique ligament, to the popliteus muscle, and to the posterior capsule.
For the preparation, three layers can be found:
The crural fascia extends from the patella to the popliteal fossa. It has connecting fibers to the anterior aspect of the tibia, and the fascicles run to the patella and superficial MCL. The sartorius tendon covers the semitendinosus and gracilis tendons, which run between layers I and II.
Layer II contains the superficial MCL and extends from the adductor tubercle anteriorly as fibers composing the medial patellofemoral ligament ( Fig. 61.13 ). It sends a slip to the undersurface of the vastus medialis obliquus and vastus intermedius.

Layer III is found underneath the superficial MCL. It is formed by the joint capsule and the deep fibers of the MCL, merging with the meniscus and the posterior oblique ligament, also described as the oblique popliteal ligament . There is a strong contribution from the attachment of the semimembranosus tendon insertion, with fibers merging into the oblique popliteal ligament, the deep layer of the MCL, and the popliteus muscle belly ( Fig. 61.14 ).

The knee is categorized as a complex hinge joint with six degrees of freedom: translational, rotational, and varus/valgus displacements are limited by the joint facets and the adjacent cartilage, ligaments, and muscles.
The ACL consists of an anteromedial and posterolateral bundle.
The PCL has the strongest caliber of all knee ligaments and is divided into an anterolateral and a PM bundle.
The anterolateral complex consists of the iliotibial band, including the Kaplan fibers, the anterolateral capsule, and a capsular structure recently named the anterolateral ligament.
The posterolateral side of the knee is complex and includes dynamic elements, such as the iliotibial tract, the biceps tendon, and the popliteus tendon, and static structures, such as the lateral collateral ligament, fabellofibular ligament, short lateral ligament, popliteofibular ligament, arcuate ligament, posterolateral capsule, posterior horn of the lateral meniscus, and lateral coronary ligament.
Medial stabilizing structures are the MCL, the posterior oblique ligament, the flexor tendons (sartorius, semitendinosus, and gracilis), and the semimembranosus tendon.
Knee dislocations account for approximately 0.02% of all musculoskeletal injuries and 0.2% to 0.3% of all joint dislocations.
The diagnosis can easily be missed due to normal radiographs, the absence of joint effusion due to ruptures of the joint capsule, and concomitant fractures of the distal femur and proximal tibia, which hamper the evaluation of ligamental stability. The mean age of patients is reported as 28 to 34 years, and the distribution between sexes is reported as 75% male and 25% female.
Typical accident patterns are reported as 82% traffic accidents and 18% football injuries. Among polytraumatized patients, around 19% sustain severe fractures or ligamental injuries of the knee. The mean age of patients is reported to be 31 to 34 years.
Concomitant vascular damage can easily be missed because relying on abnormal pedal pulses on initial examination is not sensitive enough to detect vascular injury. The incidence of popliteal vessel injury is reported as ranging from 29% to 33%, with the need for amputation in 9% to 20% of the cases. High-risk injuries to the peroneal and tibial nerve are reported as ranging from 30% to 43%.
Rates of accompanying fractures are reported above 60%. Generally speaking, there is a high risk of around 50% of associated injuries in patients with knee dislocation. This rate can increase up to 80% in maximum care trauma centers.
The incidence of popliteal vessel injury is reported as ranging from 16% to 32%. This is because of the firm anatomic fixation of the popliteal artery in the hiatus tendineus proximally and the arcus tendineus musculi solei distally. However, in a cohort of 19 cases from 1992, no injury to the popliteal artery (0%) was reported. Concomitant vascular damage can easily be missed because relying on abnormal pedal pulses on initial examination is not sensitive enough to detect vascular injury. In the case of nonpalpable pedal pulses, Doppler sonography should be performed.
Arteriography is not routinely recommended in all patients but is recommended in those with a history or clinical findings of ischemia.
In the case of evident traumatic vascular damage, the loss of time for preoperative angiography has to be evaluated compared with the benefit of early surgical intervention with intraoperative angiography. Even in patients with normal arteriograms, limb ischemia in the dislocated position is reported in 10%, with improvement only by reduction of the knee dislocation. Furthermore, lesions of the intima caused by overextension of the artery can lead to the delayed formation of thrombi within several hours or days. This underlines the importance of continuous clinical controls of perfusion of the limb, including pulse oximetry.
Continuous control of the limb perfusion, especially in cases of Schenck type III and Schenck type IV, is mandatory.
As an alternative to angiography, magnetic resonance angiography (MRA) can be performed, particularly as a diagnostic tool in the postacute phase. This method combines the possibility for diagnostics of soft tissue and vascular injuries at the same time. Injuries of the popliteal artery can be repaired by saphenous vein bypass grafts, with a reported success rate of 89%. Intraoperative angiography is of particular importance to detect stenoses and thrombi of the anastomosis, requiring revision in 22% to 55%.
Green and Allen emphasized the need for completion of vascular repair within 6 to, at the latest, 8 hours from the time of injury for limb salvage. Eighty percent of their patients undergoing revascularization within 8 hours after trauma maintained their limb. In their cohort of 245 cases, 86% of the patients not treated in this time period required amputation. Among the remaining 14%, two-thirds had ischemic changes.
In the case of revascularization, the development of compartment syndrome caused by reperfusion edema is highly likely. Therefore prophylactic dermatofasciotomy of the lower leg and foot should be generously indicated. Global rates of eventual need for amputation in all patients with knee joint dislocation are indicated as ranging between 9% and 20%.
In 1969 Reckling and Peltier reported rates of nerve damage caused by knee joint dislocation in 14% to 35% of patients. Nowadays, injuries to the peroneal and tibial nerve are reported as ranging from 22% to 43%. Nerve damage can be caused by either overextension of the nerve, traumatic disruption, or compartment syndrome. Accompanying injuries to the menisci occur in approximately 73% of all knee dislocations.
Rates of accompanying fractures beyond bony avulsions of ligaments are reported as ranging from 24% to 64%. Combined intraarticular or metaphyseal fractures of both the distal femur and proximal tibia are referred to as “floating knee” injuries. According to Krettek and colleagues, three types of complex knee injuries can be distinguished: type 1 consists of the “floating knee” injury, as explained earlier. Type 2 is defined as the combination of distal femoral fractures or proximal tibial fractures with second- or third-degree open or closed soft tissue injury. Knee dislocations are defined as type 3.
The incidence rate of compartment syndrome caused by knee joint dislocation is described as around 5%. In all doubtful cases and particularly in the comatose patient, invasive measurement of compartment pressure is recommended.
To date, most studies only report small numbers of patients with heterogeneous study populations. This is of particular importance regarding the major impact of injury patterns, for example, expressed by the Schenck classification, on the clinical outcome. There have not yet been any studies with level I evidence comparing different treatment options for knee dislocation. There has been only one prospective cohort study at the time of this writing, which compared repair and reconstruction of the posterolateral corner (PLC). However, this study included not only patients with knee dislocation (77%) but also those with isolated PLC injuries.
Recommendations result from meta-analyses and systematic reviews. Compared with nonoperative treatment, surgical treatment reveals a higher percentage of excellent or good International Knee Documentation Committee (IKDC) scores (58% vs. 20%) and higher rates for return to work (72% vs. 52%) and return to full sport (29% vs.10%). It can be concluded that conservative treatment yields poor results in knee dislocation and cannot be recommended.
Comparing primary repair with reconstruction of damaged structures, similar mean Lysholm scores (88 vs. 87) and excellent or good IKDC scores (51% vs. 48%) were detected.
In 1999 Mariani and colleagues reported their mean 7-year follow-up of 23 patients and recommended primary reconstruction instead of reattachment of the cruciate ligaments. Avulsions of the collateral ligaments are reported in 84% for the LCL and 46% for the MCL.
Presently, there is no study focusing on treatment options depending on the rupture site of ligaments, distinguishing avulsions and intraligamentous ruptures. Regarding injuries of the MCL in the context of knee dislocation, both repair and reconstruction yield favorable results. Special considerations apply for the PLC and the cruciate ligaments. Primary repair of the PLC resulted in higher failure rates compared with primary reconstruction (37% vs. 9%). The prospective cohort study from Stannard and colleagues found significantly higher failure rates for repair (37%) compared with reconstruction (9%) of the PLC. Regarding the cruciate ligaments, reported results are controversial. Levy and colleagues reported that primary repair showed decreased stability and ROM and a lower return to preinjury activity levels compared with primary reconstruction (0% vs. 33%). Frosch and colleagues could not find any significant difference between suture repair and reconstruction of the ACL and PCL. Excellent or good results were found for suture repair of the ACL and PCL in 77.5%, compared with 73% of excellent or good results for reconstruction of the ACL and PCL.
Owens and colleagues performed a 4-year follow-up for 30 knee dislocations treated by primary repair of all injured ligaments followed by an early rehabilitation program. They found comparable outcomes to published results of ligament reconstruction. Shelbourne and colleagues reported encouraging results regarding long-term stability and satisfaction for an “en masse surgical technique” for lateral repair combined with an ACL reconstruction. They left the PCL untreated and found a good return to high levels of activity.
As a different strategy, Yeh and colleagues performed an arthroscopic PCL reconstruction with a patellar bone-tendon–bone graft, combined with only a débridement of the ACL and repair of the collateral ligaments, with a mean Lysholm score of 84 and an almost normal ROM after a mean follow-up of 27 months.
The use of allografts can reduce donor-site morbidity and operating time. Shapiro and Freedman reported good results for a primary fresh-frozen allograft reconstruction of both the ACL and PCL. Wascher and colleagues reported satisfying results with a mean Lysholm score of 88 in 13 patients who underwent ACL and PCL reconstruction with fresh-frozen Achilles or patellar tendon allografts. However, there is no evidence for allografts achieving superior results compared with autografts or primary suture of the torn ligaments.
Early surgery within 3 weeks after trauma yielded higher mean Lysholm scores (90 vs. 82), a higher percentage of excellent or good IKDC scores (47% vs. 31%), and higher sports activity scores (89 vs. 69) on the Knee Outcome Survey, compared with delayed surgery. This is confirmed by the results of Harner and colleagues, who reported higher subjective scores and better objective restoration of knee stability in acutely operated patients compared with those operated on more than 3 weeks after trauma. To differentiate acute from chronic injury, 3 weeks after trauma is commonly applied as the threshold. Anatomic suture repair becomes impossible after this time due to retraction of ligament stumps, scar formation, and granulation tissue.
Furthermore, Richter and colleagues presented better results for suture repair within 1 week compared with suture repair later than 1 week after trauma. The two-stage management, consisting of collateral ligament sutures within 8 to 10 days, followed by reconstruction of the cruciate ligaments after 6 to 8 weeks, was reported with good clinical outcome in 70% of the patients.
Of interest, Bin and Nam reported a necessity for reconstruction of the ACL in only 20% and of the PCL in 47% of the patients after initial repair or reconstruction of the collateral ligaments. Special attention has to be put on the risk of compartment syndrome caused by fluid leaking out of the ruptured joint capsule during early arthroscopy.
The risk of arthrofibrosis appears to be increased after early ligament reconstruction and is reported as 1.7% ; however, no data about the development of arthrofibrosis after ligament suture have existed until recently.
The use of allografts has been reported to reduce the risk of arthrofibrosis in one study. Late ligament reconstruction at a mean of 32 months after injury has also been reported to achieve good or excellent results in 60% of patients.
To summarize, conservative treatment and delayed surgery cannot be recommended for the multiligament-injured knee. Early operative treatment within 3 weeks yields favorable functional and clinical outcomes, with comparable results for primary repair or reconstruction. Primary reconstruction should be considered for the PLC.
Richter and colleagues reported a 3- to 16-year follow-up of 34 patients in 1999, defining a patient age younger than 40 years, a low-energy trauma, early reconstruction of both cruciate ligaments, and initial postoperative functional treatment as positive prognostic factors.
A careful evaluation of the whole patient as well as the involved lower extremity is mandatory, especially in a polytraumatized patient. Knee dislocations may spontaneously reduce in up to 60% of the cases, and instability may be underestimated.
The original classification for knee dislocations was introduced by Kennedy in 1963. He noted five main types of dislocation: anterior, posterior, lateral, medial, and rotatory. Rotatory dislocations were classified as anteromedial, anterolateral, anteromedial, PM, and posterolateral. This classification system was carried out according to the initial radiographs. The extent of ligament damage was not reflected by the classification. Apart from ligament injuries for knee dislocation fractures, several classifications can be used: Arbeitsgemeinschaft für Osteosynthesefragen (AO), Schatzker, or Moore.
The AO classification ( Fig. 61.15 ) classifies all long bone fractures. The proximal tibia and fibula are listed as region 41, with classifications A (extraarticular fractures), B (partial articular fractures), and C (complete articular fractures). Avulsion fractures of the eminentia intercondylaris are named as A1; extraarticular fractures are A2 and A3. Simple split fractures are denominated as B1, impression fractures B2, and split-impression fractures B3. Simple complete articular fractures are denominated as C1 and C2, and complex articular fractures are classified as C3.

The AO classification is used by the Orthopaedic Trauma Association for its meetings and by the Journal of Orthopaedic Trauma for all its publications.
Schatzker and colleagues ( Fig. 61.16 ) suggested distinguishing lateral and medial tibial plateau fractures into split fractures, compression fractures, and combined split-compression fractures. Bicondylar fractures are distinguished into laterally tilted, medially tilted, and axial depressed.

A comparison of intraobserver and interobserver reliability revealed better values for the AO classification compared with the Schatzker classification.
Dislocation fractures of the tibial head are classified into five types (L1 to L5) according to Moore ( Fig. 61.17 ). L1 and L2 are fractures of the dorsomedian tibial plateau without (L1) or with (L2) fracture of the intercondylar eminence. Bony avulsions of the joint capsule and ligaments are denominated as L3. Lateral rim impression fractures with medial ligamental injury are classified as L4. L5, the “four-part fracture,” contains a bicondylar tibial plateau fracture with an additional fracture of the intercondylar eminence. This injury always goes along with capsular and ligamental injuries and a high risk of concomitant neurovascular damage.

In 2003 Robert Schenck introduced a classification system that was based on anatomic injuries. The classification is summarized in Table 61.1 , and examples of the most common types are shown in Fig. 61.18 . A type I dislocation means that one of the cruciate ligaments remains intact. A type II dislocation finds the cruciates torn, with intact collateral ligaments. This type of knee dislocation is extremely uncommon. In a type III injury, both cruciates are torn, and one collateral remains intact. This group is subclassified into IIIM with a torn MCL and IIIL with a torn LCL. In a type IV injury, all four ligaments are torn. Later, a type V was added, which is any type of periarticular fracture in conjunction with a knee dislocation.
| Grade | Anatomic Structures Injured |
|---|---|
| KDI | One cruciate intact knee dislocation |
| KDII | Both cruciate ligaments torn, collaterals intact |
| KDIII | Both cruciates torn, one collateral torn |
| KDIIIM | Both cruciates torn, medial collateral ligament (MCL) torn |
| KDIIIL | Both cruciates torn, lateral collateral ligament (LCL) torn |
| KDIV | All four ligaments torn |
| KDV | Periarticular fracture-dislocation |

Soft tissue injuries are graded according to Oestern and Tscherne with G1 to G4. G1 stands for minimal contusion; G2 for extensive contusion. G3 is any soft tissue injury that is likely to develop compartment syndrome. G4 is a compartment syndrome or a state after a compartment syndrome.
We are using a modified classification system that can be used for both instabilities and dislocations. In cases of instability without dislocation under fluoroscopy ( Fig. 61.19 ), we use the abbreviation “I” for instability. In cases of a true dislocation, we use “D” to indicate a dislocation. According to the scheme shown in Fig. 61.20 , the direction of instability or dislocation determines the type of knee dislocation. If there is only one direction, it is a type 1 dislocation/instability (D1/I1). If there are two directions, it is a type 2 (D2/I2). If there are three directions, it is a type 3 (D3/I3). If the knee is unstable in all four directions, it is a type 4 (D4/I4). Any coinciding fractures are classified according to AO. Vascular injuries are designated with a “C.” Open knee dislocations are graded as O1 to O3 according to Gustilo and Anderson. An adjacent nerve injury is classified as “N,” and a patellar tendon rupture is designated as “PT.” In our experience, this classification system allows classification of all types of knee injuries with ligament involvement. It is anatomically oriented, and therefore, the extent of surgical repair can be assessed. The classification is summarized in Table 61.2 .


| Grade | Anatomic Structures Injured |
|---|---|
| I1/D1 | Unidirectional (A/P/M/L) |
| I2/D2 | Bidirectional (AP/AM/AL/PL/PM) |
| I3/D3 | Tridirectional (APM/APL) |
| I4/D4 | All four ligaments torn (APML) |
| Fractures | Classified according to AO |
| Associated Injuries | |
| O | Open dislocation (OI–OIII, according to Gustilo and Anderson) |
| G | Closed dislocation with soft tissue injury (G0–G3, according to Oestern and Tscherne) |
| C | Arterial injury |
| N s/p | Nerve injury (s = sciatic; p = peroneal) |
| PT | Patella tendon rupture |
Soft tissue injuries are graded according to Oestern and Tscherne with G0 to G3. G0 stands for no or minimal soft tissue injury, and G1 stands for simple bruise or pressure of the fragment from the inside. G2 is any soft tissue injury that is likely to develop into a compartment syndrome. G3 is a compartment syndrome or a state after a compartment syndrome.
Depending on the extent of injury, especially in a PCL involvement, the rate of vascular injuries is increasing. For types 3 and 4 knee dislocations, the arterial involvement is up to 10%. In types 2 to 4 knee dislocations with the involvement of the lateral and/or PLC, the rate of peroneal nerve damage is increasing. Additional structures, such as the patellar tendon, may be ruptured.
If the patient is not intubated, important information comes from the description of the mechanism of injury. Both dislocations and instabilities may be described as a popping sensation in the knee joint. In knee dislocations, an abnormal position of the knee up to a full rotation may be reported. Hyperextension injury may be described. Spontaneous reposition may be noticed by the patient and can disguise the true extent of the injury. Extreme swelling or tenderness of the joint may compromise physical examination. If the dorsal capsule is ruptured, a joint effusion may not be present because of leaking outside the joint. The following steps should be included during the physical examination:
Side-to-side evaluation of joint position, swelling, and tenderness
Evaluation of joint stability (anteroposterior displacement) in 30 and 70 degrees of flexion in internal, neutral, and external rotation of the tibia
Test of patella stability and varus/valgus displacement in 0 and 30 degrees of knee flexion. Documentation of peripheral pulse quality, Doppler sonography, and/or ankle-brachial index
Checkup of neurologic status, with special emphasis on peroneal and tibial nerves
After the examination, the direction of instability should be documented, and a preliminary diagnosis is made, for example, I3 (APM). An example of a typical acute evaluation in a patient with an I3 injury is shown in .
Standard anterior-posterior (AP) and lateral radiographs must be conducted, and if these do not allow evaluation of the knee reposition, a fluoroscopic investigation should be performed. If the knee can be flexed 30 degrees, a tangential view of the patella is added. In any type 3 or 4 knee dislocation, if there is no access to immediate magnetic resonance imaging (MRI) or computed tomography angiography (CTA), the patient must be admitted, and the ankle-brachial index must be repeatedly documented over 24 hours. MRI in the coronal, sagittal, and axial plane is performed. The extent of the injury can be best visualized by fat-saturated, turbo-spin echo sequences (see ). These best document the extent of ligament injury and the location of the disruption. Care must be taken to extend the field of view so that all injured structures are visualized (quadriceps tendon, gastrocnemius heads, tibial tuberosity). The cartilage may be better evaluated using a gradient echo sequence. In the case of additional fractures, a computed tomography (CT) scan is recommended (see ).
Knee dislocations account for approximately 0.02% of all musculoskeletal injuries and 0.2% to 0.3% of all joint dislocations.
Concomitant vascular damage can easily be missed because relying on abnormal pedal pulses on initial examination is not sensitive enough to detect vascular injury.
Rates of accompanying fractures are reported at above 60%.
Conservative treatment yields poor results in knee dislocation and cannot be recommended.
Comparing primary repair with reconstruction of damaged structures, similar outcome scores were detected.
There is a large controversy regarding the right treatment of knee dislocation or multiligament injuries. There are two competing concepts: one is to repair what is torn, and the other is to replace what is injured. There are insufficient data in the literature to support one of these two strategies. If a primary repair of the ligaments is performed, the best conditions are found early (within 14 days). Before repair of the ligaments, the most devastating condition is an arterial injury and/or a compartment syndrome, which is found in up to 11% of all Schenck type III and IV knee dislocations.
On any Schenck type III or IV or JaK D3/D4 injury, we recommend an arteriogram. This can be done by CTA or MRA, with MRA having the advantage of delivering images of the knee cartilage and ligaments at the same time. If there is no availability of either CTA or MRA, the vascular status (Doppler signal of the pedal pulses or ankle-brachial index) must be monitored for at least 24 hours. Because of the possibility of intima lesions, a control ultrasound image examination is recommended within 24 hours before surgery.
In the case of an arterial injury, there must be rapid admission to the operating room. A femorotibial external fixator should be placed laterally or anterolaterally. This should anticipate the approach for the vascular reconstruction. Vascular reconstruction usually uses the medial approach, with adequate exposure of a popliteal artery in segment three.
Most vascular surgeons perform a resection of the dissected popliteal segment and perform a greater saphenous vein interposition ( Fig. 61.21 ). The patency rates of polytetrafluoroethylene (PTFE) prostheses are significantly inferior to those of autologous venous grafts ( Fig. 61.22 ). At the present time, vascular stents have been used in acute ischemic traumatic popliteal artery lesions, but occlusion may occur, and there is no prospective, randomized study comparing stent implantation with vascular stents in traumatic popliteal artery dissection. For the best exposure of the popliteal artery, the position of the fixator pins should be lateral or anterior on the femoral side and anterior on the tibial side. Arterial reconstruction can be achieved with a similar technique as has been published for popliteal artery aneurysms.


After arterial reconstruction, there must be close follow-up of the pedal pulses and monitoring for compartment syndrome. In the case of a compartment syndrome, the posterior compartments can be opened by the medial incision used for the arterial reconstruction, and an additional lateral incision for the anterior and peroneal compartments is recommended.
There is much controversy regarding the usefulness of repair or reconstruction. The advantages of repair are that autografts are being preserved and no allografts are necessary. The assessment, if a direct repair of the ligament structures is possible, should include the following analysis:
Quality of the ruptured ligament: The substance of the ligament should be intact, and there should be the possibility of implementing sutures that supply an anatomic reduction without tourniquet effect on the vascular supply. The volume of the ligament should be large enough to get good compression of the rupture site.
Timing: The lesion should be addressed within less than 3 weeks after the injury. Bony avulsion ruptures are more likely to get good healing conditions than are intraligamentary ruptures. This is especially true for the lateral side.
Suture material: For the ligament repair, several sutures have been recommended. For the ACL and PCL, 2-0 nonabsorbable sutures seem most appropriate, but failure forces are limited to 60 N, which is far below what has been found during activities such as passive hyperextension or during isokinetic exercising. Fiber-enforced suture material is useful. Biomechanical data show that sutured ligaments withstand approximately one-fourth of an augmented suture. The elongation of the sutures is substantial. New implants have been developed that may lessen the degree of elongation.
Fig. 61.23 shows different ligament suture repairs, and Fig. 61.24 shows the mechanical properties of these constructs with different suture materials. No matter what suture technique is used, the surgeon should ensure that the joint stays in a stable position during flexion-extension after the repair. This may be enforced by application of an external fixator with a ROM device (e.g., Compass elbow universal hinge, Smith & Nephew, Memphis, TN; Fig. 61.25 ; see ).



Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here