Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We define a complication as a disease process that occurs in addition to a principal illness. In the lexicon of diagnosis-related groupings, complications are comorbidities. However, a broken implant complicating the healing of a radius shaft fracture hardly seems to fit either of these definitions. In orthopaedic trauma terminology, the term complication has come to mean an undesired turn of events specific to the care of a particular injury.
Complications can be local or systemic and are caused by, among other things, physiologic processes, errors in judgment, or fate. Codivilla described complications as “inconveniences.” A colleague once described a pin tract infection with external fixation as a problem, not a complication. Preventing pin tract infection is indeed a problem that needs a solution, but when it occurs in a patient, it becomes a complication. Since 1995 there has been mandatory reporting of “sentinel events,” a type of major complication that involves unexpected occurrences such as limb loss, surgery on the wrong body part, and hemolytic transfusion reaction. Another term, “never events,” was introduced in 2001 by Ken Kizer and includes medical errors that should never occur (e.g., wrong site surgery, a retained foreign object after surgery, intraoperative or postoperative death in an American Association of Anesthesiologists Class I patient, any stage 3 or 4 pressure ulcer acquired after admission). These definitions beg the question, Is death from any arrhythmia caused by a congenital irritable cardiac focus any more preventable on an operating table than it is on a basketball court?
Patients with broken bones expect perfection in their treatment outcomes. These unrealistic expectations have led in part to the current adverse medicolegal climate surrounding the care of broken bones. There are many grading systems for assessing the quality of these results but none for complications. As a starter, operative misadventures can be classified as follows:
Unexpected events that slow down the healing process, such as contaminating a reamer
Events that change the operation but have no long-term consequences, such as breaking a drill bit
Events that cause long-term harm, such as cutting a nerve
This chapter presents current knowledge about three systemic complications (fat embolism syndrome [FES], thromboembolic disorders, and multiple organ system dysfunction and failure) and four local complications (soft tissue damage, vascular problems, posttraumatic arthrosis, and peripheral nerve injury).
FES is the occurrence of hypoxia, confusion, and petechiae a few days or even hours after a long bone fracture. FES is distinct from posttraumatic pulmonary insufficiency, shock lung, and adult respiratory distress syndrome (ARDS). When known etiologic factors of posttraumatic pulmonary insufficiency such as pulmonary contusion, inhalation pneumonitis, oxygen toxicity, and transfusion lung are excluded, there remains a group of patients who have FES with unanticipated respiratory compromise several days after a diaphyseal fracture.
Fat embolism was first described by Zenker in 1861 in a railroad worker who sustained a thoracoabdominal crush injury. It was initially thought that the fat from the marrow space embolized to the lungs and caused the pulmonary damage. This hypothesis in part may still be the best one. Fenger and Salisbury believed that fat from fractures embolized to the brain, resulting in death. Von Bergmann first clinically diagnosed fat embolism in a patient with a fractured femur in 1873. The incidence of this now recognized complication of long bone fracture was extensively documented by Talucci and colleagues in 1913 and subsequently studied during World Wars I and II and the Korean conflict.
Although the embolic fat in the lungs comes from bone, other processes are required to produce the physiologic damage to lung, brain, and other tissues. The term fat embolism syndrome may not describe the entire pathomechanics of this condition as was originally hypothesized; embolization of active substances and fat from the injured marrow space has been thought to be the source of embolic fat. Recent studies suggest otherwise. Mudd and associates did not observe any myeloid tissue in any of the lung fields at autopsy in patients with FES and suggested that the soft tissue injury, rather than fractures, was the primary cause of FES. Husebye and colleagues also noted that FES might also result from “an abnormal patient reaction to the fat intravasation.” In a review of the literature, ten Duis stated that “future attempts to unravel this syndrome … should pay full attention to differences in the extent of accompanying soft tissue injuries that surround a long bone fracture.” In a laboratory rabbit model, Aydin and colleagues found that pulmonary contusion had more deleterious effects than fractures in the formation of cerebral fat embolism.
Although there are many unanswered questions about FES, several issues are apparent. It strikes young patients; older patients with femoral fractures do not seem at risk. It usually occurs after lower, not upper, limb fractures, and is more frequent with closed fractures. McDermott and colleagues reported three cases of patients with tibial fractures from football injuries who also had dehydration and developed FES, and they concluded that adequate preoperative hydration, especially if injuries were sustained during heavy exercise, may reduce the risk of developing FES. In a prospective study, Chan and associates found an incidence of 8.75% of overt FES in all fracture patients, with a mortality rate of 2.5%. The incidence rose to 35% in patients with multiple fractures. Other investigators reported the incidence of FES between 0.9% and 3.5% in patients with long bone fractures.
Early recognition of the syndrome is crucial to preventing a complex and potentially lethal course. Clinically, FES consists of a triad of hypoxia, confusion, and petechiae appearing in a patient with fractures. The disease characteristically begins 1 to 2 days after fracture, after what has been called the latent or lucid period. Sixty percent of all cases of FES are seen in the first 24 hours after trauma, and 90% of all cases appear within 72 hours. Gurd and Wilson's criteria for FES are commonly used, with the clinical manifestations grouped into either major or minor signs of FES, as shown in Box 24.1 . The diagnosis of FES is made when one major and four minor signs are present (see Box 24.1 ), along with the finding of macroglobulinemia. The best laboratory test is the measurement of arterial oxygenation on room air. When the P o 2 is less than 60 mm Hg, the patient may be in the early stages of FES.
* A positive diagnosis requires at least one major and four minor signs.
Lindeque and colleagues believe that Gurd and Wilson's criteria are too restrictive and should also include the following:
P co 2 of more than 55 mmHg or pH of less than 7.3
Sustained respiratory rate of more than 35 breaths per minute
Dyspnea, tachycardia, and anxiety
If any one of these is present, the diagnosis of FES is made. Other supportive findings include ST segment changes on electrocardiography and pulmonary infiltrates on chest radiography. In 2013 Prakash and colleagues suggested that early elevation of interleukin-6 (IL-6) correlated with higher risk of FES development in those with isolated skeletal trauma. IL-6 is a small protein secreted by lymphocytes and macrophages in response to trauma. As IL-6 measurements are not readily available at all institutions, published literature on this topic remains in its infancy.
Neurologic changes have been noted in up to 80% of patients. It is important to assess the neurologic status of the patient to differentiate between fat embolization, frontal concussion/contusion, and intracranial mass lesions. Although hypoxia alone can cause confusion, in FES, petechial hemorrhages, particularly in the reticular system, may alter consciousness. These changes persist despite adequate oxygen therapy. Focal neurologic findings should be investigated to rule out lesions caused by associated head trauma. Persistent alterations of consciousness or seizures are a bad prognostic sign.
Clinically, fat embolism is a diagnosis of exclusion. In the first few days, sudden pulmonary compromise can also result from pulmonary embolism (PE), heart failure, aspiration, and medication reaction. When these possible causes have been excluded along with many other less likely conditions, fat embolism becomes the leading cause of morbidity in the injured patient with a long bone lower limb fracture.
Although the precise pathomechanics of FES are unclear, Levy found many nontraumatic and traumatic conditions associated with FES. The simplest and earliest hypothesis is that broken bones liberate marrow fat that embolizes to the lungs. Marrow fat globules produce mechanical and metabolic effects culminating in FES. The mechanical theory postulates that fat droplets from the marrow enter the venous circulation via torn veins adjacent to the fracture site.
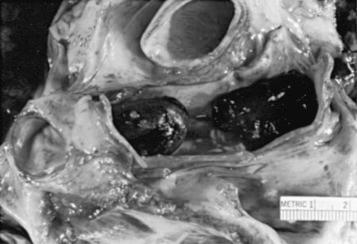
Peltier coined the term intravasation to describe the process whereby fat gains access to the circulation. The conditions in the vascular bed that allow intravasation to take place also permit marrow embolization. Indeed, marrow particles are found in the lungs when fat is found ( Fig. 24.1 ).

Mechanical obstruction of the pulmonary vasculature occurs because of the absolute size of the embolized particles. In a dog model, Teng and colleagues found 80% of fat droplets to be between 20 and 40 µm. Consequently, vessels in the lung smaller than 20 µm in diameter become obstructed. Fat globules of 10 to 40 µm have been found after human trauma. Systemic embolization occurs either through precapillary shunts into pulmonary veins or through a patent foramen ovale.
The biochemical theory suggests that mediators from the fracture site alter lipid solubility causing coalescence, because normal chylomicrons are less than 1 µm in diameter. Many of the emboli have a histologic composition consisting of a fatty center with platelets and fibrin adhered. Large amounts of thromboplastin are liberated with the release of bone marrow, leading to activation of the coagulation cascade.
Studies of the physiologic response to the circulatory injection of fats have shown that the unsaponified free fatty acids are much more toxic than the corresponding neutral fats. Peltier hypothesized that elevated serum lipase levels present after the embolization of neutral fat hydrolyzes this neutral fat to free fatty acids and causes local endothelial damage in the lungs and other tissues, resulting in FES. This chemical theory might in part explain the latency period seen between the arrival of embolic fat and more severe lung dysfunction. Elevated serum lipase levels have been reported in association with clinically fatal FES. Alternative explanations are also possible for the toxic effect of fat on the pulmonary capillary bed. The combination of fat, fibrin, and (possibly) marrow may be sufficient to begin a biochemical cascade that damages the lungs without postulating enzymatic hydrolysis of neutral fat. Bleeding into the lungs is associated with a fall in the hematocrit level. The resulting hypoxemia from the mechanical and biochemical changes in the lungs can be severe, even to the point of death of the patient.
Autopsy findings in patients dying of FES do not, however, show a consistent picture. This may be caused by a lack of clear-cut criteria that define patients included in a given series, but may also be because manifestations of FES depend on a wide number of patient, accident, and treatment variables.
In light of the incidence of fat emboli and FES in trauma patients, it is likely that other precipitating or predisposing factors such as shock, sepsis, or disseminated intravascular coagulation are needed for the phenomenon of embolized fat to cause FES. Müller and associates summarized that “fat embolism syndrome is likely the pathogenetic reaction of lung tissue to shock, hypercoagulability, and lipid mobilization.”
Two clinically related treatment questions arise: (1) Is there an association between intramedullary (IM) nailing, FES, and other injuries? (2) Is there an effect from different nailing methods on the incidence of FES? In 1950 Küntscher described FES as a complication of IM nailing. Pape and associates found that early operative fracture fixation by nailing was associated with an increased risk of ARDS in patients with thoracic injury. These results are in contrast to those of the group without thoracic injury. Thoracic trauma is associated with direct pulmonary injury. The pathogenic mechanisms were examined by Lozman and colleagues. Thus the timing and the associated injuries are crucial in deciding when to use a nail.
In a prospective study, Pape and associates showed a significant impairment of oxygenation in multiple trauma patients who underwent reamed nailing. A group of similar patients who had unreamed nailing did not have the same signs of pulmonary dysfunction. These investigators reasoned that the most likely difference between the two groups was a lower degree of fat embolization in the unreamed group. In sheep, Pape and colleagues demonstrated intravasation of fat associated with reaming of the IM canal. They concluded that the unreamed procedure caused substantially less severe lung damage than the reamed procedure. However, Heim and associates found that there was a significant increase in IM pressure associated with unreamed nail insertion and that both reamed nailing and unreamed nailing lead to bone marrow intravasation ( Fig. 24.2 ). Thus the use of an unreamed nail does not solve the problem of bone marrow embolization and resultant pulmonary dysfunction.

The question of what the degree of fat embolization has not been fully answered. High IM pressures have been linked with fat embolization and FES. Wozasek and colleagues looked at the degree of fat intravasation during reaming and IM nailing and correlated this with IM pressure changes and echocardiographic findings. They found peak IM pressures in both the tibial and the femoral nailings in the first two reaming steps. Insertion of the nail caused only minimal pressure rises, but this was after reaming. Echocardiography, however, demonstrated that the maximal infiltration of particles occurred when the nail was inserted. They concluded that the phenomenon of fat intravasation did not depend on the rise in IM pressure. Pinney and associates studied 274 patients with isolated femur fractures and found that waiting more than 10 hours after injury was associated with a 2.5-fold increase in FES. Bulger and colleagues noted that early IM fixation did not seem to increase the incidence or severity of FES.
The risk of FES can be decreased by several measures. Proper fracture splinting and expeditious transport, use of oxygen therapy in the preinjury period, and early operative stabilization of long bone fractures of the lower extremities are three important measures that can be taken to reduce the incidence of this complication. Blood pressure, urinary output, blood gas values, and in the more critically injured, pulmonary wedge pressures should be monitored to evaluate fluid status and tissue perfusion more precisely. Dramatic advances in emergency medical transport have resulted in an increased rate of survival in increasing patients with complex polytrauma and high injury severity scores. In some instances, this has led to a tendency to “scoop and run” without traction splinting. Unsplinted long bone fractures in patients transported over long distances are a setup for intravenous (IV) fat intravasation. Oxygen therapy by mask or nasal cannula can help mitigate the decrease in arterial oxygenation after fracture and appears to have value in the prevention of FES.
Although fracture fixation and medullary nailing in particular cause a transient decrease in oxygenation, the immediate stabilization of fractures before the development of low arterial saturation may prevent the occurrence of FES. In a prospective randomized study of 178 patients, Bone and associates confirmed that early fracture stabilization, within the initial 24 hours after injury, decreased the incidence of pulmonary complications. Likewise, Lozman and colleagues, in a prospective randomized study, concluded that patients receiving immediate fixation had less pulmonary dysfunction after multiple trauma and long bone fractures than did those patients receiving conservative treatment.
Although IM nailing is the preferred method of stabilization, the timing of nailing is a point of controversy. There was concern that immediate nailing of long bone fractures early in the postinjury period would increase the incidence of pulmonary complications, including FES. External fixation of long bone fractures can be used as a temporizing alternative to IM nailing. Earlier studies showed no evidence to support the view that the effect of reaming on intravascular fat is additive or that immediate reamed IM fixation causes pulmonary compromise. In fact, the opposite is true, most likely because fracture stabilization removes the source of intravascular marrow fat and decreases shunting in the lung, because the patient can be mobilized to an upright position.
No cases of FES were seen in a retrospective study by Talucci and associates in which 57 patients underwent immediate nailing. Similarly, Behrman and colleagues reported a lower incidence of pulmonary complications for patients undergoing early fixation among 339 trauma patients who underwent either immediate or late fixation of femoral fractures. In the study by Lozman and colleagues, patients who had delayed fracture fixation had a higher intrapulmonary shunt fraction compared with that in the early fixation group.
Early IM nailing of long bone fractures is not without complications. Pell and colleagues, using intraoperative transesophageal echocardiography, demonstrated varying degrees of embolic showers during reamed IM nailing. FES developed postoperatively in three patients, and one patient died. Other studies showed an increased number of pulmonary complications associated with early, reamed IM nailing of femoral shaft fractures.
Specific therapy has been used in an attempt to decrease the incidence of FES. No clinical effect on the rate of FES has been found with increased fluid loading, the use of hypertonic glucose, alcohol, heparin, low molecular weight dextran, and aspirin. Various studies have looked at the efficacy of corticosteroids in reducing the clinical symptoms of FES. Large doses of steroids immediately after injury do have a beneficial effect. Corticosteroids most likely decrease the incidence of FES by limiting the endothelial damage caused by free fatty acids. Babalis and colleagues, in a randomized, prospective study of 87 patients with long bone fractures allocated to either a placebo group or a group treated with IV, low-dose methylprednisolone, found that methylprednisolone deceased posttraumatic hypoxemia and fat embolism in patients with isolated lower limb long bone fractures, especially when early fracture stabilization is not possible. They concluded that the prophylactic use of methylprednisolone in small doses was useful in preventing posttraumatic hypoxemia and FES. A meta-analysis performed by Bederman et al. in 2009 identified some improvements in those with long bone fractures but no change in mortality rate. Although this is encouraging, routine use of steroids is not without significant risk of complications and is not routinely employed. Current studies remain too variable; therefore no uniform conclusion can be drawn.
FES is primarily a disease of the respiratory system, and current treatment is therefore mainly with oxygen and meticulous mechanical ventilation. Treatment of FES hypoxia remains mainly supportive.
Finally, in no way should it be construed that either clinical experience or scientific investigation provides a sure pathway to prevent the appearance of this significant postinjury and potentially lethal problem. Although careful review of the medical record might suggest how things could have been done alternatively, there is no certainty, for example, that waiting until the Pa o 2 returns to normal before performing a nailing prevents the complication of FES; indeed, waiting might invite another complication.
In 1860 Virchow proposed the triad of thrombogenesis: increased coagulability, stasis, and vessel wall damage ( Fig. 24.3 ). These are all factors that are affected by trauma. Virchow also linked the presence of deep venous thrombosis (DVT) with PE and deduced that a clot in the large veins of the thigh embolized to the lungs. Laennec, in 1819, was the first to describe the clinical presentation of an acute pulmonary embolus. The pathogenesis of a proximal DVT was described by Cruveilhier in 1828. Venous thrombi have been shown to develop near the valve pockets on normal venous endothelium and are not necessarily related to inflammation of the vessel wall.

Trauma creates a hypercoagulable state. Vessel wall injury with endothelial damage exposes blood to tissue factor, collagen, basement membrane, and von Willebrand factor, which induce thrombosis through platelet attraction and the intrinsic and extrinsic coagulation pathways. Antithrombin (AT-III) activity, which decreases the activity of thrombin and factor Xa, was found to be below normal levels in 61% of critically injured trauma patients. Also, fibrinolysis is decreased, which appears to be from increased levels of plasminogen activator inhibitor type 1 (PAI-1), which inhibits tissue plasminogen activator and thus decreases the production of plasmin.
The presence of heart disease alone increases the risk of PE by 3.5 times, and this risk is further increased if atrial fibrillation or congestive heart failure is present. The risk of DVT is increased during pregnancy and is especially great in the postpartum period. Spinal cord injury is associated with a threefold increase in lower extremity DVT and PE.
A meta-analysis by Velmahos and colleagues looked at DVT and risk factors in trauma patients. The following variables studied did not have a statistically significant effect for increasing the development of DVT:
Gender
Head injury
Long bone fracture
Pelvic fracture
Units of blood transfused
The variables that were statistically significant included spinal fractures and spinal cord injury and increasing the DVT risk by twofold and threefold, respectively. They could not confirm that the widely assumed risk factors of pelvic fracture, long bone fracture, and head injury affected the incidence of DVT but did note that the multiple-trauma patients may have already been at the highest risk of DVT. Van Gent et al. reviewed 2370 patients with an 11.2% incidence of a DVT event. On multivariable regression analysis, increased age and increased injury severity score (ISS) were associated with increased risk of DVT; however, increased ISS was protective for PE. Specific injuries were not evaluated.
For immobilized trauma patients with no prophylaxis, the incidence of venography-proven thigh and iliofemoral thrombosis is between 60% and 80%. Even with full prophylaxis, the incidence of DVT may be as high as 12%. Stannard and colleagues reported a significant rate of DVT in high-energy skeletal trauma patients despite thromboprophylaxis. They noted in a series of 312 patients with high-energy trauma that 11.5% developed venous thromboembolic disease with an incidence of 10% in those with nonpelvic trauma and 12.2% in the group with pelvic trauma despite thromboprophylaxis. Some investigators have concluded that “there is no adequate prophylaxis against DVT in the trauma patient.”
Trauma to the pelvis and lower extremities greatly increases the risk of DVT and PE. In an autopsy study of 486 trauma fatalities, Sevitt and Gallagher found 95 cases of PE for an incidence of 20%. At autopsy, the rate of PE after hip fracture was 52 in 114 (46%); for tibia fractures, 6 in 10 (60%); and for femoral fractures, 9 in 17 (53%). The rate of DVT for hip fractures increased to 39 in 47 (83%) and for femur fractures to 6 in 7 (86%) when special studies of the venous system were done at autopsy.
According to a review of 56,299 orthopaedic trauma patients, multiple factors were associated with increased risk of DVT: specifically age, body mass index (BMI), lower extremity injury, and ASA score. In addition, independent risk factors were noted: ventilator use, ascites, alcohol, steroids, sepsis, and gangrene. Overall, DVT incidence was 0.84% within 30 days.
PE is a significant cause of death after lower extremity injury. Two-thirds of patients having a fatal pulmonary embolus die within 30 minutes of injury ( Fig. 24.4 ). Although the incidence of fatal PE without prophylaxis after elective hip surgery is from 0.34% to 3.4%, the incidence after emergency hip surgery is from 7.5% to 10%.
The types of DVT that are at high risk for causing a PE are those that originate at the popliteal fossa or more proximally in the large veins of the thigh or pelvis. Moser and LeMoine found the risk of pulmonary embolization from distal lower extremity DVT to be relatively low. Of DVTs that are first limited to the calf, about 20% to 30% extend above the knee. Those that extend above the knee carry the same risk as femoral and popliteal thrombi. Kakkar and colleagues speculated that thrombi in the calf are securely attached and resolve rapidly and spontaneously. However, embolization from “calf only” venous thrombi does occur. Calf vein thromboses are responsible for 5% to 35% of symptomatic PE, 15% to 25% of fatal PE, and 33% of “silent” PE.
In addition to PE, complications of DVT include recurrent thrombosis and postthrombotic syndrome. Symptoms of postthrombotic syndrome are edema, induration, pain, pigmentation, ulceration, cellulitis, and stasis dermatitis. Symptoms are present in up to 20% to 40% of those having had a DVT.
Upper extremity DVT is much less common (2.5%) and can be due to primary or secondary causes. Primary causes include effort thrombosis (Padget-Schroetter syndrome), and it may be idiopathic. Effort thrombosis is most common in athletes and laborers who do repetitive shoulder abduction and extension arcs of motion. Secondary causes are venous catheters, venous trauma, extrinsic compression or malignancy, and hypercoagulable conditions.
The clinical signs and symptoms of DVT are nonspecific. DVT was clinically silent in two-thirds of cases in which thrombosis was found at autopsy or in whom the findings on leg venography were positive.
Clinically, the diagnosis of DVT and PE is frequently difficult. With PE, although some patients experience sudden death, many more present with gradual deterioration and symptoms similar to pneumonia, congestive heart failure, or hypotension. Symptoms can be intermittent with episodes of transient pulmonary compromise caused by clusters of small emboli. Because the clinical diagnosis is difficult, diagnostic studies are necessary so that early treatment can be instituted. There are various tests, each of which has its limitations and advantages.
Although venography was the classic method for detecting DVT, noninvasive venous Doppler examination is the most used method for imaging DVTs. Continuous-wave Doppler (CWD) or Doppler ultrasound examination is easy to do and can be done at bedside, but it requires experience to reduce the false-positive result rate. Venous thrombosis is characterized by the absence of venous flow at an expected site, loss of normal fluctuation in flow associated with respiration, diminished augmentation of flow by distal limb compression, diminished augmentation of flow by release of proximal compression, and lack of change on Valsalva maneuver. Barnes and colleagues found that Doppler ultrasonography was 94% accurate, and no errors were made in diagnosis above the level of the knee. However, for isolated calf vein thrombosis, CWD is insensitive. An additional disadvantage is that CWD may fail to detect nonobstructive thrombi even in proximal lesions.
Color-flow duplex ultrasonography (CFDU) uses a Doppler component that is color-enhanced and detects blood flow by the shift in frequency from the backscatter of high-frequency sound. The frequency is shifted by an amount proportional to the flow velocity. The color saturation is proportional to the rate of flow. A black image indicates an absence of flow, flow velocities less than 0.3 cm/sec, or flow vectors at a right angle to the second beam. The addition of color allows for the easier and faster detection of vascular structures. Blood flowing away from the transducer appears blue, whereas blood flowing toward the transducer appears red. This has provided improved imaging of the iliac region, the femoral vein in the adductor canal, and the calf veins. CFDU is superior to duplex scanning and B-mode imaging in detecting nonocclusive thrombi because the flow characteristics in the vessels are readily detected. Several studies have reported high sensitivity and specificity in symptomatic patients.
Serial ultrasonography has been used as surveillance screening to detect DVT in trauma patients, but it was thought not to be cost effective. So far it appears the benefits of serial ultrasonography are not apparent and appear to only increase cost. When DVT develops in the calf, about 25% extend to the thigh if left untreated. If the initial ultrasound missed the asymptomatic DVT and no treatment is given, approximately 2% of cases will have an abnormal proximal scan on testing 1 week later.
Magnetic resonance imaging (MRI) has been recently applied to the detection of DVT in the pelvis. Rubel and colleagues have reported on the use of magnetic resonance (MR) venography to evaluate DVT in patients with pelvic and acetabular trauma. Stannard and collegues reported that ultrasound had a false-negative rate of 77% for diagnosing pelvic DVT compared with MR venography. Stover and colleagues, in a prospective study of MR venography and contrast-enhanced computed tomography (CT), reported that the false-positive rate for MR venography was 100% and the false-positive rate for contrast-enhanced CT was 50%. They stated that they cannot recommend the sole use of either CT venography or MR venography to screen and direct the treatment of asymptomatic thrombi in patients with fracture of the pelvic ring because of these high false-positive rates. An additional disadvantage of MR venography is the cost, which is typically 2 to 2.5 times the cost of an ultrasound scan and 1.4 times the cost of venography.
Multidetector computed tomography pulmonary angiography (CTPA) is now the primary imaging modality for patients suspected of having an acute PE. The data indicate that multidetector CTPA is more accurate than single-slice CT or ventilation/perfusion (V/Q) scans. In fact, CTPA has fewer nondiagnostic scans than V/Q scans. Conventional CT with contrast, not performed as a dedicated CTPA, is no longer indicated in the workup of acute chest pain presumed to be secondary to acute PE.
The concept of venous thromboembolism (VTE) prophylaxis with traumatic injuries of the musculoskeletal trauma is a misnomer. The process of clot formation has likely already begun at the time of injury. Prophylaxis is really ex post facto. The concept of DVT “protection” rather than prophylaxis is probably more accurate for the orthopaedic trauma patient.
There are four types of patients with orthopaedic trauma who should be considered for VTE (venous thrombosis and PE) protection: polytrauma patients, elderly hip or pelvis fracture patients, isolated extremity injury patients, and spinal cord injury patients. It is challenging to generalize about VTE prophylaxis for all four groups together as one. VTE prophylaxis or protection for each type of orthopaedic patient will be discussed separately.
Without prophylaxis, patients with multisystem or major trauma have a DVT rate that exceeds 50% with a fatal PE rate of 0.4% to 2.0%. PE is a common cause of death in trauma patients. VTE accounts for about 9% of readmissions to the hospital after trauma. Polytrauma patients represent a heterogenous group and present many challenges. These patients are often cared for by multiple services (e.g., general surgery, critical care, orthopaedic trauma surgery) as a team. Many of these polytrauma patients with acidosis, coagulopathy, and hypothermia are initially treated with a damage control orthopaedics (DCO) approach (e.g., temporary spanning external fixation of long bone fractures).
Although VTE prophylaxis in the treatment of these patients needs to be individualized, several concepts are now becoming fairly standard. The recommendations published by the American College of Chest Physicians (ACCP) every 2 to 3 years as a Chest journal supplement are often considered to be the best guide. Note, however, that ACCP panelists may have vested economic interests in the agents used for VTE prevention. In general, there is a trend toward preferring non–vitamin K antagonist anticoagulants over warfarin and low molecular weight heparin. Kearon and colleagues recommended dabigatran, vivaroxaban, apixaban, or endoxaban rather than vitamin K antagonist therapy as long as there is no malignancy. These authors suggest vitamin K antagonist therapy over low molecular weight heparin. Recommended treatment for a proximal DVT of the leg for PE provoked by surgery is anticoagulation for 3 months. However, these authors recommend discretion with “an unprovoked DVT of the leg” when deciding on duration more than 3 months. Routine use of compression stockings is also no longer recommended in acute DVT to prevent postthrombotic syndrome.
Warfarin, when it can be monitored, is an acceptable and economic pharmacologic alternative with a long history. For prophylaxis, an international normalized ratio (INR) of 1.4 to 1.6 is often adequate; and for treatment of thrombosis with embolism, an INR of 2.0 to 2.5 is recommended. Note that the INR for cardiac indication is higher. The role of newer agents for chemoprophylaxis is being determined.
An inferior vena cava (IVC) filter is not recommended for primary VTE prevention. Although screening has not been shown to be of value in diagnosis of asymptomatic DVT, many institutions surveyed by the OTA used screening tools for DVT in fracture patients.
Without prophylaxis, hip fracture patients have DVT rates of 50% with a proximal DVT rate of 25%. Fatal PE is more common in hip fracture patients than in total hip and knee arthroplasty patients. Recommendations include the following antithrombotic approaches (rather than no antithrombotic prophylaxis) for at least 2 to 3 weeks: low molecular weight heparin (LMWH), ondaparinux, low-dose unfractionated heparin, adjusted-dose vitamin-K antagonist, aspirin (ASA), or an intermittent pneumatic compression device. Recent literature suggests that alternative newer agents may be less effective or equivalent to the cheaper options (LMWH, warfarin). In addition, it is recommended that if hip fracture surgery would be delayed LMWH should be started. Sagi and colleagues recommend 4 weeks of therapy postoperatively. From an ethical standpoint, one might question if overly vigorous anticoagulation is appropriate in every case. Consider, for example, the elderly, demented nursing home patient who falls and has a hip fracture. Indeed, there is potentially a wide range of morbidity from the use of anticoagulation in the elderly, who may experience bleeding, stroke, and diagnostic misadventures as a result of overzealous prophylaxis. European guidelines recommend the use of ASA for total knee arthroplasty, total hip arthroplasty, and hip fracture as it is associated with less bleeding.
Isolated extremity injuries are probably the most common injuries seen by orthopaedic physicians. According to Ahmad and colleagues, the incidence of postoperative VTE after foot or ankle surgery was only 0.79% within 90 days. The recommended approach to acute isolated DVT of the leg without severe symptoms or risk factors for extension is serial imaging of the veins rather than anticoagulation. On the other hand, surveillance of patients for VTE and protection/prophylaxis seem prudent, particularly for patients with risk factors such as underconditioning, middle or old age, multiparity, and so on. At a minimum, one could consider simple measures (early mobilization, ankle pump exercises, GCS, IPC with or without GCS) or more intensive measures (preoperative and immediate postoperative GCS, followed by a short course of LMWH, synthetic pentasaccharides, or adjusted vitamin K antagonists). Office staff should be trained to inquire about VTE prophylaxis when patients call.
Acute spinal cord injury was the risk factor most strongly associated with the development of DVT in major trauma. Rogers and colleagues noted that spinal cord injuries or spinal fractures are high risk for venous thromboembolism. It is recommended that “thromboprophylaxis be provided for all patients with acute spinal cord injuries.” The recommendations for patients with acute spinal cord injury were for pharmacologic prophylaxis when safe together with mechanical prophylaxis (e.g., intermittent pneumatic compression, etc.).
After DVT or PE is suspected, the clinical impression should be confirmed by diagnostic testing. Parenteral anticoagulants can be started unless contraindicated while the diagnostic testing is pending and is the current recommendation for patients where there is a high clinical suspicion of acute VTE. If there is an “intermediate” or “low” clinical suspicion of DVT, the recommendation is not to start parenteral anticoagulants. The options for anticoagulation include parenteral anticoagulation with one of the non–vitamin K antagonist oral anticoagulants. Furthermore, there is now more focus on the specific location of the DVT (i.e., proximal or distal in the lower extremities). The new recommendations make suggestions about which patients with isolated subsegmental PE require anticoagulant therapy and which do not. The reader is referred to the text of this reference. Furthermore, for patients with acute PE associated with hypotension who do not have a bleeding risk, systemically administered thrombolytic therapy is recommended. However, in most patients with acute PE not associated with hypotension, systemically administered thrombolytic therapy is not recommended; anticoagulant therapy is recommended. Follow-up venous scans help determine when therapy can be stopped.
Although options such as thrombolytic therapy for the treatment of acute DVT without PE may be considered, the latest recommendations are for anticoagulant therapy alone over catheter-directed thrombolysis. Operative venous thrombectomy is also deemphasized, because anticoagulant therapy alone is suggested over operative venous thrombectomy. Furthermore, the recommendations are for anticoagulation of the same intensity and duration in patients who undergo thrombosis removal as in patients who are comparable who did not undergo thrombosis removal.
IVC filters are used less frequently and are no longer recommended as primary prophylaxis against VTE. IVC filters have associated complications such as venous stasis leading to edema, pain, varicose veins, and skin ulcers in a condition known as the postphlebitic syndrome. Other complications include bleeding or thrombus formation at the site of insertion, migration of the filter, and perforation of the vena cava. In addition, filters are not 100% effective.
Vena cava interruption is performed when heparinization is contraindicated, as in patients with a preexisting bleeding disorder; severe hypertension; neurologic injury; or bleeding problems of pulmonary, gastrointestinal, neurologic, or urologic etiology. If anticoagulation fails to stop pulmonary emboli, vena cava interruption is indicated. Also, if patients develop complications with anticoagulation, they can be switched to vena cava interruption. An additional approach is the preoperative use of vena cava interruption in patients who are at extremely high risk for PE, where anticoagulation is contraindicated.
The complete prevention of thromboembolism in orthopaedic trauma is impossible because trauma cannot be anticipated. Current literature and experience clearly indicate that certain trauma patients benefit from some form of VTE surveillance or prophylaxis. DVT and PE are common causes of morbidity, mortality, and litigation associated with the care of orthopaedic trauma patients. Clinicians are also advised to stay informed about the consensus recommendations that are published every 2 to 3 years in the Chest supplement.
Questions remain such as, “Is there a genetic predisposition to VTE?” Risk stratification is being used in other areas of medicine and is only beginning to be understood in orthopaedic trauma. In addition, combinations of injuries, multiple lower extremity fractures with a spinal cord injury, or a pelvic fracture together with a femur fracture likely exponentially increase the risk of VTE. Although current prophylactic regimens in trauma patients significantly reduce the relative risk for DVT, no method provides 100% protection. Interestingly, our DVT incidence has trended downward with prudent attention to prophylaxis; however, the incidence of PE has remained constant over the past decade. Further randomized, multicentered, controlled trials of DVT prophylaxis in trauma patients are necessary to obtain proper ability to understand the factors that affect PE incidence. Nonetheless, our ability to diagnose VTE and protect patients from it is constrained by acute hemorrhage and inability to tolerate anticoagulation, soft tissue contusion, and extremity injuries that prevent the placement of IPC. Prophylaxis is also impossible, and the best we can do is to try for VTE protection. Nonetheless, there are many methods of DVT surveillance and protection at our disposal, and they ought to be considered. Although the ideal method of documentation for hospital or outpatient examinations is unknown, we have found that a note such as “no signs or symptoms of PE/DVT” along with the documentation of VTE protection/prophylaxis is prudent and reasonable.
Multiple organ failure (MOF) is defined as the sequential failure of two or more organ systems remote from the site of the original insult after injury, operation, or sepsis. The organ failure can be pulmonary, renal, hepatic, gastrointestinal, central nervous, or hematologic. These systems can be monitored for objective criteria for failure, but criteria vary from series to series ( Tables 24.1 and 24.2 ). The risk of developing MOF and the severity of the MOF can also be graded by measuring the effects on specific organ systems.
| Organ or System | Dysfunction | Advanced Failure |
|---|---|---|
| Pulmonary | Hypoxia requiring intubation for 3–5 days | ARDS requiring PEEP >10 cm H 2 O and Fi o 2 >0.5 |
| Hepatic | Serum total bilirubin ≥2–3 mg/dL or liver function tests ≥ twice normal | Clinical jaundice with total bilirubin ≥8–10 mg/dL |
| Renal | Oliguria ≥479 mL/day or creatinine ≥2–3 mg/dL | Dialysis |
| Gastrointestinal | Ileus with intolerance of enteral feeds >5 days | Stress ulcers, acalculous cholecystitis |
| Hematologic | PT/PTT >125% normal, platelets <50,000–80,000 | DIC |
| Central nervous system | Confusion, mild disorientation | Progressive coma |
| Cardiovascular | Decreased ejection fraction or capillary leak syndrome | Refractory cardiogenic shock |
| Pulmonary | Need of ventilator support at Fi o 2 ≥0.4 for 5 consecutive days. |
| Hepatic | Hyperbilirubinemia >2.0 g/dL and an increase of serum glutamic-oxaloacetic transaminase. |
| Gastrointestinal | Hemorrhage from documented or presumed stress-induced acute gastric ulceration. This can be documented by endoscopy; if endoscopy is not performed, the hemorrhage must be sufficient to require 2 units of blood transfusion. |
| Renal | Serum creatinine level greater than 2.0 mg/dL. If a patient has preexisting renal disease with elevated serum creatinine level, doubling of the admission level is defined as failure. |
MOF is the end result of a transition from the normal metabolic response to injury to persistent hypermetabolism and eventual failure of organs to maintain their physiologic function. A 1991 consensus conference used the term multiple organ dysfunction syndrome (MODS) to describe this spectrum of changes. Organ dysfunction is the result of either a direct insult or a systemic inflammatory response, known clinically as the systemic inflammatory response syndrome (SIRS), which can be reversible or progress to MODS or MOF. SIRS can be caused by a variety of infectious and noninfectious stimuli ( Fig. 24.5 ). Treatment of the offending source must be undertaken early, because once organ failure has begun, treatment modalities become progressively ineffective. Fry identified the mortality rate for failure of two or more organ systems as about 75%. If two organ systems fail and renal failure occurs, the mortality rate is 98%. MOF has been described as the number one cause of death in surgical intensive care units.

The basic theory behind the development of MOF and the closely related ARDS has undergone modification since the 1970s and mid-1980s. Moore and Moore described the earlier models, which promoted an infectious basis for ARDS and MOF, with two possible scenarios: (1) insult → ARDS → pulmonary sepsis → MOF, or (2) insult → sepsis → ARDS and MOF. Current thinking promotes an inflammatory model of MOF with an inflammatory response from a number of infectious and noninfectious stimuli. Two patterns exist: the one-hit model (massive insult → severe SIRS → early MOF) and the more common two-hit model (moderate insult → moderate SIRS → second insult → late MOF). Research into the pathogenesis of MOF has focused on how the inflammatory response is propagated, independent of infection. Moore and Moore have the global hypothesis that postinjury organ failure occurs as the result of a dysfunctional inflammatory response. Deitch created an integrated paradigm of the mechanisms of MOF. In general, three broad overlapping hypotheses have been proposed in the pathogenesis of MOF: (1) macrophage cytokine hypothesis, (2) microcirculatory hypothesis, and (3) gut hypothesis. Further understanding of MOF must extend to the cellular and molecular levels.
Organ injury in MOF is largely caused to the host's own endogenously produced mediators and less due to exogenous factors such as bacteria or endotoxins ( Box 24.2 ). There is increasing evidence that biologic markers for the risk of development of MOF may be more useful than anatomic descriptions of injuries. Nast-Kolb and associates measured various inflammatory markers in a prospective study of 66 patients with multiple injuries (ISS >18) and found that the degree of inflammatory response corresponded with the development of posttraumatic organ failure. Specifically, lactate, neutrophil elastase, IL-6, and IL-8 were found to correlate with organ dysfunction. Strecker and colleagues studied 107 patients prospectively and found that the amount of fracture and soft tissue damage can be estimated early by analysis of serum IL-6 and creatine kinase and is of great importance with regard to long-term outcome after trauma. These investigators found significant correlations between fracture and soft tissue trauma and intensive care unit (ICU) stay; hospital stay; infections; SIRS; MOF score; and serum concentrations or activities of serum IL-6, IL-8, and creatine kinase during the first 24 hours after trauma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here