Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fracture healing is a complex and dynamic process that restores injured bones to prefracture conditions.
Fracture healing requires coordinated interactions among multiple cell types, cytokines, and growth factors. The healing process is influenced by the local mechanical and biologic environment, the surgical fixation device, and the reduction method.
Maintaining adequate blood flow to the affected site is the primary determinant of how well a fracture heals. To optimize fracture healing, the fracture must be reduced to a satisfactory position and stabilized.
Intramembranous ossification is the process of direct bone formation without a cartilage intermediate. This type of ossification is responsible for primary bone healing and normal bone remodeling.
Primary bone healing is favored when anatomic reduction minimizes the fracture gap and motion between two fracture surfaces is restricted through rigid internal fixation.
Intramembranous ossification begins with osteoprogenitor cells, a class of undifferentiated mesenchymal cells located in the deep layers of periosteum, endosteum, and marrow. These cells aggregate into membrane-like layers and proceed to differentiate directly to osteoblasts without the formation of cartilage. The process concludes with direct deposition of osteoid and subsequent mineralization.
Throughout this process, no clinically visible callus can be appreciated on radiographs.
Endochondral ossification is a sequential process that is responsible for secondary bone healing.
Secondary bone healing of a fracture is favored when the fracture site has some degree of mobility, unlike primary bone healing, which is favored by a rigid environment. Treatments that create favorable conditions for secondary bone healing include nonrigid fixation and casting immobilization.
The majority of fracture healing takes place via secondary bone healing. This process begins with mature chondrocytes undergoing senescence and calcification, leaving behind a scaffold of extracellular matrix. Blood vessels and osteoblasts then invade this cartilage matrix, resulting in the deposition of immature bone.
It is important to note that bone replaces cartilage in this process; cartilage is not converted to bone.
Fractures heal via primary (intramembranous) and secondary (endochondral) types of healing. The degree of fracture stability will influence which type of healing predominates.
Both types of fracture healing require a similar amount of time to restore mechanical integrity.
Rigid fixation strives for primary bone healing that is more expedient in returning to function and induces less deformity at the fracture site.
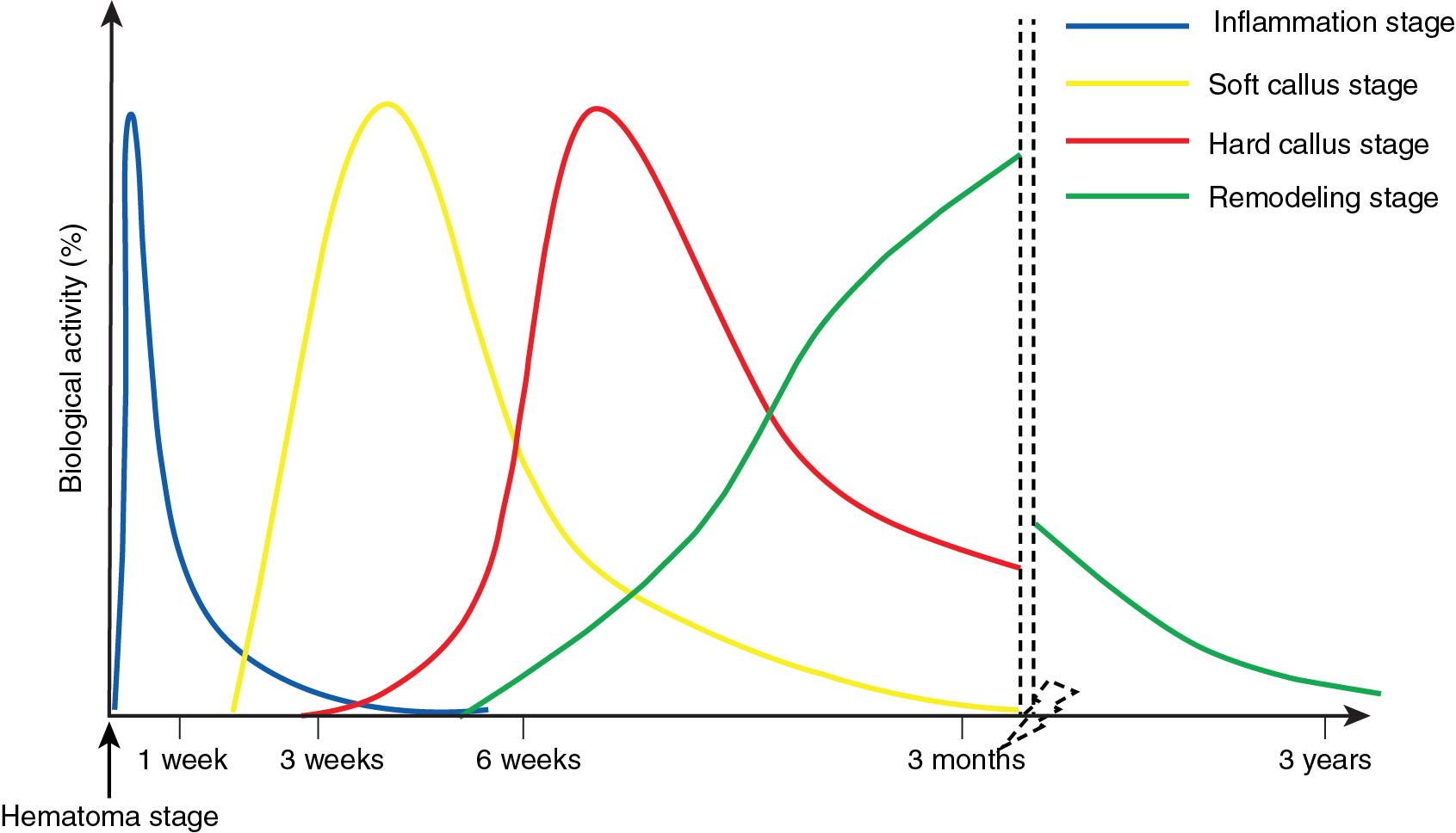
The healing process begins at the time of the injury. Although secondary bone healing is conceptualized here in stages, these processes overlap with each other in reality ( Fig. 7.1 ).
A fracture causes local disruption of blood vessels, leading to the formation of a hematoma.
The oxygen saturation inside a hematoma decreases significantly over the first 72 hours after a fracture. In response, hypoxia-inducible factor (HIF)-1 increases the production of vascular endothelial growth factor (VEGF), resulting in angiogenesis and revascularization of the affected area.
In addition, local hypoxic conditions alter the gene expression of osteoprogenitor cells, causing them to proliferate, secrete extracellular matrix, and differentiate into chondrocytes.
Several animal studies have shown that the removal of fracture hematoma during early healing (2–4 days) leads to inferior healing, highlighting the critical role of the hematoma in the healing process.
The inflammatory stage is characterized by a strong cellular response to fracture healing.
Cellular debris inside the hematoma incites an inflammatory response mediated by inflammatory cells including platelets, neutrophils, macrophages, and lymphocytes. These cells release various cytokines to modulate the healing process. Some cytokines elicit pain, encouraging the individual to immobilize the injured area.
Among these cytokines, tumor necrosis factor (TNF)-alpha plays a vital role in coordinating the inflammatory stage. A fracture-related hematoma contains a sevenfold higher concentration of TNF-alpha compared with peripheral blood. Low levels of TNF-alpha are associated with delayed fracture healing.
This stage begins a few days to 6 weeks after the injury. The process is analogous to the formation of cartilage through endochondral ossification.
Fibroblasts and chondroblasts build connective tissue and cartilage, respectively. The fibrocartilaginous callus fills the fracture gap, providing stability and supporting vascular ingrowth.
The local mechanical environment drives the differentiation of progenitor cells into either osteoblastic (stable environment) or chondroblastic (unstable environment) lineages of cells.
The conversion of cartilage into woven bone defines the hard callus stage. This occurs several weeks after a fracture and takes approximately 3 months.
Woven bone is a disorganized matrix of collagen fibers that further strengthens the callus and is visible on radiographs.
For patients receiving nonoperative treatment such as casting or traction, the hard callus stage is also accompanied by a clinically apparent reduction in pain and increased stability at the fracture site.
In this stage, woven bone is converted into lamellar bone through organized osteoblast and osteoclast activity. This phase can continue for several years.
The histology of fully healed bone is almost identical to the unbroken bone.
During remodeling, bone responds to loading stress according to Wolff’s law.
Wolff’s law states that the growth and remodeling of bone is influenced by mechanical forces that are applied to the bone. Placing moderate amounts of mechanical stress on bone during the remodeling phase can trigger an adaptive response that augments bone strength.
For a summary of factors influencing fracture healing, see Table 7.1 .
| Local factors | Systemic factors | Medications |
|---|---|---|
| Location | Age | NSAIDs |
| Fracture pattern | Traumatic brain injury | Corticosteroids |
| Comminution or bone loss | Diet and nutrition | Bisphosphonates |
| Fracture gap | Medical conditions (DM) | Quinolones |
| Soft-tissue coverage | Hormones | Antineoplastics |
| Method of fixation | HIV | |
| Neurovascular injury | Alcohol | |
| Open fracture | Smoking | |
| Infection | ||
| Radiation |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here