Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
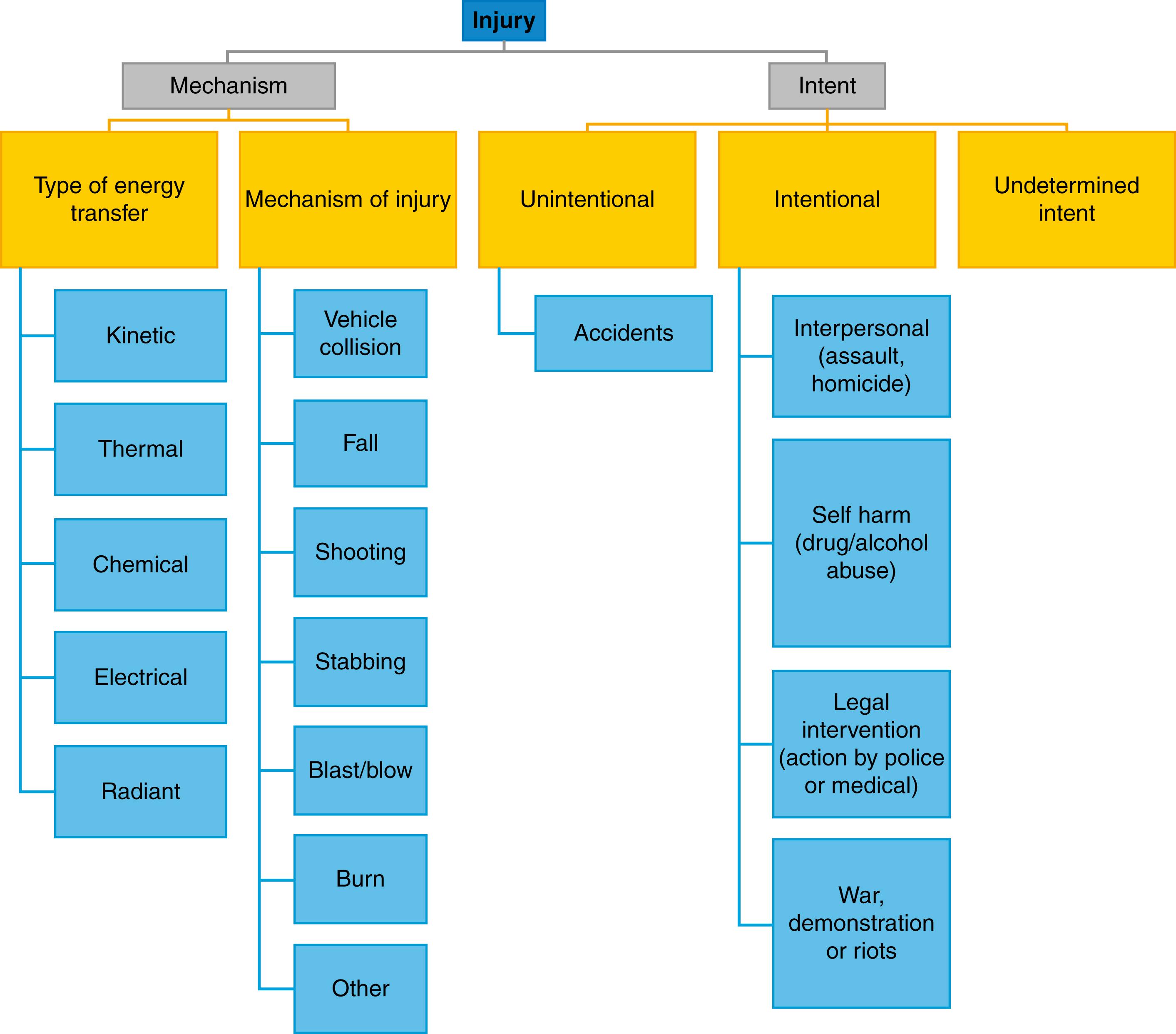
Injury is one of the major causes of death and disability in the world. The World Health Organization defines injury as physical damage that results when a human body is subjected to levels of energy that exceed tissue and physiological tolerances. Injury encompasses a wide range of causative energy sources, mechanisms and circumstances ( Fig. 15.1 ). Many factors also influence a patient’s exposure to, and tolerance of, energy transfer. These include physical, genetic, physiological, environmental, behavioural, cultural and social variables. In terms of consequences, there is also a spectrum of severity that ranges from simple self-limiting minor injury, through life changing severe injury to complex, life threatening major trauma ( Fig. 15.2 ). Conceptualising and quantifying the epidemiology of injury can therefore be challenging. One useful approach is to consider injury like any other disease process with the pathophysiology being the result of interaction of a host (patient), an injurious agent (energy) and the sociocultural and physical environment. Applying the ‘host/agent/environment’ model and seeing injury as a disease caused by energy allows a public health approach to be taken to injury prevention and control. The Haddon Matrix ( Box 15.1 ) relates the ‘host/agent/environment’ model to events before, during and after the injury. It illustrates how factors associated with the patient or victim, the energy transfer and the sociocultural or physical environment can be identified for any specific injury problem in the preevent, event and postevent phases. Understanding these factors allows them to be targeted for primary (preevent) or secondary (event) prevention in much the same way as for any other disease process. In the United Kingdom, the commonest causes of severe injuries are road traffic related injuries (typically involving younger patients) and falls (typically involving older patients). The Haddon Matrix ( Box 15.1 ) illustrates how the approach to prevention in these groups could be very different.
| Phase/Factor | Host (Injured Person) Factors | Injurious Agent (Energy) Factors | Environment Factors (Physical or Socioeconomic) |
|---|---|---|---|
| Preevent phase | Bone density, flexibility, balance and strength, comorbidity | Height of fall, contact with other surfaces/objects | Optimised medication, availability of care and supervision |
| Event phase | Protective measures, such as hip protectors | Energy absorbing flooring and surfaces | Room lighting, trip/slip hazards, handrails and supervision |
| Postevent phase | Severity of anatomical injury, physiological consequences and comorbidity | Alarm systems and supervision (to reduce long lie). | Access to emergency care. Policy on falls prevention and care |
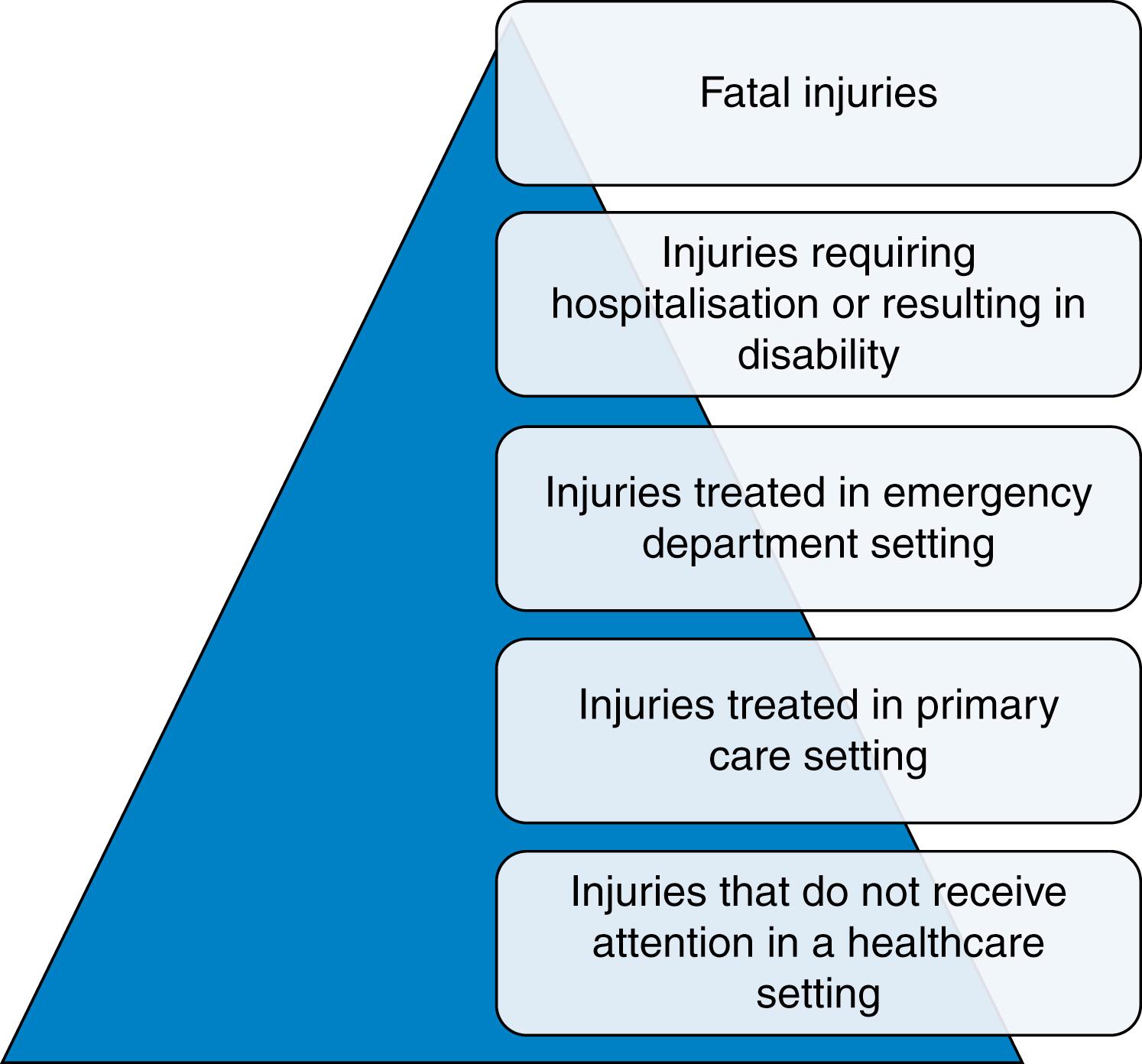
Injury severity is typically defined using anatomical scoring systems. The most widely used system is the Injury Severity Score (ISS). Application of the ISS methodology results in a score between 1 and 75—with the higher scores being associated with increased mortality and morbidity. By convention, severe injury is defined as an ISS score between 9 and 15, and major trauma as an ISS score of greater than 15. Using the ISS, the incidence of severe injury and major trauma in the United Kingdom is between 15 and 30 patients per 100,000 population per year. ISS is anatomically based and calculated retrospectively (when all injuries are known). The time-sensitive nature of injury and the limited knowledge about the extent of injury at first assessment means that the emergency services and trauma receiving hospitals must be able to quickly assess, triage, transport and treat a much larger number of patients (as illustrated in the injury pyramid) to ensure effective care for the most seriously injured.
A trauma system integrates injury prevention, prehospital care, emergency department (ED) care, acute hospital care, reconstruction, rehabilitation and reablement in a structured and organised way. The fundamental premise underpinning a trauma system is that patients with the most severe and complex injuries should be managed in designated Major Trauma Centres (MTCs) with access to the full range of specialist services and a significant caseload. There are two broad conceptual models for trauma system design. These ‘inclusive’ or ‘exclusive’ models differ, in the simplest terms, with respect to the extent to which all components of the emergency medical system (and wider healthcare system) are involved. In ‘exclusive’ systems, the focus is predominantly, or exclusively, on the MTC. The underpinning philosophy is that all patients with suspected major trauma within the area covered by the MTC are primarily transported there from the scene, bypassing all other facilities. Exclusive systems are ideally suited for urban areas with short prehospital journeys. In contrast, an ‘inclusive’ system is better suited to more dispersed populations where prehospital journeys may be much longer and primary transfer to the MTC is not necessarily a safe or publicly acceptable option. Arrangements must therefore be put in place to designate a range of smaller trauma receiving hospitals, often referred to as Trauma Units, who can undertake initial assessment, resuscitation and stabilisation before secondary transfer to an MTC where necessary. Regardless of system design, injured patients require rapid assessment and, in many cases, rapid interventions, to save life and reduce disability. In some case, prehospital interventions or care in a local hospital ED are essential before a patient can be moved to an MTC. In others, a patient’s only chance of survival might be rapid transport to the MTC, bypassing other hospitals. There is no single solution—each trauma system must be designed and tailored to the injury epidemiology of the population and managed to maintain effective care from point of injury through to rehabilitation.
A key component of any trauma system is early access to the emergency services. In most developed countries, a single national telephone activation number (112 or 999) connects callers to an Ambulance Service call handler who uses sophisticated telephone triage systems to identify the main problem, mobilise the right resources and provide prearrival care advice. Ambulance Services can deploy a range of healthcare professionals from paramedic practitioners through to physician-paramedic critical care teams and may use a range of land and air transport platforms to deploy personnel and move patients. The clinical priority for prehospital personnel is to search for immediate threats to life, initiate resuscitation as necessary and transport patients safely to hospital. In some cases, prehospital care can be very basic and rapid (e.g., a stable and alert stabbing victim in an urban area). In others, prehospital care may be particularly complex and take considerable time (e.g., the provision of prehospital anaesthesia to an unstable and trapped driver in a rural area). Time is important but the historical characterisation of prehospital personnel as undertaking either a ‘scoop and run’ or ‘stay and play’ approach to trauma care no longer applies. The key prehospital time is ‘time to meaningful intervention related to the effect of the injury’ rather than time to the nearest hospital. The challenge is to understand the nature of the meaningful intervention, provide the minimum necessary care to deliver that and then undertake safe transfer to the appropriate destination. In recognition of the challenges of prehospital care, the public are being encouraged to provide citizen aid, paramedics are becoming increasingly sophisticated healthcare practitioners and Prehospital Emergency Medicine has become a recognised medical subspecialty.
An overarching consideration for prehospital personnel is safety. Trauma patients are often in an uncontrolled, high-risk environment. Industrial accidents, falls from height, road traffic collisions, scenes of public disorder, fires, railway incidents and building collapses all have very specific hazards that place both the patient and the emergency services personnel at risk. Whatever the mechanism, it is essential for risks to be managed as far as possible. The priority in terms of patient care is to undertake an initial clinical assessment, often referred to as a primary assessment or primary survey , to identify and control any immediate threats to life. In contrast to the conventional medical model, where a detailed history leads to a focused clinical examination, trauma care cannot rely on a detailed history (or sometimes any history) and the rapid focused clinical examination takes priority. As with the initial approach to all critically ill or collapsed patients, this examination follows a standard structured sequence. The most widely used is the CACBCDE sequence ( Box 15.2 ). Prehospital personnel will undertake this primary assessment in a linear or stepwise approach—addressing specific life threats as they are identified. As more personnel become available, concurrent activity can take place both in terms of clinical care and in planning rescue (for those who require extrication) and the transport and destination hospital options.
| C | Immediate search for and control of catastrophic external bleeding with combinations, as necessary, of direct pressure, indirect pressure, wound packing, use of haemostatic dressings (i.e., dressings with procoagulant and/or mucoadhesive properties) and application of a tourniquet |
| A | Supporting the airway as necessary with manual positioning or manoeuvres, suction, basic airway adjuncts (nasopharyngeal and oropharyngeal airways), supraglottic airway devices and possibly direct laryngoscopy and tracheal intubation (with or without drugs) or surgical cricothyroidotomy |
| C | Controlling and restricting movement of the cervical spine until a deliberate decision regarding the risks and benefits of spinal immobilisation can be made |
| B | Assessment of the effectiveness of breathing, excluding or treating life-threatening thoracic injuries, such as tension pneumothorax, open pneumothorax, flail chest, and providing supplemental oxygen and/or noninvasive or invasive ventilatory support |
| C | Assessment of the circulation to identify physiological markers of shock, gaining access to the circulation and looking for signs of life-threatening injuries in the chest, abdomen, pelvis and limbs that could contribute to shock, controlling those that can be controlled (e.g., splinting the long bones and pelvis) |
| D | Assessment of neurological disability (Glasgow Coma Scale Score, pupil responses, limb weakness and lateralising neurological signs) to identify brain or spinal injuries and assist with decision making regarding the continued need for spinal immobilisation |
| E | Establishing a controlled safe environment , protecting the patient from extremes of heat or cold, exposing the patient to ensuring there are no missed or external injuries and establishing a brief history |
Resuscitative interventions should ideally be performed concurrently with the primary assessment—as CABCDE threats to life are identified, the relevant technical and therapeutic interventions can be undertaken. This is no different from the hospital setting although it is often extremely challenging, given constraints around physical access to the patient, environmental circumstances and availability of resources. Nonetheless, emergency services are able to undertake a wide range of prehospital medical interventions and doctors qualified as subspecialists in Prehospital Emergency Medicine can often be deployed to the incident scene with additional knowledge, skills and equipment.
Traumatic cardiac arrest is one circumstance where prehospital resuscitation may need to be very extensive. Regardless of mechanism, the reversible causes are hypoxia, hypovolaemia, tension pneumothorax and cardiac tamponade. Every effort is made to simultaneously control external bleeding, ventilate and oxygenate the patient, decompress the chest, splint the pelvis and any long bone fractures, and give appropriate fluids or blood if available. If the mechanism is penetrating trauma, then prehospital resuscitative thoracotomy may be necessary. If the mechanism is major pelvic and/or lower limb injury, then surgical control of the aorta, via thoracotomy or endovascular approaches, may be required. There is considerable complexity and risk associated with this level of resuscitation at the roadside and in-transit to hospital but it can be life saving. Most patients do not require such complex care but prehospital services must be prepared for the worst.
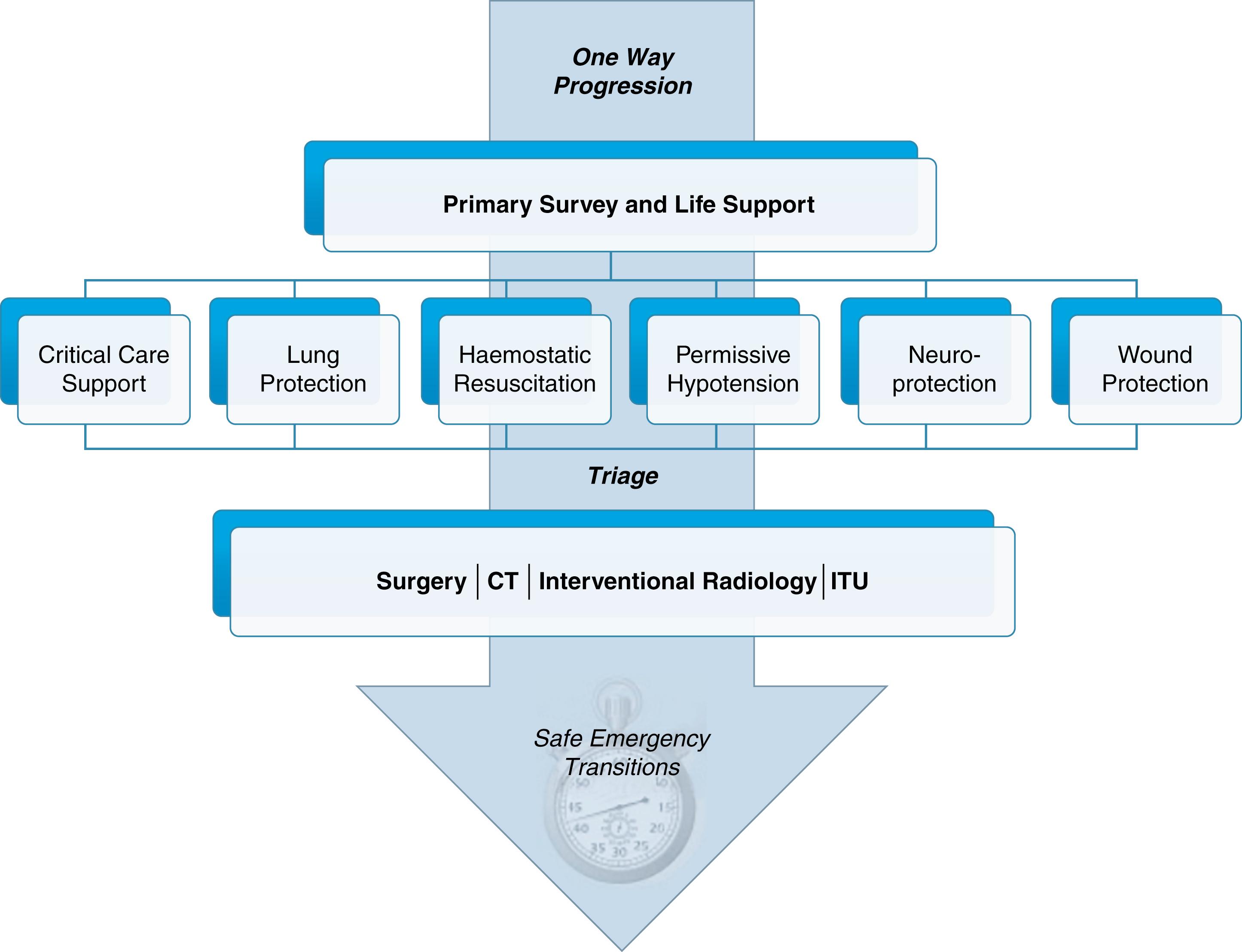
The concept of damage control is often applied to surgical interventions in hospital rather than in prehospital resuscitation. The idea is simple—do not undertake interventions that may exacerbate the effects of the injury either physically or physiologically. In surgical terms, this means limiting surgical intervention to that which is absolutely necessary and then focusing on optimising physiology. However, there are a number of nonsurgical damage control strategies that can be applied in the prehospital phase and that can reduce the risk of secondary injury from hypoxia, hypovolaemia, metabolic derangement and patient handling. Damage control resuscitation in hospital is discussed in more detail later—prehospital damage control strategies follow the same principles. Once primary assessment and resuscitation is underway, deliberate decisions should be made about which, if any, damage control strategy could be applied. The most common strategies include: permissive hypotension (see later), haemostatic resuscitation, wound-protection, neuroprotection and lung-protection ( Fig. 15.3 ).
Injured patients need to be transferred rapidly to an appropriate hospital. A key principle for safe transport is that the patient’s in-transit care should not be compromised. Once primary assessment, resuscitation and any damage control strategies have been completed to the point at which transfer is deemed safe, then it is important that good work at the scene is not undone by rough or careless handling, loss of inadequately secured tubes and lines or the physiological consequences of the transfer itself. A neuroprotective strategy might, for example, be compromised by allowing the patient to be conveyed in a head down posture (as often happens in helicopters) or subjecting them to rapid acceleration and deceleration forces during emergency driving. The destination hospital should be determined by preagreed trauma system and field triage criteria and the receiving hospital should be prealerted both the moment the emergency services identify that there is a seriously injured patient, who is likely to be transferred to them and when leaving the scene. The aim should be seamless transition from the scene to the receiving ED regardless of transport platform (helicopter or land ambulance).
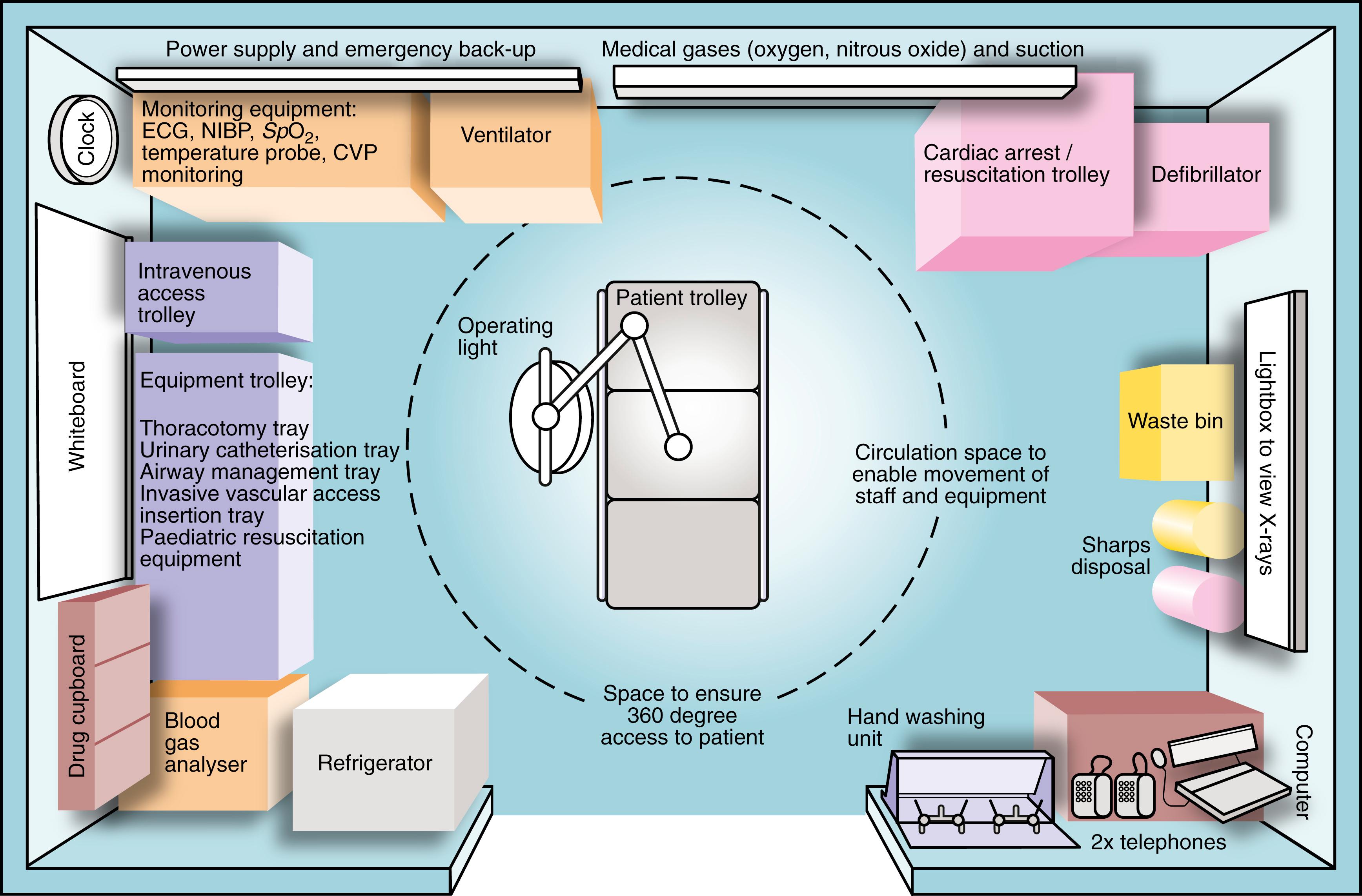
A key element of effective trauma care is preparation. Every ED should have an appropriate physical environment ( Fig. 15.4 ), a well-rehearsed process and prepared staff and equipment to manage an undifferentiated trauma patient arriving with short notice. If there is more than one patient, then predefined major incident plans should be available. Whether there are one or more patients, an early and comprehensive prealert to the receiving ED is essential. A common prealert system for a single patient is the ATMISTER system:
A ge
T ime
M echanism
I njuries
S igns
T reatment
E xpected time of arrival (ETA)
R equests (such as activation of massive blood loss protocols)
A common prealert system for a multiple casualty incident is the METHANE system:
M ajor incident declared
E xact location
T ype of incident
H azards present or suspected
A ccess—routes that are safe to use
N umber, type, severity of casualties
E mergency services present and those required
Success in managing patients with life-threatening multiple injuries depends on good preparation and organisation—as a system, department and hospital. Each ED should have protocol for managing prealert calls and mobilising the appropriate resources—both to attend the ED to receive patients and to prepare for transition of patients from the ED to imaging, operating theatres, critical care units and wards. Initial management often involves a trauma team response within the ED and concurrent activity between several disciplines throughout the initial phases of care ( Box 15.3 ). Training for the initial management of trauma patients has been standardised through Advanced Trauma Life Support (ATLS) and similar courses, now widely available throughout the world. The ATLS principles are simple—a systematic approach should be followed, using a common language and terminology, and the greatest threats to life must be treated first. Following these principles, the severity of physiological derangement is often used as the basis for triage systems that are applied when there is more than one patient to assess. Most systems differentiate ambulatory from nonambulant patients and then follow the principles of the primary assessment to identify patients with the greatest threat to life. Triage categories and their associated meaning are illustrated in Table 15.1 .
Team leader (senior doctor experienced in trauma care)
Airway manager (typically an anaesthetist)
Airway assistant (typically a trained Operating Department Practitioner or Nurse)
A primary assessment doctor (typically a surgeon or emergency physician)
A procedures doctor (typically a surgeon or emergency physician)
Two experienced ED nurses to support the team
A scribe to record contemporaneous notes related to team members, timings, history, clinical findings and any initial care decisions
Support staff (porters, healthcare assistants, radiographers, phlebotomists)
More comprehensive teams, especially in MTCs, include:
An emergency general surgeon
An orthopaedic surgeon
Specialist surgeons, such as vascular, cardiothoracic, plastics, maxillofacial, neurosurgical and plastics according to the prealert information and injuries
A radiologist (particularly when ultrasound may be required to help triage patients or where interventional radiology is being considered)
A transfusion practitioner (particularly when massive blood loss protocols have been activated)
Specialist obstetric or paediatric staff as necessary
| Category | Definition | Colour | Treatment |
|---|---|---|---|
| P1 | Life-threatening | Red | Immediate |
| P2 | Urgent | Yellow | Urgent |
| P3 | Minor | Green | Delayed |
| P4 | Dead | White/Black |
Special provisions are often made for obstetric and paediatric cases. Obstetric cases include all the elements of the trauma teams described in Box 15.3 with the addition of obstetric and neonatal expertise. Paediatric cases require additional medical paediatric, paediatric surgery, and paediatric anaesthetic or intensive care involvement.
As with the prehospital phase of care, the priority is to undertake an immediate CABCDE assessment to identify and control any immediate threats to life (see Box 15.2 ). The overarching priorities for the trauma team are:
Rapid primary assessment combined with damage control resuscitation
Prioritisation and initial treatment of identified injuries
Safe emergency transfers to computed tomography (CT), critical care and/or the operating theatre
Detailed history and secondary, head-to-toe, assessment
Consideration of the need for early transfer to an MTC or specialist unit
A team leader, often a surgeon or emergency physician, is required to coordinate the efforts of the trauma team and maintain tempo and control—particularly if there are parallel and competing priorities for care. Trauma team leaders require additional training to help them both apply the principles of effective team leadership and maintain their knowledge and skill related to the trauma care. The trauma team initial assessment (or reassessment) is often taught in a sequential manner and may be performed in a sequential manner when resources are limited. If there has been adequate preparation and prealert, they can often be undertaken in parallel by a team coordinated by the trauma team leader. Fig. 15.5 provides an outline of the components of the primary assessment in hospital.

On completion of the primary assessment, resuscitative interventions and initiation of any damage control strategy, further information should be gathered and a secondary assessment or survey should be undertaken. A useful mnemonic for gathering further history is AMPLE :
A llergies
M edicines and drugs currently taken
P ast medical and surgical history and possibility of pregnancy
L ast meal (including alcohol)
E vents leading to the injury event
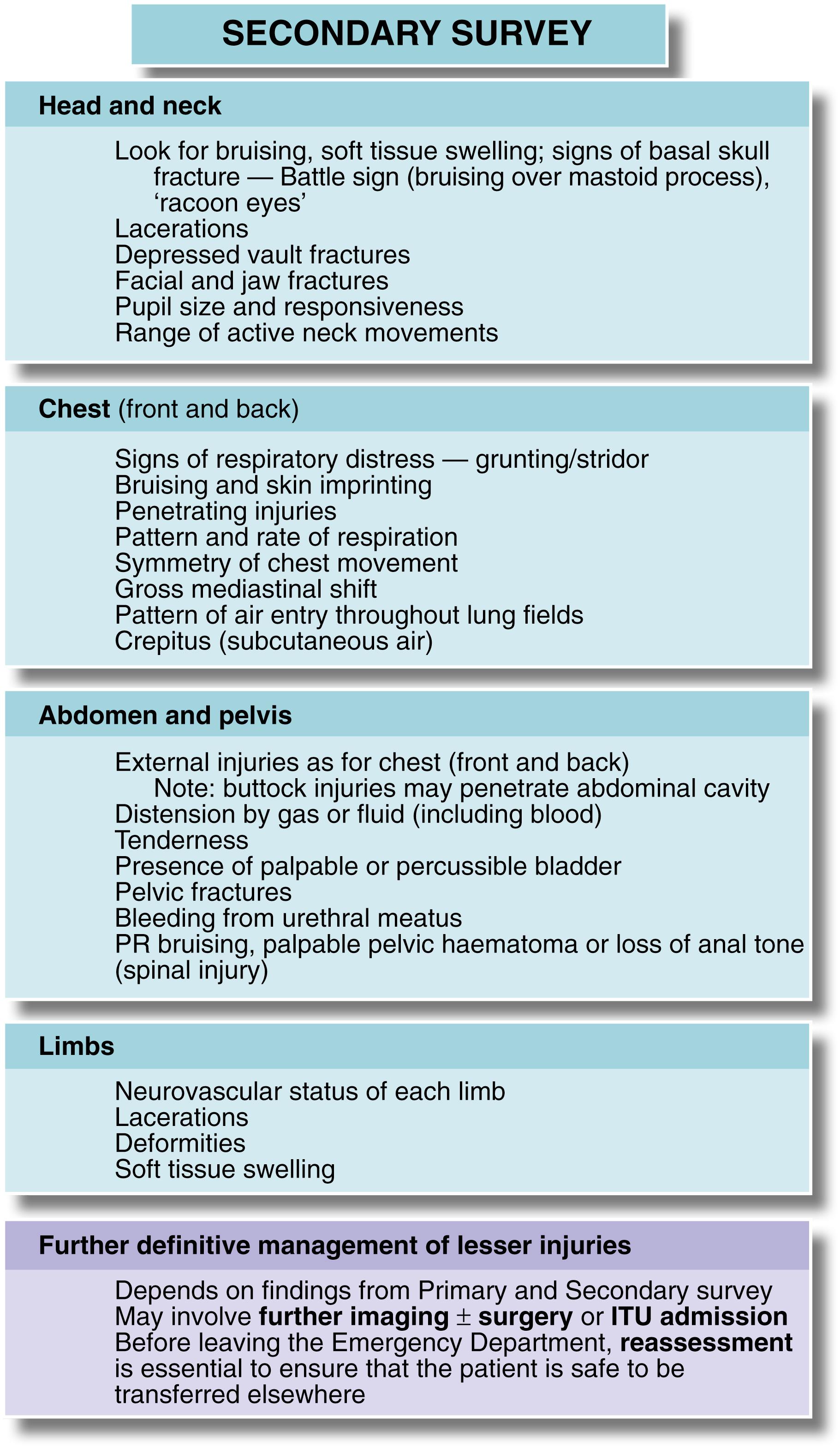
The secondary survey is a systematic head-to-toe physical examination looking for signs of physical injury in each body system and/or region. It may not be possible to progress to the secondary assessment in very unstable patients during initial care, but it is imperative that a full secondary survey is carried out as soon as possible after admission. Some examples of clinical features to be sought are given in Fig. 15.6 .
Early mortality following major trauma is predominantly associated with uncontrolled bleeding and three associated key pathophysiological changes termed the lethal triad : coagulopathy, hypothermia and acidosis. Later mortality is associated with organ failure and infection. Following primary assessment and immediate life support interventions, trauma team leaders must decide whether to use a damage control resuscitation strategy in an attempt to reduce early and late mortality and morbidity, and which particular strategy to follow to ensure ongoing organ and system support, optimise physiology and reduce risk of further tissue injury.
The concept of damage-control resuscitation, illustrated in Fig. 15.3 , acknowledges that no two trauma patients are the same and that separate injury types and physiological presentations may require very different approaches to resuscitation. A major challenge for the trauma team leader is to assimilate all the information from the primary assessment and determine whether one or more damage control strategies should be used. Some of the strategies may compete and risk further harm.
Critical care support refers to the early introduction of anaesthesia, positive pressure ventilation, invasive monitoring and use of vasoactive drugs. Almost all major trauma patients will require a period of critical care support, either in the resuscitation room, operating theatre or intensive care unit. Critical care support may also be essential to undertake other damage control strategies but there are some patients where less may be more, particularly in the early stages of resuscitation. The most striking example of this is the concept of permissive hypotension (see earlier). This strategy is not a therapeutic goal but a deliberate decision to allow the shocked patient with exsanguinating noncompressible haemorrhage (typically intraabdominal or intrathoracic) to remain shocked to prevent further bleeding. A permissive hypotensive strategy can be dangerous and is only ever utilised as a bridge to definitive surgical control of bleeding within a limited time window. Patients who remain shocked for over an hour may, for example, succumb. These patients would typically move to the operating theatre before induction of anaesthesia (similar to abdominal aortic aneurysm patients).
Haemostatic resuscitation refers to an approach that combines minimising bleeding (through meticulous attention to haemorrhage control and wound, fracture and patient handling), optimisation of coagulation (on the assumption that all trauma patients are at risk of coagulopathy and many are already coagulopathic on arrival), and early judicious replacement of volume with blood products (red cells plasma and platelets). Coagulation is supported with tranexamic acid and clotting factors whilst coagulopathy can be minimised by careful attention to the environment, avoidance of haemodilution and correction of metabolic derangement. In an emergency, universal donor packed red cells (group O, Rh negative) can be transfused but evidence from recent conflicts has demonstrated the advantages of early administration of whole blood (or combined blood products) in reducing the risk of haemodilution and coagulopathy. EDs are increasingly using massive blood loss or massive transfusion protocols, which include several units of, packed red cells, fresh frozen plasma and platelets. These may be transfused in a 1:1:1 ratio or in a goal-directed manner, guided by clinical response, coagulation testing and thromboelastography.
A lung protective strategy recognises that injured lungs, through contusion, laceration and aspiration combined with mechanical chest wall injury need support but that all invasive ventilation is harmful. Ventilation may cause further structural injury to the alveolar–capillary unit through hyperoxia, volutrauma (overdistention) and barotrauma (overpressure). Lung protective ventilation (low tidal volume, low minute volume, lower mean airway pressures) combined with thoracic decompression (drainage of haemothorax and extraalveolar gas) may reduce ventilator associated lung injury. However, a lung protective ventilation strategy may be associated with permissive hypercapnia that may be harmful in traumatic brain injury.
A neuroprotective strategy recognises that although the primary neurological injury has already occurred, there is scope to reduce the extension of injury to surrounding neurological tissues by optimising perfusion. This is achieved by reducing cerebral metabolic demand (anaesthesia) and optimising cerebral blood flow through a combination of controlling ventilation, improving cerebral venous drainage and maintaining an optimum mean arterial pressure (see Ch. 16 ). Clearly, a permissive hypotensive strategy and a neuroprotective strategy are directly competing—the patient with traumatic brain injury and a ruptured spleen who is exsanguinating must receive a balanced approach. Wound protective strategies are discussed in Chapter 17 .
In major trauma, there is now consensus that adults should undergo whole body CT or ‘trauma’ CT a soon as possible after completion of the primary survey. In some systems, patients are now received directly into hybrid imaging suites/resuscitation room/operating theatres and undergo whole body imaging within a few minutes of arrival. Trauma CT involves CT of the head and neck without contrast, followed by CT of the chest, abdomen and pelvis with contrast. Historical characterisation of the CT scanner as the ‘doughnut of death’ related to incidents where CT scanners were remote from the resuscitation room, patients were not sufficiently stable or supervised and the scanning itself took a long time. Current protocols involve the whole trauma team moving with the patient to an adjacent or colocated CT suite, continuing resuscitation throughout scanning and achieving diagnostic images within a few minutes that permit prioritisation of emergency interventions.
If a patient is considered too unstable to undergo CT, then there is still a role for ultrasound and chest and pelvic x-rays in the resuscitation room. Focused Assessment with Sonography for Trauma (FAST) reliably detects free intraabdominal fluid and concentrates on five areas, the ‘five Ps’—Perihepatic (hepatorenal space or Morison pouch), Perisplenic (splenorenal recess) and Pelvic (inferior portion of the peritoneal cavity and pouch of Douglas) in the abdomen, and Pleural and Pericardial in the chest. It is, of course, user-dependent. Chest x-rays, typically anteroposterior (AP) supine projections, can assist in confirmation of tube and line placement, identification of pneumothorax, pulmonary contusion, lung collapse and haemothorax. Pelvic x-rays can identify major pelvic disruption.
Abdominal and thoracic injuries often coexist, so it is logical to think in terms of torso trauma . Major torso injuries are a common cause of death at the scene, for example from avulsion of the thoracic aorta, cardiac injury or massive liver injury. Immediate diagnosis and urgent laparotomy or thoracotomy offers almost the only hope of survival, but the injury is often too extensive or time too short to intervene.
The site and signs of external injury provide clues to internal injuries. This is obvious with penetrating injuries but is also true of blunt injuries. For example, trauma to the left upper abdominal quadrant or lower ribs is often coupled with splenic rupture ; similarly for the liver with right-sided injuries. Lower abdominal injuries may injure the bladder , and loin trauma the kidney . Central anterior chest trauma can damage the heart whilst clavicular area injury may traumatise the brachial plexus or subclavian blood vessels .
Abdominal injuries are less common than head and chest injuries and mortality can be low with prompt and appropriate management. When death occurs, it is usually from massive haemorrhage arising from bursting of liver or spleen or from penetration of major arteries or veins, particularly with gunshot wounds. Note that unrecognised injuries are a significant avoidable cause of death.
Areas of the abdomen other than the main peritoneal cavity may be wounded; pelvic viscera lie within a bony cage but extend low enough to be injured by buttock or perineal wounds. Similarly, retroperitoneal viscera may seem protected but are vulnerable to flank or back wounds, or to deep anterior stab wounds or any gunshot wounds. This area is not easily palpated and diagnosis usually requires CT.
Overall, 20% of patients with closed abdominal trauma require operation. In penetrating injuries, 30% with stab wounds require operation and close to 100% of those with gunshot wounds.
Clinical diagnosis is unreliable in blunt injuries because overt signs of bleeding or hollow viscus perforation may not develop until hours after injury. If the patient is stable but the injury involved high-energy transfer or other significant injuries are present, early CT scanning should be performed.
If surgical intervention is not needed, regular nursing observations and serial clinical examination should be performed for signs of peritonitis or intraabdominal bleeding. Note that significant injuries almost always become manifest within 24 hours.
Stab wounds may or may not penetrate the peritoneal cavity. They often cause little damage unless the blade penetrates the retroperitoneal area and injures great vessels or pancreas. It used to be thought that all abdominal stab wounds required surgical exploration but current policy in most cases is more conservative management. A large series from Baragwanath Hospital, Soweto, South Africa, demonstrated that 70% of patients or more, could safely be managed by observation in hospital for 24 hours, and operated upon only if there were signs of deterioration. However, haemodynamically unstable patients and those with extensive or potentially contaminated penetrating wounds must be explored surgically without delay.
For most patients, the first step is to determine whether the peritoneum has been breached, by exploring the wound under local anaesthesia. If it has, then laparoscopy, ultrasonography or CT scanning can be used to explore intraabdominal viscera. If the peritoneum is intact and/or imaging is negative (as in most cases), conservative management with careful monitoring is appropriate. However, about one third who later proved to have significant injury were initially free of signs, emphasising the need for repeated clinical and radiographic reassessment.
The severity of internal injury depends on the path and mass of the missile and especially on its velocity. Low-velocity wounds (e.g., hand-gun bullets) cause damage confined to the wound track, whereas high-velocity (i.e., rifle) bullet wounds injure widely and deeply. This is because the much higher kinetic energy is dissipated in the tissues. In addition, cavitation is caused and debris is sucked into the wounds, causing contamination with clothing and soil. If a bullet hits bone, secondary missiles cause further injury. The size of the entry wound is often small because of elastic recoil of the skin and is no guide to the extent of injury. Given the unpredictable extent of injuries, all gunshot wounds must be surgically explored to check for visceral organ, intestinal and vascular damage. Buttock wounds may penetrate the pelvic cavity and should be treated in the same way.
Closed abdominal injuries usually result from road traffic collisions, falls, sporting contact injuries and accidents involving horses. Following substantial blunt injury, about 20% will require laparotomy. The spleen is the most vulnerable organ, especially in left-sided injuries ( Fig. 15.7 ). Liver injury requires greater impact force, usually from the front or right side. Pancreatic and duodenal injuries are uncommon and usually result from a heavy central abdominal impact, transecting the pancreas or retroperitoneal duodenum across the vertebral bodies. This most commonly occurs in children falling across the handlebars of bicycles. The kidneys are vulnerable to punches or kicks in the loins.
Bowel is damaged by rapid deceleration or crushing, and is particularly vulnerable at sites where freely mobile bowel becomes attached to the retroperitoneum, that is, at each end of the transverse colon, at the duodenojejunal flexure and in the ileocaecal area. A full bladder , common after a bout of heavy drinking, may rupture into the peritoneal cavity (or sometimes retroperitoneally) after abdominal impact. The bladder and urethra are also liable to be torn in displaced pelvic fractures. The clinical features and investigation of closed abdominal injuries are shown in Box 15.4 .
History
Substantial trauma to the abdomen or lower chest
Seatbelt not worn in road traffic collision (especially driver impacting steering wheel)
Abdominal pain after trauma
Haematuria, particularly following trauma to the back or loin
Physical signs
Skin bruising immediately after injury—suggests sufficient force to cause internal damage
Imprinting of cloth pattern on skin ( cloth printing )—caused by compression of skin against vertebral bodies; implies high energy transfer impact
Unexplained hypotension—suggests concealed haemorrhage into abdominal cavity or elsewhere
Abdominal distension, that is, increasing abdominal girth—from accumulating blood, urine or gas in the peritoneal cavity
Increasing abdominal tenderness, guarding and rigidity (difficult to assess if abdominal wall bruising)—possible intestinal perforation or intraabdominal bleeding
Lateral lower rib fractures—injury to spleen, liver or kidney
Pelvic fractures, especially ‘butterfly’ fractures of all four pubic rami—often bladder or urethral injury (especially in males) and pelvic vein injury
Inability to pass urine and blood at urethral meatus and/or perineal bruising—imply rupture of urethra, usually at pelvic diaphragm, that is, postmembranous urethra (avoid urethral catheterisation in favour of suprapubic); rectal examination may reveal ‘high-riding’ prostate
Damage to anus or rectum may be palpable on rectal examination; presence of blood suggests anorectal injury. If anal sphincter tone low, suggests neurological damage from spinal injury
Investigation
Raised plasma amylase suggests pancreatic injury needing CT scanning
Chest and plain abdominal x-rays (supine and erect or lateral decubitus) for free intraperitoneal or retroperitoneal gas, rib or pelvic fractures associated with specific visceral injuries and radiopaque missiles, such as bullets, shotgun pellets and glass
Ultrasound and CT scanning—particularly useful for solid organs, that is, spleen ( Fig. 15.7 ), liver, kidneys, pancreas. Intravenous contrast CT useful for large vessel injuries
Urethrography—for suspected urethral rupture
Laparoscopy—increasingly important in closed abdominal trauma in stable patients. Can be performed under local anaesthesia
The spleen is the most commonly injured organ in blunt abdominal trauma. The organ should be preserved wherever possible
because of the dangers of postsplenectomy infection and sepsis. In one study, 2.4% of all postsplenectomy patients suffered sepsis and more than 50% of those were fatal.
CT scanning enables accurate assessment and classification of the extent of injury. Haematomas and capsular tears not extending deeply can often be managed conservatively. More severe injuries are treated by urgent laparotomy and where possible, splenic repair (splenorrhaphy) by direct suture, fibrin glue or absorbable mesh bags. Segmental resection or splenic artery ligation can be done, but 50% of splenic substance must be preserved for useful function. Whenever splenic preserving techniques are used, a period of careful observation for up to 10 days is required as catastrophic secondary haemorrhage can occur.
Isolated small liver injuries may be treated by surgical repair or local resection but paradoxically, major injuries are often best treated conservatively. This is because control of deep hepatic vessels may prove impossible, particularly bleeding from hepatic veins entering the inferior vena cava (IVC). Patients with severe liver injuries should be discussed with a regional hepatopancreaticobiliary (HPB) or liver unit. Conservative management involves large-volume blood transfusions until abdominal tamponade stops the bleeding. If operation is performed and a major liver injury is found and haemorrhage cannot be arrested, the liver should be packed with large pieces of surgical gauze, the abdomen closed and the patient stabilised. Ideally the patient should be transferred to an HPB centre, as extensive liver surgery may be necessary when the packs are removed at a ‘second look’ laparotomy 24 to 48 hours later.
Pancreatic transection is treated by surgically removing the distal part and oversewing the stump. A crushing pancreatic injury may have to be treated with drainage alone. Renal injuries are usually managed conservatively unless nephrectomy is required for uncontrollable bleeding.
Injuries to small bowel are dealt with by simple suture or, if mesenteric vascular supply is impaired, by resection and anastomosis. Conventional treatment for right-sided colon injuries is resection and anastomosis of colon to ileum. Localised injuries to other parts of the colon without substantial faecal contamination can usually be resected and joined end to end. After knife or gunshot wounds, simple repair gives good results if there is minimal peritoneal contamination. Extensive injuries with contamination require exteriorisation of the damaged bowel ends to the abdominal wall (see Ch. 27 ).
High-velocity penetrating injuries wreak havoc on the gut, causing devascularisation and multiple perforations. All necrotic or ischaemic tissue must be excised. The immediate dangers are peritonitis and systemic sepsis from contamination. Exteriorisation of viable bowel ends is mandatory and planned reexploration usual.
Intraperitoneal rupture of the bladder is treated by laparotomy and suturing, with a urethral catheter left in situ for 5 to 7 days. Extraperitoneal bladder rupture is treated conservatively, with prolonged urethral or suprapubic catheterisation. Urethral tears require specialist urological management. If the urethral wall is partly intact on urethrography, it can be treated, at least initially, by suprapubic catheterisation. Complete urethral avulsion injuries are usually treated by suprapubic catheterisation, with formal repair after inflammation has settled.
Major trauma patients sometimes have extensive and complex intraabdominal injuries. In an unstable patient, prolonged surgery to manage these definitively may exacerbate the lethal triad of coagulopathy, hypothermia and acidosis. Damage control laparotomy can be used here as a life-saving procedure that should be completed within an hour. Temporary clamping, then packing or ligating vessels controls haemorrhage. Hollow viscus injuries are stapled or resected without anastomosis. The abdomen is temporarily closed, or sometimes left open, and arrangements made for definitive surgery in 24 to 48 hours following resuscitation.
The types of chest injury, their clinical features and their treatment are summarised in Table 15.2 .
| Nature of the Injury | Clinical Features | Treatment |
|---|---|---|
| Sternal fracture | Anterior chest pain and tenderness; ‘clicking’ on palpation; arrhythmia and ECG changes | Consider cardiac contusion or tamponade; FAST scan; 24-h ECG; cardiac enzymes |
| Rib fractures | Localised pain on respiration or coughing; tenderness over fractures; usually visible on chest x-ray | Analgesia, intercostal blocks, physiotherapy, prophylactic antibiotics in chronic bronchitis |
| Flail chest , that is, multiple rib fractures producing a mobile segment | Respiratory embarrassment; ‘paradoxical’ indrawing of the flail segment on inspiration | Intercostal block analgesia; endotracheal intubation and ventilation if hypoxic |
| Pneumothorax , that is, air in pleural cavity causing lung collapse | Unilateral signs: loss of chest movement and breath sounds, percussion note resonant; sometimes chest wall emphysema; confirmed by chest x-ray | Intercostal drain with underwater seal |
| Sucking chest wound , that is, open pneumothorax with mediastinum ‘flapping’ from side to side with each respiration | Gross respiratory embarrassment, audible sucking of air through chest wound | Sealing of chest wound with impermeable dressing; intercostal drainage |
| Tension pneumothorax , that is, expanding pneumothorax causing progressive mediastinal shift to the opposite side and tracheal deviation | Signs of pneumothorax with disproportionate and increasing respiratory distress and hypoxaemia | Urgent chest drainage |
| Lung contusion | Deteriorating respiratory function; opacification of affected lung field on chest x-ray | Oxygenation, physiotherapy, mechanical ventilation if severe |
| Rupture of bronchus (uncommon) | Respiratory distress, surgical emphysema in the neck; suggested by air in mediastinum on chest x-ray (see Fig. 15.9A ); confirmed by bronchoscopy | Operation by thoracic trained surgeon |
| Rupture of oesophagus (very rare) | May have surgical emphysema in neck and pneumomediastinum on chest x-ray but diagnosis often missed until mediastinitis or empyema develops | Surgical repair if recognised early; surgical drainage and diversion if late |
| Haemothorax , that is, blood in the pleural cavity Usually arises from chest wall injury—rib fracture lung parenchyma or minor venous injury. Most are self-limiting. Arterial injuries less common and likely to need thoracotomy |
Dull percussion note, breath sounds absent, tachycardia and hypotension caused by blood loss | Most have stopped bleeding by the time of examination and only tube drainage is required Dark, venous bleeding more likely to cease spontaneously than bright arterial bleeding Tube must be large enough to drain without clotting, ideally 32F–36F; placed in sixth intercostal space in midaxillary line If patient haemodynamically stable, admit and observe. If continuing drainage of 200 mL+ per hour over 4 h, should undergo thoracotomy |
| Cardiac tamponade , that is, bleeding into pericardial cavity (usually penetrating trauma) | Hypotension, inaudible heart sounds, distended neck veins with systolic waves; enlarged, rounded heart shadow on chest x-ray; confirmed with ultrasound | Long needle aspiration via epigastric approach; operation if tamponade recurs |
| Cardiac contusion | Often arrhythmia or ECG changes similar to myocardial infarction | Conservative management |
| Rupture of aorta (usually from deceleration injury)—fatal unless false aneurysm develops in mediastinum | Back pain, hypotension; systolic murmur or signs of tamponade in some cases; characteristic widening of mediastinum on chest x-ray; diagnosis confirmed by arteriography | Urgent thoracotomy and Dacron graft or minimal-access stent graft if available |
| Rupture of diaphragm , linear split usually in left diaphragm with herniation of gut into chest (penetrating or abdominal crush injury) | Respiratory distress, bowel sounds heard in the chest; diagnosis by chest x-ray and confirmed by barium meal; many cases only discovered much later; diagnosis may be made by laparoscopy or at laparotomy | Repair of diaphragm, usually via abdominal approach |
Chest injuries are common in patients with multiple injuries. They are also common in road traffic collisions where, in some cases, the extent of the energy transfer to the chest can be revealed by seatbelt injury (see Fig. 15.8 ). Injuries that may pose an immediate life threat and which should be sought in the primary assessment include significantl tension pneumothorax, massive haemothorax, open pneumothorax, and tracheal or bronchial injuries. Signs of more subtle, but serious thoracic injuries should be sought in the secondary assessment. These include simple pneumothorax, simple haemothorax, fractured ribs, flail chest and pulmonary contusion.
Serious chest injuries may be present without external injury, particularly tearing of mediastinal contents (aorta, bronchi and oesophagus). Early imaging, typically with trauma CT, is required to exclude these injuries (see Fig. 15.9 ).
Mechanisms of chest injury include penetrating trauma, blunt impact and crushing injuries, deceleration injuries and rupture of the diaphragm caused by abdominal compression. Less than 10% of chest injuries require surgical intervention but early recognition of these may be life saving.
Following trauma, chest drains are usually placed in the fourth or fifth intercostal space on the midaxillary line. The technique is shown in Fig. 31.6 , p. 426. Bilateral open thoracostomy is often used to decompress the chest in emergency care in ventilated patients as a precursor to formal chest drain insertion.
Veins and arteries may be damaged by penetrating or blunt trauma. Gunshot wounds are more likely to damage vessels than stab wounds, and blunt injuries can damage arterial walls causing occlusion. Iatrogenical injuries are becoming common with the rise in radiological and minimal access procedures, and hip replacements. Damaged vessels bleed or impair distal circulation, or both.
Bleeding may be revealed (visible), or concealed and the rate of loss determines the presentation and risk of death. Concealed haemorrhage often occurs in the chest, abdomen or pelvis or in limb muscles with fractures; blood from facial fractures may be swallowed and unrecognised.
Trauma that interrupts arterial flow to a limb or organ causes ischaemia, leading to potential limb or organ loss, stroke, bowel necrosis, and consequent multiple organ dysfunction. Skeletal muscle can survive ischaemia for 3 to 6 hours and still recover but peripheral nerves are sensitive and deficits can result from brief ischaemia.
If arterial supply is restored after delay, the release of inflammatory mediators, lactic acid and potassium into the circulation can cause reperfusion syndrome . In limbs, this can lead to compartment syndrome, and can also initiate a systemic inflammatory response with myocardial and other organ dysfunction.
Laceration is the most common. Completely severed arteries contract, limiting haemorrhage, but partially transected arteries continue bleeding. Veins are unlikely to retract.
Blunt trauma causes crushing, stretching or shearing injuries to vessels. Intimal flaps can lead to thrombosis or dissection. Thrombosis may propagate down the vessel or embolise distally. Arterial ‘spasm’ alone does not cause limb ischaemia—the cause is thrombosis or vessel wall damage and requires urgent investigation.
An arterial puncture (e.g., femoral artery catheterisation or a stab wound) may cause bleeding that is enclosed by connective tissue, forming a pulsatile mass of clot known as a false aneurysm. This often presents days or weeks later. Distal flow is usually conserved and diagnosis can be confirmed by duplex Doppler ultrasound. First-line treatment is usually ultrasound-guided compression to cause thrombosis of the leak. If this fails, or for larger defects, suturing or patching may be needed.
An injury to an artery and an adjacent vein can cause an arteriovenous (AV) fistula, which may eventually rupture or lead to cardiovascular compromise. These present some days or weeks after the injury. In a limb, the patient complains of a swelling with dilated superficial veins. On examination, there is a machinery-type murmur heard and diagnosis is confirmed by angiography. Treatment is by dividing the fistula and repairing the vein and artery, sometimes with a flap of fascia interposed.
Visible bleeding from a traumatic wound with signs of hypovolaemia makes the diagnosis obvious. In limbs, palpable distal pulses are the most reliable sign of intact distal circulation. Hand-held Doppler probes can be misleading in detecting distal pulses and in ankle pressure measurement.
The following ‘hard’ signs of vascular injury indicate a need for urgent operative intervention, often without prior investigation.
Audible bruit or palpable thrill
Active, especially pulsatile haemorrhage
An expanding haematoma
Distal ischaemia (cold, pale, pulseless limb)
Patients with the following ‘soft’ signs of vascular injury do not require urgent investigation or exploration; they should be admitted, investigated as needed and observed over 24 hours.
Haematoma
History of haemorrhage at the accident scene
Unexplained hypotension
Peripheral nerve deficit
Reduced but definitely palpable pulse
Injury site near a major artery
In expert hands, Duplex ultrasound can detect intimal tears, thrombosis, false aneurysms and AV fistulae, but angiography by direct puncture, CT angiography or on the operating table remains the gold standard for investigation and mapping of vascular injury. Arteriography may be needed to show the extent of injury in a stable patient with equivocal signs; to exclude injury where there are no hard signs but suspicion of vascular injury; in limb fractures with absent pulses; in injury by high-velocity missiles or multiple fragment injuries; and in blunt trauma. Angiography may be used to treat certain injuries by embolisation or stenting where expertise is available.
The priorities in managing vascular injury are arrest of life-threatening haemorrhage and restoration of normal circulation. Temporary bleeding control is usually achievable by pressure over the site of injury.
Direct exploration of an actively bleeding wound is usually inadvisable because of poor visibility and the technical difficulty of achieving control. Far better to obtain proximal control, if necessary via a separate incision, isolating and clamping the artery before exploring the wound. Sometimes the artery distal to the wound needs clamping remotely too.
With proximal and distal clamps in situ, the wound is explored, debrided and the extent of arterial and venous damage assessed. In general, large veins should be repaired first to allow drainage as soon as the artery is repaired. A cut or lacerated artery may be amenable to direct repair or to patching, if direct suturing would narrow the lumen. More extensive damage may require an interposition graft, usually autologous long saphenous vein, or sometimes synthetic graft material. Before repair, proximal then distal clamps are released in turn to check for adequate blood flow. If inadequate, a Fogarty balloon catheter is passed to extract thrombus and heparinised saline instilled.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here