Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The incidence of otitis media (OM) is highest in the first years of life and declines as children grow older and the functions of the immune system and eustachian tube mature.
The cause of OM is multifactorial; risk factors are genetic, social, and environmental.
The presence of middle ear effusion (MEE) is an important prerequisite for diagnosing acute OM (AOM) and OM with effusion (OME). AOM is an acute infection with distinct bulging of the tympanic membrane (TM) that is often accompanied by rapid onset of signs and symptoms that may include fever, otalgia, and TM erythema. In OME these symptoms may be absent, and hearing loss caused by MEE is the most prominent symptom.
Diagnostic modalities for OM include (pneumatic) otoscopy, otomicroscopy, tympanometry, and audiometry.
Symptomatic management of otalgia and fever is the cornerstone of AOM treatment, with immediate antibiotics indicated for children with severe or persistent infections, and with observation with close monitoring (watchful waiting) with delayed antibiotics (if needed) for milder infections.
Topical nasal or oral decongestants, antihistamines, and corticosteroids are ineffective for AOM and OME and therefore not recommended for treatment.
Management of OME usually starts with observation with close monitoring, with tympanostomy tubes indicated primarily for children with persistent MEE and hearing loss, speech and language delay, or learning difficulties.
Adenoidectomy is considered in children aged 4 years or older with recurrent OME or AOM and in children of any age with OM and nasal symptoms.
Topical antibiotics are the recommended treatment for tympanostomy tube–associated otorrhea.
Otitis media (OM) or inflammation of the middle ear is among the most common diseases in young children. Despite reports of a declining incidence over time, OM continues to be a leading cause for medical visits, antibiotic prescribing, and surgery worldwide. OM can present as acute otitis media (AOM), otitis media with effusion (OME), and chronic suppurative otitis media (CSOM) ( Table 15.1 ). In this chapter, we will focus on AOM and OME.
| Preferred Term | Definition | Comment |
|---|---|---|
| Otitis media (OM) | Inflammation of the middle ear without reference to etiology or pathogenesis | Nonspecific umbrella term for any condition associated with middle ear inflammation |
| Acute otitis media (AOM) | Rapid onset of signs and symptoms of inflammation in the middle ear | Diagnosed when there is moderate to severe bulging of the eardrum; mild bulging of the eardrum and recent (<48 hr) onset of ear pain or intense erythema of the eardrum; or acute ear discharge unrelated to otitis externa (inflammation of the external ear canal) ∗ |
| Recurrent AOM (rAOM) | ≥3 well-documented and separate AOM episodes in the preceding 6 months or ≥4 episodes in the preceding 12 months with >1 episode in the last 6 months | Children without persistent MEE tend to have a good prognosis and often improve spontaneously; children with persistent MEE have a poorer prognosis and might benefit from ventilation tubes |
| Otitis media with effusion (OME) | Fluid in the middle ear without signs or symptoms of acute ear infection | Diagnosed by one or more of the following: reduced eardrum mobility on pneumatic otoscopy, reduced eardrum mobility on tympanometry, opaque eardrum, or a visible air-fluid interface behind the eardrum on otoscopy |
| Chronic OME | OME persisting for ≥3 months from date of onset (if known) or from date of diagnosis (if onset is unknown) | Chronic OME has much lower rates of spontaneous resolution compared with OME of new onset or after an episode of AOM |
| Chronic suppurative otitis media (CSOM) | Chronic inflammation of the middle ear and mastoid mucosa with a nonintact eardrum (perforation or ventilation tube) and persistent ear discharge | No consensus on duration of ear discharge needed for diagnosis, with recommendations ranging from 2 weeks to at least 3 months |
| Middle ear effusion (MEE) | Fluid in the middle ear from any cause | MEE is present with both OME and AOM and might persist for weeks or months after the signs and symptoms of AOM resolve |
∗ The degree of bulging does not reflect AOM severity. Severe AOM is defined as having moderate-to-severe ear pain, ear pain for at least 48 hours, or temperature 39°C or higher.
The presence of fluid in the middle ear, also called middle ear effusion (MEE), is an important prerequisite for diagnosing AOM and OME. The middle ear space is normally air-filled, but MEE may develop secondary to infection, inflammation, or eustachian tube dysfunction. Compared with adults, the eustachian tube in young children is short, floppy, and horizontal, which can lead to underventilation of the middle ear space and contamination from reflux of nasopharyngeal secretions.
AOM is an acute infection characterized by distinct bulging of the tympanic membrane (TM) that is often accompanied by rapid onset of signs and symptoms of middle ear inflammation. Ear-specific symptoms such as otalgia and nonspecific symptoms including fever, irritability, and sleep disturbance obtained by history ( Table 15.2 ) can raise suspicion for AOM but are insufficient for accurate diagnosis because symptoms may be subtle (e.g., tugging, rubbing, or holding the ear) or absent. Typical signs of AOM seen on otoscopy include bulging or fullness of the TM ( Fig. 15.1 ), TM erythema, and acute onset of otorrhea exclusive of otitis externa or CSOM (see Table 15.2 ).
| Modality | Description | Comment |
|---|---|---|
| Signs and symptoms (obtained by history) | Includes ear-specific symptoms (ear pain, hearing loss), nonspecific symptoms (nausea, irritability, sleep disturbance, anorexia) and signs (fever, vomiting) | The hallmark of AOM and OME are ear pain and hearing loss, respectively, but signs and symptoms alone have poor diagnostic accuracy |
| Symptom severity scales | Parent-reported measures of AOM severity using categorical responses or a faces scale | Not useful for AOM diagnosis, but can be used to rate severity, follow the course of disease, and assess outcomes |
| Otoscopy | Visual examination of the ear canal and tympanic membrane with an otoscope | Bulging tympanic membrane is characteristic of AOM; opaque or cloudy tympanic membrane is characteristic of OME |
| Pneumatic otoscopy | Examination of the middle ear using an otoscope to create an airtight (hermetic) seal in the ear canal and then gently squeezing (or releasing) the attached rubber bulb to change the pressure in the ear canal and observe the tympanic membrane | A normal tympanic membrane moves briskly with applied pressure, but the movement is minimal or sluggish when there is fluid in the middle ear; no motion is observed if tympanic membrane is not intact |
| Otomicroscopy | Examination of the ear canal and tympanic membrane using the binocular, otologic microscope to obtain a magnified view with good depth perception | Primary use is to assess tympanic membrane abnormalities (atrophy, sclerosis, retraction pockets) and to help distinguish surface findings from middle ear pathology |
| Tympanometry | An objective measure of middle ear function that requires an airtight seal in the ear canal Tympanometry provides a graph showing how energy admitted to the ear canal is reflected back to an internal microphone while the canal pressure is varied from negative to positive (pressure admittance function) and can be performed with a portable (handheld) unit or a desktop machine |
If the middle ear is filled with fluid, tympanic membrane vibration is impaired and the result is a flat, or nearly flat, tracing If the middle ear is filled with air but at a higher or lower pressure than the surrounding atmosphere, the peak on the graph will be shifted in position based on the pressure (to the left if negative, to the right if positive) |
| Acoustic reflectometry | Uses a transducer and microphone at the entrance of the ear canal, without an airtight seal, to measure how much sound is reflected off the tympanic membrane | Higher reflectivity levels indicate a greater probability of effusion, but unlike tympanometry it only assesses the probability of effusion and cannot measure middle ear function |
| Computed tomography | An imaging procedure, using ionizing radiation, to create a detailed scan of the temporal bone | Useful in surgical planning for CSOM but not useful for primary diagnosis of AOM, OME or CSOM |
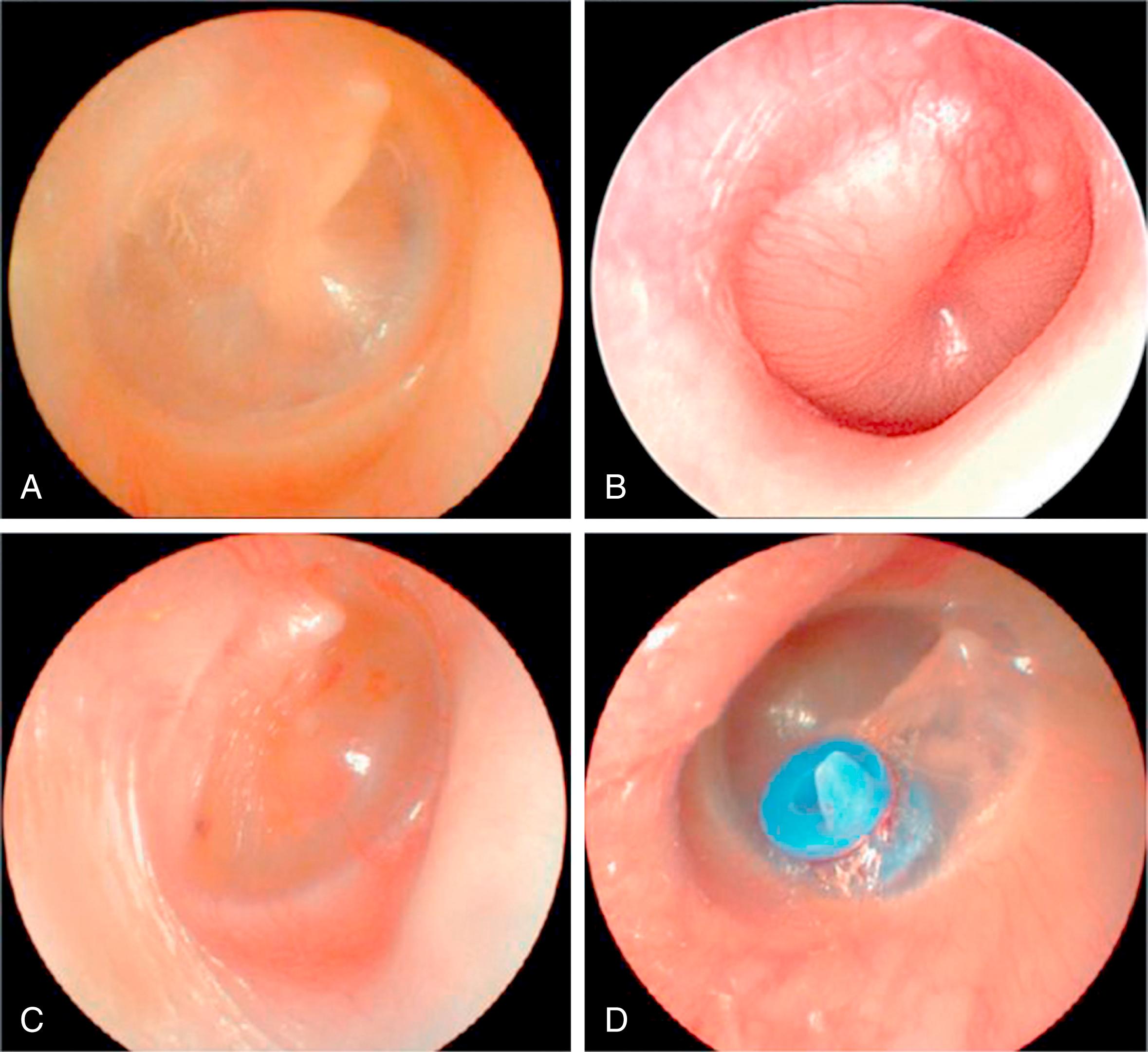
OME is similar to AOM in that MEE is also present, but unlike AOM there are no signs or symptoms of an acute infection. The most prominent symptom of OME is conductive hearing loss caused by impaired transduction of sound waves in the middle ear caused by MEE. Hearing difficulties may be especially pronounced in challenging acoustic environments (e.g., soft voice, increased distance from speaker, background noise). Persistent or chronic OME may also be accompanied by ear discomfort; poor balance; gross motor delays; sleep disturbance; school difficulties; and delays in speech, language, or learning.
In addition to examination of the ears, a comprehensive head and neck examination is invaluable to identify conditions that may predispose the patient to OM (see the section “Risk Factors”). Facial features should be assessed for possible craniofacial anomalies. Examination of the oropharynx may show a cleft palate or a bifid uvula that raises suspicion for a submucous cleft palate.
Otoscopy is the primary diagnostic method for AOM (see Table 15.2 ). To adequately visualize the TM, the external ear canal must be cleared of cerumen and debris. The assessment of the TM should note color, degree of translucency, position, integrity, and landmarks.
Image-based scales have been developed to standardize recording and interpretation of otoscopic findings. , A computerized peer-reviewed curriculum containing validated images and expert content has been demonstrated to enhance residents’ abilities to interpret still and video images of TMs and improve pediatric otoscopy skills in real patients compared with routine immersion exposure learning only.
The normal TM is pearly gray, translucent, and concave (see Fig. 15.1A ). A visible landmark is the handle (manubrium) of the malleus, which is attached to the TM, with the umbo in the center of the TM. A red but translucent TM is a typical finding in a crying infant, secondary to engorgement of blood vessels in the TM. In contrast, a red TM that is full or bulging often is a sign of AOM (see Fig. 15.1B ). A pink, yellow, or blue TM is often associated with a lack of translucency (e.g., an opaque or cloudy TM), which is highly predictive of MEE.
The position of the TM may range from severely retracted to normal to bulging. Mild to moderate retraction indicates negative middle ear pressure, which may or may not be associated with MEE. A severely retracted TM usually indicates a prolonged history of middle ear dysfunction causing atrophy and collapse of the TM, which may become adhesive to the underlying bone; MEE may or may not be present. Bulging of the TM is a key diagnostic feature of AOM (see Table 15.2 ), , consistent with MEE containing a high level of bacterial pathogens. As AOM resolves and the bulging subsides, the TM may have a cobblestoned appearance (shagrination). ,
Pneumatic otoscopy, which allows assessment of the TM and its mobility, is recommended as the primary diagnostic tool for OME, although experienced otoscopists may not find it necessary. , The normal TM moves briskly with application of slight positive and negative pressure (e.g., barely pressing the pneumatic bulb). Almost any TM may move regardless of presence of MEE if greater degrees of pressure are applied. To ascertain the mobility of the TM, a good airtight seal must be obtained between the speculum and the ear canal. The largest speculum that fits comfortably should be used. A bulb through which air is puffed should be attached to the otoscope, allowing for visualization of TM mobility.
Distinctly impaired mobility of the TM on pneumatic otoscopy is highly predictive of MEE. , Total absence of mobility of the TM may also reflect a perforation in the TM or a patent tympanostomy tube. Other features, such as fluid levels or bubbles, may be more easily discerned with pneumatic otoscopy.
Otomicroscopy provides superior illumination and magnification and allows for more detailed visualization of features associated with chronic OM such as atrophy, tympanosclerosis, atelectasis, retraction pockets, and perforation. It also gives the clinician the freedom of both hands to remove cerumen or perform a minor procedure, like tympanocentesis or unblocking a tympanostomy tube. Otoendoscopes are increasingly useful for diagnosing and managing ear disorders and likely provide comparable information to otomicroscopy when assessing TM integrity and the presence or absence of MEE.
Tympanometry provides information about TM mobility and the presence or absence of MEE. It estimates the equivalent ear canal volume, defined as the amount of air in front of the probe, normally 0.3 to 0.9 mL in children (see Table 15.2 ). A small probe that emits a tone is placed in the ear canal with an airtight seal. Tympanometry is generally performed using a 226-Hz tone, but a 1000-Hz probe tone is best for young children age less than 6 months because of increased compliance of the external auditory canal. The tympanogram is obtained by plotting the immittance (acoustic energy of the reflected tone) of the middle ear as a function of varying pressure in the external ear canal, ranging from −400 to +200 daPa (decapascals) ( Fig. 15.2 ). The instrument provides measures such as peak compensated (static) admittance, tympanometric peak pressure, acoustic reflex, and tympanometric width (TW; a measure of gradient).
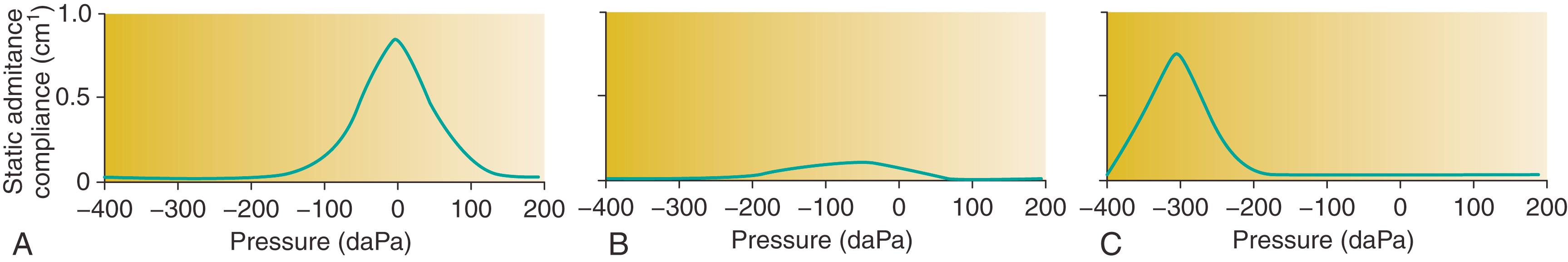
In a normal air-filled middle ear with equal pressure on both sides of the TM, the peak pressure of the tympanogram is 0 daPa. A low equivalent volume (<0.3 mL) may indicate an inaccurate reading because the probe is pressed against the canal wall or because of cerumen impaction, whereas a high equivalent volume (1 to 5.5 mL) suggests a perforation or a patent tympanostomy tube. However, a very small equivalent volume may be in fact an accurate measurement in children with stenotic external auditory canals (e.g., Down syndrome).
Compared with pneumatic otoscopy, tympanometry is easier to perform and has a comparable sensitivity but a lower specificity for diagnosing OME. ,
Acoustic reflectometry measures the amount of sound reflected off the TM, with higher values indicating a greater probability of MEE (see Table 15.2 ). Advantages over tympanometry include ease of use, no requirement for an airtight seal, and the availability of an inexpensive consumer version, which can be used reliably by parents to monitor the middle ear status of their child. Reflectometry has been shown to be less sensitive and specific than tympanometry in detecting MEE. Its high specificity and negative predictive values, however, make reflectometry useful for ruling out MEE.
MEE usually results in a mild to moderate conductive hearing loss. The assessment of the child’s hearing is essential to OME management, because prolonged hearing impairment can predispose the affected child to delays in speech and language development and learning difficulties.
Behavioral audiometry requires cooperation of the child with the examination, and the test is adapted to the age of the child. Visual reinforcement audiometry is typically used for children 6 months to 2 years of age and involves presentation of a sound stimulus in the sound field with observation of the child’s conditioned head turn response. This reaction is rewarded with a visual reinforcement such as an animated toy or video. Conditioned play audiometry, for children older than 2 years, is similar to conventional audiometry (for children >5 years), but the child places toys in a bucket rather than raising a hand to acknowledge hearing the sound. Hearing thresholds are determined at 0.25, 0.5, 1, 2, 4, and 8 kHz and may be ear specific or measured in the sound field, depending on the age of the child.
Auditory brainstem response (ABR) and transient-evoked otoacoustic emissions are useful methods for testing children who do not cooperate with behavioral hearing evaluation because of their young age or developmental delay. Except for infants young enough to be tested during natural sleep, sedation or general anesthesia is usually necessary for ABR testing in younger children.
Otoacoustic emissions (OAEs) testing is an objective assessment that measures function of the cochlear outer hair cells. In many countries, it is used for detection of congenital and early acquired sensorineural hearing loss in newborns because it is fast and easy to perform. It can also be used for hearing screening in children who do not cooperate with behavioral testing. However, the presence of MEE may result in absent OAE responses. Therefore if the OAEs are absent, tympanometry should be performed to assess the middle ear. For children who “fail” OAE testing, especially after repeat examination, follow-up with behavioral audiometry or ABR must be done to assess the type and degree of hearing loss.
AOM is among the most common infectious diseases in young children. Almost all children experience at least one AOM episode during their early years ; by 6 months of age, 20% of children have had two or more AOM episodes and by 3 years of age 50% have had three or more episodes. ,
The average global AOM incidence rate is estimated at 10.8 new episodes per 100 people per year. This rate ranges from an average of 3.6 in central Europe to an average of 43.4 for sub-Saharan West Africa and central Africa, reflecting that the burden of AOM varies with economic status ( Fig. 15.3 ). Global AOM incidence rates are highest in children aged 1 to 4 years (61 new episodes per 100 children per year).

Because OME is often asymptomatic and may go undetected, its incidence and prevalence has been difficult to establish accurately. The most reliable data on the epidemiology of OME come from large cohort studies of children from the United States and Western Europe, mostly performed in the 1980s and 1990s, showing a point prevalence of OME on screening tests of up to 20%.
The highest incidence of OME is around 1 year of age; by age 3 years, nearly all children have experienced at least one OME episode. ,
The risk of OM is influenced by a range of host-related and environmental factors ( Fig. 15.4 ). Onset of first AOM before 12 months of age is a powerful predictor of recurrence. , Additional host-related factors that increase risk of OM include male sex, Aboriginal ancestry, genetic predisposition, craniofacial abnormalities such as cleft palate, immunodeficiency, and hypertrophy of the adenoids. , Environmental factors that increase risk of OM include low socioeconomic status, recurrent upper respiratory tract infections (URTIs), fall and winter season, , daycare attendance, , having older siblings, tobacco smoke exposure, and pacifier use. , Breastfeeding protects against OM. , There is ongoing debate regarding the role of prematurity, , allergy, obesity, and gastroesophageal reflux in the pathogenesis of OM.
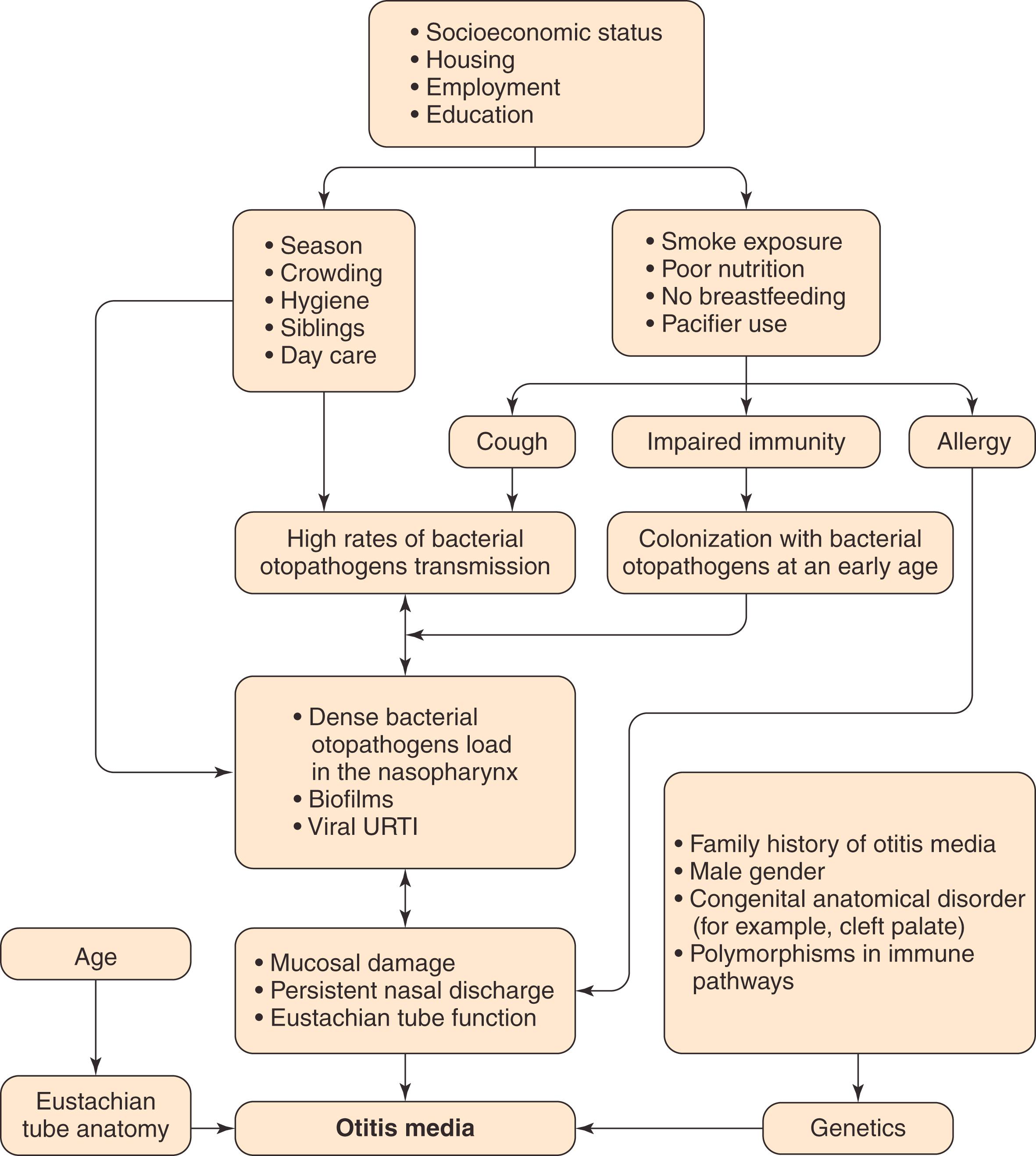
The pathophysiology of OM is multifactorial (see Fig. 15.4 ), with the developing immune system and eustachian tube dysfunction playing central roles in the young child’s susceptibility to middle ear infection and effusion.
OM pathogenesis starts with early and dense bacterial colonization of the nasopharynx, early-onset AOM, and the establishment of an acute inflammatory cycle in the middle ear as a result of continuing exposure to infective agents, including viruses and bacterial persistence in the middle ear through biofilm formation. The final result may be severe chronic ear disease.
An anatomic and functioning eustachian tube not only protects the middle ear against the influx of respiratory viruses and bacterial otopathogens, but it is also essential for draining secretions from the middle ear space and for equalizing pressure. The anatomy of the developing eustachian tube in infants has a central role in the susceptibility to infections of the middle ear (see Fig. 15.4 ). The eustachian tube epithelium is the frontline defense against the passage and colonization of otopathogens from the nasopharynx. The eustachian tube epithelium predominantly consists of ciliated respiratory epithelial cells, which produce antimicrobial proteins (such as lysozyme), interspersed with goblet cells, which produce both mucoid and serous mucus. The direction of mucociliary flow from the middle ear through the eustachian tube to the nasopharynx in combination with antimicrobial proteins secreted by the epithelium protect against bacterial colonization of the middle ear.
Anatomically, the eustachian tube is shorter, wider, floppier, and more horizontal in infants than in adults, which facilitates transmission of pathogens from the nasopharynx to the middle ear and increases the risk of OM. Frequent supine positioning of infants may also enhance infection risk. As children grow, the skull base extends downward, increasing the angle of the eustachian tube gradually from approximately 10 degrees at birth to 45 degrees in adults; concurrently, the eustachian tube length increases from 13 to 35 mm. These anatomic changes and functional maturation of the immune system contribute to a reduced risk of OM as children age, even in children at highest risk of OM.
Early bacterial colonization of the nasopharynx predisposes children to early-onset and recurrent OM. , Streptococcus pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis are the most common bacteria reported globally, but individual species and strain dominance vary across countries and are influenced by the use of pneumococcal conjugate vaccines (PCVs). , Until 2000, S. pneumoniae was the single most common bacterial pathogen in AOM in the United States. Routine vaccination of infants and young children with 7-valent (PCV7, Prevnar) and 13-valent PCVs (PCV13, Prevnar 13), approved in 2000 and 2010, respectively, changed AOM epidemiology substantially; the overall prevalence of S. pneumoniae decreased, S. pneumoniae vaccine serotypes were replaced by nonvaccine serotypes, and the prevalence of H. influenzae and M. catarrhalis increased.
Bacterial biofilms are sessile communities of interacting bacteria encased in a protective matrix of exopolysaccharides and adherent to a surface. The matrix protects bacteria against the host’s immune response, and the reduced metabolic rate of bacteria in the biofilm renders them resistant to antibiotics. , Mucosal biofilms have been isolated from the middle ear of patients with persistent OME, , CSOM, and cholesteatoma.
AOM is generally preceded by a viral infection of nasopharyngeal and eustachian tube epithelium, the so-called common cold or viral URTI. Predisposing viruses include respiratory syncytial virus (RSV), rhinovirus, adenovirus, coronavirus, bocavirus, influenza virus, parainfluenza virus, enterovirus, and human metapneumovirus. , Bacterial otopathogens that colonize the nasopharynx are generally harmless until the virus initiates a local inflammatory process ( Fig. 15.5 ). Viral infection changes the nasopharyngeal mucosa by modifying host immune function, inducing cytokine activity and inflammatory mediators and increasing bacterial colonization and adherence through the upregulation of host cell surface antigens that serve as bacterial receptor sites. , It also alters the properties of mucus and diminishes the normal mucociliary clearance by mucosal cells of the nasopharynx and eustachian tube. This causes eustachian tube dysfunction, , leading to negative middle ear pressure, which occurs more severely in young children. , Negative middle ear pressure in turn facilitates an influx of respiratory viruses and/or bacteria into the middle ear. Concurrent nasal obstruction, from chronic rhinitis or an obstructing adenoid, can worsen the negative pressure because of the Toynbee phenomenon. The risk for AOM development after viral URTI depends on the density of bacterial colonization of the nasopharynx; the risk is lowest in the absence of colonized bacteria and highest with dense bacterial colonization.
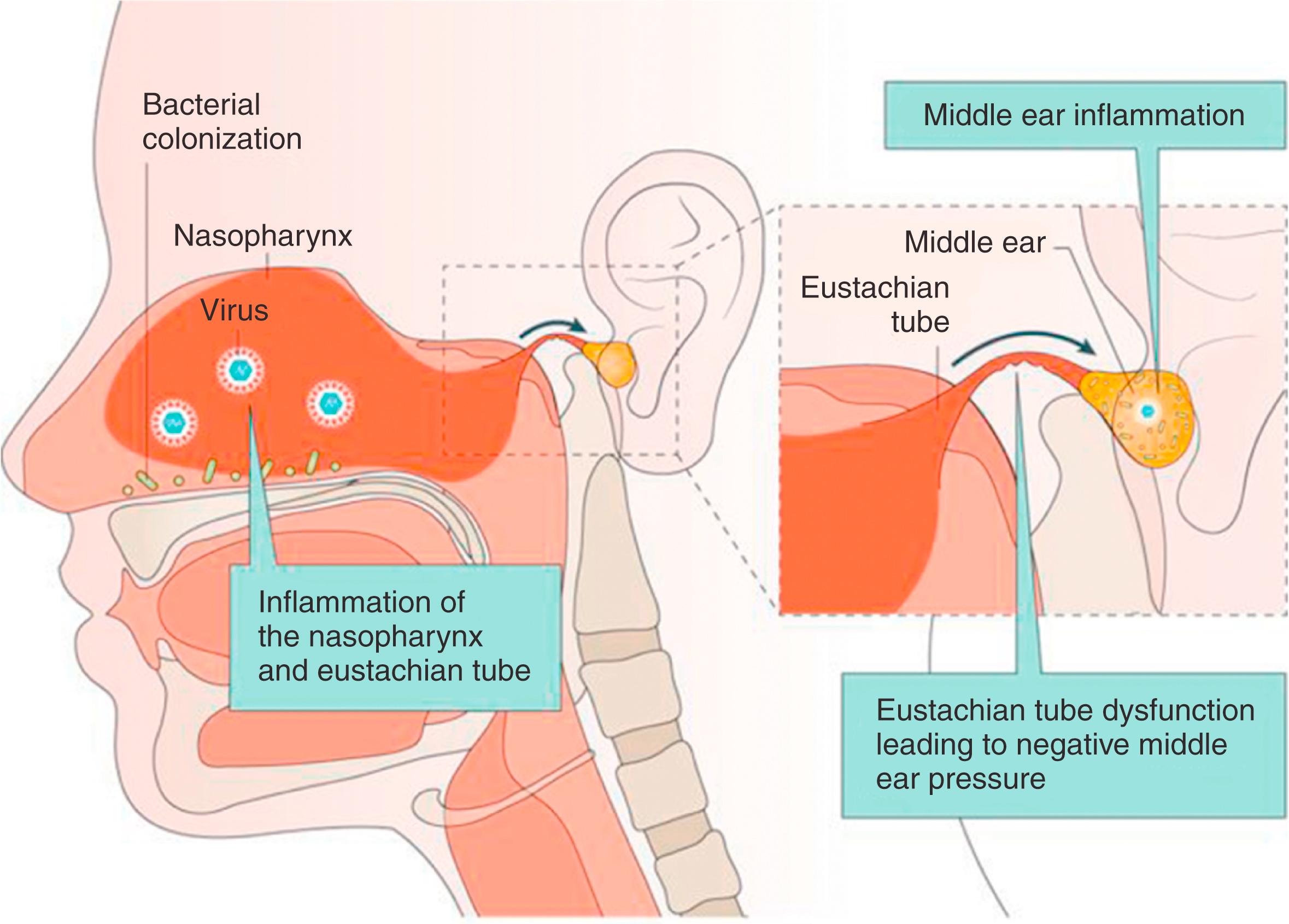
AOM after viral URTI typically occurs when the infection is severe enough to cause URTI symptoms and associated tubal dysfunction. Asymptomatic viral infection does not lead to AOM. AOM may result from viral infection alone, as approximately 5% of the MEE isolated from children with AOM contain only viruses. Viral infection not only leads to AOM but also to new-onset OME; in children aged 6 to 47 months, the rate of AOM and OME after viral URTI was 37% and 24%, respectively.
Innate immune systems are responsible for initiating frontline responses to pathogens in the nasopharynx, eustachian tube, and middle ear. They include physical barriers, such as mucociliary-generated flow of mucus, and innate defense molecules such as lysozymes, cytokines, chemokines, defensins, and complement factors. , Activation of pattern recognition receptors, especially Toll-like receptors (TLRs), by invading pathogens triggers the release of antimicrobial proteins and proinflammatory cytokines. , Upregulation of these mechanisms is essential for the rapid resolution of OM.
The middle ear is an effective immunocompetent site that maintains essentially a sterile environment. Adaptive immunity consists of both mucosal and systemic immune responses. In response to infection, antigen-specific secretory IgA and IgG antibodies and IgA-producing cells have been detected in the middle ear fluid and mucosa, respectively. Research on middle ear cell-mediated responses to infection is only just commencing, but it has been suggested that regulatory T cells play a key role in controlling inflammation. Current evidence as to whether deficiencies in humoral immunity contribute to susceptibility to OM is incomplete. Low IgA, IgG2, and mannose-binding lectin levels have been found in children with chronic recurrent OM, but more research is needed to explore for aberrations in adaptive immune responses as potential risk factors for OM.
Estimates of heritability of AOM and OME vary from 40% to 70%, with boys at slightly higher risk than girls. Numerous genes that regulate the immune response have been associated with a predisposition to OM. Some of the heritable risk for OM may result from pathogen-specific cytokine polymorphisms; interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF) polymorphisms are predictive of OM coincident with RSV and rhinovirus infection, whereas polymorphisms in several signal transduction pathways, such as TLR signaling, have been associated with increased risk of OM. , Most polymorphisms described thus far disrupt the establishment of an effective innate immune response, but transforming growth factor-β (TGFβ) can be pathophysiological through interference with moderation of proinflammatory responses. , Although data with respect to deficiencies in specific antibiotic responses to otopathogens in OM-prone children are conflicting, the role of possible cell-mediated dysfunction is becoming clearer. The genetic contribution to these observations is, however, unknown and further research is required to fully understand the role of these genetic factors in OM pathogenesis.
There is still debate on the role of allergy in the pathogenesis of OM. Atopic conditions, such as allergic rhinitis, are common among children with OM, , , but there is no evidence to show that antiallergic medication is effective in children with AOM or OME without proven allergies.
Gastroesophageal reflux has been suggested as a causative factor in OM with a potential role for antireflux therapy in the management of this condition. Gastroesophageal reflux disease has been reported in half of children with persistent OME and two in three of those with recurrent AOM, which may be higher than the overall prevalence of this condition among children. Also, pepsin/pepsinogen has been detected in the middle ear fluid of children with persistent and/or recurrent OM. , However, most studies were uncontrolled, and the pH level required to activate pepsin and for occurrence of mucosal damage is unknown. Therefore a cause-effect relationship between gastroesophageal reflux and OM remains unclear. Current evidence on the role of antireflux therapy in the management of persistent and/or recurrent OM is limited to uncontrolled observational studies among children and adults with coexisting gastroesophageal reflux disease, which do not show significant benefit. , As such, antireflux treatment is currently not recommended for OM.
As the pathophysiology of OM is multifactorial, various strategies can be used for prevention. These strategies mainly focus on reducing modifiable risk factors, such as environmental risks and viral and bacterial infections. Antibiotic prophylaxis and surgical interventions aimed at reducing the burden of OM in children are discussed in the Treatment section.
Breastfeeding protects against OM for the first 2 years, with largest effects observed in children who were exclusively breastfed and in those who were breastfed for at least 6 months. , Avoidance of environmental risk factors such as large-group daycare attendance (six or more children), daytime pacifier use, and exposure to tobacco smoke, in particular during the first 2 years of life, has also been associated with a reduction of OM. , ,
Current guidance recommends exclusive breastfeeding for at least 6 months, avoidance of tobacco smoke exposure, and discussion of other lifestyle changes such as reducing pacifier use and altering child care arrangements so that the child is exposed to fewer children.
The aim of these vaccines is to reduce or eliminate nasopharyngeal colonization of S. pneumoniae, nontypeable H. influenzae, and M. catarrhalis.
PCV7 directed against S. pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F was licensed for use in the United States in 2000 and recommended for use in children younger than 6 years of age by the American Academy of Pediatrics. The vaccine was added to the primary series of universal vaccinations at 2, 4, and 6 months, with a booster dose at 12 to 15 months. PCV7 is associated with a 6% to 7% relative risk reduction in overall AOM episodes and a 20% reduction in tympanostomy tube insertions for chronic recurrent OM.
In 2010 PCV7 was replaced by PCV13 in the United States, which included six additional S. pneumoniae serotypes (1, 3, 5, 6A, 7F, and 19A). Rigorous data are currently lacking, but PCV13 has been associated with a further reduction of AOM, mastoiditis, and tympanostomy tube insertions in the United States.
Although the use of PCVs has led to a shift from S. pneumoniae vaccine to nonvaccine serotypes and nontypeable H. influenzae in the nasopharynx in vaccinated children, , PCVs are thought to decrease pneumococcal-associated AOM because the replaced serotypes are considered less otopathogenic. It has also been suggested that the prevention of OM caused by the pneumococcal serotypes targeted by the vaccine in young children results in a reduction of subsequent and more complex disease caused by nonvaccine serotypes and nontypeable H. influenzae. PCVs might therefore disrupt the continuum of evolution from pneumococcal-associated OM toward chronic recurrent OM. Importantly, the use of PCVs in children who have already experienced frequent AOM episodes will not prevent additional OM episodes.
Because nontypeable H. influenzae is commonly isolated from middle ear fluid in AOM, much effort has been channeled into developing an effective vaccine. Many different approaches using animal models have been used to develop vaccine antigens for nontypeable H. influenzae –associated OM, including outer membrane protein P5, 26, P2, P6, Htr protein, protein D, phosphorylcholine, detoxified lipooligosaccharides, and type 1V Pili protein.
The PHiD-CV10 vaccine is a 10-valent PCV with protein D, an outer membrane protein of H. influenzae, that is available in Europe. Although effective for pneumococcal-associated AOM, PHiD-CV10 may be less protective for nontypeable H. influenzae than an 11-valent pneumococcal capsular polysaccharides vaccine conjugated to protein D. Currently, no other licensed vaccine against nontypeable H. influenzae exists.
M. catarrhalis is the third most common bacterium isolated from the middle ear, but there is ongoing debate on its role in AOM pathogenesis. Evidence suggests that M. catarrhalis is associated with a stable bacterial community composition and respiratory health.
Candidate vaccine antigens that have been shown to induce potentially protective responses against M. catarrhalis include outer membrane protein OlpA, CopB, filamentous hemagglutinin (FHA-like protein), and lipooligosaccharides. However, little progress has been made in defining the protective immune response, and currently no licensed vaccine against M. catarrhalis exists.
Because viruses play a key role in the pathogenesis of AOM, , viral vaccines may affect AOM incidence.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here