Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Olfaction is important for hazard avoidance and quality of life.
Olfactory dysfunction affects 20% of the general population and is most commonly caused by sinonasal disease, upper respiratory tract infections, head trauma, normal aging, and neurodegeneration.
Olfactory dysfunction should be diagnosed through clinical history, examination, and psychophysical testing.
Treatment of dysfunction should be in line with currently available guidelines.
The sense of smell is evolutionarily ancient. In humans, it guides food intake, environmental hazard avoidance, and social communication. Moreover, olfaction acts as a marker of disease and can predict mortality. In this chapter, the anatomy and physiology of smell are discussed, followed by the clinical presentation, pathophysiology, investigation, and treatment of olfactory dysfunction.
The perception of smell requires activation of the olfactory nerve (CN I) by volatile chemicals within the nose. Depending on odor type, this is accompanied to varying degrees by activation of the trigeminal nerve (CN V), which imparts sensations of coolness, heat, irritation, or pungency. Following peripheral activation in this way, signals are transmitted to the central nervous system, where processing and integration lead to formation of the odor percept.
In mammals, odorants may either activate the main or accessory olfactory system. The latter is an anatomically and functionally distinct system used by many species to detect odorants with low volatility, including pheromones. This system consists of a peripheral vomeronasal organ (VNO), which contains specialized chemosensory cells that project via the vomeronasal nerve to the accessory olfactory bulb (OB). In humans, despite the presence of a VNO-like structure in the septum of some patients, this has no apparent central connection, and is thought to be a nonfunctional vestige. Therefore, only the main olfactory system will be discussed in this chapter.
The olfactory neuroepithelium (OE) is a pseudo-stratified columnar epithelium, which contains three main cell types: basal cells, olfactory receptor neurons (ORN), and supporting (sustenacular) cells. In addition to these, microvillar cells and duct cells can be found in smaller numbers. Deep to the OE, and separated by the basement membrane, the lamina propria contains a dense vascular network as well as Bowman glands, connective tissue, and olfactory ensheathing cells (a specialized form of glia). Taken together, the lamina propria and OE constitute the olfactory mucosa.
Numbering between 6 and 30 million in young adults, ORN are specialized bipolar neurons. With cell bodies located within the OE, ORN extend a single dendrite toward the epithelial surface, from which multiple nonmotile cilia pass into the overlying nasal mucous layer. These cilia contain olfactory receptors (ORs), so their presence greatly increases available surface area for odorant binding. At their basal pole, ORN extend single, unmyelinated axons that pass through the basement membrane and into the lamina propria, where they come together to form bundles (fila) surrounded by olfactory ensheathing cells. These fila (which collectively form the olfactory nerve) then pass through the foramina of the cribriform plate, where they synapse with second order neurons in the ipsilateral OB. ORN are therefore unique first-order excitatory sensory neurons in their simultaneous contact with the outside environment (ciliary contact with nasal mucus layer) and central nervous system ( Fig. 36.1 ).
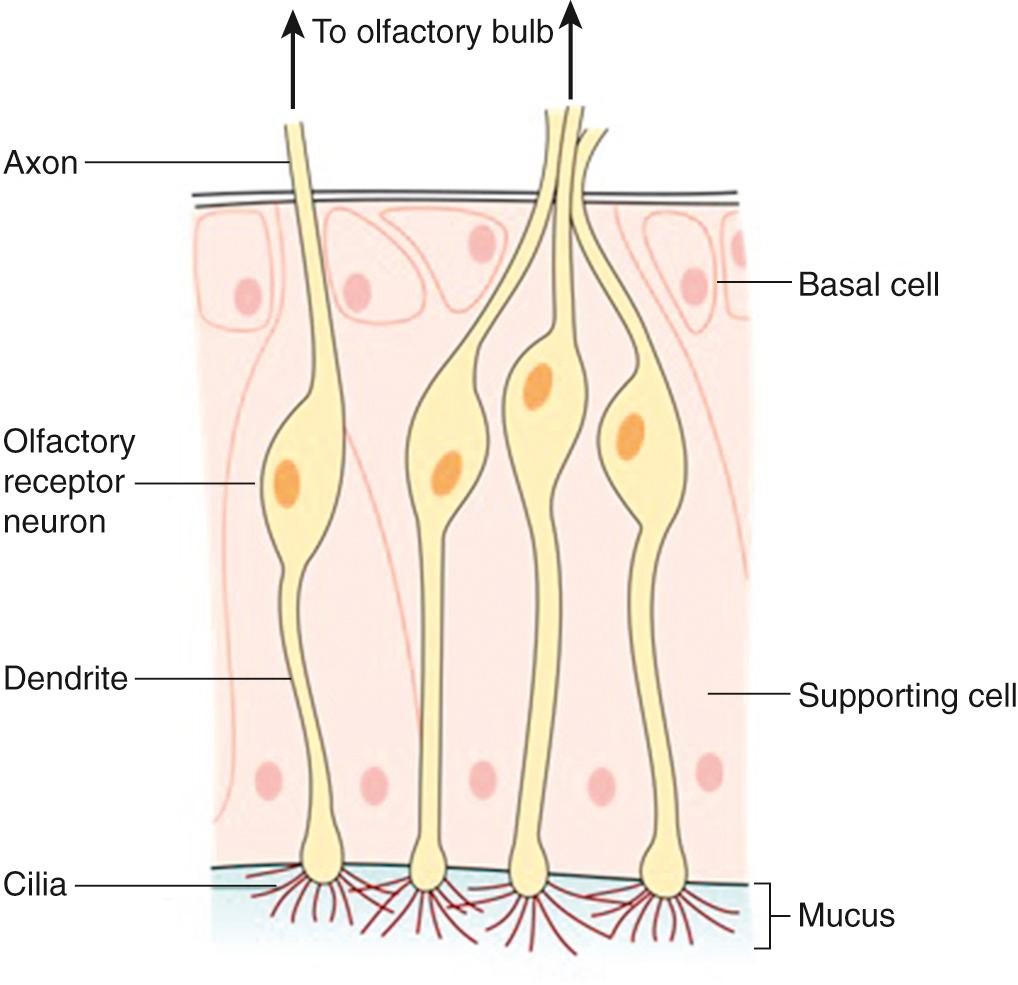
As a consequence of this external contact, ORN are prone to damage from a variety of exogenous pathogens, dusts, and other toxins. Accordingly, the OE has developed another unusual feature: adult neurogenesis. Following damage, ORN are replaced from the basal cell population of the OE. Whilst it is thought that the global ORN population undergoes complete regeneration every 4 to 6 weeks in animals, the rate of turnover in humans is unknown and may be dependent on both the degree and cause of injury. This process is facilitated by olfactory ensheathing cells, which are present in the OE as well as the OB and help guide axonal regenertation. In keeping with this, olfactory ensheathing cells have been shown to support axonal outgrowth and repair in models of nerve injury. Techniques have been developed allowing safe harvesting of olfactory mucosa and, therefore, olfactory ensheathing cell populations, and it has been suggested that autologous transplantation may be a potential therapeutic option for nerve injury in the future.
With increasing age, cumulative or extensive damage, the regenerative capacity of the OE may, however, become exhausted. At this point the epithelium undergoes respiratory or squamous-type metaplastic change. Histologic studies suggest that the OE forms one continuous sheet at birth but that cumulative damage and failed neurogenesis lead to metaplastic change from as early as 2 years. Consequently, adult OE is characterized by varying degrees of inhomogeneity—with more marked cases displaying greater OE replacement, which can assume ultimately a checkerboard appearance.
The olfactory cleft is an anatomic region demarcated by the cribriform plate superiorly, superior turbinate laterally, and superior septum medially. While the location of the OE was traditionally thought to be limited to the area of the olfactory cleft, it is now thought to be more anterior in young people. In keeping with this, mature, functional ORN can be found at the anterior insertion of the middle turbinate in young adults. With age and progressive metaplasia, this distribution becomes more variable, with cadaveric tissue from older adults only consistently containing OE in the area of mucosa directly underneath the cribriform plate. This variability in location and metaplastic interspersion is important to remember when attempting to either biopsy the OE or avoid its damage during surgical procedures (this is of particular importance given that the OE does not appear macroscopically different from respiratory epithelium). With regards to the former, biopsy success rates seem to be higher posteriorly than anteriorly. In young people, the OE is thought to span an area of approximately 2 to 5 cm 2 , although with increasing age and metaplasia, as described, this surface area likely decreases.
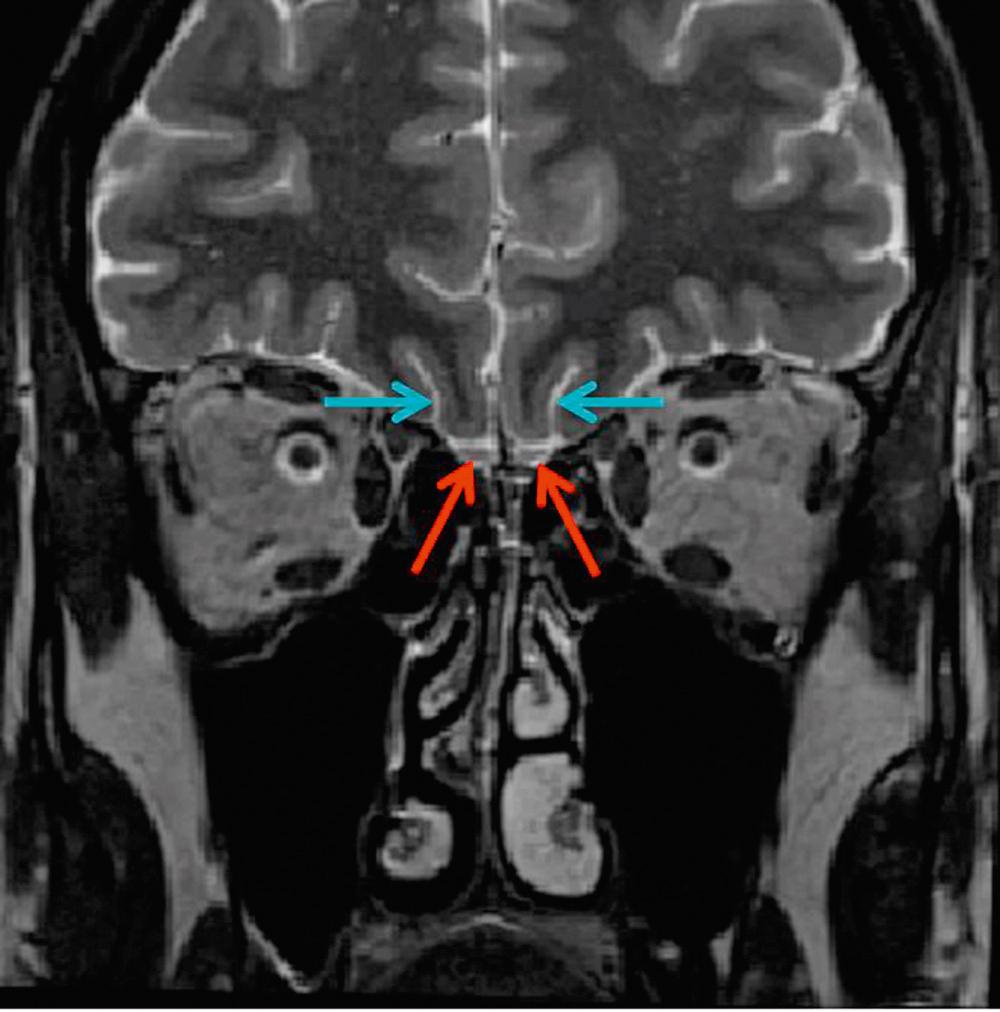
The OB is the first relay in the olfactory system and forms the most important part of the primary olfactory brain network. These paired structures are found immediately dorsal to the cribriform plate and ventral to the orbitofrontal cortex. Under normal circumstances the OBs can be seen as paired structures on T2-weighted coronal magnetic resonance images of the anterior cranial fossa ( Fig. 36.2 ).
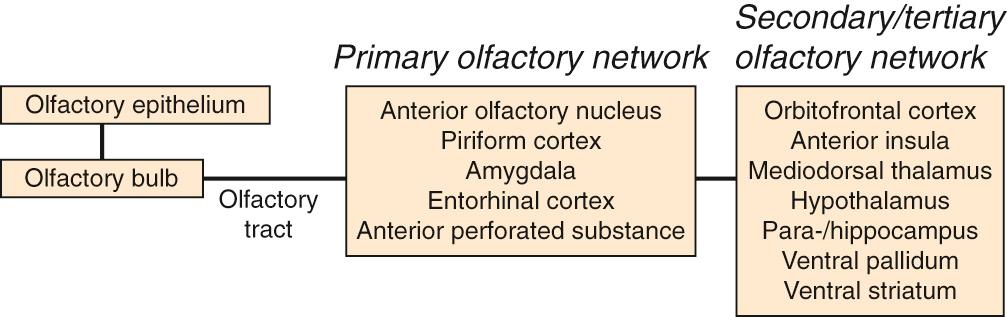
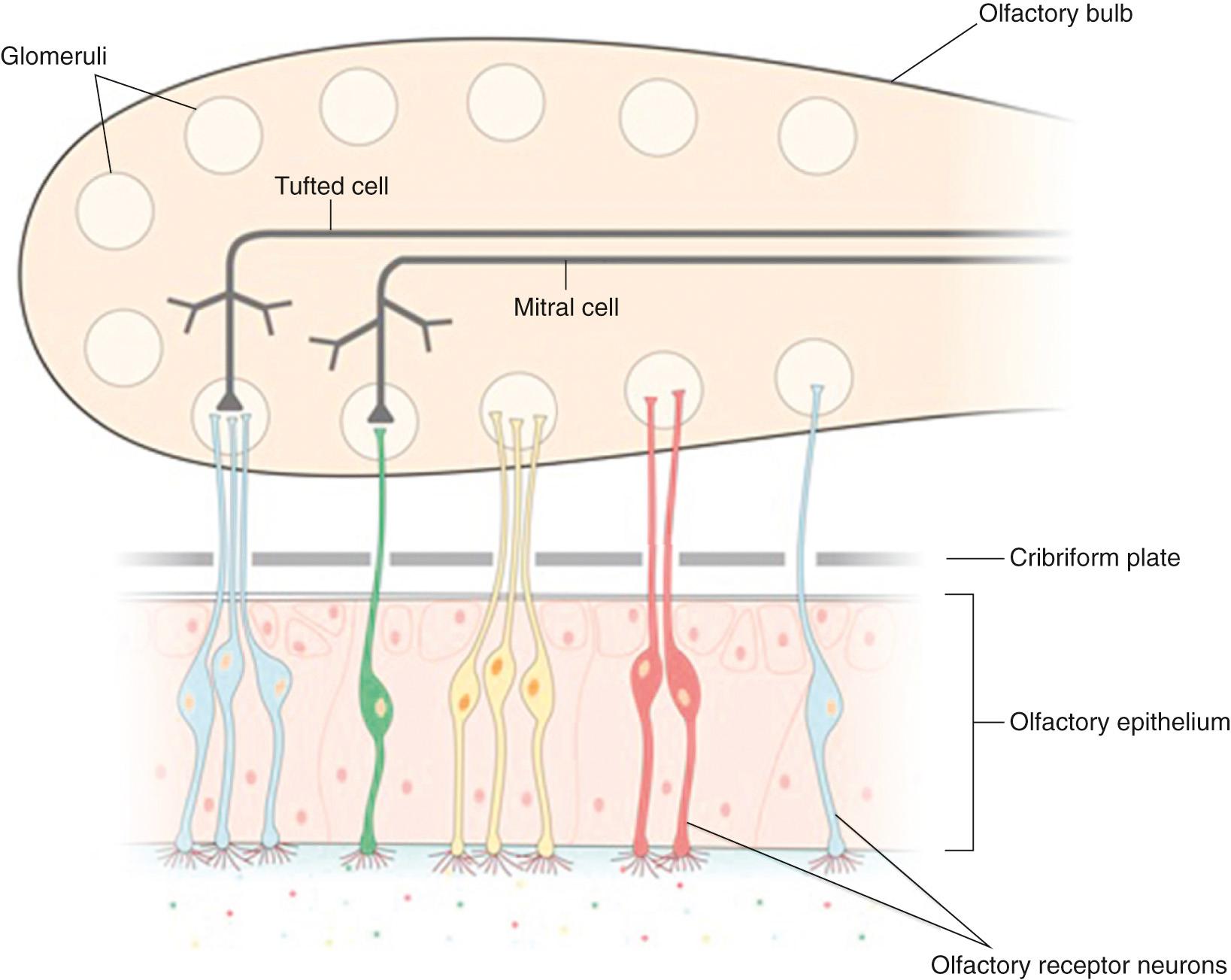
Axons from the ORN enter the ipsilateral OB and synapse with second-order neurons, called mitral and tufted cells, within specialized regions called glomeruli. Glomeruli are circular tangles of ORN axons intertwined with dendrites from mitral and tufted cells, as well as from periglomerular interneurons. Axons from these second-order neurons then extend to regions of the primary olfactory network: tufted cells extend to the anterior olfactory nucleus and anterior perforated substance and mitral cells extend to the anterior olfactory nucleus, piriform cortex, anterior cortical nucleus of the amygdala, and the rostral entorhinal cortex ( Fig. 36.3 ). Collectively, these structures make up the primary olfactory network. Odor processing also involves “secondary” and “tertiary” brain areas, including structures such as the hippocampus, parahippocampal gyrus, insular cortex, and orbitofrontal cortex. Many of these structures are important components of the limbic system, which regulates emotion and motivation and is important for learning and memory. As a result of this anatomic and functional overlap, olfactory dysfunction is associated with reduced quality of life, including depression and anxiety.
Though odorants can reach the OE through diffusion alone, in practice access is facilitated by nasal airflow. This airflow may either be anteroposterior in direction, as occurs in orthonasal olfaction, or posteroanterior, as occurs in retronasal olfaction ( Fig. 36.4 ).
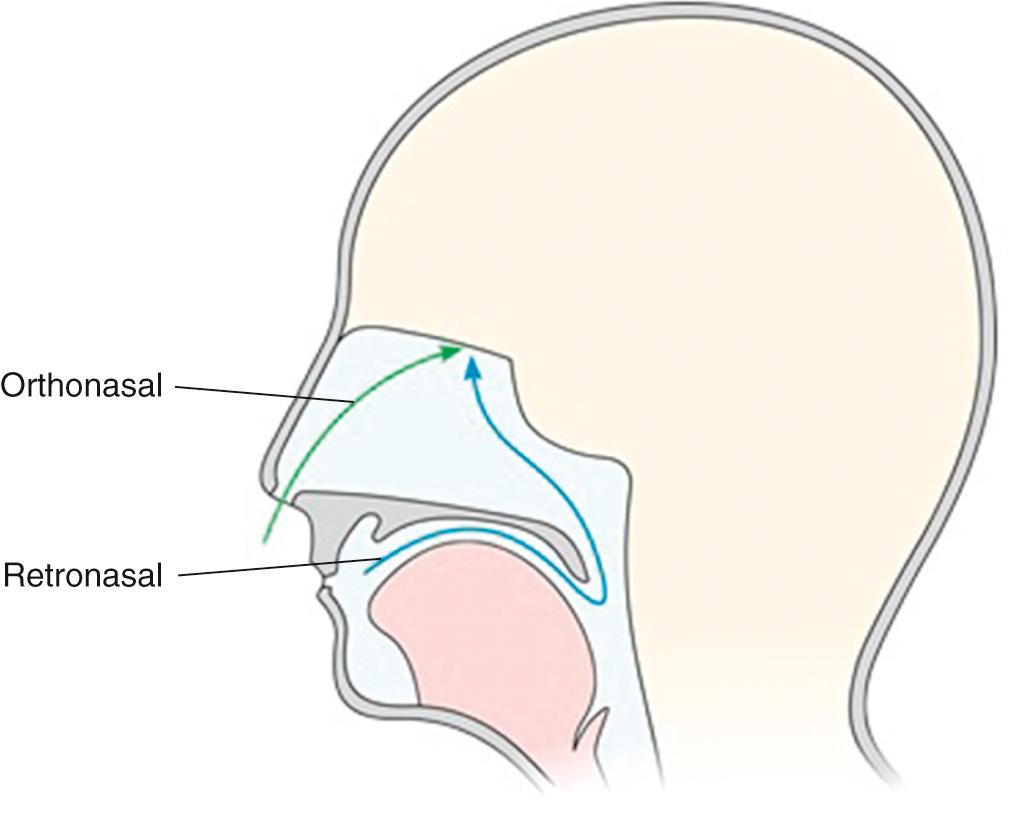
Assuming that the majority of OE is found within the classical confines of the olfactory cleft, during orthonasal flow, odorants must traverse the narrow nasal cavity to reach this remote area beneath the cribriform plate. Consequently, during normal breathing, less than 15% of the total inspired nasal air will reach the OE. Furthermore, the combined irregularity of nasal cavity anatomy and high velocity airflow results in nonlinear aerodynamics and complex odorant distribution patterns.
Total nasal airflow or nasal resistance, as measured using standard rhinometric techniques, correlates poorly with olfactory function. In line with this, evidence from anatomically accurate 3D modeling techniques has shown that small changes in nasal anatomy can lead to significant changes in airflow distribution without concurrent change in overall flow rate. For example, small anatomic changes within pivotal regions (e.g., nasal valve or olfactory cleft) that do not affect total nasal airflow/resistance can result in marked changes in airflow within the olfactory cleft (>700% change). Rhinomanometry (which measures total flow/resistance) may therefore be an inadequate tool for the assessment of nasal airflow, where olfactory outcomes are of interest.
The nasal cycle describes the physiologic and cyclical alternation of mucosal congestion between right and left nasal cavities. This can increase unilateral nasal resistance by up to four times, although several studies have demonstrated minimal effect on monorhinal olfactory function, as measured using odor threshold. Conversely, it has been proposed that under normal conditions, differential flow rates between right and left sides may actually lead to improved range of odorants that are simultaneously detectable, suggesting an olfaction-related functional purpose for the nasal cycle.
Retronasal olfaction can occur during normal nasal exhalation but is more marked during eating and drinking. During normal swallowing, the oral and pharyngeal stages cause secondary retrograde airflow (which can be felt by holding a finger in front of the nostrils as one swallows). This retrograde flow carries odorants from food or drink within the oral cavity and oropharynx toward the olfactory cleft, via the nasopharynx. As a result of multimodal integration with gustatory and somatosensory inputs from the mouth, the resultant smell is located to the mouth. This integration is the basis of the flavor percept.
A thin layer of mucus (approximately 50 µm thick) can be found overlying the OE, into which odorants must diffuse prior to their activation of the ORs. This mucus is secreted from Bowman glands within the lamina propria of the olfactory mucosa but likely mixes with that from goblet cells in the neighboring respiratory mucosa. It is largely composed of water but also contains mucopolysaccharides (glycosaminoglycans), proteins, and salts. As a result of the movement established by the mucociliary apparatus of the respiratory mucosa (remembering that ORN dendritic cilia are nonmotile), olfactory mucus is periodically and mechanically cleared and replaced.
For an odorant to be perceived, it must first enter the olfactory mucous layer. This entry depends on the sorptive qualities of the odorant, which are affected by the odorant's psychochemical properties, the properties of the mucous layer (particularly water content), and the nasal airflow. Odor sorption is also modulated by the presence of odorant binding proteins (OBPs), which are small, soluble proteins (lipocalins) that are found within the olfactory mucus. OBPs are thought to act as passive odorant-transporters, in particular, helping to concentrate hydrophobic odorants in the aqueous nasal mucus for OR-binding; however, they may also play a more active role in facilitating odorant-OR binding and clearing reactive oxidants involved in oxidative stress. Following binding of an odorant to its receptor, the olfactory mucus must also provide the appropriate ionic microenvironment for downstream signal transduction cascades.
In addition to OBPs, the protein compartment of the olfactory mucus also contains various enzymes and antibodies. Given the direct pathway that ORN provide to the brain, one of the main functions of the mucous layer is protective. Interestingly, the relative concentration of mucus constituents varies in different disease states.
Three types of receptor are believed to be involved in olfaction and can be found within the main OE: the OR, a putative pheromone receptor, and the trace amine-associated receptor (TAAR). Five subtypes of TAAR can be found within the OE and neurons that express TAARs do not appear to express OR. While it is thought they may be involved in the detection of social cues, further work is required to fully delineate their expression and function in humans. Unless otherwise stated, the term “OR” is used only to describe ORs found within the OE, not pheromone receptors or TAARs.
ORs are a large multigene family of G protein–coupled receptors that are found in the surface membrane of the ORN's dendritic cilia. Odorant-OR binding initiates a cascade of downstream signaling that ultimately results in the firing of an action potential within the parent ORN. More specifically, following binding of an odorant within the OR-binding pocket, an olfaction-specific G-protein (G olf ) is activated, which then interacts with intracellular adenylyl cyclase III, increasing its activity. Increased cytoplasmic cAMP concentrations, in turn, leads to the opening of cation-specific cyclic nucleotide-gated (CNG) channels, which allow influx of Ca 2+ and Na + . This cation influx results in depolarization of the cell. As intracellular Ca 2+ concentration rises, calcium-gated chloride channels open, resulting in an outward Cl − current that potentiates cell depolarization ( Fig. 36.5 ). Once depolarization reaches threshold, an action potential is generated, which is then propagated down the ORN axon toward the OB.
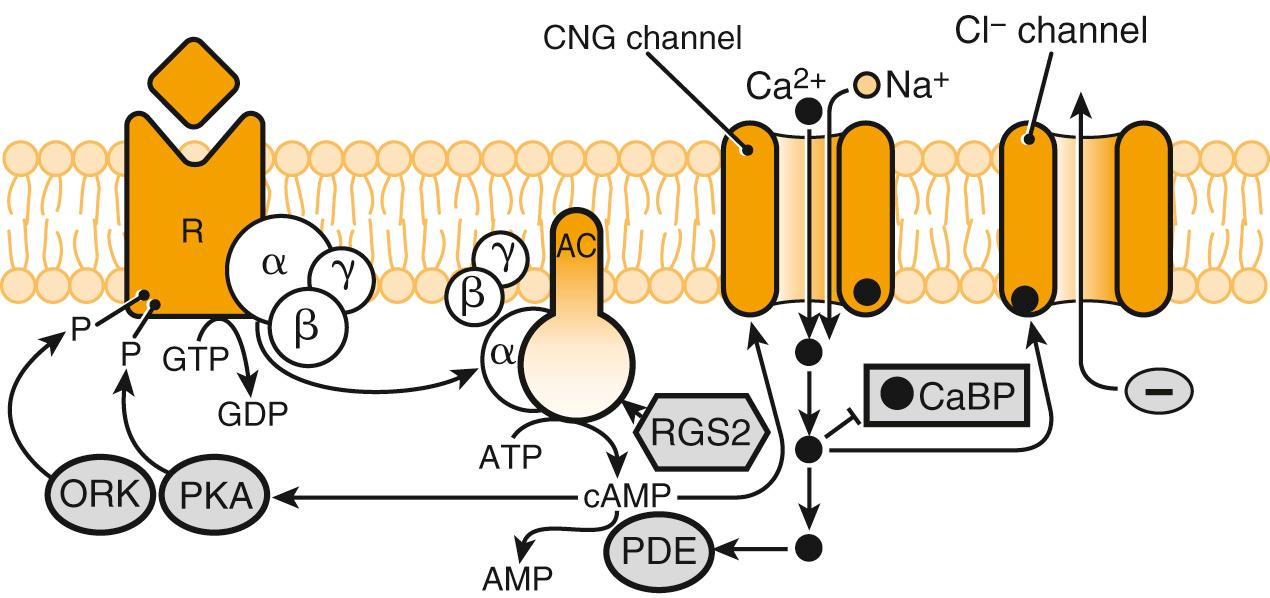
To allow for temporal encoding of a stimulus, as well as ORN adaptation to prolonged odorant exposure, several feedback inhibition pathways are initiated following OR activation. Two of these pathways are triggered by rising levels of intracellular Ca 2+ : first, calcium–calmodulin interacts directly with CNG cation channels, causing reduced channel sensitivity to cyclic nucleotides and, therefore, reduced cation influx. Second, calcium-dependent phosphorylation of adenylyl cyclase causes reduced production of intracellular cAMP and, in turn, reduced CNG cation channel activation. Other mechanisms involved in feedback inhibition include removal of intracellular Ca 2+ via the Na + –Ca 2+ exchanger and degradation of intracellular cAMP by phosphodiesterases.
The human OR gene family contains approximately 1000 genes, distributed across the genome except for chromosomes 20 and Y. Of these, it is thought that around 600 are nonfunctional pseudogenes and 400 functional genes. This is, therefore, the largest gene family within the mammalian genome (constituting approximately 1% of the total), highlighting the evolutionary importance of olfaction.
ORN express OR-genes in a monoallelic and mutually exclusive fashion. Consequently each neuron displays only one receptor type; however, owing to broad molecular binding ranges, many ORs are capable of detecting multiple odorants. In turn, each odorant is recognized by multiple receptors. Importantly, as part of the mechanism by which odor perception is neurally encoded, odorants are detected by a unique combination of OR (and therefore ORN). Such combinatorial encoding facilitates creation of neural fingerprints for odorants, thereby enabling the detection and discrimination of many more distinct odors than would otherwise be possible given the number of functional OR genes. Combinatorial encoding is made more complex by ligand-binding behavior: some odorants act as agonists, some partial agonists, and some antagonists.
The neural fingerprint of an odorant may be further enhanced through some degree of spatial encoding. In rodents, the main OE can be divided into four rough zones. In turn, ORN expression varies according to zone, although within a particular zone neurons expressing the same OR-type are distributed randomly. This rudimentary “rhinotopy” may contribute to odor discrimination, although zonal ORN distribution in humans has yet to be delineated. In addition to this static OR-topography, the physicochemical properties of odorants may affect their spatiotemporal distribution pattern across the OE. This is because the physicochemical properties of an odorant determine its relative sorption into the olfactory mucus—with high and low sorption odors being deposited in different areas of the epithelium. In vivo, this sorption pattern is affected by nasal airflow. The voluntary modification of nasal airflow through sniffing may, therefore, modulate odor encoding and in turn perception.
Further spatial encoding has also been suggested at the level of the OB. Experimental work, again in animals, has shown that axons from ORNs expressing the same receptor type come together to synapse within one of two a
a Note that this 1 : 2 ratio was established in rodents; in humans the ORN-type to glomeruli ratio may be up to 1 : 16.
glomeruli in the ipsilateral OB. Consequently, each glomerulus in the OB receives axonal input from only one type of OR ( Fig. 36.6 ). In this way, the spatial activation map of glomeruli within the bulb is dependent on the receptor-binding properties of an odorant. Furthermore, the temporal dynamics of stimulus presentation likely contribute to odor encoding within the OB.
After initial encoding at the peripheral level and at the level of the OB (where inhibitory interneurons also play a significant role), olfactory signals are further integrated at the level of the primary and secondary olfactory brain networks, ultimately resulting in formation of the odor percept. While the true discriminatory ability of humans is unknown, estimates have ranged from the order of thousands to hundreds of thousands of different odors. This being said, people are often unable to smell specific odors, with otherwise intact olfaction. This is thought to be a normal physiologic trait and is termed specific anosmia (this will be discussed in more detail in later sections).
Most odorants activate both the olfactory system and the intranasal trigeminal system, and these two systems interact through mutual suppression and enhancement. There are several possible sites of interaction between trigeminal and olfactory information, including (1) the central nervous system, for example, the mediodorsal thalamus or the piriform cortex, (2) the OB, (3) the olfactory epithelium, and (4) trigeminal reflexes, for example, changes of nasal patency or mucous secretion. Importantly, trigeminal afferents also mediate sensations of airflow (see above).
Olfactory function is modulated by numerous factors. The major modulators include:
Age: olfactory function deteriorates with normal aging.
Sex: women generally have superior olfactory function to men.
Environmental factors/smoking: pollution and smoking can negatively impact olfactory function.
Olfactory dysfunction can be broadly classified as either quantitative or qualitative. Quantitative dysfunction involves alteration in the strength of perceived odors but not their quality, whereas qualitative dysfunction involves alteration in the quality of an odor. It is unusual to find qualitative dysfunction in the absence of quantitative dysfunction ( Table 36.1 ).
| Normosmia | Normal olfactory function. |
| Hyposmia (or microsmia) | Quantitatively reduced olfactory function. |
| Functional Anosmia | Quantitatively reduced olfaction to the extent that the subject has no function that is useful in daily life. |
| Anosmia | Absence of all olfactory function. |
| Specific Anosmia (or partial anosmia) | Quantitatively reduced ability to smell a specific odor despite preserved ability to smell most other odors. Thought to be a normal physiologic trait with little clinical significance. |
| Hyperosmia (or superosmia) | Quantitatively increased ability to smell odors to abnormal level. This form of olfactory dysfunction is extremely rare but has been described, for example, in association with migraine. |
| Parosmia (or dysosmia, cacosmia, euosmia, or troposmia) | Qualitative dysfunction in the presence of an odorant (i.e., distorted perception of an odor stimulus). |
| Phantosmia | Qualitative dysfunction in the absence of an odorant (i.e., an odorant is perceived without concurrent stimulus, an “olfactory hallucination”). |
| Orthonasal Olfaction | The perception of odorants anteriorly caused by airflow from the nostrils to the olfactory clefts, for example, during sniffing. |
| Retronasal Olfaction | The perception of odorants located within the oropharynx, caused by airflow to the olfactory clefts via the nasopharynx during swallowing or nasal exhalation. Retronasal olfaction forms the basis of flavor perception. |
Epidemiologic estimates of olfactory dysfunction vary markedly. This variation can be attributed to differences in definitions of impairment, sample demographics, and assessment techniques used. Of particular note, studies using subjective patient report tend to produce lower prevalence estimates than studies using some form of psychophysical testing. b
b Psychophysical tests involve assessment of sensory function through the use of corresponding stimuli (e.g., audiogram). In olfaction, psychophysical tests involve recording participant response to presentation of an odor stimulus. In doing so, different aspects of olfaction can be assessed, for example, odor identification or threshold. Commonly used tests include the Sniffin' Sticks (which tests for odor threshold, discrimination, and identification) and the University of Pennsylvania Smell Identification Test (UPSIT), which tests for odor identification. Psychophysical tools are discussed in detail in later sections.
Furthermore, the prevalence of olfactory dysfunction increases with age, making comparisons of epidemiologic data only possible where the sample demographics are similar. Despite these difficulties, the emerging consensus estimates for the prevalence of hyposmia and functional anosmia in the general population are approximately 20% and 5%, respectively (for review, see Yang and Pinto and Hummel et al. ). Accordingly, olfactory dysfunction is probably the commonest form of sensory impairment; using the aforementioned consensus estimates, it is more common than blindness or profound deafness.
Approximately 5% to 10% of patients with olfactory dysfunction spontaneously report qualitative impairment (e.g., see references ); focused history and investigations show an even higher incidence.
The pathogenesis of parosmia or phantosmia is unclear. One theory is that partial but incomplete loss of ORNs allows affected patients to perceive a stimulus, but as a result of interference with encoding (see previous section), its quality is altered. Another possibility involves changes in the central nervous processing of olfactory information.
With regard to phantosmia, abnormally active ORNs or loss of inhibitory neurons at the level of the OB may cause distorted sensations. A central cause is also hypothesized, where hyperactive neurons might create the impression of an odor percept. Qualitative olfactory dysfunction is typically associated with quantitative impairment and appears to be an indicator of change within the olfactory system. Phantosmia may be a deafferentiation syndrome and, therefore, indicate poor prognosis. In contrast, parosmia could be seen within the context of recovery.
It is known that most cases of qualitative olfactory impairment gradually deteriorate with time. In the case of phantosmia, endoscopic surgical excision of the olfactory epithelium or its cocainization has been suggested as a possible therapeutic approach.
Social anxiety, nutritional disturbances, and depression are well acknowledged consequences of smell disorders. Moreover, it has been suggested that olfactory function and depression are interdependent, with reduced OB volume predisposing toward depression, which, in turn, leads to impaired olfaction.
Olfactory dysfunction has also been associated with epilepsy, cerebrovascular accident, and a variety of neurodegenerative diseases, most notably Alzheimer dementia and Parkinson disease (PD). Olfactory dysfunction also increases with age, with anosmia being an independent predictor of mortality. These associations will be discussed in greater detail in later sections.
Given the above, it would follow that the investigation and, where possible, treatment of olfactory dysfunction should be undertaken as required.
Intact olfactory function is of particular importance for certain professions, including, for example, chefs, perfumers, and sommeliers. Olfactory dysfunction in this group may, therefore, have significant economic effects in addition to other psychosocial effects.
Our sense of smell serves to warn us about certain environmental hazards, including fire, spoiled food, and gas leaks. For this reason, patients with impaired olfaction are at greater risk from these dangers and should be counseled accordingly.
Olfactory dysfunction has previously been classified according to the suspected anatomic location of pathology. Accordingly, disorders were categorized as in Table 36.2 ; however, this classification system is increasingly disencouraged because it provides an overly simplistic and restrictive approach to pathophysiology. For example, hyposmia and anosmia secondary to chronic rhinosinusitis (CRS) may be caused in part by conductive, sensorineural, and/or central dysfunction. Conductive dysfunction may result where mucosal edema or polyps lead to mechanical obstruction of odorant transmission to the olfactory cleft. In keeping with this, olfactory function is correlated with the degree of olfactory cleft opacification found on computed tomography (CT). Inflammation within the neuroepithelium may also lead to short-term, reversible OR-odorant binding interference. Accordingly, multiple studies have demonstrated a link between olfactory dysfunction in CRS and eosinophilia. When longer established, such inflammatory change can also lead to histologic remodeling of the OE and replacement with respiratory or squamous type epithelium. Finally, areas of the primary and secondary olfactory cortex (including the OB and orbitofrontal cortex) are structurally altered in established disease. Therefore, sensorineural and central mechanisms may contribute to the pathophysiology of olfactory dysfunction in CRS, in addition to conductive mechanisms. Similar arguments can be made for other disease etiologies, for example, in posttraumatic impairment, which can also be the result of combined conductive, sensorineural, and central mechanisms. As a result of these limitations, olfactory research has evolved away from anatomic classification, instead using presumed underlying etiology to classify dysfunction. Accordingly, the most common causes and, therefore, classifications of olfactory dysfunction are outlined in Box 36.1 .
| Conductive | Obstructed transmission of odorants to the ORN. |
| Sensorineural | Damage to the ORN. |
| Central | Damage to, or loss of areas of, the central nervous system involved in olfactory processing. |
Sinonasal olfactory dysfunction
Postinfectious olfactory dysfunction (PIOD)
Posttraumatic olfactory dysfunction (PTOD)
Neurologic/neurodegenerative olfactory dysfunction
Congenital olfactory dysfunction
Endocrine-associated olfactory dysfunction
Drug/toxin-associated olfactory dysfunction
Age-related olfactory dysfunction
Idiopathic olfactory dysfunction
Other a
a Includes iatrogenic dysfunction, dysfunction caused by neoplasia, and dysfunction caused by multiple systemic comorbidities
There are estimated to be approximately 200 different etiologic causes for olfactory dysfunction ; however, work from several specialist centers has demonstrated that when age-related dysfunction is excluded, approximately two thirds of cases can be attributed to sinonasal, postinfectious, or posttraumatic causes (e.g., see references ) ( Table 36.3 ).
| Etiology | Goodspeed et al. ( n = 441) |
Seiden and Duncan ( n = 428) |
Miwa et al. ( n = 345) |
Temmel et al. ( n = 278) |
Brämerson et al. ( n = 292) |
|---|---|---|---|---|---|
| Sinonasal | 30.2% | 14% | 21.4% | 21% | 14.3% |
| Postinfectious | 18.6% | 18% | 17.1% | 36% | 27.4% |
| Posttraumatic | 8.6% | 18% | 17.1% | 17% | 10.3% |
| Idiopathic | 21% | 18% | 28.4% | 18% | 33.6% |
| All other causes | 21.6% | 32% | 16% | 8% | 14.4% |
Sinonasal disease is a common cause of quantitative olfactory dysfunction and most frequently refers to chronic inflammatory conditions of the nose and paranasal sinuses. Accordingly, quantitative olfactory dysfunction is a key diagnostic symptom for CRS, as outlined in both the European Position Paper on Chronic Rhinosinusitis and Nasal Polyps and the American Academy of Otolaryngology-Head and Neck Surgery Guidelines. Given that a large proportion of the general population is thought to have CRS (e.g., 10.9% of the European population ), and that such conditions are often treated outside of the specialist chemosensory clinic (e.g., primary care or general ENT/rhinology clinics), it is likely that the true burden of sinonasal olfactory dysfunction is greater than is reflected in specialist case series. Nevertheless, such series demonstrate a prevalence of 7% to 56% of cases seen.
Within this heterogenous group of conditions, the degree of olfactory impairment is greatest in CRS with nasal polyposis followed by CRS without polyps, nonallergic rhinitis, atrophic rhinitis, and allergic rhinitis. Patients frequently describe a gradual onset of impairment with a fluctuating course, and as the dysfunction experienced is predominately quantitative, parosmia and phantosmia are rare. Some epidemiologic studies have shown a slight female preponderance, and when left untreated it is unlikely that sinonasal olfactory dysfunction will resolve spontaneously.
As outlined above, the pathophysiologic mechanism underlying olfactory dysfunction in CRS involves a combination of factors. First, mechanical obstruction of odorants may occur owing to edema, discharge ± polyps. Consequent changes in nasal aerodynamics and reduced access to the OE have been shown to contribute to olfactory impairment. Once odorants have reached the OE, their perception may be further impaired through inflammatory mediated OR dysfunction. Work in transgenic animal models has demonstrated short-term TNF-α dependent OR interference. With time, and as inflammation becomes more established, it appears that ORN death occurs, coupled with subsequent impaired neuronal regeneration. This animal work is in keeping with findings from human studies. For example, the link between eosinophilia and reduced olfactory function is well established and inflammatory infiltrates have been histologically demonstrated within the olfactory mucosa of patients with CRS. Other studies have demonstrated remodeling and replacement with metaplastic epithelium or fibrosis in human biopsies. Finally, structural neuroimaging studies have demonstrated reduced volume in areas of the primary and secondary olfactory networks, including the OBs, medial orbitofrontal cortex, and insula in patients with CRS, compared to controls.
Clinical studies have demonstrated superior general and olfactory specific outcomes with earlier treatment of CRS. Histologic change at the level of the neuroepithelium and structural change within the brain may underlie these findings. Sinonasal olfactory dysfunction should, therefore, ideally be treated as early as possible.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here