Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Albinism, derived from the Latin albus , meaning white, is an inherited disorder of melanin biosynthesis, which results in absent or reduced melanin production and causes a set of phenotypically heterogeneous conditions characterized by pigment deficiency. Melanogenesis requires complex interactions of enzymes and proteins within melanosomes to produce eumelanin (brown or black pigment) or pheomelanin (yellow or red pigment). This is coded for by a set of genes, whose mutations are known to cause albinism ( Fig. 40.1 ). Oculocutaneous albinism (OCA) and syndromes associated with OCA are inherited in an autosomal recessive manner, whereas ocular albinism (OA1) is associated with X-linked inheritance. Current terminology is based on the specific gene in which mutations can be found to define a particular type of albinism. Older terms, such as imperfect, incomplete, or complete albinism and tyrosinase-positive or tyrosinase-negative albinism, which were based on results of hair bulb incubation with l -tyrosine, are no longer used. Several laboratories provide panels for genetic testing of individuals with the phenotype of albinism.
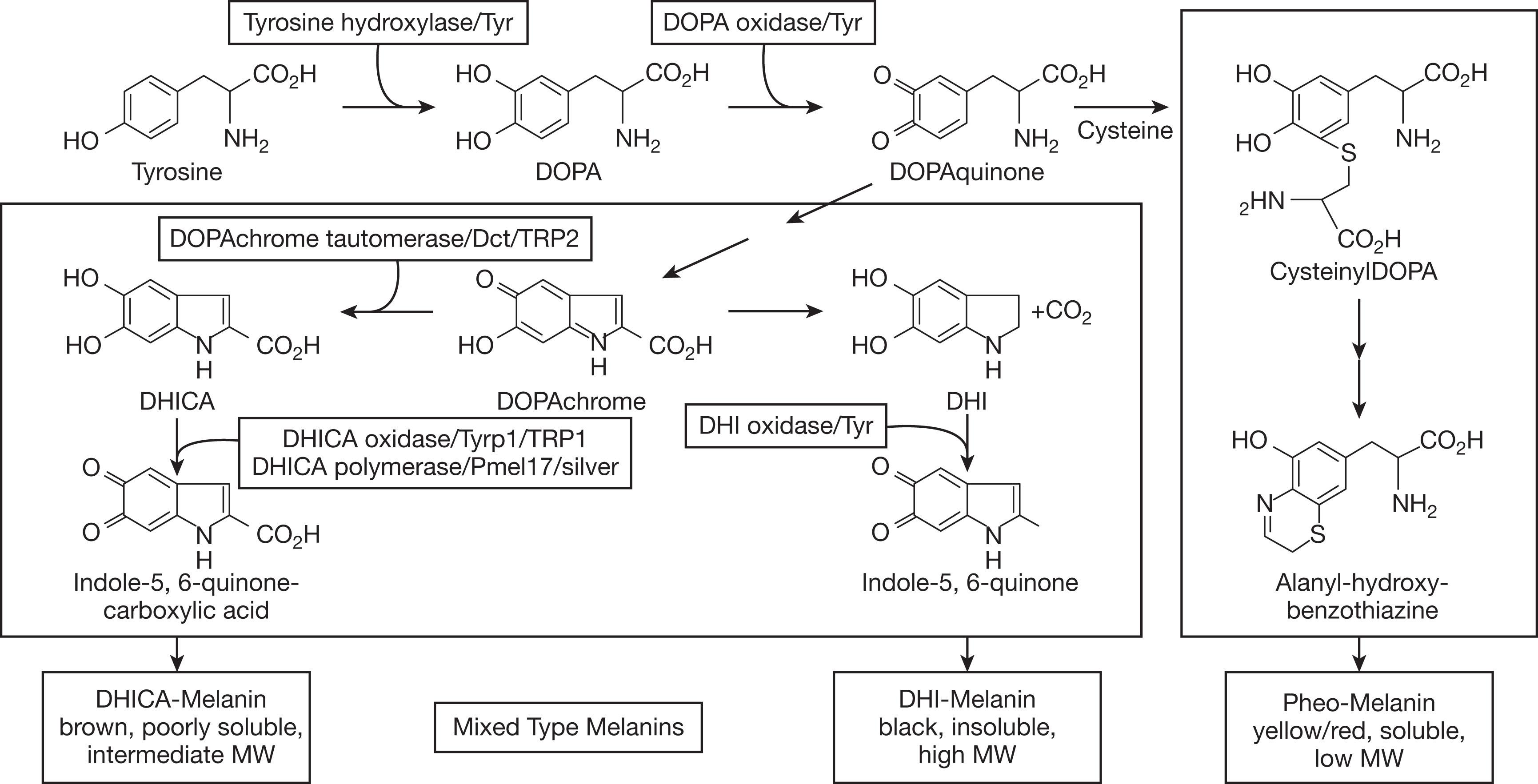
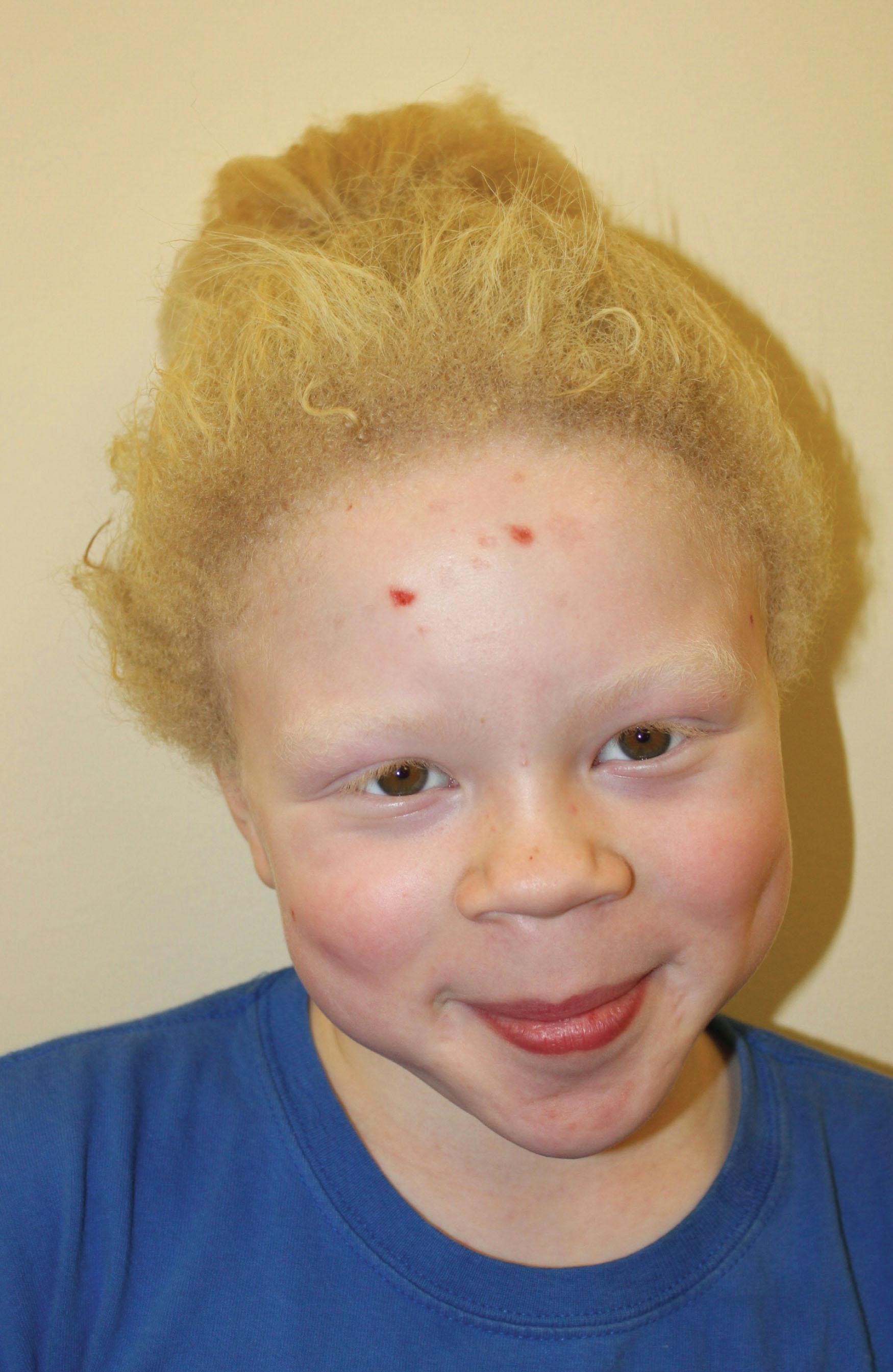
Although the rate of albinism varies geographically, albinism occurs with a frequency of approximately 1 in 20,000 worldwide, and more than 1% of the population carries a heterozygous mutation in a gene that causes albinism. The most common types are OCA1 and OCA2. OCA1 is due to mutations in the tyrosinase ( TYR ) gene, which is essential in the initial and rate-limiting steps of melanin biosynthesis. Persons with OCA1A (previously tyrosinase-negative) produce no melanin pigment in their skin, hair, or eyes over their lifetime. Those with OCA1B have some residual enzyme activity so they produce some melanin pigment in their hair, their eyelashes darken, their skin develops a slight tan, and melanin pigment can be detected in the posterior iris epithelium and occasionally in the macular retinal pigment epithelium (RPE) ( Fig. 40.2 ). OCA1 includes those diagnosed with minimal pigment or temperature-sensitive albinism, and those previously classified as having yellow albinism. Those with OCA2, due to the mutations in the OCA2 gene, often have blond or red hair at birth ( Fig. 40.3 ). Persons with OCA2 can often be difficult to distinguish from persons with OCA1B due to their pigmenting phenotype. In sub-Saharan Africa, there is a predominance of OCA2 due to a 2.7-kb interstitial deletion in OCA2 , related to a founder effect. Other types of OCA include OCA3, OCA4, OCA5, OCA6, OCA7, and OCA8 ( Table 40.1 ).

| Type of albinism | OMIM# | Gene | Locus | Encoding function | Comments |
|---|---|---|---|---|---|
|
|
TYR | 11q14–21 | Tyrosinase, which catalyzes several steps in melanogenesis | OCA1A: no melanin; OCA1B: varying amounts of melanin are present |
| OCA2 | 203200 | OCA2 (previously called P gene) | 15q11.2–12 | Melanosomal membrane protein | Common in sub-Saharan Africa due to 2.7-kb deletion; includes brown OCA |
| OCA3 | 203290 | TYRP1 | 9p23 | Stabilizes tyrosinase and regulates eumelanin production | Previously called red or rufous OCA |
| OCA4 | 696574 | SLC45A2 (previously called MATP and AIM1 ) | 5p13.2 | Membrane transport protein | Minimal to near normal melanin; phenotype similar to OCA2; common in Japan |
| OCA5 | 615312 | Unknown | 4q24 | Unknown | Described in Pakistani family |
| OCA6 | 609802 | SLC24A 5 | 15q21.1 | Melanosome maturation | Described in Chinese family |
| OCA7 | 615179 | C10orf11 | 10q22.2–q22.3 | Melanocyte differentiation | Described in families in Faroe Islands and Denmark |
| OCA8 | NA | DCT/TYRP2 | 13q32.1 | Enzyme catalyst in melanogenesis | Described in a French girl and a North African woman |
| OA1 | 300500 | GPR143 | Xp22.2–22.3 | Regulation of melanosome distribution | Nettleship–Falls OA; pigmentary mosaicism in obligate carrier |
| HPS-1 | 203300 | HPS1 | 10q23.1–23.3 | Transmembrane protein in BLOC-3 within BLOC-4 and BLOC-5 | Common in northwestern Puerto Rico due to founder effect; progressive pulmonary fibrosis and colitis |
| HPS-2 | 608233 | ADTB3A , AP3B1 , HPS2 | 5q14.1 | Lysosomal trafficking | Neutropenia and/or immune defects; pulmonary fibrosis, conductive hearing loss, dysplastic hip |
| HPS-3 | 614072 | HPS3 , BLOC2S1 | 3q24 | Vesicle-related protein | Found in central Puerto Rico; mild systemic findings; colitis |
| HPS-4 | 614073 | HPS4 , BLOC3S2 | 22q11.2–12.2 | Involved in BLOC-3 and BLOC-4 | Pulmonary fibrosis, colitis |
| HPS-5 | 614074 | HPS5 , RU2 , KIAA1017 , BLOC2S2 | 11p15–p.13 | Organelle biogenesis | Mild phenotype; hyperlipidemia, elevated creatinine clearance in some |
| HPS-6 | 614075 | HPS6 , RU , BLOC2S3 | 10q24.32 | Organelle biogenesis | Mild systemic findings |
| HPS-7 | 614076 | DTNBP1 , HPS7 , BLOC1S8 | 6p22.3 | Component of BLOC-1 | Mild lung disease |
| HPS-8 | 614077 | BLOC1S3 , HPS8 | 19q13.32 | Vesicular transport protein | Mild systemic findings |
| HPS-9 | 614171 | BLOC1S6 , PLDN , PA , HPS9 | 15q21.1 | Intracellular vesicle trafficking | Few cases described |
| HPS-10 | 617050 | AP3D1 , HPS10 | 19q13.32 | Lysosomal biogenesis | Microcephaly, seizures, immunodeficiency |
| HPS-11 | NA | BLOC1S5 | 6p24.3 | Lysosomal biogenesis; cargo transport | Few cases described |
| CHS | 214500 | LYST (previously CHS1 ) | 1q42.1–42.2 | Lysosomal trafficking regulator protein | Frequent infections due to immunodeficiency; may have bleeding diathesis |
* OCA1 includes previously described temperature-sensitive OCA, minimal pigment OCA, and yellow OCA.
Syndromes associated with OCA are less common than non-syndromic albinism and include Hermansky–Pudlak syndrome (HPS), occurring with a frequency of approximately 1–9 per 1,000,000 persons worldwide, although certain types have a greater prevalence in particular areas (e.g. 1 in 1800 in northwest Puerto Rico due to founder mutations in HPS-1). In HPS, affected individuals have absent dense bodies (delta granules) in their platelets, which interferes with the secondary phase of aggregation to exogenous stimuli. Thus, individuals with HPS have easy bruising, epistaxis, and prolonged bleeding after procedures such as dental extraction, childbirth, and surgery. Eleven types of HPS have been identified to date, and all have autosomal recessive inheritance causing abnormalities in proteins involved in the biogenesis of lysosome-related organelles (e.g. melanosomes, dense granules in platelets). Some types are associated with pulmonary fibrosis and intestinal accumulation of ceroid, causing granulomatous colitis/inflammatory bowel disease (see Table 40.1 ). Whole-mount electron microscopy of platelets without dense bodies can identify those with HPS, but genetic testing identifies the specific type of HPS and the potential for other systemic abnormalities. The phenotype for OCA in HPS is variable and can overlap with non-syndromic types of albinism. Genetic testing may be necessary to establish the diagnosis.
Chédiak–Higashi syndrome (CHS), inherited as an autosomal recessive disorder, is also associated with OCA. Persons with CHS have frequent infections and can progress to a lymphoproliferative phase. They are treated with hematopoietic stem cell transplantation, preferably prior to the accelerated phase. If untreated, they will also develop neurological abnormalities and eventually succumb to an overwhelming infection. Although these individuals have the ocular and cutaneous features of albinism, their hair has a subtle silvery sheen, often prompting investigation to establish the diagnosis. Peripheral blood smear shows giant intracellular granules in neutrophils.
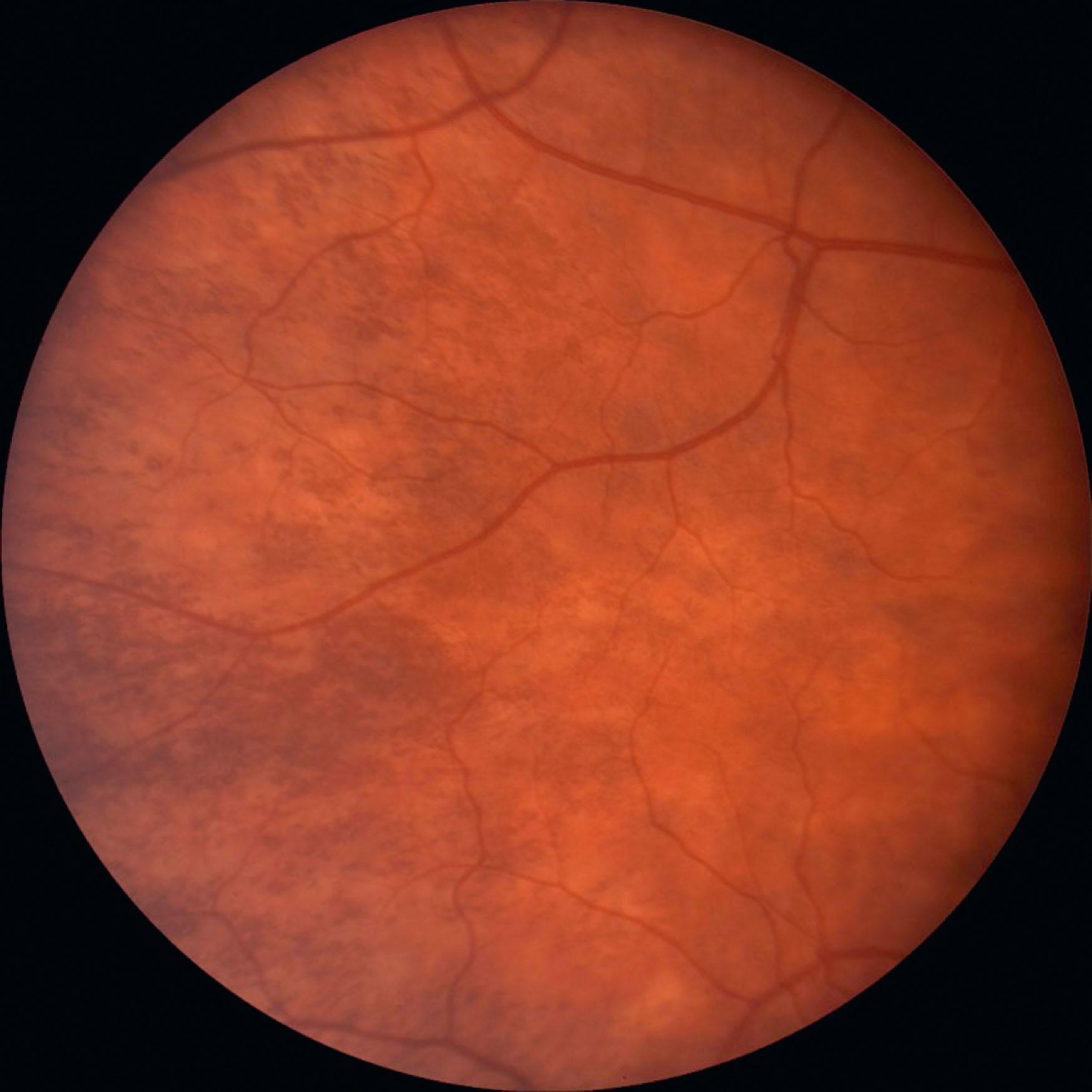
In ocular albinism (OA1), which occurs less frequently than OCA, there is a mutation in the GPR143 gene on the X chromosome. Males with OA1 have the typical ocular features of albinism, but their cutaneous and hair pigment is nearly normal. Some may have hypopigmented macules; skin biopsy may show macromelanosomes, but they are not pathognomonic. Without careful eye examination, these individuals may be identified as having uncomplicated infantile nystagmus syndrome. Mothers of children with OA1 will typically show some iris transillumination and pigmentary mosaicism in the retina ( Fig. 40.4 ), unless the child has a new mutation; the obligate carrier is rarely symptomatic. Pigmentary mosaicism, noted in 80%–90%, represents expression of Lyonization with pigmented RPE adjacent to non-pigmented areas, most notable in the mid-periphery of the fundi. Some males and females with almost normal pigmentation have been given a diagnosis of autosomal recessive ocular albinism, but many have been found to have OCA with genetic testing, most often due to mutations in the TYR gene. If a deletion in GPR143 occurs, contiguous genetic disorders may be associated with OA1, e.g. ichthyosis of the skin ( STS ), Kallman syndrome ( KAL1 ), and chondrodysplasia punctata ( ARSL ).
The ocular features in albinism vary from one individual to another, within a family, and within a specific type of albinism, due to interaction of heterogeneity in the type of albinism and the constitutional genotypes. A clinical diagnosis of albinism is based on a constellation of findings and may be confirmed with genetic testing.
Many parents report that their infants with albinism close their eyes when exposed to bright light and do not appear to be as visually attentive as their children without albinism, but most agree that visual attentiveness improves by 5–6 months of age. Vision often continues to improve as the child matures but rarely becomes normal. The spectrum of best-corrected visual acuity in persons with albinism ranges from 20/20 to worse than 20/400. A spectrum of visual acuity is noted across and within the different types of albinism, and persons with OCA1A generally have worse visual acuity than persons with other types of albinism. High refractive errors are common in albinism, with large amounts of astigmatism being particularly noted in OCA1A. Early correction of large refractive errors and careful re-evaluation of the refractive error over time maximizes the visual outcome by reducing ametropic amblyopia and improving binocular alignment. Other reasons for reduced visual acuity include foveal hypoplasia, light scattering, nystagmus, and possibly retinostriate misrouting. Certain features associated with relatively better visual outcomes include demonstration of some stereoacuity and the presence of granular melanin pigment in the macula with clinical evidence of macular differentiation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here