Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intrauterine infection is defined as an infection transmitted to the fetus any time during pregnancy, except for the last week (this is a perinatal infection). Intrauterine infections contribute to adverse pregnancy outcomes, such as stillbirth, preterm birth, intrauterine growth restriction and congenital abnormalities. Approximately 2% of live-born infants have an intrauterine infection, which translates to approximately 2.5 million infants per year globally, with the low- and middle-income countries (LMIC) affected disproportionately. These infants may be asymptomatic or born with symptomatic disease. The mechanism of infection is almost always transplacental hematological spread via the umbilical vein. This results in widespread disease involving multiple organs. Usually, the earlier in pregnancy a fetus is infected, the more severe the sequelae. In 1971, the now widely used acronym “ToRCH complex/syndrome” was coined to include four congenital infections (TOxoplasmosis, Rubella, CMV, and Herpes), which were thought to have similar clinical presentations. Subsequently the “O” in TORCH has come to represent a long list of “Other” infections, including syphilis, parvovirus, human immunodeficiency virus (HIV), tuberculosis, malaria, listeriosis, Zika virus, and many others. The term “TORCH” is further compromised by the fact that true congenital herpes is rare, as it is usually acquired perinatally. Diagnostic investigations should therefore not be batched as a “TORCH screen,” but rather targeted towards the most likely cause. As infection is transplacental, histological and/or microbiological placental specimens may be extremely helpful when the etiology is unclear. Many intrauterine infections share similar clinical signs, with hepatosplenomegaly being an important and frequent finding ( Table 11.1 ). Isolated growth restriction is not an indication for further investigations. The specific systemic manifestations of each organism will be discussed in the respective chapter subsection. The relative global health burdens of intrauterine infections are summarised in Table 11.2 , which helps guide readers as to their impact and importance.
| Growth restriction | Maculopapular rash | Hydrops fetalis |
|---|---|---|
| Preterm infant | Pneumonitis | Bony changes |
| Hepatosplenomegaly | Petechiae/purpura | Adenopathy |
| Jaundice (conjugated) | Pallor | Microcephaly |
| Disease | Worldwide cases per year | Symptomatic at birth | Notes |
|---|---|---|---|
| Congenital cytomegalovirus | 1 million | 10%–15% | 10% of asymptomatic infants will develop hearing loss |
| Congenital syphilis | 600,000 | 40% |
|
| Congenital toxoplasmosis | 200,000 | 10%–30% |
|
| Congenital rubella syndrome | 100,000 | Majority | Vaccine preventable |
| Congenital Zika syndrome | <2000 | Majority | Endemic cases with sporadic outbreaks, predominantly in tropical regions |
| Congenital herpes simplex | <500 | All | Intrauterine transmission is rare |
Cytomegalovirus (CMV) is a member of the Herpes virus family. It is a ubiquitous virus and the most common cause of congenital infections in the world. Infection in healthy children or adults is usually asymptomatic, or non-specific. For immunocompromised individuals, especially transplant patients and those infected with HIV, CMV disease can be severe. Infection during pregnancy poses significant risks to the fetus. As the virus establishes latency, a current infection may therefore be primary, a reactivation or re-infection. Infection as a result of reactivation or re-infection tends to be milder, due to the presence of antibodies. Seroprevalence in adults ranges from 40% to 100%, with higher rates found in countries with lower socioeconomic development. Contributory factors include densely populated areas and limited access to sanitation.
It is estimated that 0.2%–2% of all infants are born with congenital CMV – approximately one million infants per year, of which 10%–20% (approximately 150,000) suffer disability or death. Congenital CMV is the leading cause of non-genetic deafness and imposes a significant financial burden ($3 billion per year in the United States alone). Transmission to the fetus occurs in approximately 40% of cases when the mother has primary CMV infection during pregnancy. Although transmission is more common later in pregnancy, the earlier it occurs, the more severely the fetus may be affected. If the mother has a re-infection or reactivation, the risk of transmission is only 1%. Although for the individual this is low, in populations with high seroprevalence, more infants are infected due to re-infection or reactivation than due to primary infection. Between 85% and 90% of congenitally infected infants do not readily exhibit clinical symptoms of CMV infection at birth. However, 10% of these asymptomatic infants may be born with, or develop sensorineural hearing loss over time. The remaining 10%–15%, described as having symptomatic congenital CMV infection, often demonstrate one or more clinically observable abnormalities, such as hepatosplenomegaly (60%) and growth restriction (50%). Other signs that are more typical of congenital CMV include petechiae (50%–70%), microcephaly (35%–50%), hearing loss and chorioretinitis. A head ultrasound may reveal abnormalities such as periventricular calcification, lenticulostriate vasculopathy, dilated ventricles, and white matter changes. A higher rate of congenital CMV has been noted in HIV-exposed infants, both in the pre- and post-highly active antiretroviral therapy (HAART) era, and this was found to be independently associated with advanced maternal immunosuppression (maternal CD4 count <200 cells/μL).
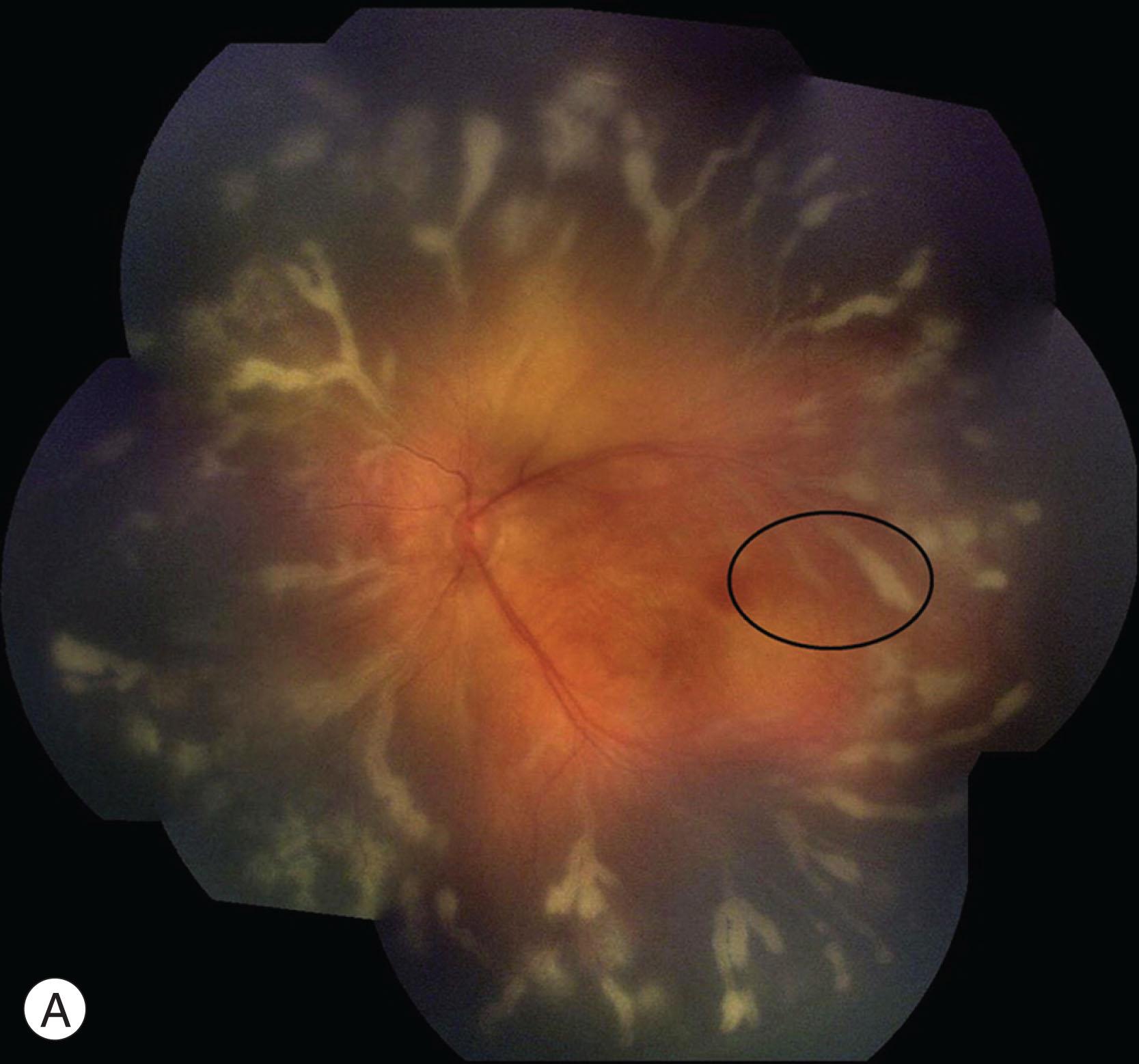
The retina and brain have the highest density of vascular pericytes in the human body. Laboratory research has shown CMV to induce proinflammatory and angiogenic cytokines within the pericytes of the inner blood–retinal barrier, which likely serves as an amplification reservoir contributing to retinal vasculitis and angiogenesis. In a long-term, prospective study on congenital CMV conducted in the United States, a similar prevalence and severity of anterior segment findings were found among the symptomatic, asymptomatic, and control groups, suggesting CMV may play a limited role in damaging non-neuron-associated cell lines. The anterior segment findings in congenital CMV patients, such as corneal stromal scar, anterior polar cataract, microcornea, superficial punctate keratitis, and iris heterochromia, were therefore unlikely to be caused by the infection, and were also not associated with significant visual impairment. By contrast, the posterior segment of the eye was more severely affected, resulting in chorioretinal scars, optic atrophy and subsequent severe visual loss (best-corrected visual acuity worse than 20/200) ( Table 11.3 ). Active and progressive CMV retinitis may occur in children with symptomatic congenital CMV infection, who are not otherwise immunocompromised. Immaturity of the immune response in the fetus and young infants is believed to be the major determinant of virulence. Even so, the exact nature and extent of this immune deficiency in congenitally-infected infants have yet to be resolved. A delayed presentation of congenital CMV retinitis may occur, as well as progression or recurrence, despite systemic anti-viral treatment ( Fig. 11.1A,B ). Cyclopia, anophthalmia, microcornea, chorioretinal hypoplasia, and optic nerve aplasia (characterized by the absence of the optic nerve, retinal blood vessels, and retinal ganglion cells) have also been described. Although not a feature in asymptomatic infants, the posterior visual pathways may be affected in symptomatic infants, with cerebral visual impairment found to be present in 14%.
| Characteristics | Symptomatic | Asymptomatic | Control | Significance (P) | ||
|---|---|---|---|---|---|---|
| S-A | S-C | A-C | ||||
| Number of patients ( n ) | 77 | 109 | 51 | |||
| Vision (at most recent examination, n (%) | ||||||
| Normal | 52 (67.5) | 90 (82.6) | 44 (86.3) | 0.017 | 0.017 | 0.554 |
| Moderate impairment | 5 (6.5) | 5 (4.6) a | 0 (0) | 0.743 | 0.156 | 0.178 |
| Severe impairment | 10 (13.0) | 0 (0) | 0 (0) | <0.001 | 0.006 | n/a |
| Not evaluated | 10 (13.0) | 14 (12.8) | 7 (13.7) | 0.977 | 0.904 | 0.878 |
| Optic atrophy, n (%) | 8 (10.4) | 0 (0) | 1 (2.0) | 0.001 | 0.085 | 0.319 |
| Unilateral | 3 (3.9) | 0 (0) | 0 (0) | |||
| Bilateral | 5 (6.5) | 0 (0) | 1 (2.0) b | |||
| Retinal lesions, n (%) c | 20 (26.0) | 4 (3.7) | 1 (2.0) | <0.001 | <0.001 | 1.000 |
| Unilateral | 11 (55) | 3 (75) | 1 (100) d | 0.273 | 1.000 | n/a |
| Bilateral | 8 (40) | 0 (0) | 0 (0) | |||
| Not noted | 1 (5) | 1 (25) | 0 (0) | |||
| Retinal detachment | 1 (1.3) | 0 (0) | 0 (0) | 0.170 | 0.517 | n/a |
a Four of these five patients’ visual acuities were taken without correction, one had glaucoma.
b Leber’s hereditary optic neuropathy.
c Retinal lesions include active chorioretinitis, retinal scars, and all non-CMV-related lesions.
Antenatally, amniocentesis and PCR on amniotic fluid is the preferred method of diagnosing congenital CMV. Detection by PCR or virus culture of the urine or saliva within the first 21 days of life confirms the diagnosis. IgM serology and viral load are less reliable, due to false-negative results.
Infants who have central nervous system (CNS) involvement or significant end-organ damage should be treated. Ganciclovir (intravenous), or its prodrug valganciclovir (oral) may be used. Ganciclovir is preferred initially in more serious cases. Treatment should be started within 28 days of birth and continued for 6 months. Side effects, which are milder with valganciclovir, include neutropenia and thrombocytopenia, and blood counts should be monitored carefully. The effectivity and treatment duration for congenital CMV retinitis is not well-established. However, macular proximity, active progression or recurrent retinitis in resistant strains have prompted clinicians to pursue an extended course of IV ganciclovir and/or foscarnet, or targeted therapy with intravitreal injections of ganciclovir and/or foscarnet.
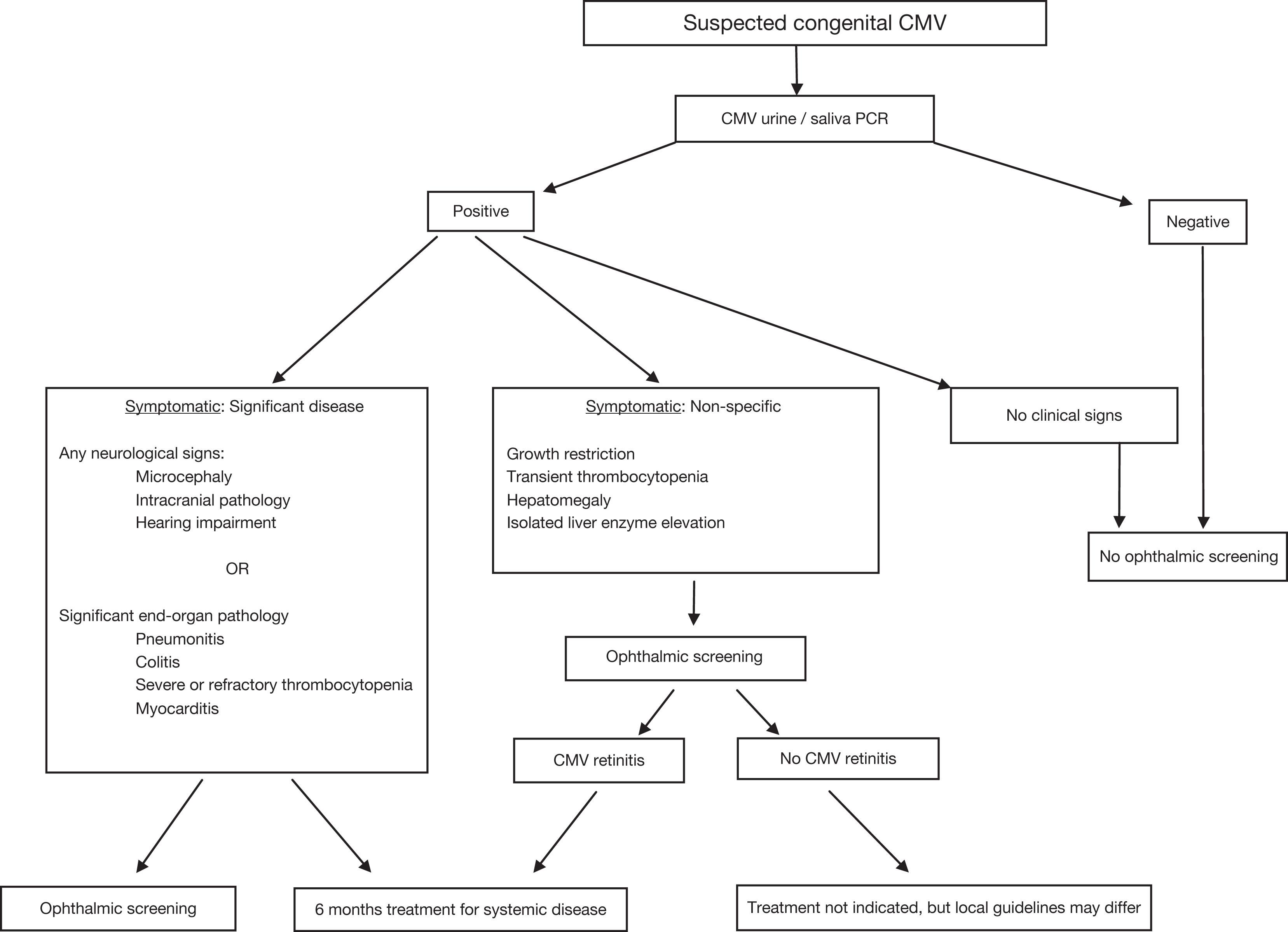
Currently there are many unanswered questions surrounding the optimal management of congenital CMV. These include the cost-efficacy of screening programs, the management of asymptomatic infants, the treatment of preterm infants, and whether starting treatment after 28 days of life is indeed beneficial. Ophthalmic screening of symptomatic infants helps to support the diagnosis of congenital CMV and may provide further motivation for the initiation of treatment. Apart from reducing sensorineural hearing loss, treatment has been shown to improve secondary markers, such as increased growth parameters, resolution of abnormal liver functions, and fewer neurological sequelae, and may cause resolution of chorioretinitis in some cases. An example of the screening algorithm used at our institution is reproduced in Fig. 11.2 .
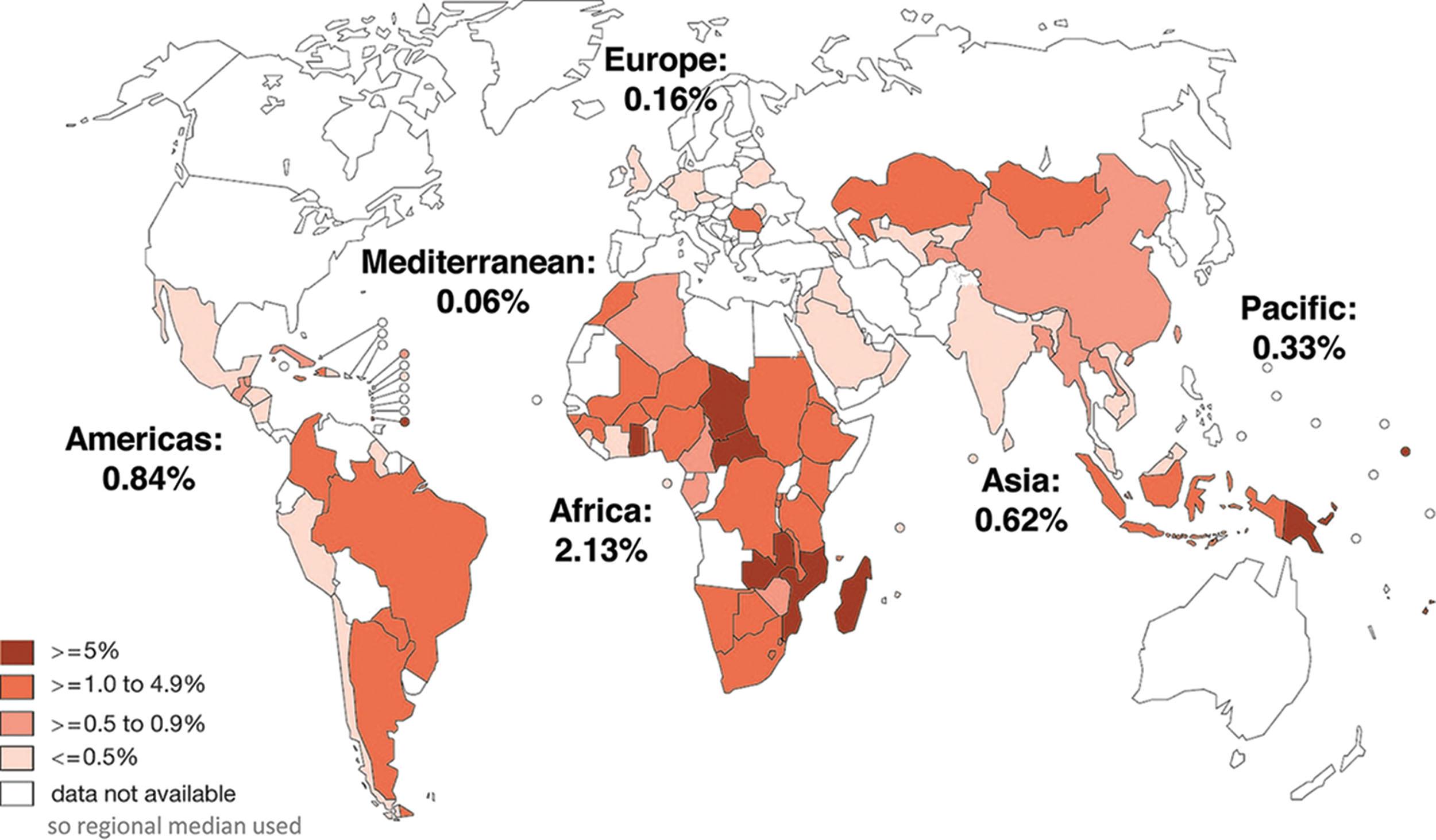
Syphilis is an infection caused by the spirochete Treponema pallidum (T. pallidum) . Humans are the only natural host. Mother-to-child transmission during pregnancy can lead to serious fetal outcomes in the second or third trimester, including preterm birth, low birth weight, congenital infection, and early fetal or neonatal death. Despite the availability of affordable preventive measures for more than 50 years and World Health Organization (WHO) strategies to eliminate congenital syphilis, limited progress has been made. The estimated global maternal syphilis prevalence in 2016 was 0.69%, resulting in a global congenital syphilis rate of 473 per 100,000 live births and 661,000 total cases. The greatest burden of syphilis in pregnancy occurs in Africa (over 50%). In some areas, including the United States, rates have actually increased. Figure 11.3 geographically depicts syphilis seropositivity among antenatal care attendees, reported by countries through the WHO HIV Universal Access System in 2008 or 2009.
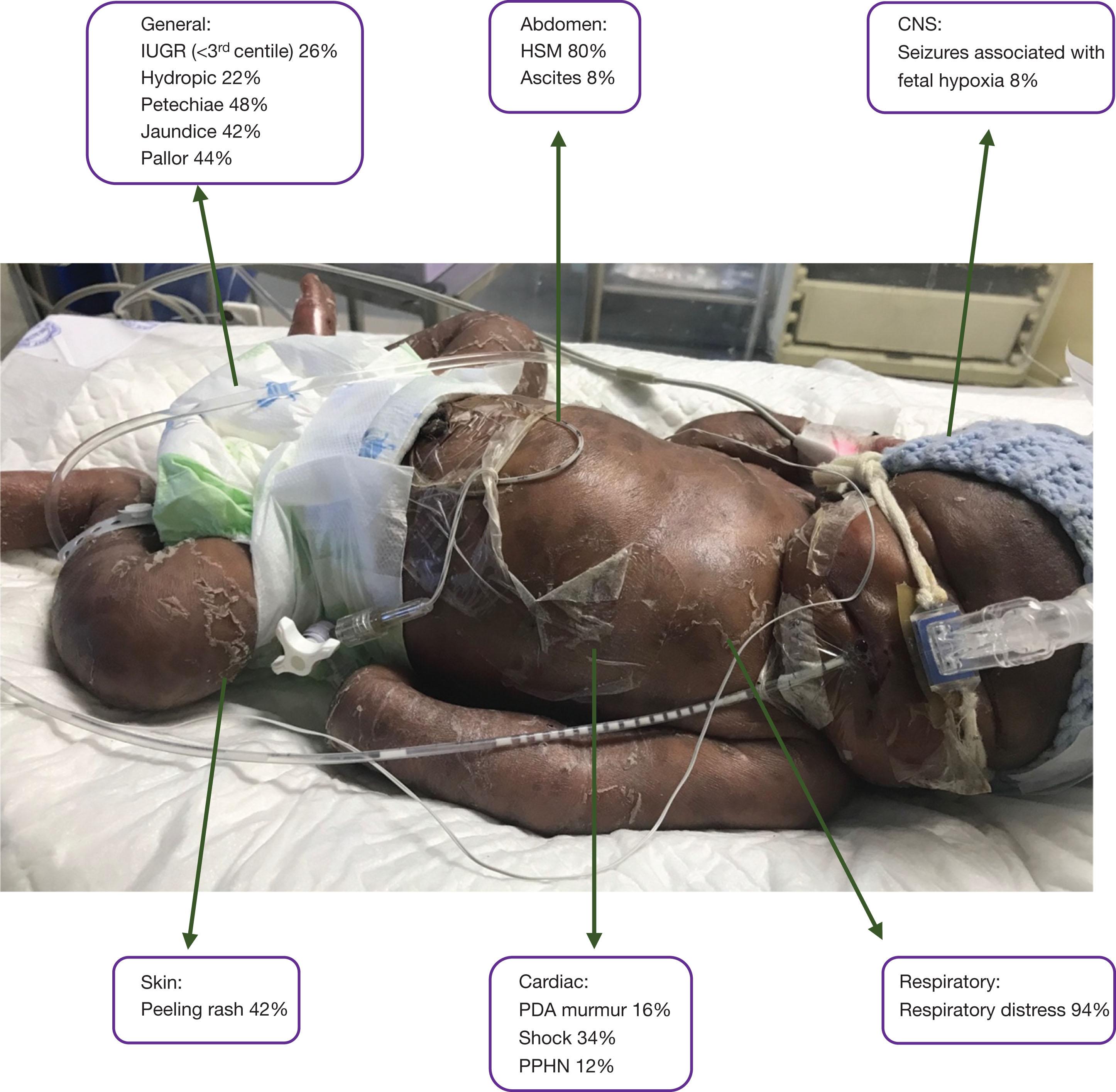
The disease is acquired through transplacental transmission of spirochetes, which occurs with increasing frequency as gestation advances. Women with untreated primary or secondary syphilis are more likely to transmit it to their fetuses than those with latent disease. Of infections acquired later in pregnancy, 40%–67% may be asymptomatic at birth. Syphilis is known as the great mimicker because of its protean clinical manifestations. When congenital, it usually manifests within the first month of life. If symptomatic at birth, infants may be extremely unwell and require intensive care. The clinical signs of symptomatic neonates in a cohort of 50 South African infants are shown in Fig. 11.4 . Hydrops fetalis was found to be an independent risk factor for mortality. Along with the generalised signs outlined in Table 11.1 , it is important to be aware of the following systemic manifestations of congenital syphilis:
The placenta is usually large, edematous, and pale.
Hepatomegaly is almost universal (80%–90%), and is often associated with splenomegaly.
A maculopapular or vesicular rash, which usually involves the palms and soles, may be present at birth, or develop later. Within a few weeks, skin crusts form and desquamate.
Syphilitic rhinitis (“snuffles”) usually develops within the first week of life. The nasal discharge is persistent and may be white, bloody (secondary to mucosal erosion), or purulent.
Abnormal long bone radiographs are a common manifestation of early congenital syphilis (30%–80%). Lucent metaphyseal bands are the most common finding, although demineralization and osseous destruction may occur. Periosteal reactions may occur once treatment has commenced. Occasionally pain or fractures may mimic paralysis (pseudoparesis).
Frontal bossing, mulberry molars, Clutton joints and Hutchinson’s triad (Hutchinson teeth, interstitial keratitis, and sensorineural hearing loss) are relatively specific for the late presentation of congenital syphilis.
Congenital syphilitic keratitis is typically a non-ulcerative and non-suppurative corneal inflammation. It is characterized by sectoral or diffuse corneal edema, infiltrated with interstitial vessels. It may be accompanied by iridocyclitis, iridoschisis, and iris atrophy. Untreated, the inflammatory process may slowly regress, leaving a thinned, scarred cornea with narrowed, midstromal “ghost” vessels. In more than 75% of cases both eyes are affected, either simultaneously or sequentially. The exact pathogenesis of syphilitic interstitial keratitis is unknown. An immunological mechanism, such as an antigen–antibody complex, has been proposed, as very few spirochetes have been identified in eyes with active keratitis, yet antibodies against treponemal antigens have been identified in these corneas. Often a long latency is observed between infection and ocular inflammation. The keratitis is typically not responsive to penicillin, but improves with steroids. The chronic use of topical steroids does not prevent inflammatory recurrences, however, and is known to be associated with dependence and complications such as cataract and glaucoma. For this reason, systemic immunosuppressive combination therapy (oral cyclosporin and steroids), with topical steroids used only during episodes of active inflammation, has been shown to be effective. Intracorneal hemorrhage (keratohematoma) leading to acquired posterior keratoconus has been described as a late complication of corneal neovascularization secondary to congenital syphilic keratitis. Anterior uveitis is usually non-specific and may be granulomatous or non-granulomatous. Congenital and acquired cataracts may form as a consequence of this inflammation. The chorioretinitis typically affects peripheral areas and resolves with pigment mottling. Severe cases may result in extensive retinal pigmentary changes and optic atrophy, resembling retinitis pigmentosa.
There is currently no method of culturing T. pallidum on laboratory media. Diagnosis may be established or suspected by dark-field microscopy, silver staining, or histopathological examination. PCR testing is still in development and not widely available. The combination of treponemal and non-treponemal serological tests remains the gold standard.
The protocol for the management of syphilis-exposed neonates depends on maternal treatment status, neonatal symptoms, and laboratory investigations. Asymptomatic neonates born to untreated or partially treated mothers receive a single intramuscular dose of benzathine penicillin. Neonates with signs or investigations indicating congenital syphilis receive a 10-day course of either intravenous penicillin G, or intramuscular procaine penicillin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here