Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The postoperative care of the limbal transplant patient is perhaps the most critical part of the care. Problems such as persistent inflammation or epitheliopathy should be addressed early by increasing immunosuppression or using adjunctive measures such as punctal occlusion, bandage lens, and tarsorrhaphy.
Acute rejection can be severe and present with limbal graft swelling and injection, but can also be low grade and have minimal inflammation and just an epithelial rejection line. Chronic inflammation and injection of the ocular surface undoubtedly will lead to chronic rejection of the graft.
Systemic immunosuppression has clearly been shown to improve long-term graft survival. The dose and duration of therapy is tailored to each clinical scenario. Close observation with laboratory monitoring is mandatory while on systemic immunosuppression, and collaboration with clinicians, particularly organ transplant specialists experienced in the use of such agents, is highly recommended.
The risk of adverse effects from immunosuppression in ocular surface transplant patients who tend to be younger and have no other systemic comorbidities is significantly lower than what has previously been reported for organ transplant recipients.
The postoperative management of patients after ocular surface reconstruction is often the most important aspect of the care that determines the success of the procedure. Besides maximizing the health of the ocular surface and tear film, the most critical aspect of the postoperative care is to prevent immunologic rejection of the limbal allografts. The planning of postoperative immunosuppression begins before the transplant even occurs, with the careful choice of donor tissue selection and screening, etc.
One key to long-term ocular surface stem cell transplantation (OSST) success is prevention of the first rejection episode owing to the fact that outcomes after a rejection episode can be poor overall. In addition to dual systemic immunosuppression (SI), the authors have found that preoperative screening for donor selection to minimize the antigenic burden of transplanted tissue (i.e., living-related conjunctival-limbal allograft (LR-CLAL)) maximizes the success of the procedure by identifying the best living-related donor candidate. Potential candidates for OSST surgery must first be willing to comply with long-term regular follow-up and the SI regimen. Relative contraindications to SI include a history of malignancy within 5 years, nonadherence with clinical or laboratory follow-up or nonadherence to medications, and the presence of significant comorbidities (i.e., uncontrolled diabetes, uncontrolled hypertension, congestive heart failure, other organ failure, and advanced age).
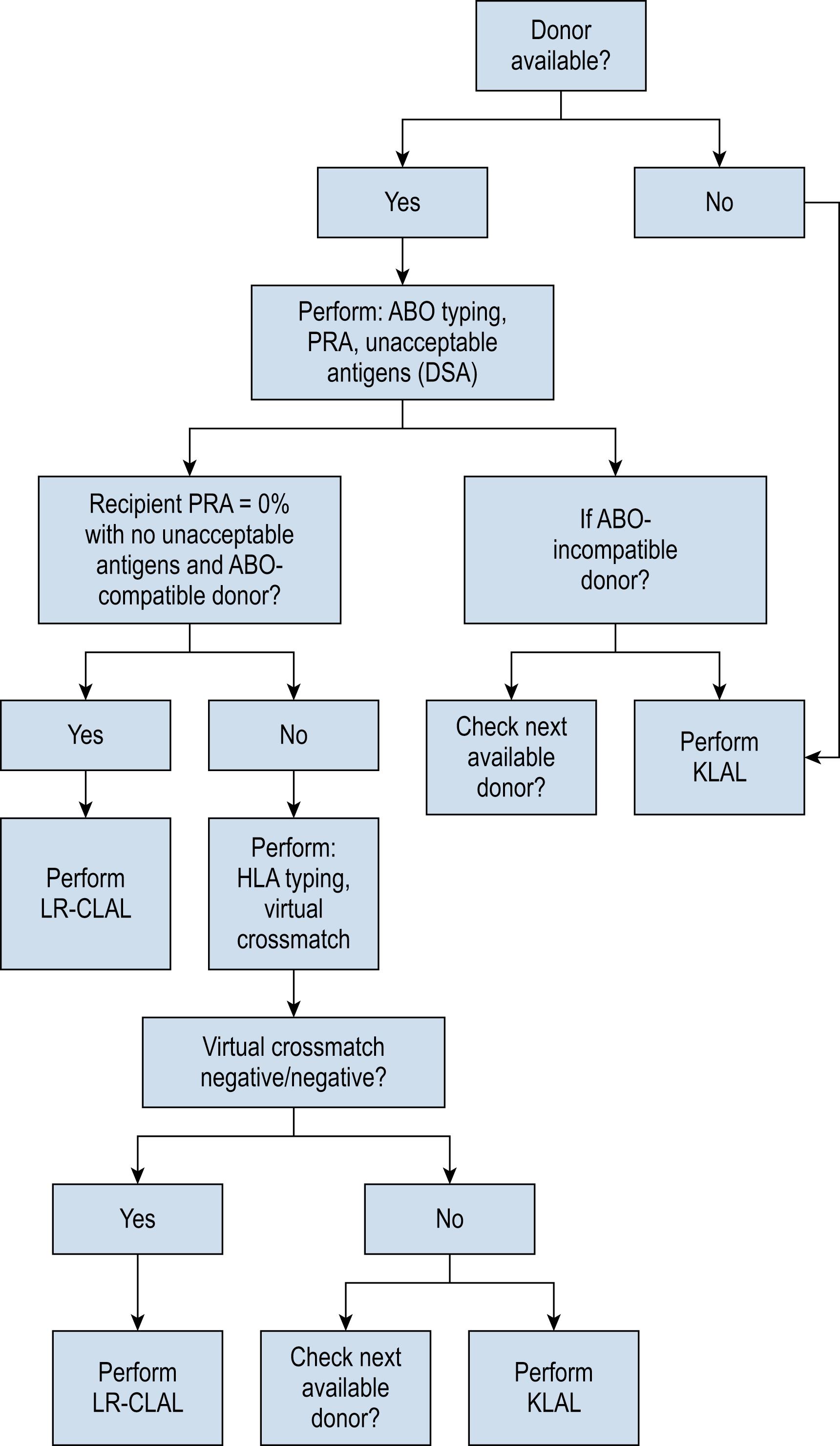
For eyes requiring an allograft OSST, patients are asked to identify potential first-degree donors. Siblings have the potential to be a human leukocyte antigen (HLA)-identical match, whereas parents and children are often at least HLA-haplo-identical (50% identical). The potential donor–recipient pair is first matched by blood type A, B, or O (i.e., ABO typed) to determine compatibility. If they are incompatible, another donor is considered (if available). If they are ABO compatible, HLA typing, donor-specific antibody (DSA), panel reactive antibody (PRA), and virtual crossmatch testing are performed.
HLA antigens are genetically determined molecules (typically proteins) found on the surface of cells that stimulate the production of antibodies. If a recipient has developed antibodies against a specific HLA antigen from prior sensitization (e.g., pregnancy, blood transfusion, and previous transplant), these specific antigens (e.g., A1 and B5) are considered unacceptable antigens (or mismatches). The number of unacceptable antigens are identified (antibodies detected above the 1500 mean fluorescence intensity threshold) for each recipient and are used to determine the calculated PRA. The potential donor is then tested for the specific unacceptable antigens through HLA typing. For each broad HLA class (class I: HLA-A, HLA-B; class II: HLA-DR, HLA-DQ), the least number of mismatches (ideally 0–2) for a donor–recipient pair identifies the closest match. Next, a virtual crossmatch for class I and class II HLA is performed to assess the immunologic compatibility based on a recipient’s alloantibody profile compared with a donor’s HLA antigen typing to predict the results of a physical crossmatch (i.e., serologic/specimen testing). A negative result for each HLA class (i.e., negative/ negative) is necessary to identify a compatible donor-recipient pair. DSAs are recipient anti-HLA antibodies specifically generated against donor cells and are also identified through testing.
PRA (reported as 0%–100%) is a screening test for a range of known unacceptable antigens and calculated based on antigen frequency regardless of donor-directed antibodies. Those recipients with higher PRA levels would be expected to potentially react to (i.e., reject) a greater proportion of the population. With no DSAs and an ABO-compatible parent or child (at least HLA-haplo-identical), a full HLA typing is not always necessary. If a recipient has PRA ≥50%, they are considered high-risk and will undergo augmented induction therapy (i.e., intravenous basiliximab). If there is a recipient PRA of 0% without any unacceptable antigens and an ABO compatible donor, full HLA typing and virtual crossmatch testing are not necessary. If a specific unacceptable antigen (i.e., DSA) is uncovered in a recipient with 0% PRA owing to a low-frequency antigen, only HLA typing for that specific HLA locus is necessary. Fig. 169.1 summarizes the decision tree for donor selection.
After identification of the best-matched available living-related donor, serologic testing for human immunodeficiency virus I/II and hepatitis B and C viruses is conducted in all potential donors. The donor eyes are screened for pertinent ocular history (e.g., previous long-term contact lens wear or previous eye surgery) and are evaluated preoperatively with a full ophthalmologic examination to assess for occult ocular disease.
Previous studies have clearly demonstrated the importance of SI in maintaining graft survival following limbal stem cell transplantation. Many earlier studies did not use an appropriate level of immunosuppression after keratolimbal allograft (KLAL), which led to a high rejection and failure rate. , For instance, short-term oral cyclosporine A (CsA) as a single agent is not adequate for preventing KLAL rejection. Similar to the experience of organ transplantation, a multidrug regimen is necessary to achieve adequate immunosuppression. Its advantage is that it allows lower doses of individual medications to be used, therefore reducing the potential risks and side effects. SI is necessary in all patients who receive limbal allografts, even in those who receive HLA-matched living-related tissue; however, minimal dosing with a multidrug approach is ideal.
Rejection is broadly divided into acute and chronic, and each can be low grade or severe. Acute rejection typically presents as regional or 360-degree KLAL swelling and hyperemia, accompanied by moderate-to-severe conjunctival injection. A progressive, advancing epithelial rejection line can be present. A new subconjunctival hemorrhage may also be visible ( Figs. 169.2, 169.3, and 169.4 ). Symptoms may include redness, light sensitivity, or pain; however, many low-grade acute rejections often present with minimal or no inflammation with an epithelial rejection line.
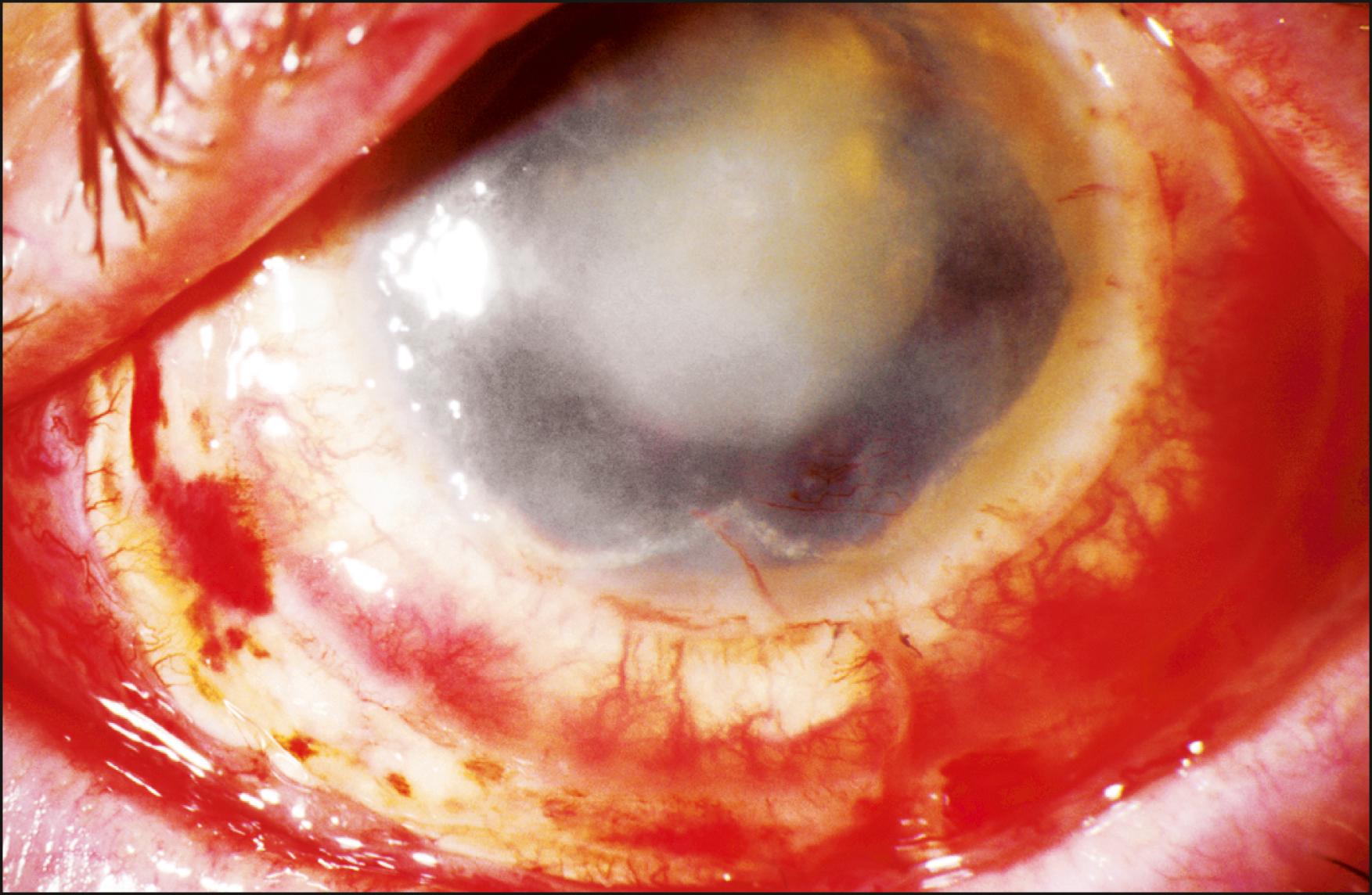
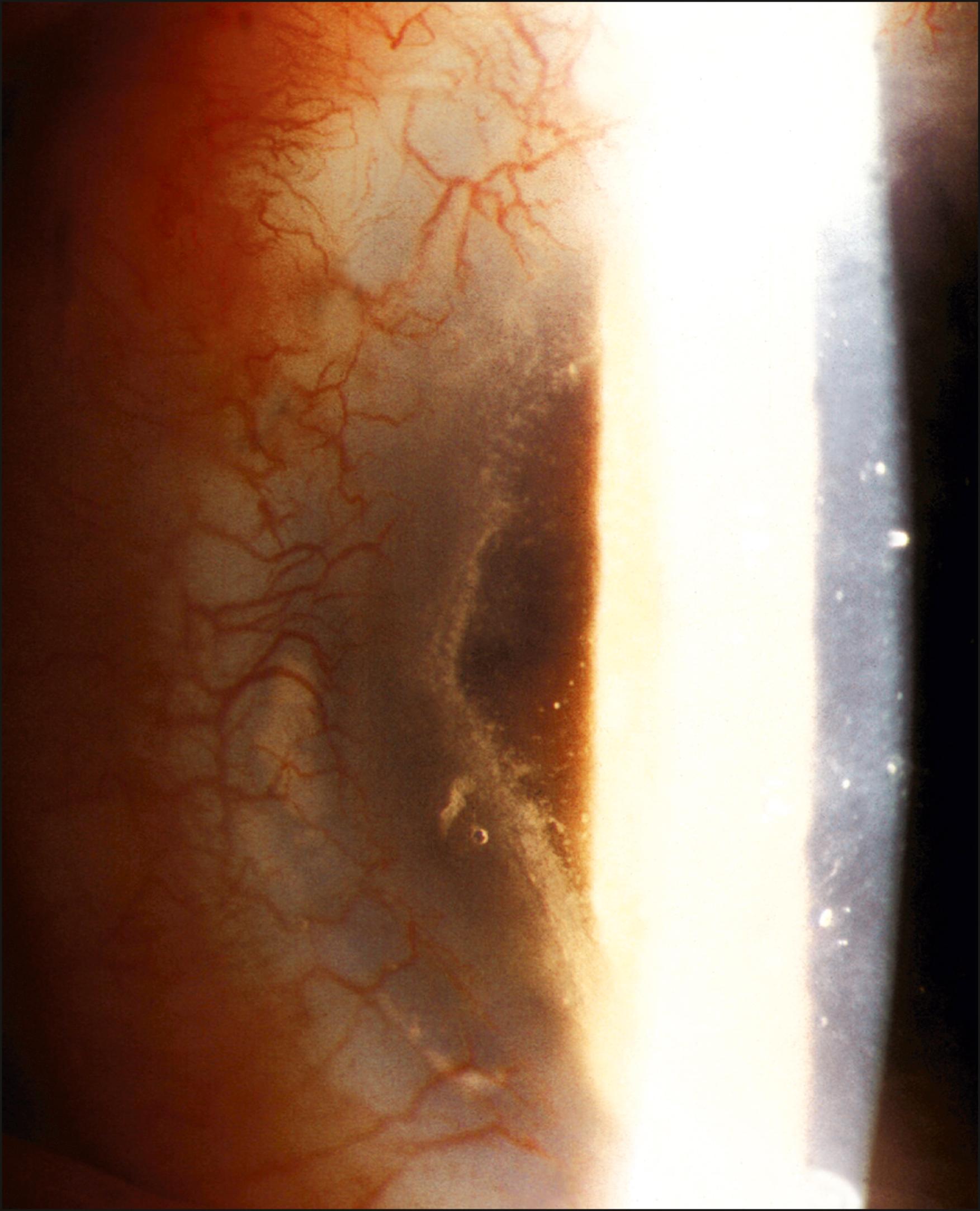
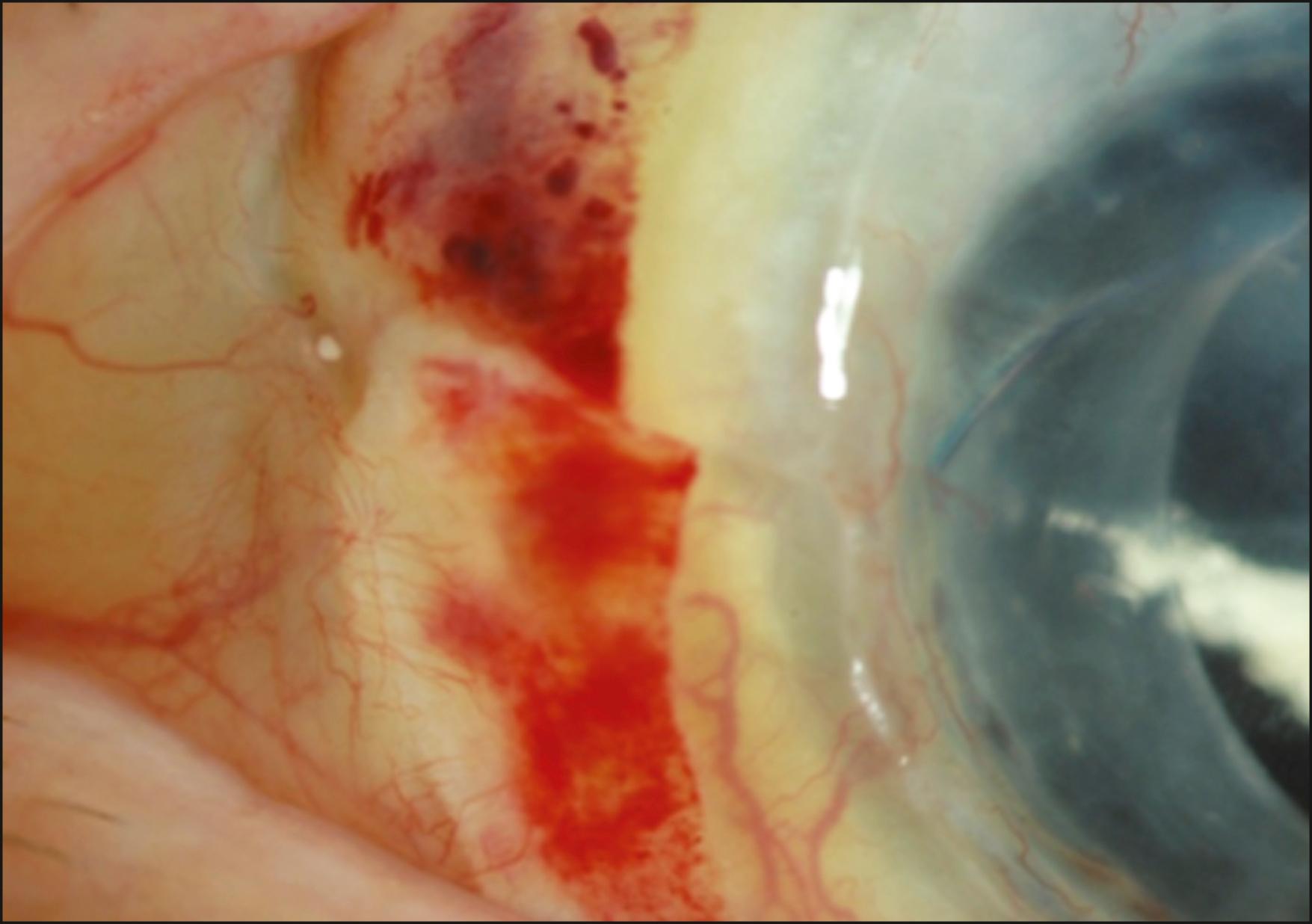
Acute rejection occurs in approximately 10%–30% of cases. It is most common in the first year, although it has been observed as late as 7 years after KLAL. Patients with underlying inflammatory diseases such as Stevens-Johnson syndrome and ocular cicatricial pemphigoid appear to be at higher risk for limbal allograft rejection. In a study by Holland et al., severe rejection occurred in 43 eyes (19.4%) with a mean time to rejection of 15.2 months (range 0.2–93.1 months). Low-grade rejection occurred in 26 eyes (11.7%) with a mean time to rejection of 26.2 months (range 1.3–64.9 months). At the final follow-up, 36.6% of eyes in the rejection group achieved a stable ocular surface compared to 71.9% of eyes in the nonrejection group. Risk factors associated with increased risk of rejection were younger age, KLAL alone, and noncompliance with immunosuppression.
In a study by Baradaran et al. involving 66 KLALs, acute epithelial rejection was diagnosed 16 times in eight eyes, whereas chronic rejection was observed in 24 eyes. At last follow-up, 12 cases (26.6%) had failed because of recurrent acute rejection (4), chronic rejection (5), refractory herpetic keratitis (1), exposure (1), and refractory papillomavirus keratitis. ,
In a series of 31 eyes with aniridic keratopathy treated with KLAL by Holland et al., all eight failures were secondary to acute or chronic stem cell rejection. Of the 21 eyes receiving SI, 19 eyes (90.5%) achieved a stable ocular surface. In contrast, only four of 10 eyes (40.0%) that did not receive immunosuppression achieved a stable ocular surface.
Daya et al. reported four eyes that experienced acute rejection out of the 27 eyes that had undergone KLAL. The patients presented at a median of 7.5 months after transplant (range, 2–12 months). In addition to injection and edema of the graft, they reported epithelial defects of the rejecting grafts. Three of the cases underwent a biopsy of the grafts, which demonstrated a T-lymphocyte infiltrate and strong MHC class II expression. All patients were treated with aggressive oral and topical immunosuppression, but ultimately they all required repeat KLAL.
Eslani et al. reported on 6 eyes that experienced late-onset acute rejection, at an average of 67.8 months (maximum 98.4 months). All patients were determined to be insufficiently immunosuppressed due to medication tapering or poor compliance, despite the recommended SI protocol. The strongest risk factor for rejection was younger age at time of transplant. In addition to providing information about a subset of eyes with acute rejection, this also suggests that the transplanted limbal stem cells can survive longer than was once suggested, particularly with the use of SI. Moreover, it supports the need for long-term immunosuppression, albeit at low doses.
Acute limbal graft rejection should be treated aggressively by increasing or restarting systemic steroids (e.g., prednisone 60–80 mg/day) as well as hourly topical steroids with or without subconjunctival steroid injection. This treatment should continue until there is clinical improvement. With timely diagnosis and proper management, almost all episodes can be reversed; however, in the long run, a patient who experiences an acute rejection is at greater risk for developing surface failure.
Chronic rejection presents with no visible signs of rejection other than inflammation with gradual surface failure. There is a subcategory of chronic rejection that is manifested by a circumferential perilimbal engorgement, stagnation, and tortuosity of vessels with mild chemosis of the KLAL conjunctival mantle (“smoldering” rejection) ( Fig. 169.5 ). This subgroup of rejection is occasionally accompanied by mild pain and photophobia.
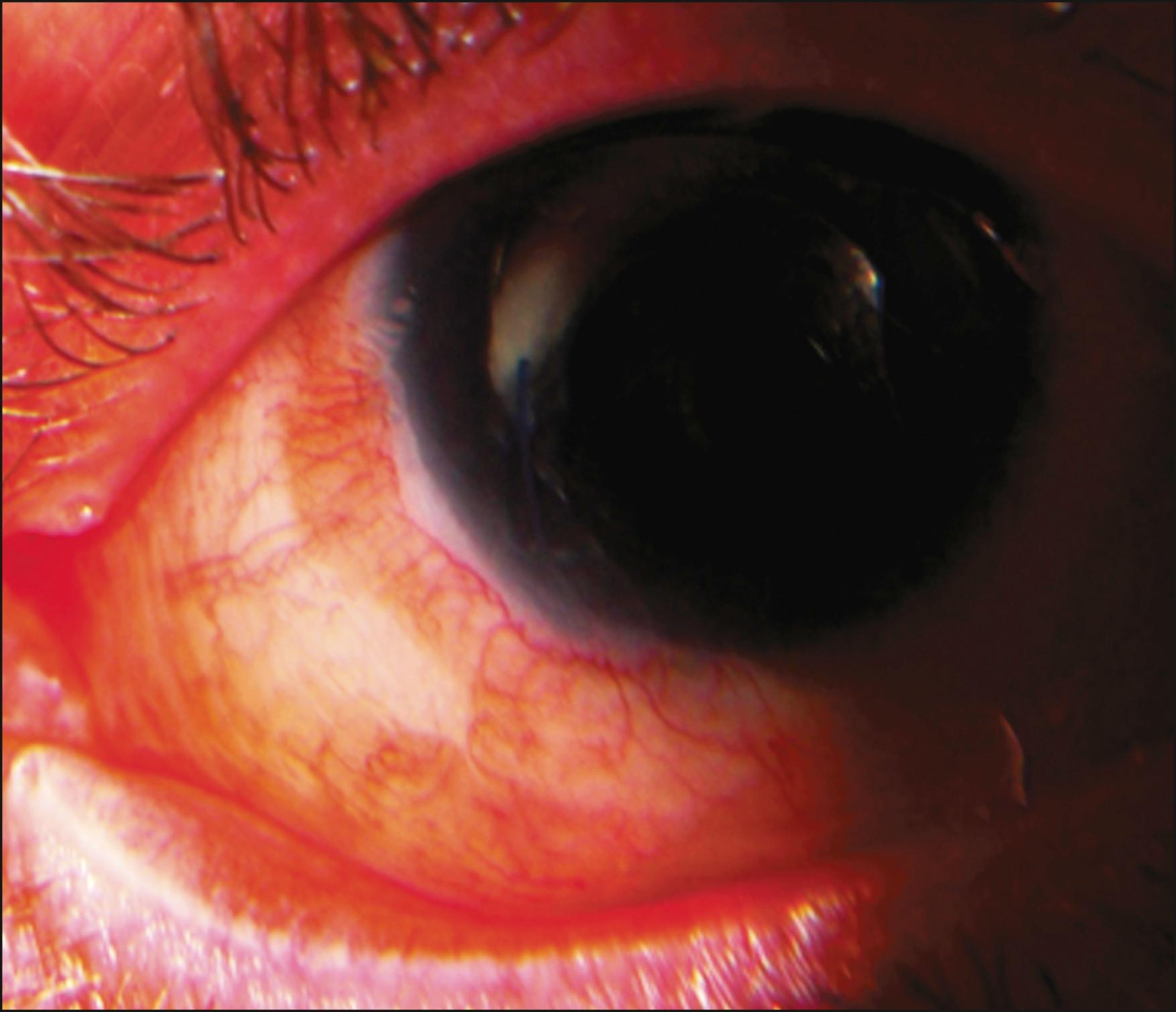
Chronic graft rejection is more difficult to diagnose but should be suspected in patients who demonstrate persistent inflammation on or near the limbal grafts (smoldering rejections). Smoldering rejections should be aggressively treated with increasing topical or subconjunctival steroids, and SI. Patients with chronic rejection may simply present with progressive epitheliopathy, loss of discernible KLAL borders, and neovascularization. A clinical response to increased immunosuppression also supports the possibility of chronic rejection. However, many cases may be subclinical and only present with late stem cell failure.
Maruyama-Hosoi et al. concluded that patients with at least one of the following three criteria—epithelial defects, acute edema, or vascular engorgement after transplantation—had significantly worse long-term outcomes than patients who did not display these findings. They also found that graft rejection could be evoked by nonspecific inflammation. Persistent inflammation stimulates inflammatory cells to migrate to the ocular surface, thereby increasing the chance for antigen recognition. Therefore careful monitoring of inflammatory changes post-KLAL is necessary to identify and prevent long-term complications. In the future, it may be possible to determine beforehand which patients may be more susceptible to rejection. A recent study in limbal transplant patients found that patients with certain genotypes causing higher production of IL-6 and TNF-α had a significantly worse outcome.
The use of SI is essential after ocular surface reconstruction involving allograft tissue. However, immunosuppressive agents can be associated with potential side effects, and therefore careful monitoring and knowledge of these side effects is necessary for proper medication management of KLAL patients. The authors strongly advocate working closely with clinicians who routinely manage patients with solid organ transplants.
Recent experience has indicated that the toxicity profile for KLAL patients on immunosuppressive therapy may be significantly lower than those reported in the literature with these immunosuppressive agents. A study conducted at the University of Cincinnati compared toxicity profiles of renal transplant versus ocular surface transplant patients based on the immunosuppressive regimen. Ocular surface transplant recipients demonstrated significantly lower toxic side effects (0%–8%) than renal transplant patients (6%–63%). This most likely reflects the fact that ocular surface transplant patients are significantly healthier than organ transplant patients, and therefore the likelihood of developing major adverse events is considerably lower.
The importance of combined immunosuppressive agents in the long-term maintenance of a clear stable ocular surface after transplant has long been established. Holland et al. report a stable ocular surface in 105 of 136 (77.2%) patients (225 eyes) with mean follow-up time of 54 months. In a series where single-agent immunosuppression protocols were used, cadaver donor success rates were significantly lower.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here