Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Various forms of organic and inorganic matter can accumulate in the post-lens tear film. These include intrinsic matter, such as desquamated epithelial cells, inflammatory cells and microorganisms, and extrinsic matter, such as dust particles that may have entered the eye from the atmosphere. Most of this matter is flushed away during daily lens wear because of the blink-activated tear pump. The accumulation of such debris during extended lens wear is potentially more problematic because it can be retained at the corneal surface for longer periods – such as overnight during sleep. An important requirement of extended-wear lenses is that such matter is flushed out from beneath the lens as soon as the patient begins to blink upon awakening in the morning.
A characteristic form of debris known as ‘mucin balls’ is observed in patients wearing silicone hydrogel contact lenses. Although the appearance of mucin balls was first formally reported in the literature in 2000 – corresponding to the market release of silicone hydrogel lenses – earlier anecdotal reports suggest that this phenomenon had been observed previously. Other descriptive terms that have been used include lipid plugs, tear microspheres, microdeposits and spherical post-lens debris. Some authors have suggested that a small number of mucin balls can be observed in patients using conventional hydrogel lenses on an extended-wear basis.
Mucin balls are observed within minutes of lens insertion between the posterior lens surface and the corneal epithelium. Observed under direct white light at low illumination, they appear as a mass of discrete grey dots that seem to be fixed in position; that is, they do not move in synchrony with the contact lens after a blink. Observed at higher magnification under direct white light illumination, mucin balls appear as cream-grey, round or ovoid inclusions that may be near-spherical or somewhat flattened ( Fig. 10.1 ).
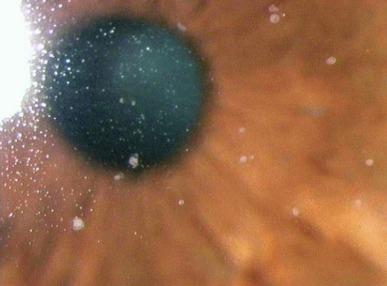
When viewed by using indirect retro-illumination, mucin balls display reversed illumination, which indicates that the material from which the mucin ball is composed (presumably mucin; see later) is of a higher refractive index than the surrounding medium (tear aqueous). Under these illumination conditions, some mucin balls take on a distinct doughnut-like appearance, with a thick annular rim and membrane across the centre ( Fig. 10.2 ).
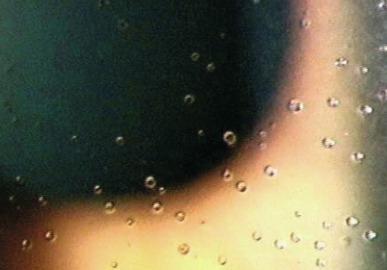
Tan et al. and Craig et al. suggested that mucin balls are more commonly observed in the superior cornea, although this characteristic has not been reported by other authors. Mucin balls can also become embedded in the conjunctival epithelium close to the limbus.
Although the term ‘balls’ has been used to describe these mucinous features – which implies a round or spherical shape – Grupcheva et al. described mucin balls as being round (70%), oval (28%) or irregular (2%) in shape. Mucin balls were also classified in terms of reflectivity, with 92% classified as being bright (homogeneous) and 8% classified as comprising of dark areas (heterogeneous).
Estimates of the size (diameter) of individual mucin balls vary; Fonn et al. and Dumbleton et al. reported a diameter of 20 to 200 μm, and Craig et al. made a similar estimate of 10 to 200 μm. Bourassa and Benjamin and Tan et al. suggested a smaller diameter, at 40 to 120 μm. Sweeney et al. reported that mucin balls fall into two size categories: small (10–20 μm) and large (20–50 μm).
Matsuzaki et al. reported an atypical case of mucin ball formation in a 25-year-old male wearing extended-wear silicone hydrogel lens (PureVision; balafilcon A (Bausch & Lomb)) for therapeutic use. Mucin balls were found between the cornea and contact lens and stained with rose bengal dye. One of them was atypically larger than usual, and the major axis measured approximately 1.5 mm. Lens wear was stopped, and all mucin balls and corneal staining disappeared within 1 day.
Some authors have used corneal confocal microscopy to measure the size of mucin balls. Ladage et al. measured the size of mucin balls in three patients and reported that the sizes ranged from 33.9 to 78.8 μm (mean 57.9 ± 14 μm). Grupcheva et al. assessed the mean diameter of mucin balls in patients wearing silicone hydrogel lenses for therapeutic and cosmetic reasons and reported these to be 77.4 and 45.8 μm, respectively. These estimates are quite large in comparison with the thickness of the tear film beneath silicone hydrogel lenses (1–2 μm) and the corneal epithelium (about 50–70μm).
Various factors will govern the number of mucin balls present at any given time. In their respective case reports, Bourassa and Benjamin observed 20 to 30 mucin balls, and Pritchard et al. observed up to 50 mucin balls over a number of visits. In clinical trials of patients wearing silicone hydrogel lenses, Fonn et al. reported that 95% of patients displayed fewer than 50 mucin balls over a 3-month period, and Sweeney et al. and Tan et al. reported that the mean number of mucin balls over a 12-month period did not exceed an average of 20. All seven patients wearing silicone hydrogel lenses in the clinical trial performed by Craig et al. demonstrated between 60 and 100 mucin balls within 22 days of commencing lens wear. Sometimes, in excess of 100 mucin balls may be observed. Craig et al. reported 230 mucin balls in one subject. Grupcheva et al. observed an average of 15 and 20 mucin balls in patients wearing silicone hydrogel lenses for cosmetic and therapeutic purposes, respectively.
After lens removal, the majority of mucin balls are blinked away, leaving depressions (also termed ‘imprints’ or ‘pits’) in the epithelial surface. These depressions fill with tear aqueous and appear as transparent spherical inclusions displaying un-reversed illumination when viewed at high magnification with a slit lamp biomicroscope using retro-illumination ( Fig. 10.3 ). Some mucin balls appear to remain lodged in the surface of the epithelium; these continue to display reversed illumination, thus allowing mucin balls to be differentiated from aqueous-filled pits.
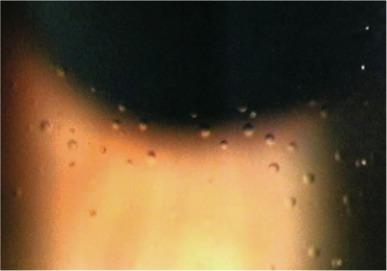
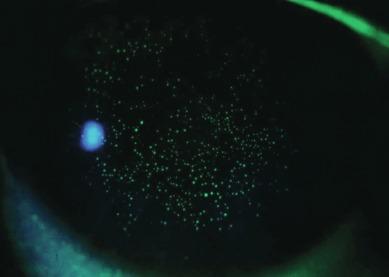
Upon instillation of fluorescein into the eye, both the remaining mucin balls and the aqueous in the epithelial depressions stain with fluorescein, resulting in a pattern of punctate spots over the cornea. In the presence of fluorescein, it is virtually impossible to distinguish between mucin balls and the aqueous-filled epithelial pits; both appear as similar-sized, discrete, solid, fluorescent-green spots when viewed under fluorescent light ( Fig. 10.4 ). Mucin balls do not stain with rose bengal.
Sweeney et al. and Tan et al. reported that mucin balls increase in number and size over the initial few months of silicone hydrogel lens extended wear. Morgan and Efron conducted a randomised, crossover clinical trial in which 30 subjects each wore a pair of PureVision (Bausch & Lomb) and Focus Night & Day (CIBA Vision) silicone hydrogel contact lenses for 8 weeks on an extended-wear basis. The percentage of patients presenting with mucin balls increased to 37% and 54% for PureVision and Focus Night & Day, respectively, after 4 weeks and began to decline thereafter ( Fig. 10.5 ). Dumbleton et al. did not detect a change in the mean grade of mucin ball appearance over a 6-month period among 92 patients wearing Focus Night & Day lenses.

As stated earlier, Morgan and Efron reported that 37% to 54% of patients wearing silicone hydrogel lenses will display mucin balls after about 4 weeks. Tan et al. observed that the percentage of all subjects wearing silicone hydrogel lenses with mucin balls varied from 37% to 82% during the year. Dumbleton et al. reported that mucin balls were observed in 70% of patients at one or more visits of their clinical trial, and in 29% of subjects at all three visits, while patients were wearing Focus Night & Day lenses. Over half (54.2%) of a group of subjects wearing Focus Night & Day lenses were observed by Szczotka-Flynn et al. to display some presence of mucin balls during at least one visit and about one-third (32.8%) displayed repeated episodes over a 12-month period.
Morgan and Efron analysed the results of their clinical trial to see if the presence of mucin balls was related to any other clinical results. Paradoxically, high-contrast visual acuity was demonstrated to be about one letter better in the presence of mucin balls ; this is consistent with the report of Dumbleton et al., who showed that subjective appreciation of vision was superior in the presence of mucin balls after 6 months of wear. There was no difference for low contrast visual acuity. It is not clear why vision should be better in the presence of mucin balls.
Some biomicroscopic signs appeared to be related to mucin balls. An unexpected finding in the study by Morgan and Efron was that conjunctival and limbal redness were both reduced and the number of microcysts appeared to increase in subjects exhibiting mucin balls. The apparent association between the appearance of mucin balls and epithelial microcysts, which has also been observed by Tan et al., may be an observational artefact relating to the difficulty in differentiating mucin balls from microcysts.
An association was revealed between the presence of mucin balls and increased corneal fluorescein staining ; this is likely to be caused by the remaining mucin balls and fluid-filled epithelial pits staining with fluorescein. The previously described associations concerning mucin balls contrast with those of other authors who found no relationship between biomicroscopic signs and mucin balls.
Naduvilath demonstrated an association between the appearance of mucin balls and the development of contact lens–induced peripheral ulcers. Specifically, patients displaying mucin balls have a × 1.6 increased probability of developing contact lens–induced peripheral ulcers compared with patients who do not display mucin balls. Contrary to this, Szczotka-Flynn et al. reported that the presence of mucin balls was significantly associated with a decreased incidence of corneal infiltrative events and that the effect was greatest when they occurred repeatedly over time.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here