Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The age of menopause has been constant over several centuries and is influenced by genetic, ethnic, and environmental variables.
The prediction of the age of menopause based on antimüllerian hormone (AMH) trajectories is possible but impractical.
Menopause is defined by the last menstrual period. Because cessation of menses is variable and many of the symptoms associated with menopause may occur prior to the cessation of menses, there is seldom a precise timing of this event. Other terms used are perimenopause , which refers to a variable time beginning a few years before and continuing after the event of menopause, and climacteric , which merely refers to the time after the cessation of reproductive function.
As life expectancy increases beyond the eighth decade worldwide, particularly in developed countries, an increasing proportion of the female population is postmenopausal. With the average age of menopause occurring around 51 years, more than one-third of a woman’s life is now spent after menopause. Here symptoms and signs of estrogen deficiency merge with issues encountered with natural aging. As the world population increases and a larger proportion of this population is made up of individuals over 50, medical care specifically directed at postmenopausal women becomes an important aspect of modern medicine. Between the years 2000 and 2005, the world population older than 60 years old increased from 590 million to 1 billion and is estimated to be 1.2 billion in 2030. In the United States, the number of women entering menopause will almost double in the 30 years between 1990 and 2020 ( Table 14.1 ).
| Year | Population |
|---|---|
| 1990 | 10.8 million |
| 2000 | 12.1 million |
| 2010 | 17.1 million |
| 2020 | 19.3 million |
Age of menopause, which is a genetically programmed event, is subject to some variability. The age of menopause in Western countries (between 51 and 52 years old) , is thought to correlate with general health status. Lower socioeconomic status is associated with an earlier age of menopause. Higher parity, on the other hand, has been found to be associated with a later menopause. Smoking has consistently been found to be associated with menopause onset taking place 1 to 2 years earlier. , While body mass has been thought to be related to age of menopause (greater mass with later menopause), the data have not been consistent. Malnourishment and vegetarianism have both been found to be associated with earlier onset of menopause. , However, physical and athletic activity has not been found consistently to influence the age of menopause. Hysterectomy (without oophorectomy) and bilateral salpingectomy have both been suggested to be associated with an earlier cessation of ovarian function because of alterations in ovarian blood flow, although these data are not consistent.
There also appear to be ethnic differences in the onset of menopause. In the United States, African-American and Hispanic women have been found to undergo menopause approximately 2 years earlier than White women. , Malay women have menopause at approximately age 45, Thai women at age 49.5, and Filipina women between the ages of 47 and 48. Indian women have also been reported to have menopause by age 46.2 years. Women in countries at higher altitude (Himalayas or Andes) have been shown to have menopause 1 to 1.5 years earlier. , Because the average age of menopause in the United States is 51 to 53 years, with an age distribution weighted toward White women, menopause prior to age 40 is considered premature. Conversely, 97% of women will have gone through menopause by age 58.
The primary determinate of age of menopause is genetic. Based on family studies, heritability for the age of menopause averaged 0.87—suggesting that genetics explain up to 87% of the variance in menopausal age. Prediction models for the age of menopause have been based on the downward trajectory of values of AMH with age, as shown in several studies, noted below. In a recent review of the literature, maternal age of menopause does contribute somewhat to values of AMH in the prediction model, but AMH is thought to be the most valuable marker.
Multiple genic loci have been identified by genome-wide association studies, which are associated with the age of natural menopause as well as ovarian insufficiency. , In a meta-analysis of 22 genome-wide association studies, candidate genes identified appear to be involved in DNA replication and damage repair, hormone production and action, and immune function. These include EXO1, HELQ, U1MC1, FAM175A, FANCI, TLK1, POLG, MCM8, and PRIM1 associated with DNA repair , and IL11, NLRP11, and PRRC2A in the immune function domain. Nevertheless, in spite of these advances, the mechanisms of action of these genes and their interactions with environmental factors and possible gonadotoxic factors, which may affect ovarian reserve and reproductive lifespan, remain to be determined. A more in-depth discussion will follow in the subsequent section on aging.
Premature ovarian insufficiency (POI), previously known as premature ovarian failure (POF), occurs in up to 10% of young amenorrheic women and has multiple etiologies, but is often idiopathic.
Women with established POI have increased morbidity and mortality unless they receive hormonal replacement therapy.
Spontaneous pregnancies may occur and long-term management should include hormonal replacement, at least until the average age of natural menopause.
POI is defined as hypergonadotropic ovarian failure occurring prior to age 40. It has been suggested that POI is the correct term for this entity, more accurately reflecting its heterogeneous nature. POI occurs in 5% to 10% of women who are evaluated for amenorrhea ; thus, the incidence varies according to the prevalence of amenorrhea in various populations. Estimates of the overall prevalence of POI in the general population were suggested to range between 0.3% and 0.9% of women , ; however, recent global data point to a much higher prevalence of 3.7%.
There is substantial morbidity and an increased mortality associated with untreated POI, which will be further elaborated on in this chapter. Apart from increased cardiovascular and metabolic risks and increased mortality, there are data suggesting impaired cognitive ability as well as an increased risk of Parkinson. Some of these data, however, have been generated in women with premature oophorectomy as opposed to those who developed spontaneous POI.
Throughout life, there is an ongoing rate of atresia of oocytes (see Chapter 8 ). Because this process is accelerated with various forms of gonadal dysgenesis due to defective X chromosomes, one possible etiology of POI is an increased rate of atresia that has yet to be explained. A decreased germ cell endowment or an increased rate of germ cell destruction can also explain POI. Nevertheless, some 1000 (of the original 2 million) primary follicles may remain. , While most of these oocytes are likely to be functionally deficient; occasionally spontaneous pregnancies occur in young women in the first few years after the diagnosis of POI.
There are several possible etiologies of POI ( Box 14.1 ). Defects in the X chromosome may result in various types of gonadal dysgenesis with varied times of expression of ovarian failure. Even patients with classical gonadal dysgenesis (e.g., 45,XO) may undergo a normal puberty, and pregnancy will occasionally ensue as a result of genetic mosaicism. Very small defects in the X chromosome may be sufficient to cause POI. Familial forms of POI may be related to either autosomal-dominant or sex-linked modes of inheritance. Overall, about 30% of cases of POI have some history of familial occurrence.
Genetic
Enzymatic
Immune
Gonadotropin defects
Ovarian insults
Idiopathic
Mutations in the gene encoding the follicle-stimulating hormone (FSH)-receptor (e.g., mutation in exon 7 in the gene on chromosome 2p) have been described, but these are extremely rare outside of the Finnish population in which these mutations were originally described.
An expansion of a trinucleotide repeat sequence in the first exon on the FMR1 gene (Xq 27.3) leads to fragile X syndrome, a major cause of developmental disabilities in males. The permutation in fragile X syndrome has been shown to be associated with POI. , There is a spectrum in findings based on the number of repeats in the premutation, and women with an “intermediate” number of repeats may have a lower ovarian reserve, which is often identified during an investigation for infertility.
Type 1 blepharophimosis/ptosis/epicanthus inversus syndrome, an autosomal-dominant disorder due to mutations in the forkhead transcription factor FOXL2, includes POI. Triple X syndrome has been associated with POI, and so has dystrophic myotonia, although the mechanism underlying this relationship is unclear.
Under the category of enzymatic defects, galactosemia is a major cause of POI that is related to the toxic buildup of galactose in women who are unable to metabolize the sugar. Even in women with fairly well-controlled galactose-free diets, POI tends to occur. , Another enzymatic defect linked to POI is 17α-hydroxylase deficiency. This rare condition manifests differently from the other causes discussed here because the defect in the production of sex steroids leads to sexual infantilism and hypertension.
Because of the prevalence of autoimmune disorders in women, the degree to which autoimmunity may be responsible for POI is unclear. One study has suggested an association in 17.5% of cases. Virtually all autoimmune disorders have been found to be associated with POI, including autoimmune polyendocrinopathies like autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, which is caused by mutations in the autoimmune (AIRE) gene on 21q22.3. The presence of the thymus gland appears to be required for normal ovarian function as POI has been associated with hypoplasia of the thymus. In patients who have undergone ovarian biopsy as part of their evaluation, lymphocytic infiltration surrounding follicles has been described, as well as the resumption of menses after immunosuppression.
Immunoassays using antibodies directed at ovarian antigens have been developed and have demonstrated positive findings in some patients with POI, although the relevance of these findings remains unsettled. Ovarian autoantibodies could also conceivably be a secondary phenomenon to a primary cell-mediated form of immunity. Specific enzymes such as 3β-HSD may also be the target of ovarian autoimmunity.
From a practical standpoint, screening for the common autoimmune disorders is appropriate in women found to have POI. Antithyroid antibodies are the most frequently encountered elevated antibody levels. Although relatively rare, as many as 2% to 4% of women with POI have anti-adrenal antibodies and may be at risk for adrenal failure. Commercial antibodies to 21-hydroxylase are available and agree with other assays for adrenal cortex antibodies. It has been suggested that screening women using dehydroepiandrosterone sulfate (DHEAS) also may be useful to detect signs of adrenal insufficiency. Since levels are age-related, young women with POI would be expected to have higher levels than women at the natural age of menopause.
More from a theoretical standpoint, abnormalities in the structure of gonadotropins, in their receptors, or in receptor binding could be associated with POI. While abnormal urinary forms of gonadotropins have been reported in women with POI, these data have not been replicated. Abnormalities of FSH-receptor binding, as mediated by a serum inhibitor, have been described. A genetic defect that may lead to alterations in FSH receptor structure was mentioned previously.
Under the category of ovarian insults, POI may be induced by ionizing radiation, chemotherapy, or overly aggressive ovarian surgery. Although not well documented, viral infections have been suggested to play a role, particularly mumps. A dose of 400 to 500 rads is known to cause ovarian failure 50% of the time, , and older women are more vulnerable to experiencing permanent failure. A dose of approximately 800 rads is associated with failure in all women. , Ovarian failure (transient or permanent) may be induced by chemotherapeutic agents, although younger women receiving this insult have a better prognosis. Alkalyzing agents, particularly cyclophosphamide, appear to be most toxic.
By exclusion, the majority of women are considered to have idiopathic POI because no demonstrable cause can be pinpointed. Among these women, small mutations in genes lying on the X chromosome, or yet-to-be identified autosomal genes, may be the cause.
Evaluation of women under age 30 who have POI should include screening for autoimmune disorders and a karyotype; detailed recommendations for screening of such women are available. In addition, vaginal ultrasound may be useful for assessing the size of the ovaries and the degree of follicular development, which, if present, may signify an immunological defect.
Treatment usually consists of estrogen replacement. If fertility is a concern, the most efficacious treatment is oocyte donation. Various attempts at ovarian stimulation are usually unsuccessful, and the sporadic pregnancies, that may occur, are just as likely to occur spontaneously (∼5%) as with any intervention. , These spontaneous pregnancies are frequently encountered while receiving estrogen replacement.
Noting irregular or unscheduled bleeding (while receiving hormonal treatment [HT]) may be an important sign of endogenous sex steroid production. Serum AMH has been used to characterize women with ovarian insufficiency to determine where they may be in the spectrum of ovarian failure. However, AMH levels cannot help predict the chance of a spontaneous pregnancy. It has been shown in a large cohort of women ( n = 358) followed for POI, that spontaneous ovarian function may ensue in 24% of women, usually within a year of the diagnosis, for at least some period of time. In this longitudinal follow-up, 4.4% of women had spontaneous pregnancies.
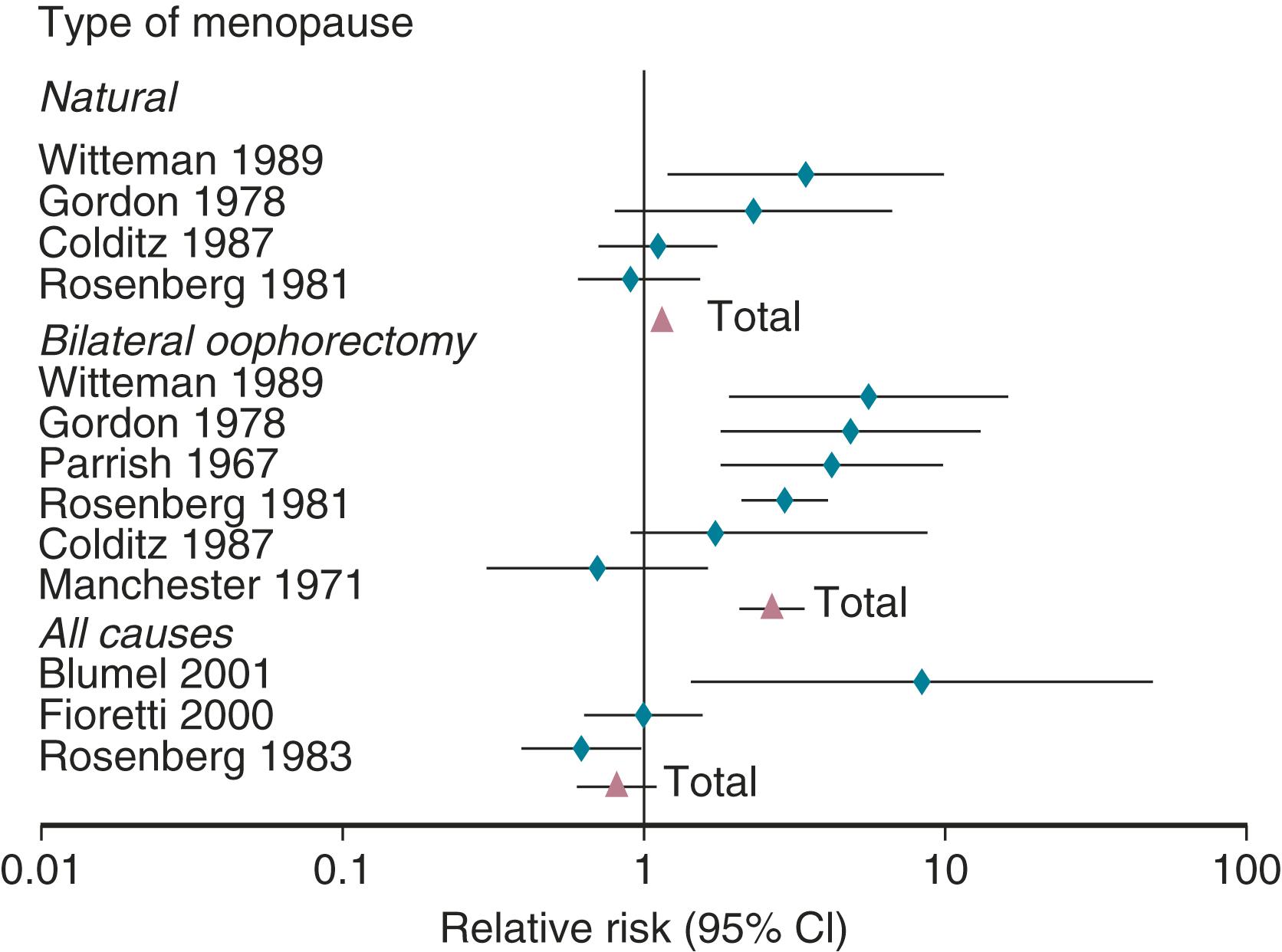
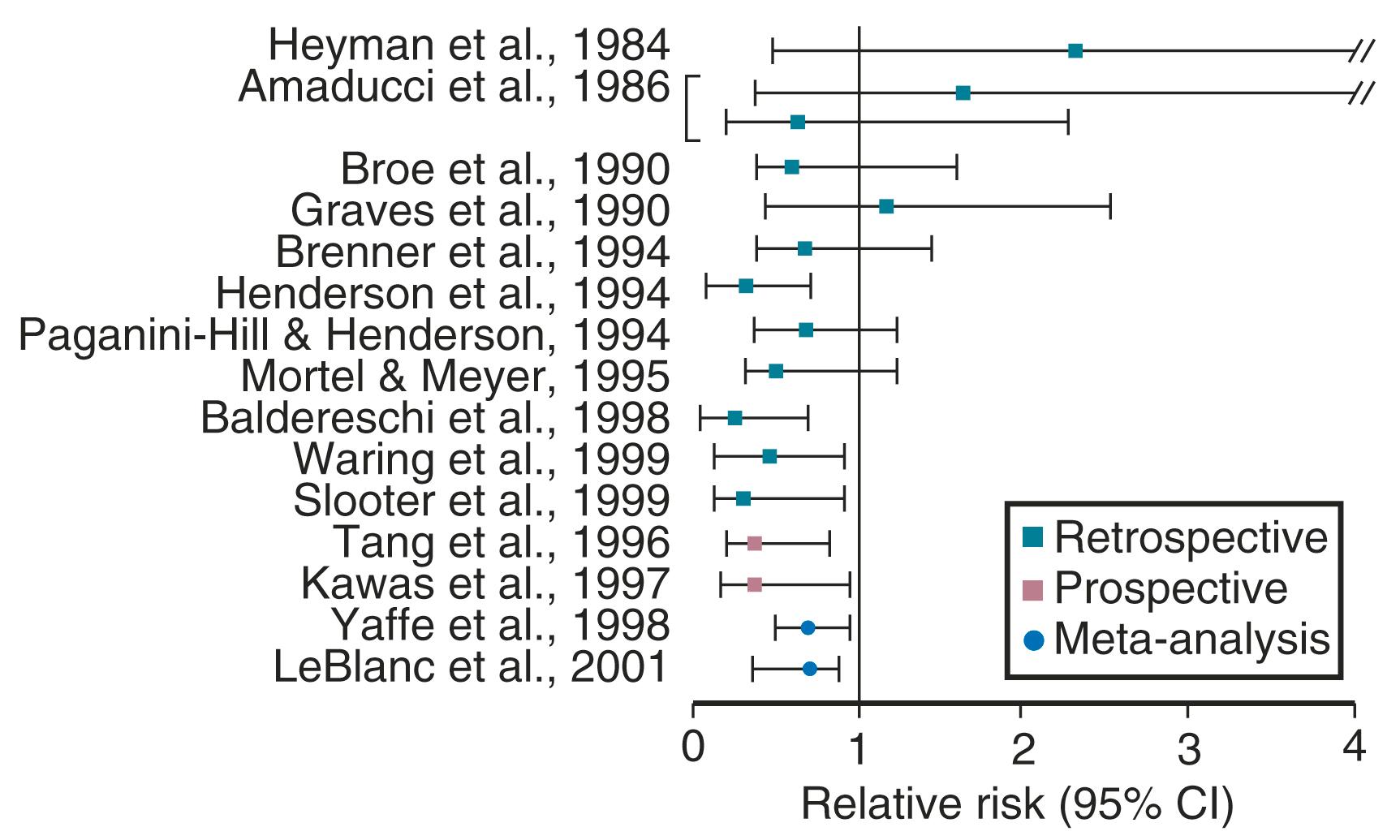
It has been well established that POI, as well as bilateral oophorectomy prior to the usual age of menopause, is associated with an increased risk of cardiovascular disease (CVD) as well as increased mortality ( Fig. 14.1 ). CVD mortality specifically is increased twofold to fourfold with early oophorectomy, and observational studies have suggested that using HT reduces this risk. A recent meta-analysis of women with POI suggested an increased risk of all-cause mortality, relative risk (RR): 1.39 (1.1 to 1.77). Early estrogen intervention has been shown to decrease all-cause mortality, which will be discussed later. Accordingly, unless there are contraindications, estrogen should be considered in all young women with POI, at least until the age of natural menopause.
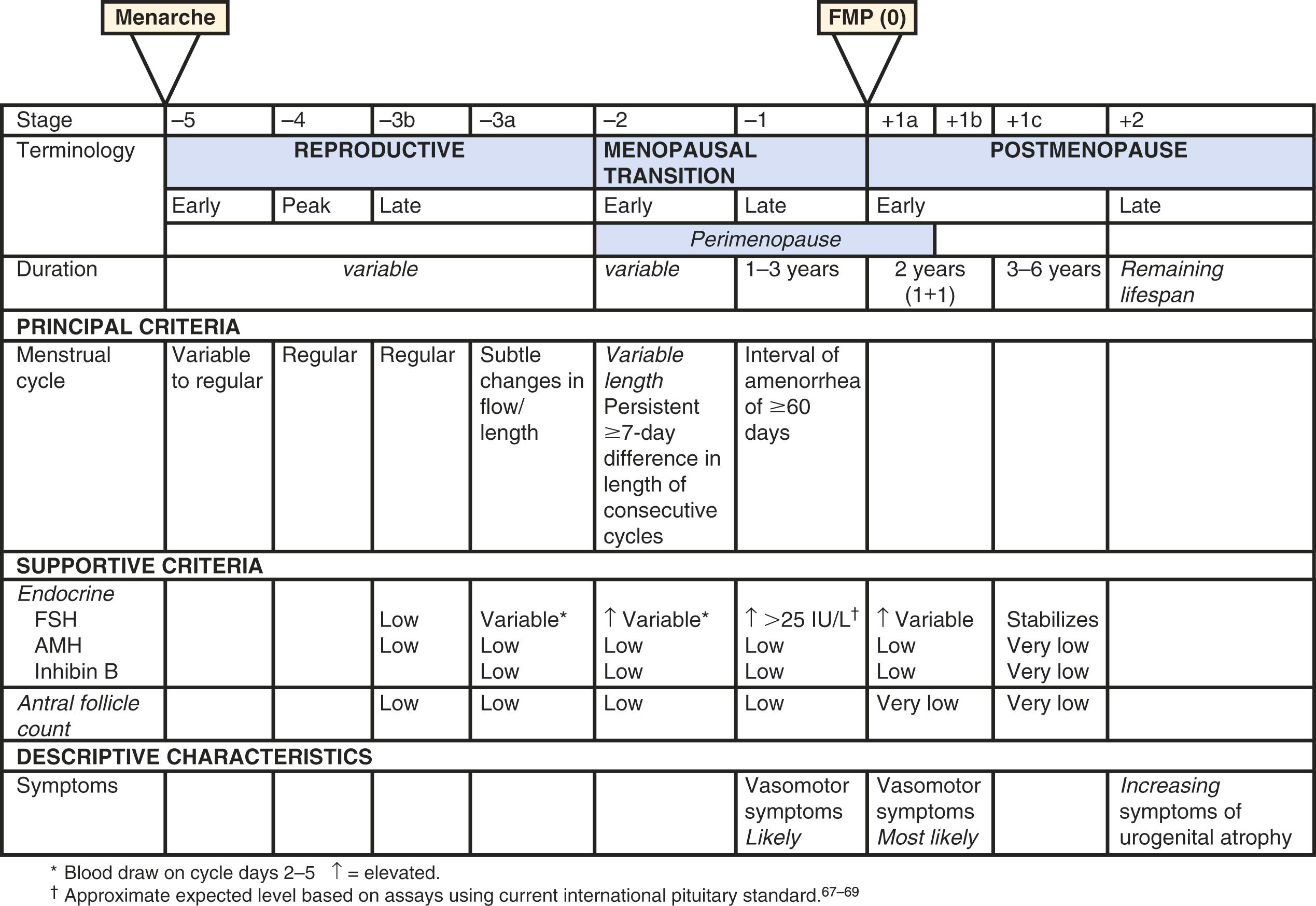
Great variations in hormonal and menstrual findings occur around the final menstrual period.
This is best described by STRAW+ 10, a workshop designed to describe these changes.
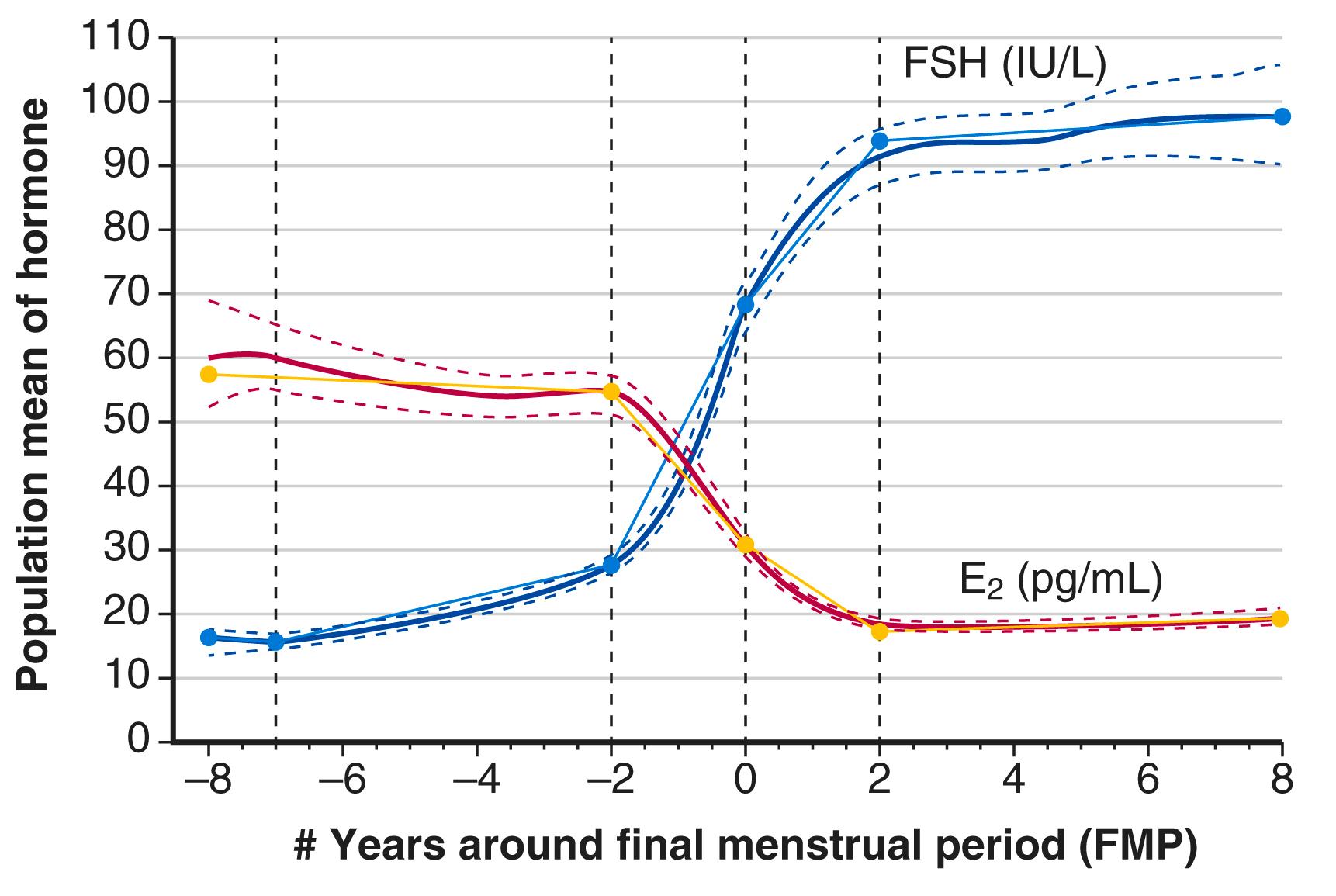
Prospective data have been generated by the Study of Women Across the Nation (SWAN) to describe the temporal changes of increasing FSH and decreasing estradiol around menopause.
The menopausal transition has now been recognized by the American Heart Association as a time that heralds an increased cardiovascular risk, warranting closer surveillance and prevention strategies.
A workshop was convened in 2001 to build a consensus on describing various stages of the menopausal transition. Ten years later, another workshop was convened to update the staging system with more recent data. The Stages of Reproductive Aging Workshop + 10 (STRAW + 10) simplified bleeding criteria for the early and late menopausal transition and modified criteria for the late reproductive stage (stage −3) and the early postmenopausal stage (stage +1; Fig. 14.2 ). Then the late reproductive stages were expanded to include information on decreasing levels of AHM and inhibin B to reflect recent longitudinal data on fluctuating hormonal levels at the time of menopause, which will be described in more detail below.
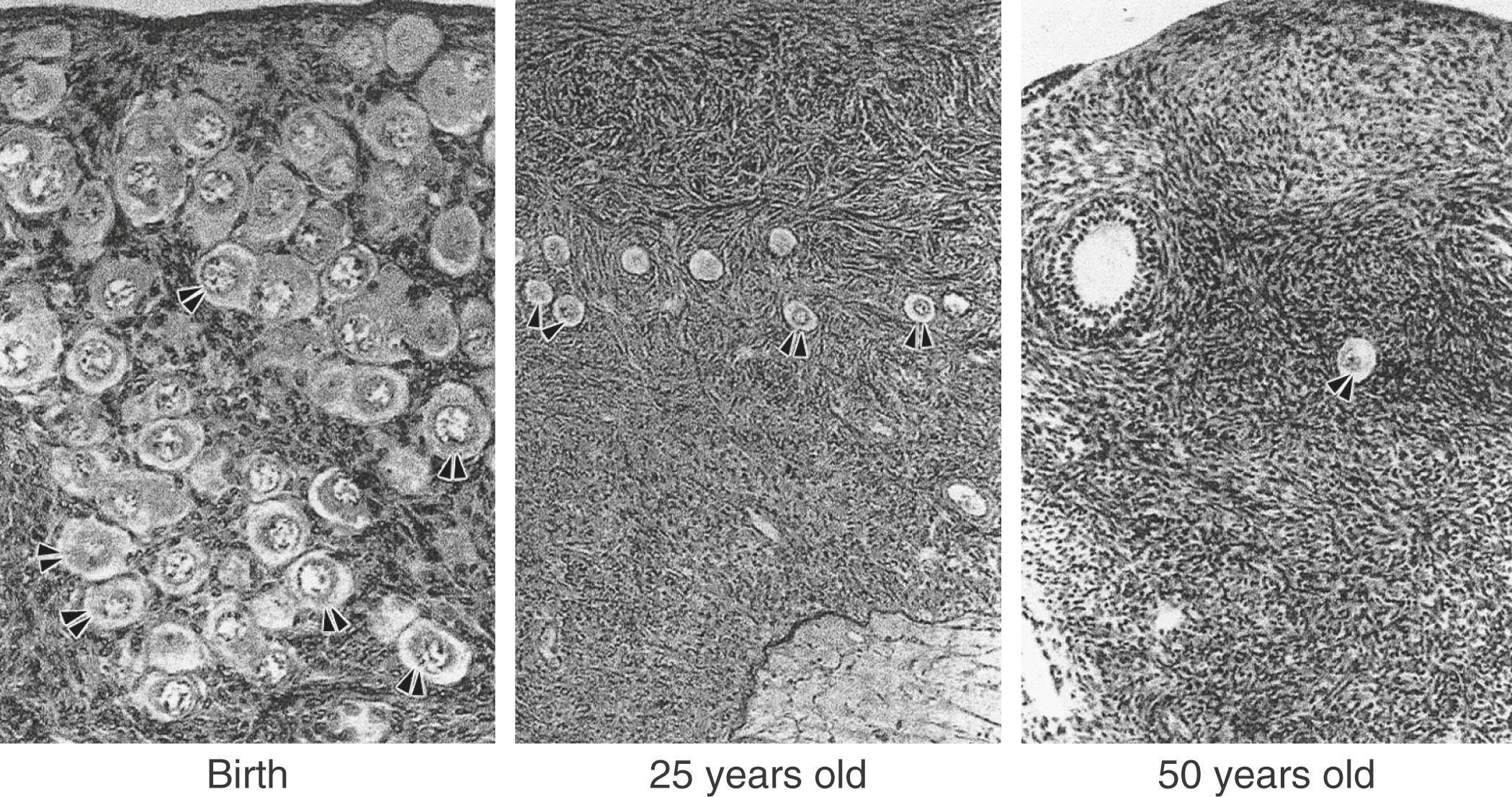
The ovary changes markedly from birth to the onset of menopause ( Fig. 14.3 ). The greatest number of primordial follicles is present in utero at 20 weeks’ gestation and undergoes a regular rate of atresia until around the age of 37. After this time, the decline in primordial follicles appears to become more rapid between age 37 and menopause ( Fig. 14.4 ) when no more than a thousand follicles remain. These remaining follicles are primarily atretic in nature.
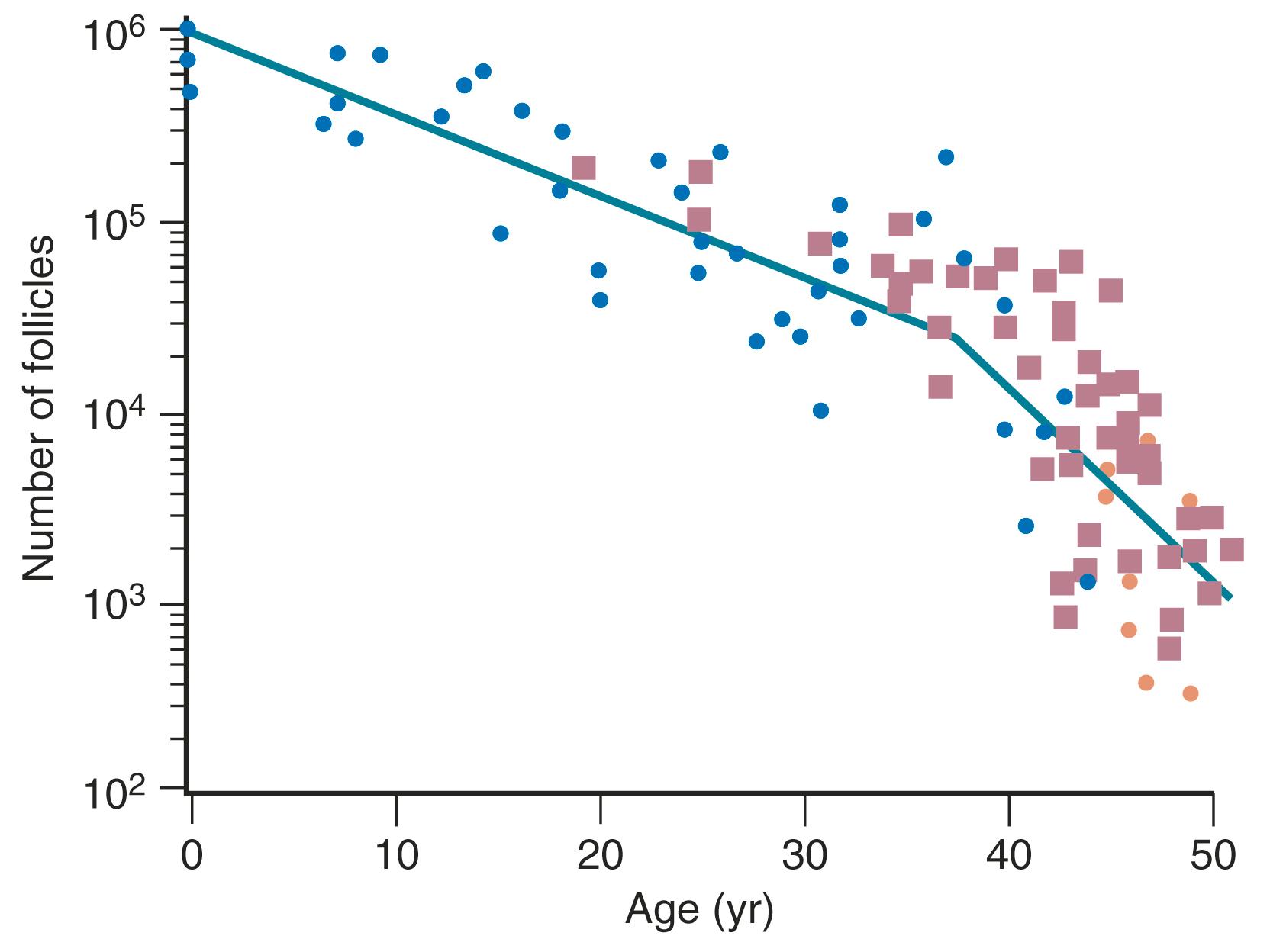
Although perimenopausal changes are generally thought to be endocrine in nature and result in menstrual changes, a marked diminution of reproductive capacity precedes this period by several years. This decline may be referred to as gametogenic ovarian failure. The concept of dissociation in ovarian function is appropriate. Gametogenic failure is signified by reduced early follicular phase inhibin secretion, rising serum FSH levels, reduced antral follicle counts on ultrasound, decreased levels of AMH, and a marked reduction in fecundity. These changes may occur with normal menstrual function and no obvious endocrine deficiency, however, and they may occur in some women as early as age 35 (10 or more years before endocrine deficiency ensues).
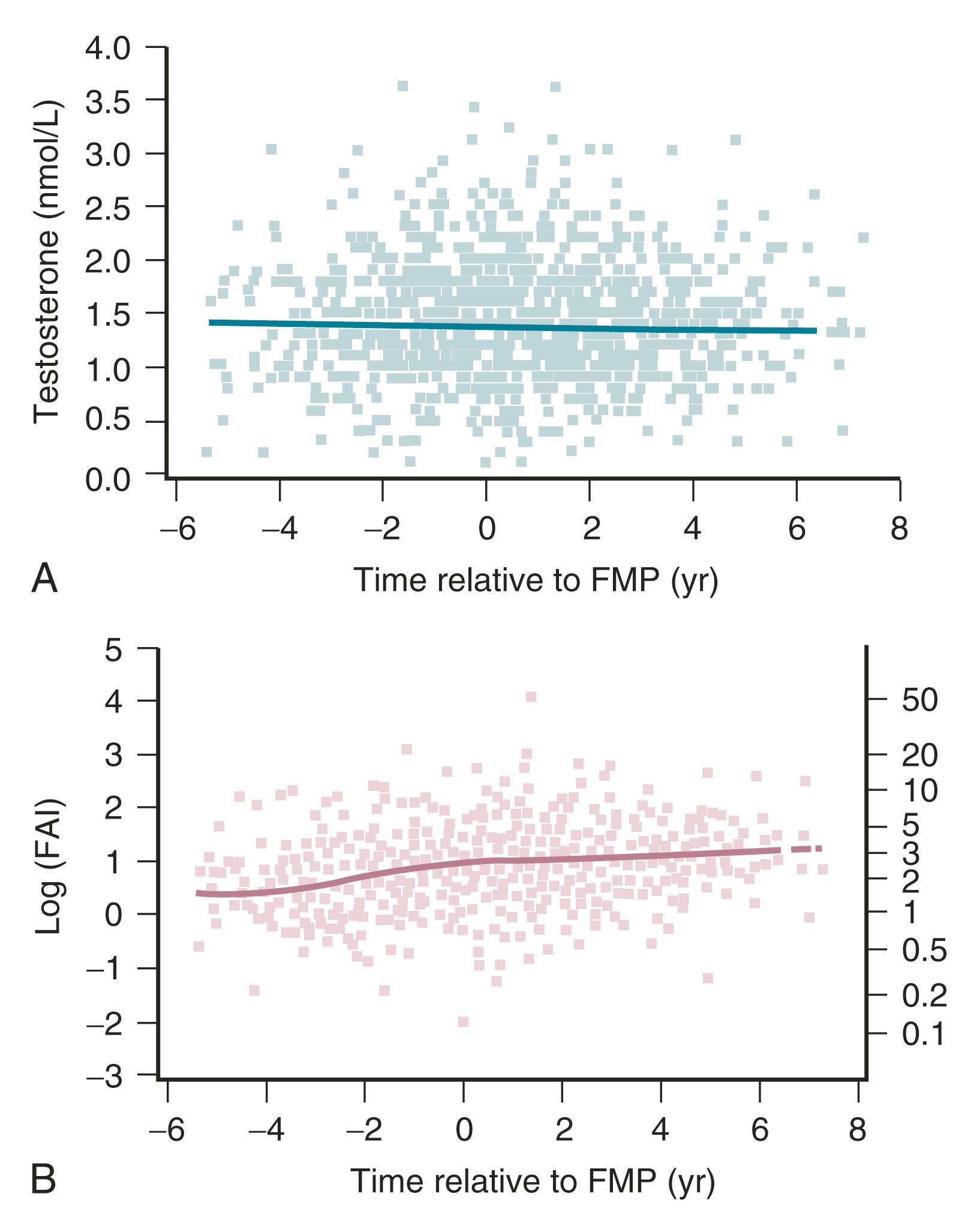
Recent longitudinal data obtained from the Study of Women Across the Nation (SWAN) have shown that estrogen levels begin to decline 2 years before the last menstrual period ( Fig. 14.5 ). Older data had shown that this only occurred during the last 6 months. In this time of transition because of the dysregulation of ovarian and hypothalamic-pituitary feedback, estradiol levels might be elevated at times, with early follicular development. The rise in FSH levels occurs several years before menopause but increases substantially in the last 2 years, and then stabilizes to steady-state levels 2 years after menopause. These steady-state levels then decrease in the late menopause (seventh decade). There is also a very slow decline in androgen status (i.e., androstenedione and testosterone), which cannot be adequately detected at the time of the perimenopause.
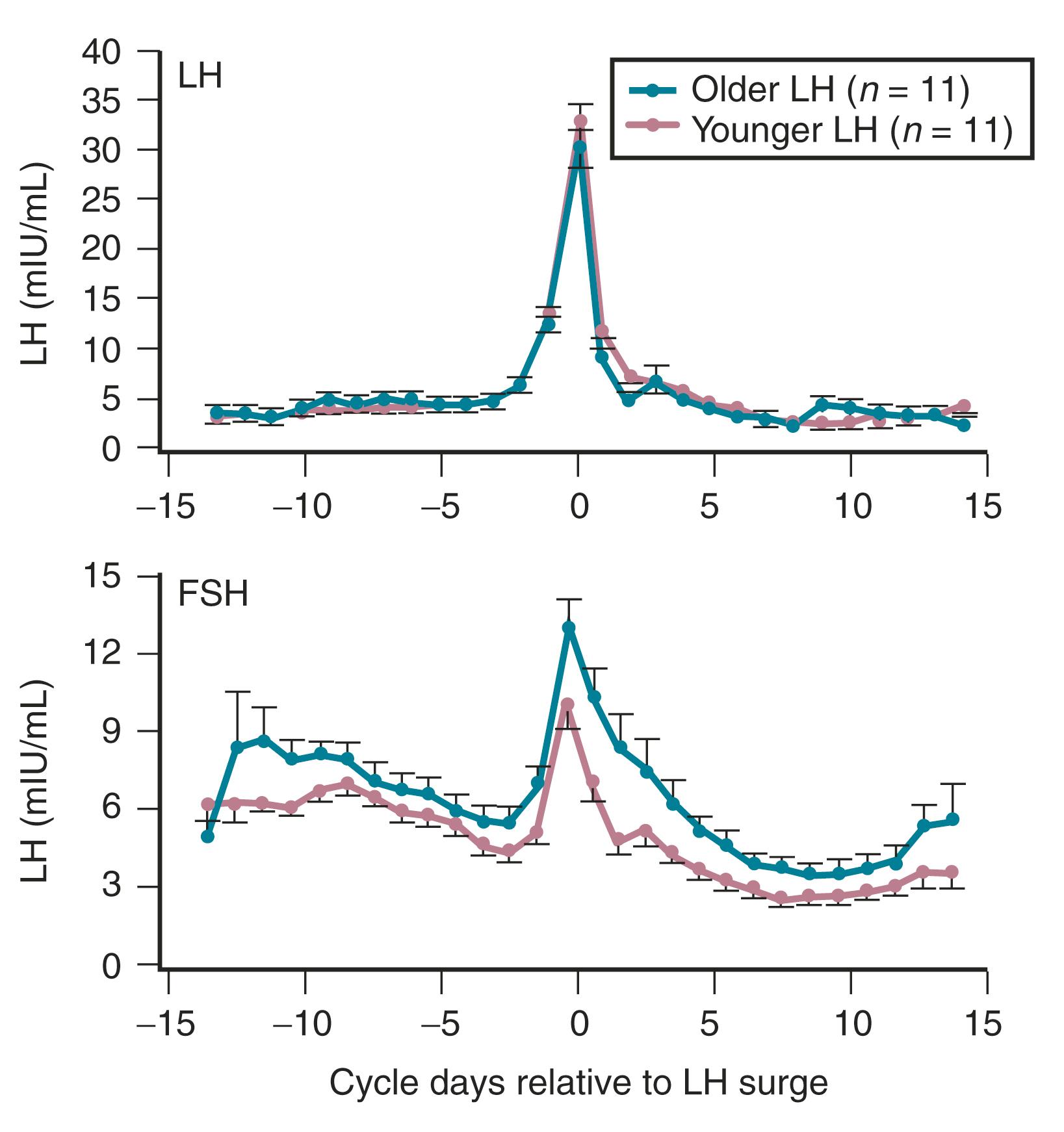
Products of the granulosa cell are most important for the feedback control of FSH. As the functional capacity of the follicular units decrease, secretion of substances that suppress FSH also decreases. Most notably, inhibin B levels are lower in the early follicular phase in women in their late 30s ( Fig. 14.6 ). , Indeed, FSH levels are higher throughout the cycle in older ovulatory women than in younger women ( Fig. 14.7 ).
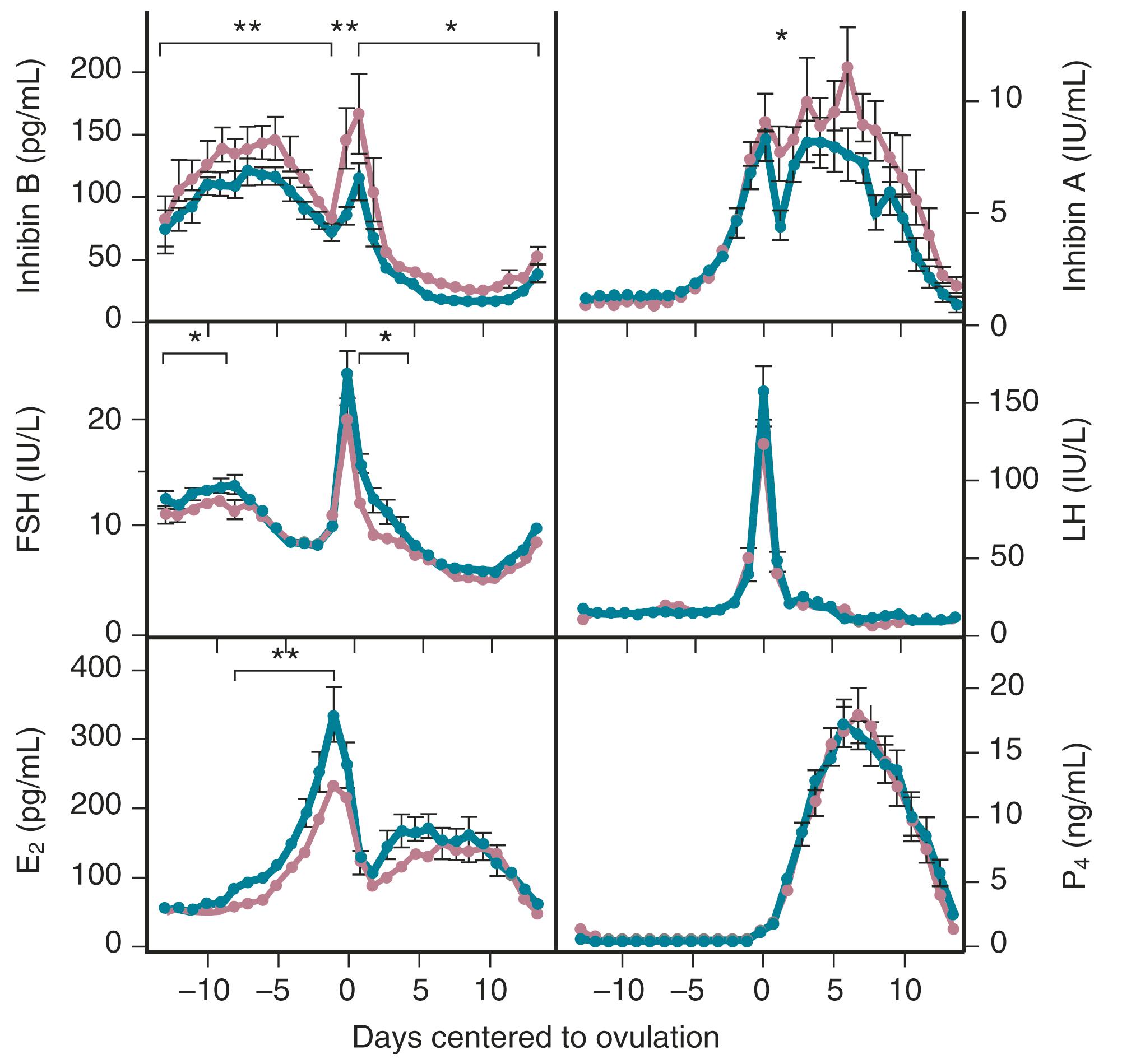
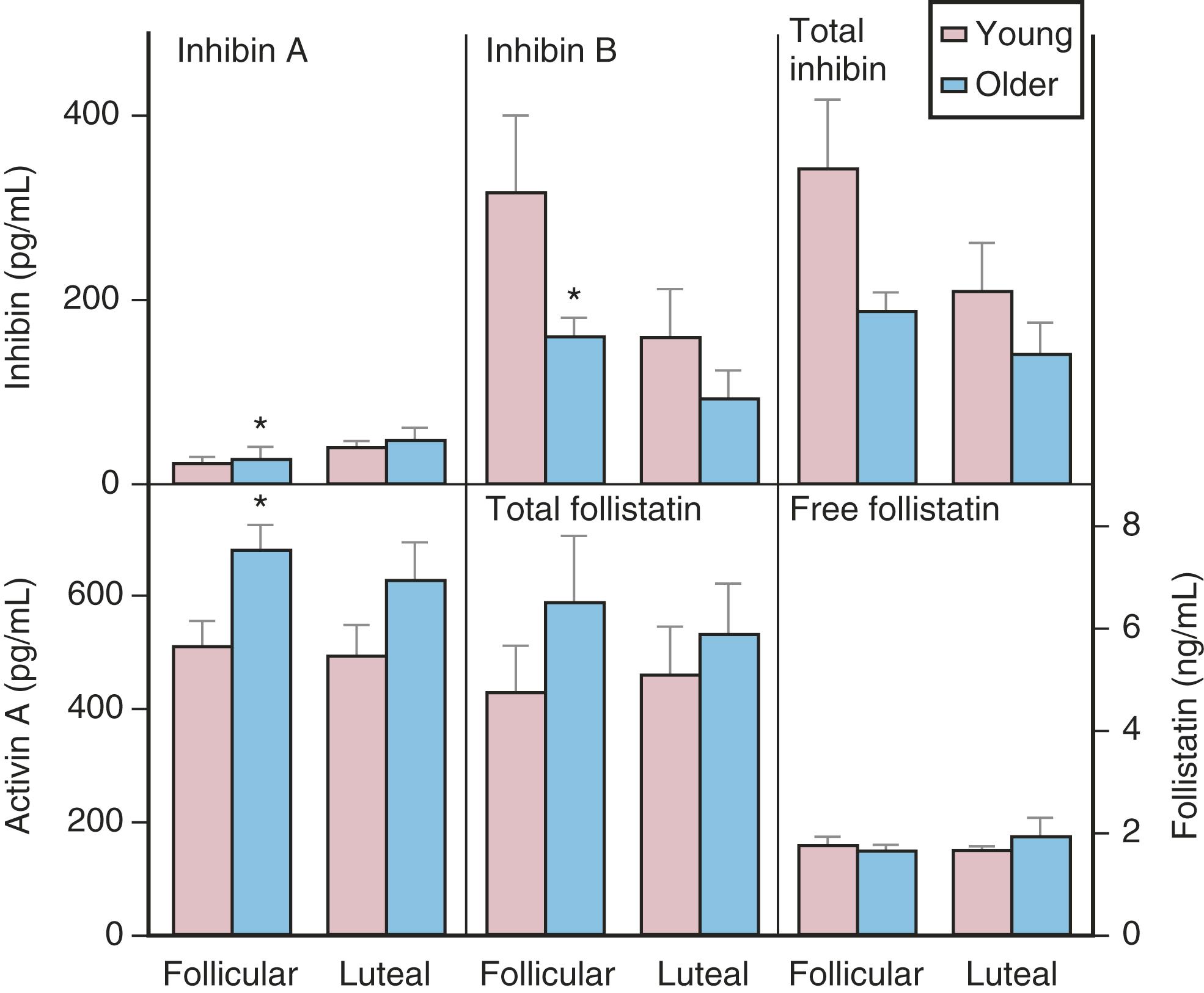
The functional capacity of the ovary is also diminished as women enter into the perimenopause. With gonadotropin stimulation, while estradiol (E 2 ) levels are not very different between younger and older women, total inhibin production by granulosa cells is decreased in women over age 35. From a clinical perspective, subtle increases in FSH on day 3 of the cycle, increases in the clomiphene challenge test, and lower AMH levels, correlate with decreased ovarian responses to stimulation and decreased fecundability. ,
Although there is a general decline in oocyte number with age, an accelerated atresia occurs around age 37 or 38 (see Fig. 14.4 ). While the reason for this acceleration is not clear, one possible theory relates to activin secretion. Because granulosa cell-derived activin is important for stimulating FSH-receptor expression, the rise in FSH levels could result in more activin production, which, in turn, enhances FSH action. A profile of elevated activin with lower inhibin B has been found in older women ( Fig. 14.8 ). This autocrine action of activin, involving enhanced FSH action, might be expected to lead to accelerated growth and differentiation of granulosa cells. Further, activin has been shown to increase the size of the pool of preantral follicles in the rat. At the same time, these follicles become more atretic.
Clinical management of women in the perimenopause should address three general areas of concern: (1) irregular bleeding; (2) symptoms of menopause, such as hot flushes; and (3) the inability to conceive. In general, because this is a time of flux, it is prudent to observe these changes and not treat transient conditions, unless they are significant and concerning.
Treatment of irregular bleeding is complicated by the fluctuating hormonal status. Estrogen levels may be higher than normal in the early follicular phase and progesterone secretion may be normal, although not all cycles are ovulatory. For these reasons, short-term use of an oral contraceptive (usually 20 μg ethinyl estradiol) may be an option for otherwise healthy women who do not smoke to help them cope with irregular bleeding.
Early symptoms of menopause, particularly vasomotor changes, may occur as the result of fluctuating hormonal levels. In this setting, an oral contraceptive again may be an option if symptoms warrant therapy. Alternatively, lower doses of estrogen used alone may be another option.
Reproductive concerns often require more aggressive treatment because of decreased cycle fecundity. Once day 2 or 3, FSH levels increase, and AMH levels are lower than the established normative data; the prognosis for pregnancy is markedly reduced. A more detailed discussion of AMH levels may be found in the next section.
In 2020, the American Heart Association published a scientific statement which stated that the menopausal transition is a risk factor for cardiovascular health. Some of the associations include the prevalence of vasomotor symptoms, sleep disturbances, depressive mood, increases in central/visceral, as well as paracardial fat, lipid changes, increases in blood pressure, as well as insulin resistance. These all evolve with time, at a variable rate in different women. Women with early ovarian aging along with POI or early surgical menopause are at greatest risk. Interventions beginning at this time are considered to be warranted and will be discussed later.
Depicted in Fig. 14.9 are the typical hormonal levels of postmenopausal women compared with those of ovulatory women in the early follicular phase. The most significant findings are the marked reductions in E 2 and estrone (E 1 ). Serum E 2 is reduced to a greater extent than E 1 . Serum E 1 , on the other hand, is produced primarily by peripheral aromatization from androgens, which decline more slowly, principally as a function of age. Levels of E 2 average 15 pg/mL and range from 10 to 25 pg/mL but are 10 pg/mL or less in women who have undergone oophorectomy. Serum E 1 values average 30 pg/mL but may be higher in obese women because aromatization increases as a function of the mass of adipose tissue. In recent year there has been a concerted effort to standardize and improve the sensitivity of estrogen assays, particularly in postmenopausal women. Assays should be carried out using mass spectrometry and normal mean estradiol levels after menopause should be approximately 10 pg/mL, rather than 15 to 25 pg/mL as suggested above and in the Fig. 14.9 .
![Fig. 14.9, Circulating levels of pituitary and steroid hormones in postmenopausal women compared with levels in premenopausal women studied during the first week (days 2 to 4 [D 2–4 ]) of the menstrual cycle. Fig. 14.9, Circulating levels of pituitary and steroid hormones in postmenopausal women compared with levels in premenopausal women studied during the first week (days 2 to 4 [D 2–4 ]) of the menstrual cycle.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MenopauseandAging/8_3s20B9780323810074000144.jpg)
Estrone sulfate (E 1 S) is an estrogen conjugate that serves as a stable circulating reservoir of estrogen, and levels of E 1 S are the highest among estrogens in postmenopausal women. In premenopausal women, values are usually above 1000 pg/mL; in postmenopausal women, levels average 350 pg/mL.
Apart from elevations in FSH and luteinizing hormone (LH), other pituitary hormones are not affected. The rise in FSH, beginning in stage −3 as early as age 38 (see Fig. 14.2 ), fluctuates considerably until approximately 4 years after menopause when values are consistently greater than 20 mlU/mL. Specifically, growth hormone (GH), thyroid-stimulating hormone, and adrenocorticotropic hormone (ACTH) levels are normal. Serum prolactin levels may be very slightly decreased because prolactin levels are influenced, in part, by estrogen status.
Both the postmenopausal ovary and the adrenal gland continue to produce androgen. The ovary continues to produce androstenedione and testosterone but not E 2 , , and this production has been shown to be at least partially dependent on LH. Androstenedione and testosterone levels are lower in women who have experienced bilateral oophorectomy, with values averaging 0.8 ng/mL and 0.1 ng/mL, respectively. The adrenal gland also continues to produce androstenedione, dehydroepiandrosterone (DHEA), and DHEAS; primarily as a function of aging, these values decrease somewhat (adrenopause), although cortisol secretion remains unaffected. Some data suggest that much “ovarian” testosterone production may actually arise from the adrenal. Most likely, this production is by indirect mechanisms due to the adrenal supplying precursor substrate (DHEA and androstenedione).
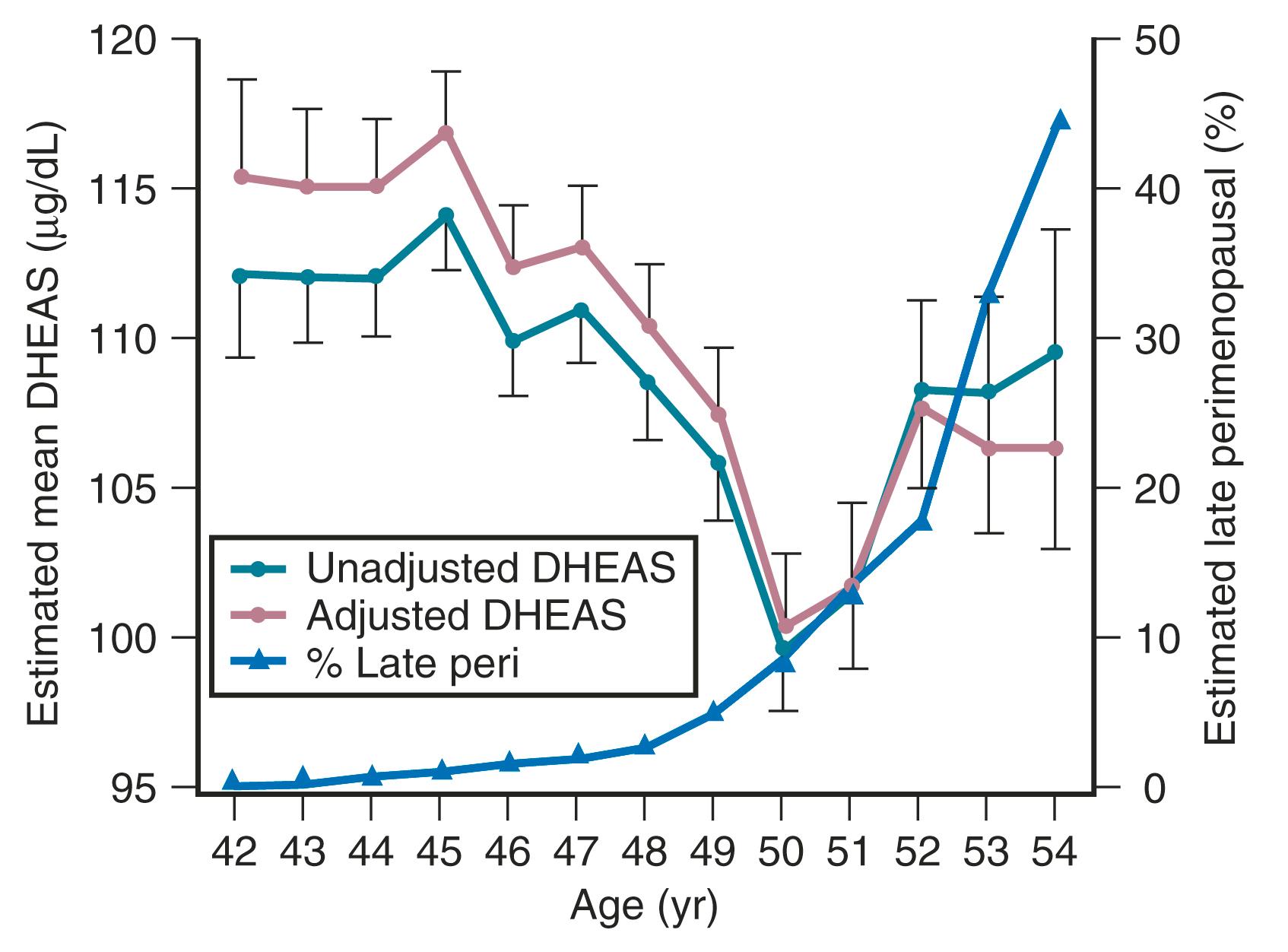
Although DHEAS levels decrease with age (approximately 2% per year), recent data have suggested that levels transiently rise in the perimenopause before the continuous decline thereafter ( Fig. 14.10 ). This interesting finding from the SWAN study also suggested that DHEAS levels are highest in Chinese women and lowest in African-American women.
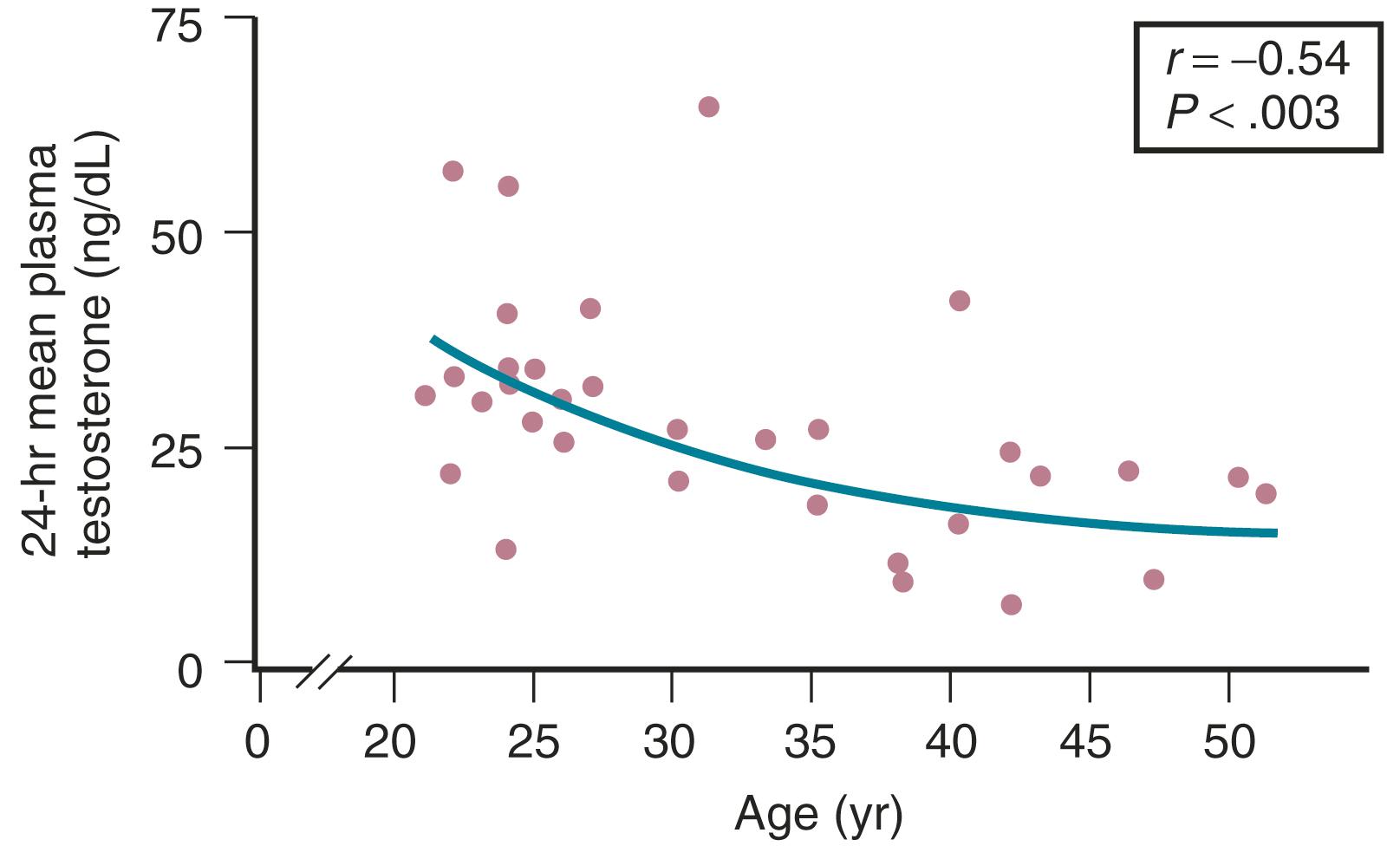
Testosterone levels also decline as a function of age, which is best demonstrated by the reduction in 24-hour mean levels ( Fig. 14.11 ). Because of the role of the adrenal in determining levels of testosterone after menopause, adrenalectomy or dexamethasone treatment results in undetectable levels of serum testosterone. Compared with total testosterone, the measurement of bioavailable or “free” testosterone is more useful in postmenopausal women. After menopause, sex hormone-binding globulin (SHBG) levels decrease, resulting in relatively higher levels of bioavailable testosterone or a higher free androgen index ( Fig. 14.12 ). In women receiving oral estrogen, bioavailable testosterone levels are extremely low because SHBG levels are increased. How this relates to the decision to begin androgen therapy in postmenopausal women will be discussed later in this chapter.
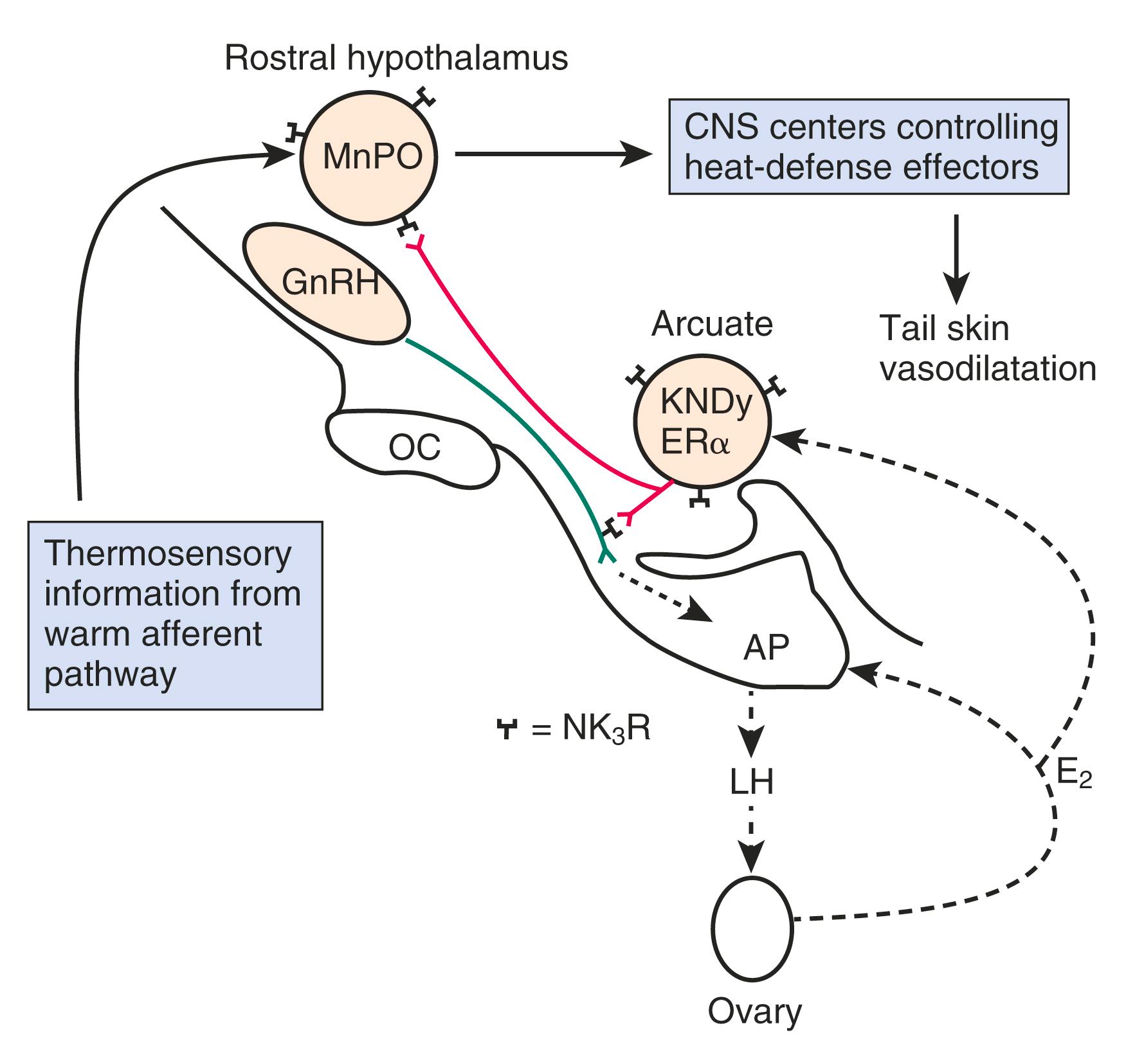
Elevated gonadotropin (FSH-LH) levels arise from reduced secretion of E 2 and inhibin, as described earlier. Estrogen is important in controlling the production of gonadotropin-releasing hormone (GnRH) mRNA in type 1 neurons. , In addition, the increase in gonadotropins observed at menopause appears to be enhanced by substance P, as well as by tachykinins produced in hypertrophied neurons, , which result from the decrease in E 2 .
Unlike the rodent, where there is evidence for a hypothalamic factor involved in ovarian senescence, , , no such clear evidence exists for women. The hypothesis proposed by Wise suggested that the effects of aging in the brain affect neurotransmitter systems that regulate GnRH, disrupting ovarian folliculogenesis, and ultimately promoting senescence. Thus, the accelerated follicular loss that is apparent in the late 30s is postulated to be due to age-related desynchronization in the rhythmicity of GnRH secretion.
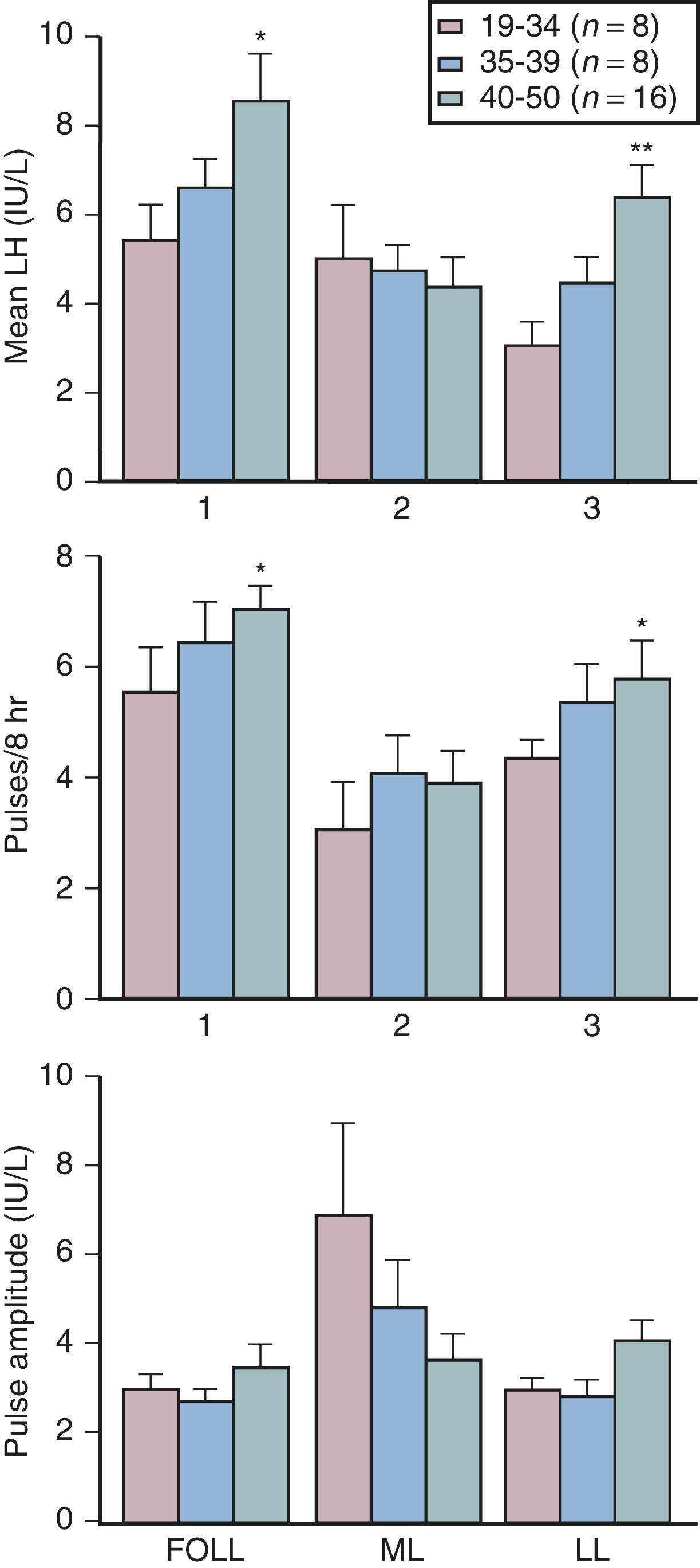
Although some aging effects of the brain are likely to exist, there is abundant human evidence for an ovarian-induced menopause. While there is slowing of LH pulsatility with aging in rodents, LH pulse frequency and amplitude increase with age as menopause approaches in women ( Fig. 14.13 ). A sleep-entrained alteration in GnRH pulse dynamics has been observed in postmenopausal women, namely the inability to increase GnRH pulse amplitude at night. There is some evidence that the high frequency and amplitude pulses of LH observed in the first few years in postmenopausal women slows down in late menopause ; the latter effect is clearly related to aging.
Ovarian aging is a programmed event, and the return of atresia, accelerating at around age 37.5, until the natural age of menopause, has now been shown to occur in almost the exact way in the chimpanzee. Ovarian aging from a hormonal standpoint is best characterized by small elevations in serum FSH occurring at the beginning of the menstrual cycle (days 2–3), reductions in inhibin B, as well as steep declines in serum müllerian inhibiting substance or AMH was introduced previously ( Fig. 14.14 ). It has been confirmed that once a level of inhibin B or AMH becomes undetectable, menopause will ensue in 4 to 5 years (see Fig. 14.14A ). There have been several prediction models of the age of natural menopause based on levels of AMH. A recent meta-analysis showed that AMH has good predictability with a hazard ratio of 5.6 to 9.2. Nevertheless, there is no absolute precision with this prediction and it is questionable whether this knowledge has any practical value.
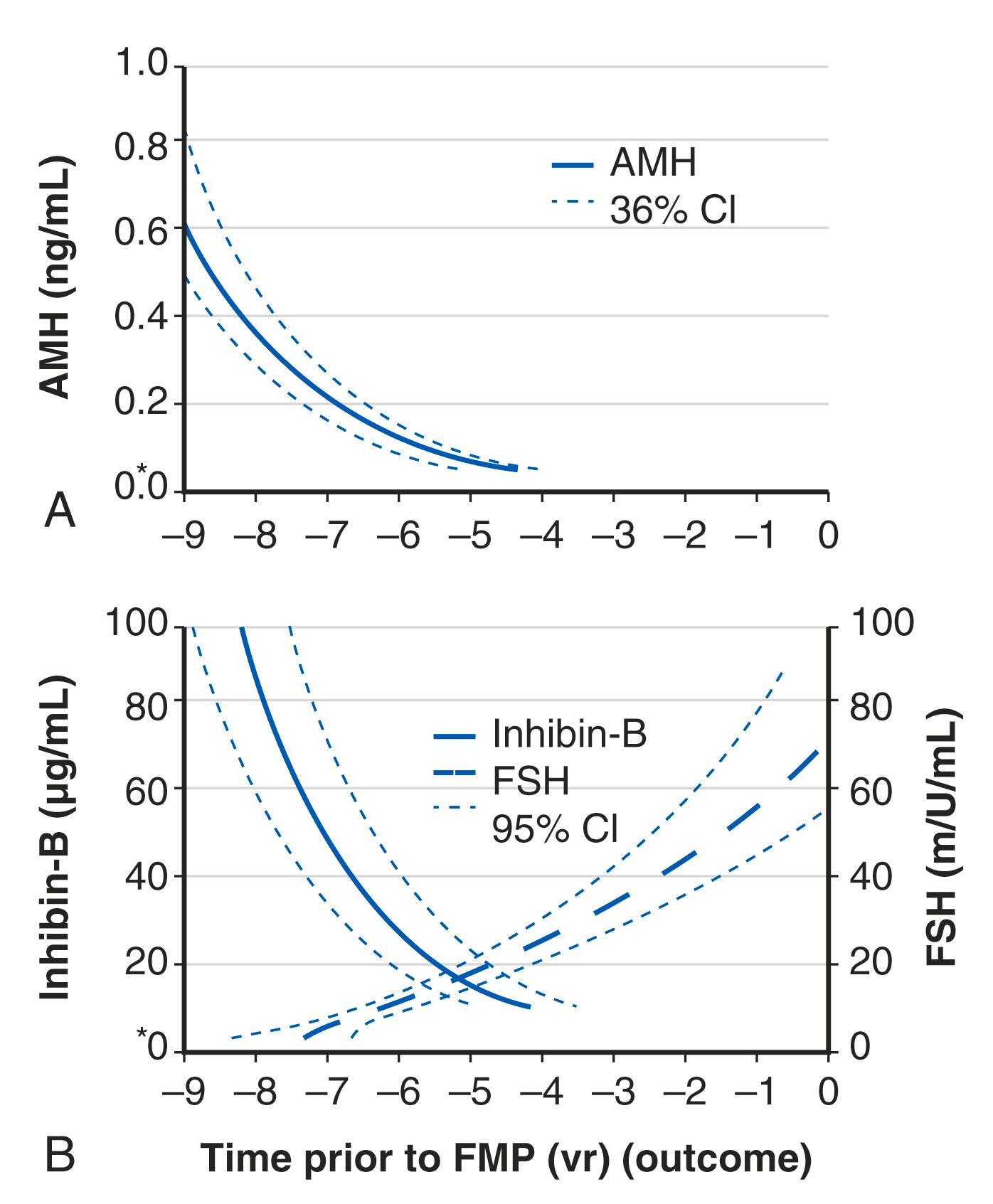
AMH is a very useful and practical determinant in that it tends to undergo less cycle-to-cycle variation compared to FSH or inhibin B and can be measured during any phase of the cycle. However, it should be kept in mind that the use of oral contraceptive pills can reduce AMH levels by approximately 20%.
Estrogen has many powerful effects on brain anatomy and function that are mediated through specific receptors.
The hallmark feature of the drop in estrogen at the time of menopause is the hot flush or vasomotor instability.
Recent data point to alterations in KNDy neurons in the hypothalamus at the time of menopause, which are linked to thermoregulatory centers in the brain.
Collagen content decreases after menopause and is, at least in part, responsive to estrogen.
Vulvovaginal and urinary complaints (the genitourinary syndrome of menopause [GSM]) are common after menopause and increase with age. There are several validated treatments for GSM.
Osteoporosis after menopause is occasioned by an increase in inflammatory factors that increase bone resorption in the face of estrogen decline.
There are several biochemical and radiographic methods to assess bone turnover and bone density, but bone strength is most important in determining fracture risk.
Many agents are now available for osteoporosis prevention and treatment.
Degenerative arthritis (osteoarthritis) correlates with aging but it may also be related to estrogen insufficiency.
CVD is the leading cause of death in women, and the risk begins to increase from the time of the menopausal transition.
Estrogen protects against atherosclerosis prior to menopause and in younger women after menopause, before the development of established atherosclerosis.
Estrogen treatment in younger healthy postmenopausal women decreases coronary disease and mortality but is ineffective in older women and may be harmful.
Stroke risk in women increases with age and is compounded by obesity and hypertension.
The risk of ischemic stroke in younger postmenopausal women is not due to atherosclerotic changes but is thrombotic in nature and may be due to underlying thrombotic risk factors.
Estrogen has powerful effects on the brain mediated by estradiol receptors α and β as well as membrane effects. Most data point to neuroprotective effects of estrogen and older observational data point to a benefit of postmenopausal estrogen of cognitive decline and Alzheimer disease (AD), but this has not been confirmed in prospective studies. There are well-defined physiological and hormonal changes that occur during the hot flush—the hallmark feature of menopausal symptoms. A narrowing of the thermoregulatory zone is a theory for the etiology of the hot flush, but new data also point to alterations in hypothalamic kisspeptin, neurokinin B , and dynorphin (KNDy) neurons at menopause.
The brain is an active site for estrogen action and estrogen formation. Estrogen activity in the brain is mediated via estrogen receptors (ER)α and ERβ receptors. Whether or not a novel membrane receptor (non-ERα/ERβ) exists is still being debated. However, both genomic and nongenomic mechanisms of estrogen action clearly exist in the brain. Fig. 14.15 illustrates the predominance of ERβ in the cortex (frontal and parietal) and the cerebellum, based on work in the rat. , While 17β-E 2 is a specific ligand for both receptors, certain synthetic estrogens have a greater affinity for ERβ.
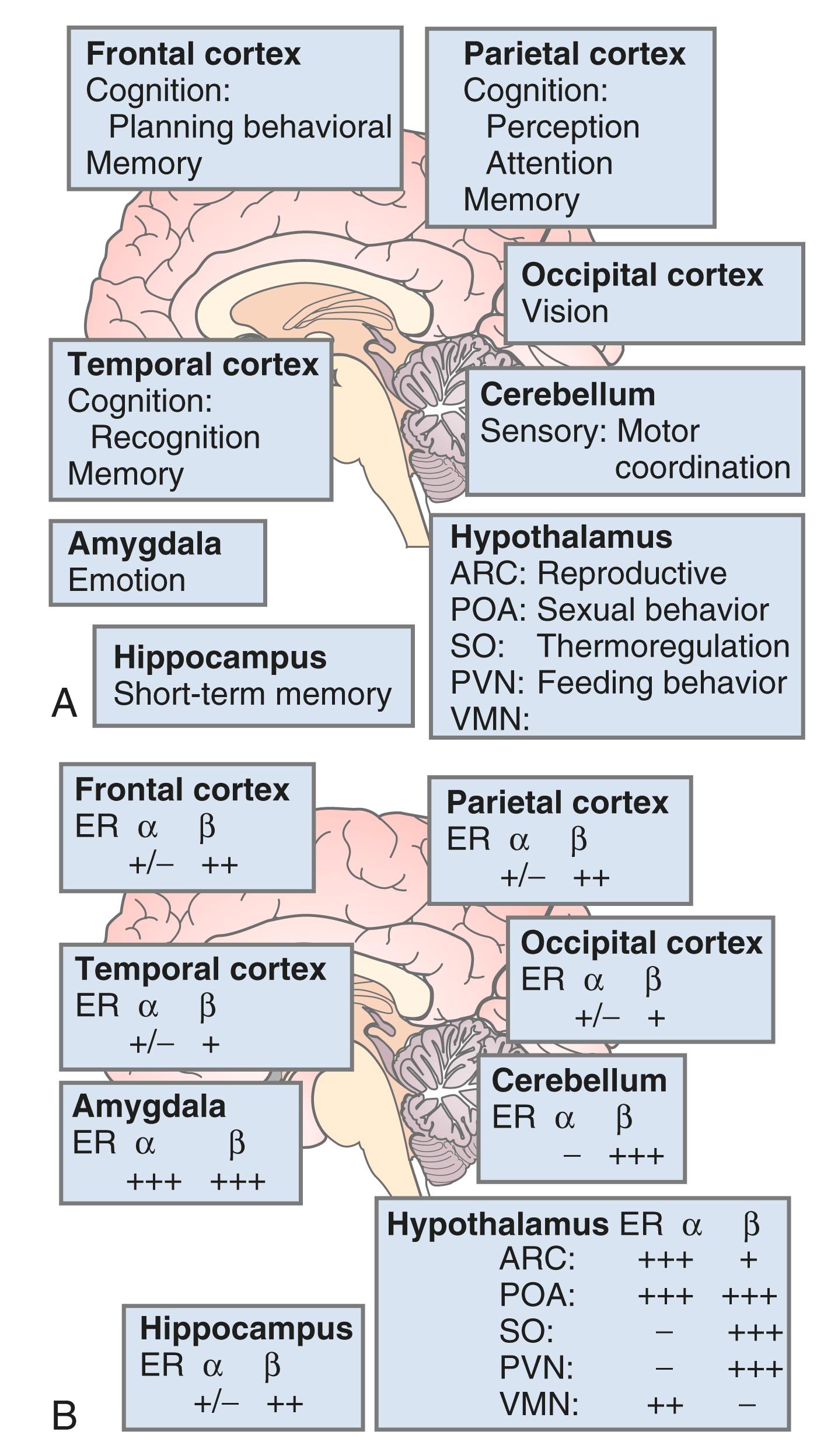
There are multiple actions of estrogen on the brain, as reviewed by Henderson ( Box 14.2 ) ; thus, there are important functions linked to estrogen that contribute to well-being in general and, more specifically, to cognition and mood. The hallmark feature of declining estrogen status in the brain is the hot flush, which is more generically referred to as a vasomotor episode.
Organizational actions
Effects on neuronal number, morphology, and connections occurring during critical stage of development
Neurotrophic actions
Neuronal differentiation
Neurite extension
Synapse formation
Interactions with neurotrophins
Neuroprotective actions
Protection against apoptosis
Antioxidant properties
Antiinflammatory properties
Augmentation of cerebral blood flow
Enhancement of glucose transport into the brain
Blunting of corticosteroid response to behavioral stress
Interactions with neurotrophins
Effects on neurotransmitters
Acetylcholine
Noradrenaline
Serotonin
Dopamine
Glutamate
γ-Aminobutyric acid
Neuropeptides
Effects on glial cells
Effects on proteins involved in Alzheimer disease
Amyloid precursor protein
Tau protein
Apolipoprotein E
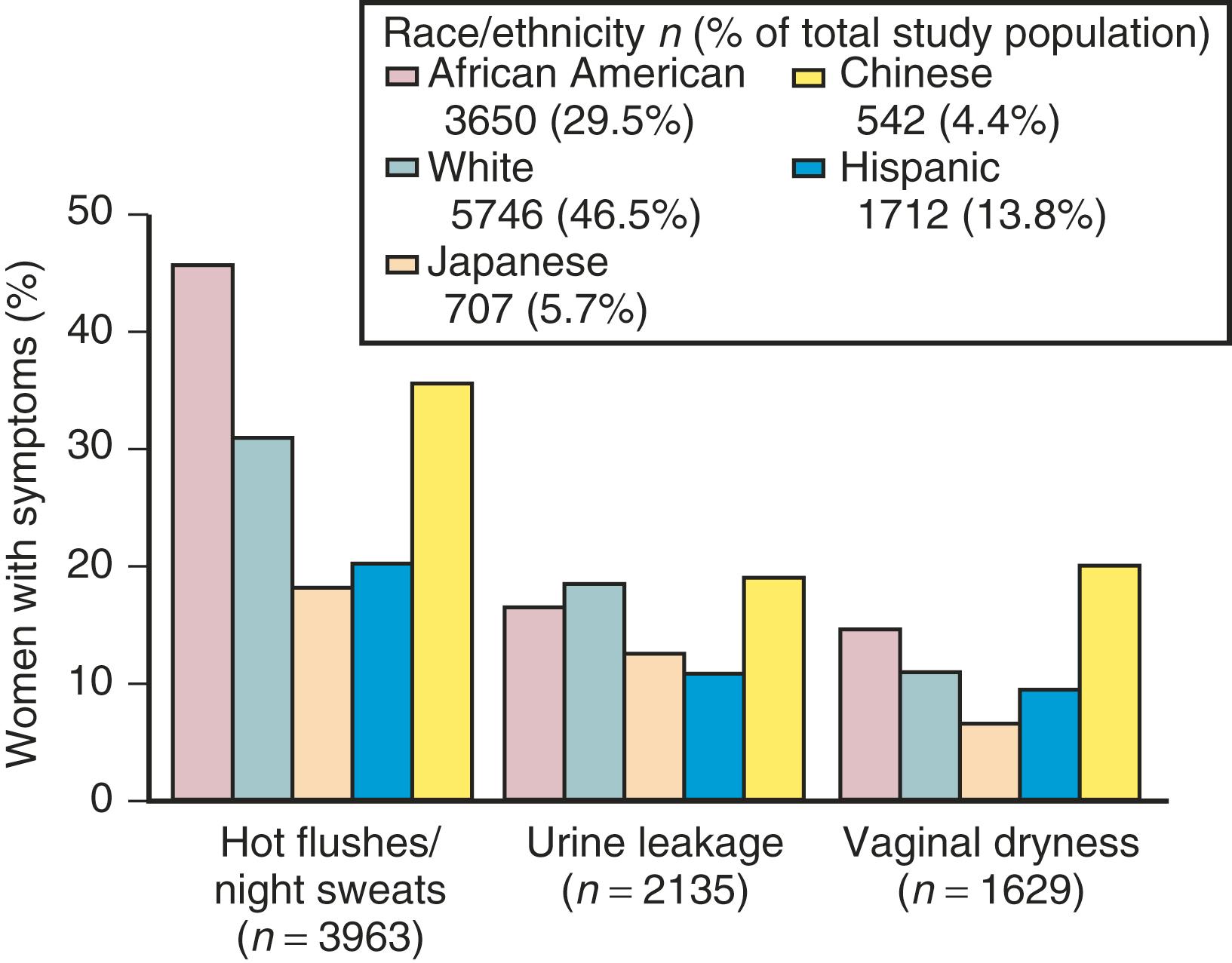
The hot flash usually refers to the acute sensation of heat, while the flush or vasomotor episode includes changes in the early perception of this event and other skin changes (including diaphoresis). Hot flushes usually occur for 2 years after the onset of estrogen deficiency but can persist for 10 or more years. , A recent longitudinal study from SWAN showed that the median duration of significant vasomotor symptoms is 7.4 years. In 10% to 15% of women, these symptoms are severe and disabling. , In the United States, the incidence of these episodes varies in different ethnic groups. Symptoms are most prevalent in Hispanic and African-American women, intermediate in White women, and lowest in Asian women ( Fig. 14.16 ). Color coding in this figure is not correct
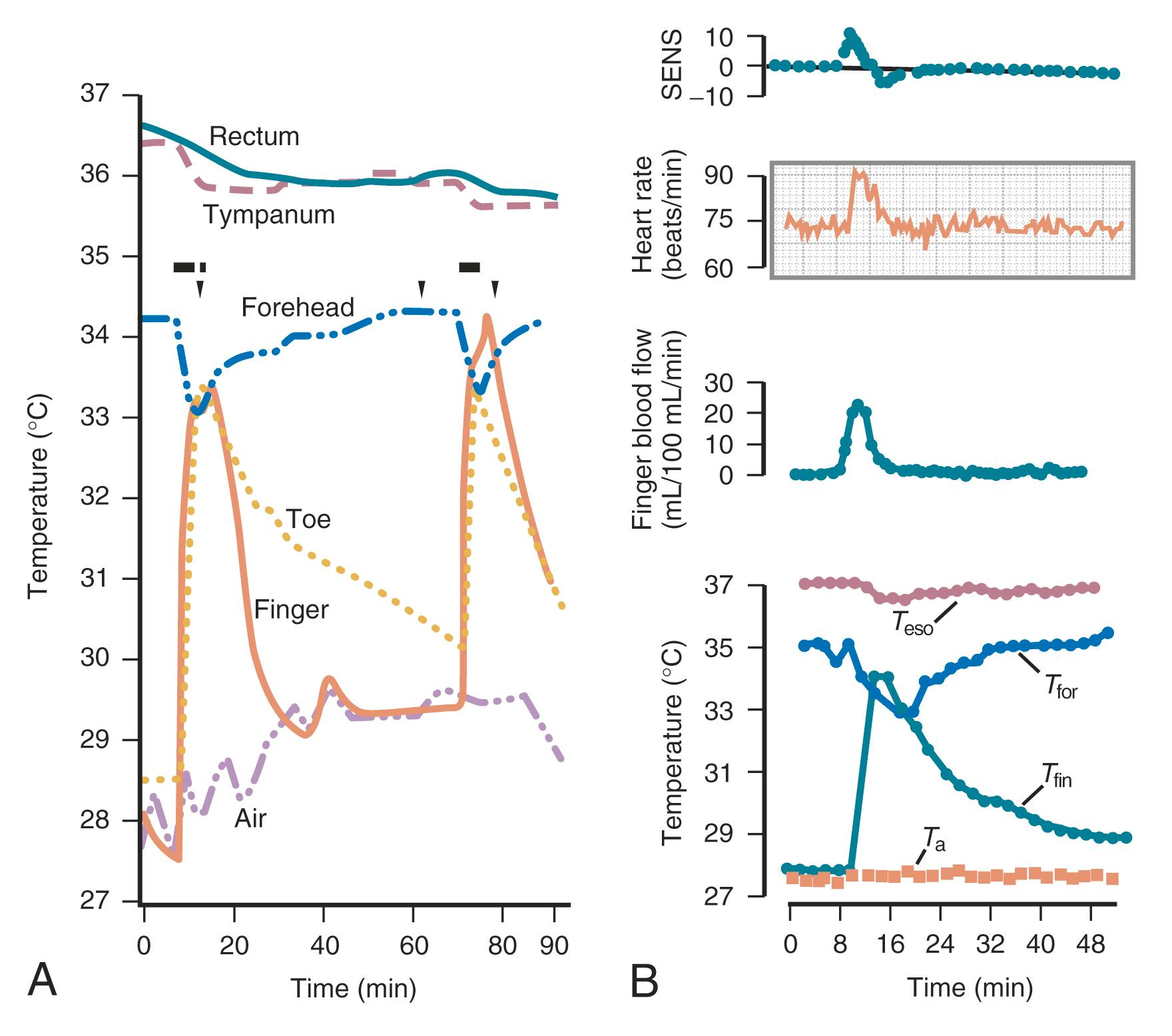
The fall in estrogen levels precipitates the vasomotor symptoms. Although the proximate cause of the flush remains elusive, the episodes result from a hypothalamic response (probably mediated by catecholamines) to the change in estrogen status. The flush has been well characterized physiologically. It results in heat dissipation, as witnessed by an increase in peripheral temperature (finger, toe); a decrease in skin resistance, associated with diaphoresis; and a reduction in core body temperature ( Fig. 14.17 ). There are hormonal correlates of flush activity, such as an increase in serum LH and in plasma levels of pro-opiomelanocortin peptides (ACTH, β-endorphin) at the time of the flush, but these occurrences are thought to be epiphenomena and not the proximate cause of the flush. Nevertheless, there may be interplay in the hypothalamus between the thermoregulatory center and release of GnRH/LH. It is now understood that the GnRH and opioid-related hormonal release, which occur at the time of the flush, are related to activation of the KNDy neurons, which communicate with the thermoregulatory center in the hypothalamus.
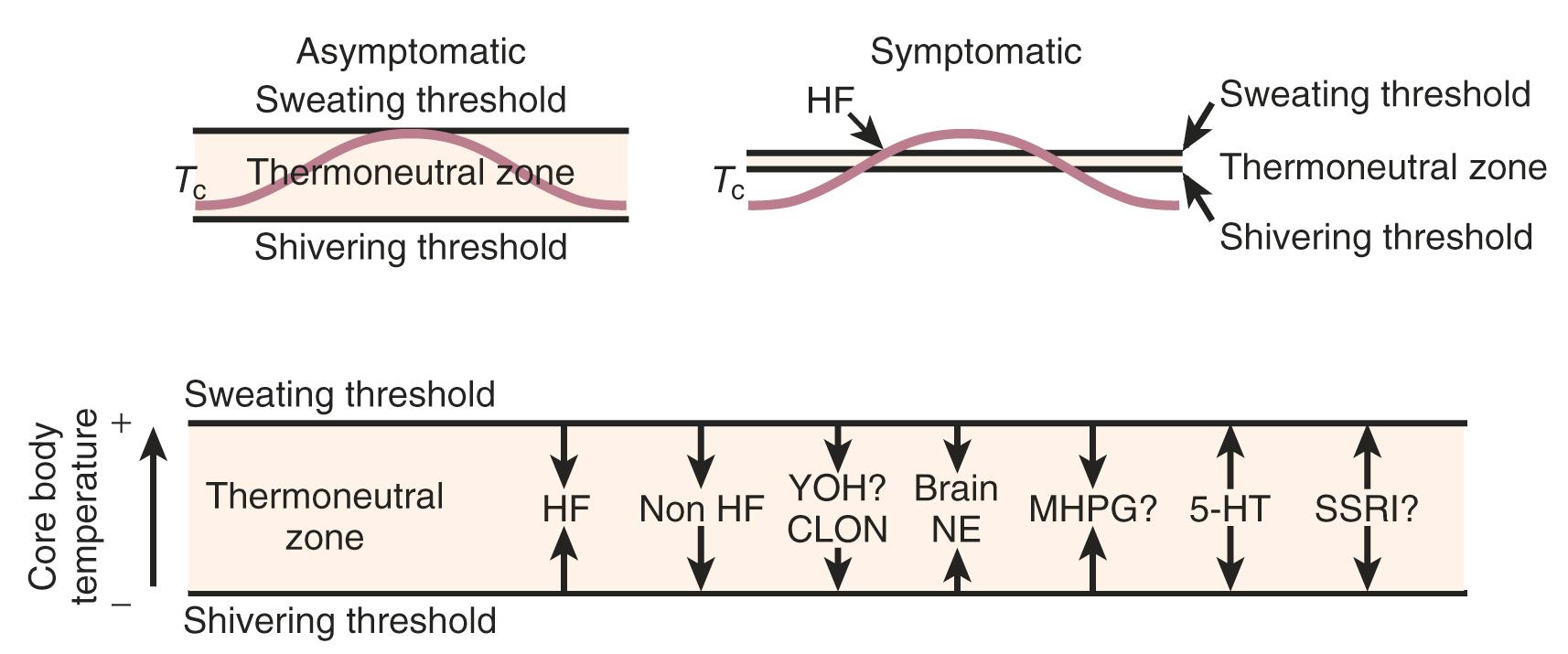
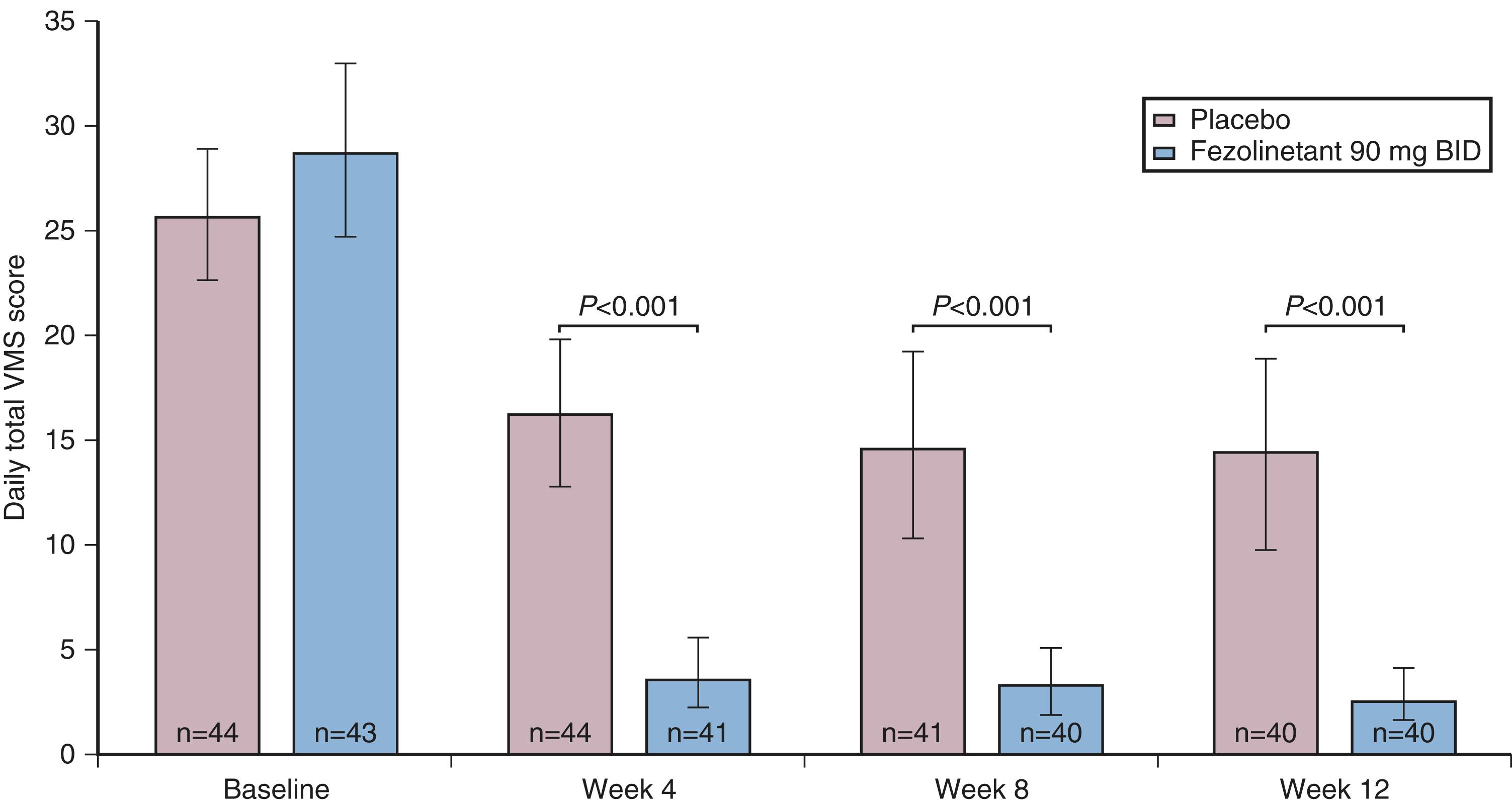
The other theory regarding the origin of the hot flush may be deduced from data from Freedman, who suggested that the major physiological finding in postmenopausal women with and without hot flushes is a narrowing of the temperature threshold for sweating and shivering in symptomatic women ( Fig. 14.18 ). While these physiological observations are valid, they may be thermoregulatory changes that are mediated by alterations in the KNDy neurons. As noted above, the KNDy neurons in the hypothalamus activate kisspeptin and other receptors on GnRH neurons which releases GnRH. At the same time, KNDy neurons also impinge upon the thermoregulatory center in the hypothalamus. Estrogen loss has been shown to increase the size of KNDy neurons and to activate the genes for neurokinin B and kisspeptin, as suggested in Fig. 14.19 . Experimentally, neurokinin B, activating the neurokinin 3 receptor, has been shown to induce hot flushes in postmenopausal women. This activation of the KNDy neuronal system would also release GnRH/LH, which is what has been observed during a hot flush. The potential exists, therefore, that specific neurokinin 3 (NK3) receptor antagonists (which are available for oral use) may be able to inhibit hot flushes in women. Several clinical trials using different NK3 receptor antagonists have shown efficacy for the treatment of hot flushes in postmenopausal women , ( Fig. 14.20 ).
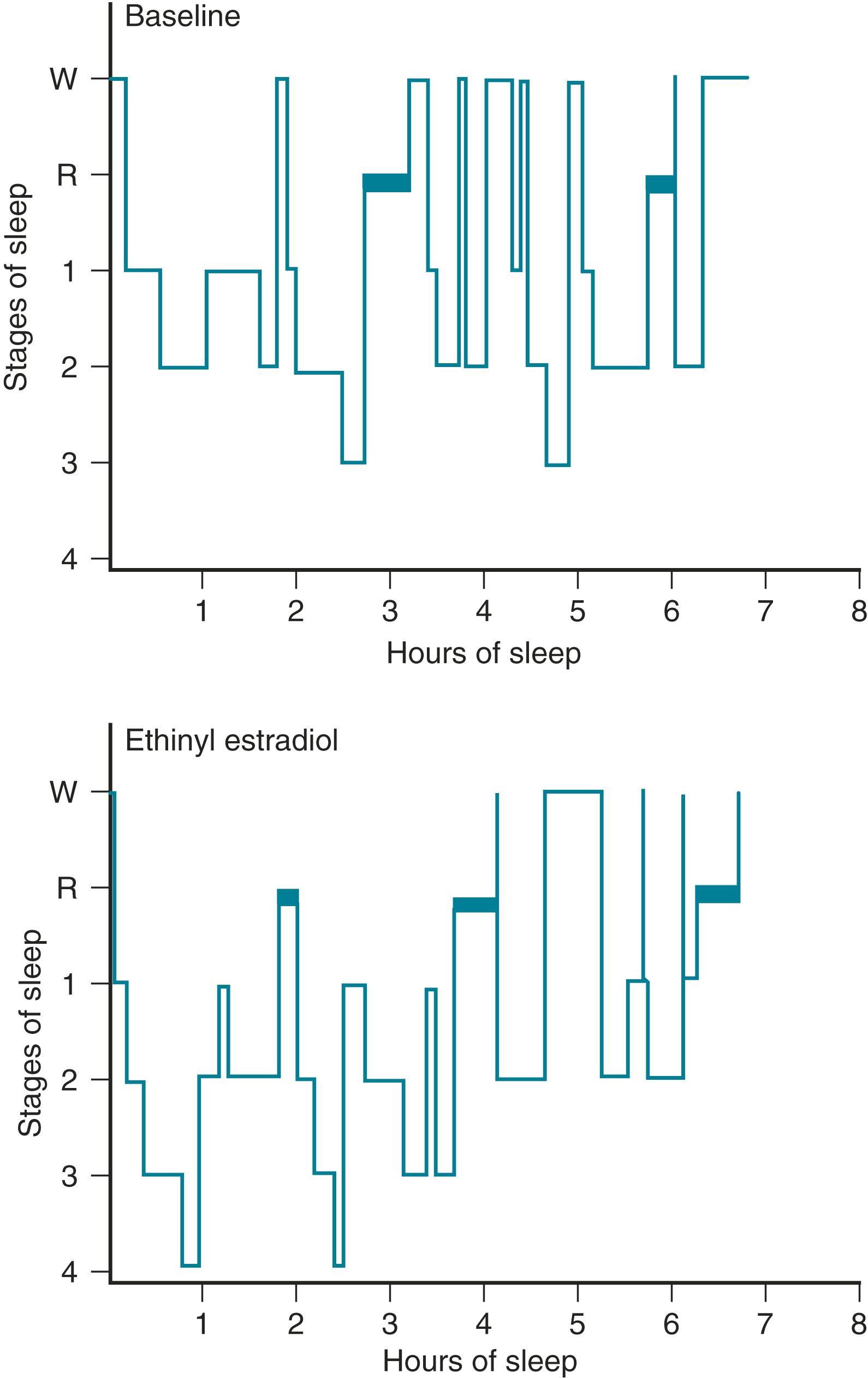
One of the primary complaints of women with hot flushes is sleep disruption. They may awaken several times during the night and require a change of bedding and clothes because of diaphoresis. Nocturnal sleep disruption in postmenopausal women with hot flushes has been well documented by electroencephalographic (EEG) recordings. Sleep efficiency is lower, and the latency to rapid eye movement sleep is longer in women with hot flushes compared with asymptomatic women. , Some of the beneficial effect of estrogen on sleep may be independent of vasomotor symptoms. This disturbed sleep often leads to fatigue and irritability during the day. The frequency of awakenings and of hot flushes are reduced appreciably with estrogen treatment ( Fig. 14.21 ). , , Sleep may be disrupted even if the woman is not conscious of being awakened from sleep. In this setting, EEG monitoring has indicated sleep disruption in concert with physiological measures of vasomotor episodes.
In postmenopausal women, estrogen has been found to improve depressed mood regardless of whether or not this is a specific complaint. (Critics of some of this work point out that mood is affected by the symptomatology and by sleep deprivation.) Blinded studies carried out in asymptomatic women have also shown benefit. In an estrogen-deficient state, such as occurs after the menopause, a higher incidence of depression (clinical or subclinical) is often manifest. However, menopause per se does not cause depression, and while estrogen does generally improve depressive mood, it should not be used for psychiatric disorders. Nevertheless, very high pharmacological doses of estrogen have been used to treat certain types of psychiatric depression in the past. Progestogens as a class generally attenuate the beneficial effects of estrogen on mood, although this effect is highly variable. ,
Cognitive decline in postmenopausal women is related to aging as well as to estrogen deficiency. The literature is somewhat mixed in showing whether there are benefits of estrogen in terms of cognition. Longitudinal data from the Study of Women Across the Nation have shown that there is a transient decline in cognitive function in perimenopausal women but that this returns to normal soon after menopause, without any intervention. Abrupt cessation of estrogen status as with bilateral oophorectomy leads to a significant reduction in cognitive activity, particularly in verbal memory in the short term. In general, particularly in the model of acute estrogen loss following oophorectomy, verbal memory appears to be enhanced with the administration of estrogen, and some of these effects have been found to correlate with acute changes in brain imaging, signifying brain activation.
Dementia increases as women age, and the most common form of dementia is Alzheimer disease (AD). Listed in Box 14.2 are several neurotrophic and neuroprotective factors that relate to how estrogen deficiency may be expected to result in the loss of protection against the development of AD. In addition, estrogen has a positive role in enhancing neurotransmitter function, which is deficient in women with AD. This function of estrogen has particular importance and relevance for the cholinergic system that is affected in AD. ,
Estrogen use after menopause has been shown in observational studies to decrease the likelihood of developing or delay the onset of AD ( Fig. 14.22 ). However, there are no randomized prospective studies as yet on this issue. It is clear, however, that if estrogen has a beneficial role, it is only in those women who receive estrogen at the onset of menopause; in older women it may be detrimental. , A recent short-term study could not show a cognitive benefit in women who received estrogen within 6 years of menopause and those who received it 10 years later. However, recent brain imaging studies have suggested that women treated early after menopause with estrogen may have less amyloid deposition, particularly if they have the Apo-E allele, a risk factor for AD. Once a woman is affected by AD, estrogen is unlikely to provide any benefit. In the Women’s Health Initiative (WHI) study, women over the age of 65 years receiving estrogen and progestogen for the first time had a decrease in cognition, which was not statistically significant for the group of women receiving estrogen alone.
Putting this together, it is likely that early initiation of estrogen is protective for long-term benefits in preventing AD and cognitive decline, similar to such a protective effect on coronary disease (discussed below). Observational data which focused on treating younger symptomatic women at the onset of menopause are supportive of this notion. , Basic studies in the rodent also suggest that there is loss of ERα soon after loss of ovarian function but that estrogen replacement may restore this functionality. To date, there are still no compelling prospective data in younger postmenopausal women to confirm these earlier observational studies on the benefits of estrogen in preventing cognitive decline or AD risk.
Estrogen has a positive effect on collagen , which is an important component of bone and skin and serves as a major support tissue for the structures of the pelvis and urinary system. Both estrogen and androgen receptors have been identified in skin fibroblasts. Nearly 30% of skin collagen is lost within the first 5 years after menopause, and collagen decreases approximately 2% per year for the first 10 years after menopause. This statistic, which is similar to that of bone loss after menopause, strongly suggests a link between skin thickness, bone loss, and the risk of osteoporosis.
Although the literature is not entirely consistent, estrogen therapy (ET) generally improves collagen content after menopause and improves skin thickness substantially after about 2 years of treatment. , There is a possible bimodal effect with high doses of estrogen causing a reduction in skin thickness. The supportive effect of estrogen on collagen has important implications for bone homeostasis and for the pelvis after menopause. Here, reductions in collagen support and atrophy of the vaginal and urethral mucosa have been implicated in a variety of symptoms including prolapse and urinary incontinence. ,
GSM is now the preferred term which encompasses urinary complaints as well as what has been known as vulvovaginal atrophy. Symptoms of urinary incontinence and irritative bladder symptoms occur in 20% to 40% of perimenopausal and postmenopausal women. Uterine prolapse and other gynecological symptoms related to poor collagen support, as well as urinary complaints, may improve with ET. While estrogen generally improves symptoms, urodynamic changes have not been shown to be altered. , Estrogen has also been shown to decrease the incidence of recurrence of urinary tract infections. These data relate to the use of vaginal estrogen rather than the use of estrogen systemically. Somewhat paradoxically, systemic estrogen may increase stress urinary incontinence, while local vaginal therapy may improve urge incontinence.
In Sweden, the restoration of bladder control in older women with estrogen has been shown to decrease the need for admission to nursing homes. Estrogen may also have an important role in normal wound healing. In this setting, estrogen enhances the effects of growth factors, such as transforming growth factor β (TGFβ). ,
Vulvovaginal complaints are often associated with estrogen deficiency. During the perimenopause stage, symptoms of dryness and atrophic changes occur in 21% and 15% of women, respectively. However, these findings increase with time, and by 4 years postmenopause, these incidences are 47% and 55%, respectively. , With this change, an increase in sexual complaints also occurs, with an incidence of dyspareunia of 41% in sexually active 60-year-old women. Estrogen deficiency results in a thinner and paler vaginal mucosa. The moisture content is low, the pH increases (usually greater than 5), and the mucosa may exhibit inflammation and small petechiae.
Vaginal cytology changes have been documented with estrogen treatment, transforming from a cellular pattern of predominantly parabasal cells to one with an increased number of superficial cells. Along with this change, the vaginal pH decreases, vaginal blood flow increases, and the electropotential difference across the vaginal mucosa increases to that found in premenopausal women.
Treatment is highly effective with various forms of vaginal estrogen. Most preparations available today do not significantly increase systemic levels of estradiol, with estrogen action occurring locally. In this regard, vaginal insertion of estrogen in the lower third of the vagina (rather than high in the vaginal fornix) attenuates systemic absorption and is preferable for vulvo-urinary complaints. Recent studies have suggested that intravaginal DHEA (0.25%–1%) is efficacious for altering vaginal cytology and symptoms of atrophy, presumably by allowing for local conversion to other androgens and estrogen. This product (prasterone) has been approved by the US Food and Drug Administration (FDA) for symptoms of dyspareunia in postmenopausal women. A selective estrogen receptor modulator (SERM), ospemifene (60 mg taken orally), with estrogen agonistic effects in the vagina, also has been approved by the FDA for dyspareunia.
Apart from dyspareunia, the most common finding in women with sexual dysfunction is hypoactive sexual desire disorder (HSDD). While a detailed description of this disorder is beyond the scope of this review and it is not specifically an issue for women after menopause, treatment options will be outlined alongside discussion of testosterone therapy.
Estrogen deficiency has been well established as a cause of bone loss. This loss can be noted for the first time when menstrual cycles become irregular in the perimenopause. From 1.5 years before the menopause to 1.5 years after menopause, spine bone mineral density (BMD) has been shown to decrease by 2.5% per year, compared with a premenopausal loss rate of 0.13% per year. , Loss of trabecula bone (spine) is greater with estrogen deficiency than is loss of cortical bone.
Postmenopausal bone loss leading to osteoporosis is a substantial healthcare problem. Thirty-five percent of all postmenopausal White women have been estimated to have osteoporosis based on BMD. Further, the lifetime fracture risk for these women is 40%. The morbidity and economic burden of osteoporosis is well documented. Interestingly, there are data to suggest that up to 19% of Caucasian men also have osteoporosis.
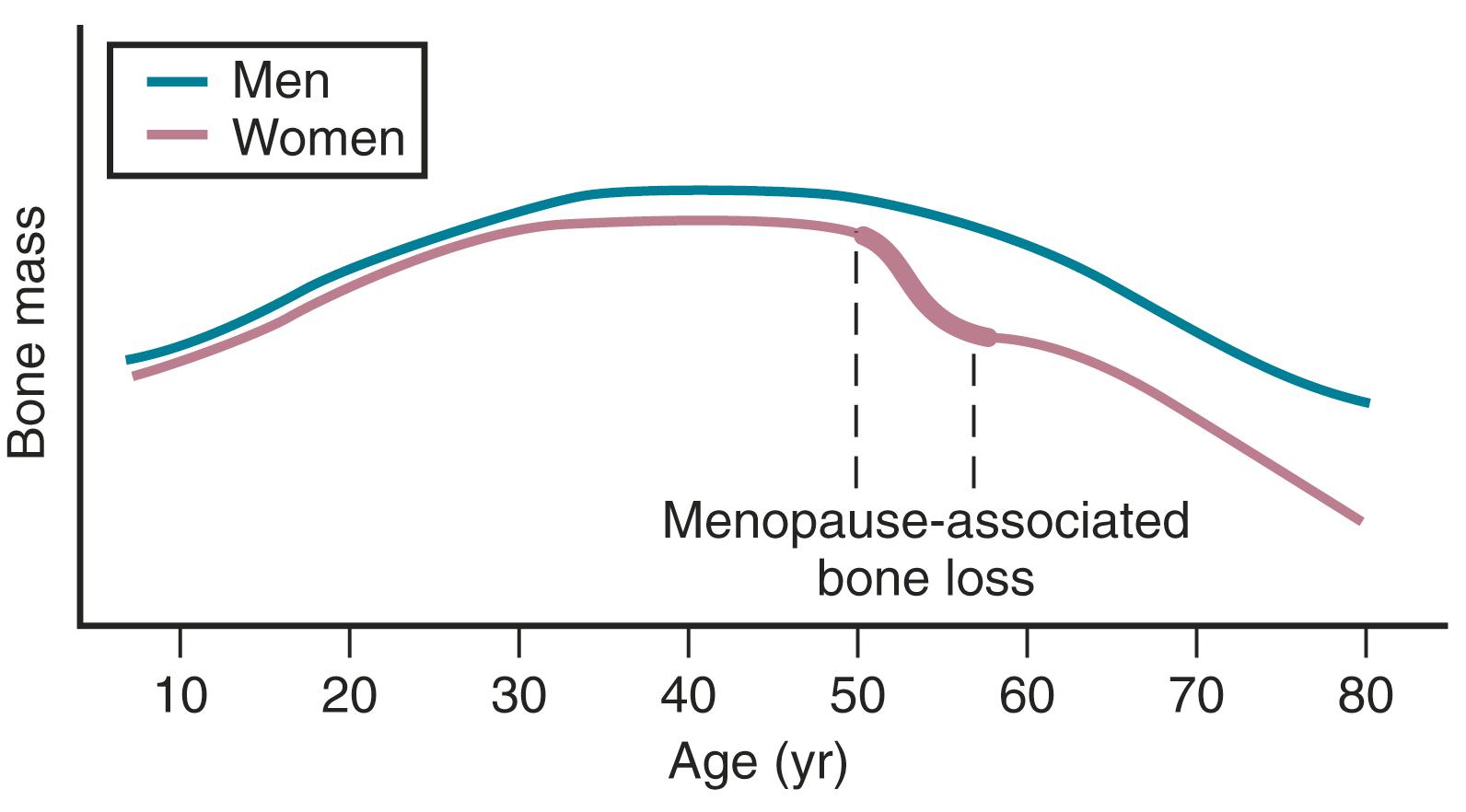
Bone mass is substantially affected by sex steroids through classic mechanisms to be described later in this chapter. Attainment of peak bone mass in the late second decade ( Fig. 14.23 ) is key to ensuring that the subsequent loss of bone mass with aging and estrogen deficiency does not lead to early osteoporosis. Estradiol, combined with GH and insulin-like growth factor-1, acts to double bone mass at the time of puberty, thereby beginning the process of attaining peak bone mass. Postpubertal estrogen deficiency (amenorrhea from various causes) substantially jeopardizes peak bone mass. Adequate nutrition and calcium intake are also key determinants. While estrogen is of predominant importance for bone mass in both women and men, testosterone is important in stimulating periosteal apposition; as a result, cortical bone is larger and thicker in men. ,
ERs are present in osteoblasts, , osteoclasts, , and osteocytes. , Both ERα and ERβ are present in cortical bone while ERβ predominates in cancellous or trabecular bone. However, the more important actions of estradiol are believed to be mediated via ERα.
Estrogens suppress bone turnover and maintain a certain rate of bone formation. Bone is remodeled in functional units, called bone multicenter units (BMUs), in which resorption and formation should be balanced. Multiple sites of bone go through this turnover process over time. Estrogen decreases osteoclasts by increasing apoptosis and thus reduces their lifespan. The effect on the osteoblast is less consistent, but E 2 antagonizes glucocorticoid-induced osteoblast apoptosis. , Estrogen deficiency increases the activities of remodeling units, prolongs resorption, and shortens the phase of bone formation. It also increases osteoclast recruitment in BMUs; resorption thus outstrips formation.
The molecular mechanisms of estrogen action on bone involve the inhibition of production of proinflammatory cytokines including interleukin-1, interleukin-6, and tumor necrosis factor, which inhibits bone resorption. Receptor-activation of nuclear factor kappa-B ligand (RANKL) is responsible for osteoclast differentiation and action. A scheme for how all these factors interact has been proposed by Riggs ( Fig. 14.24 ).
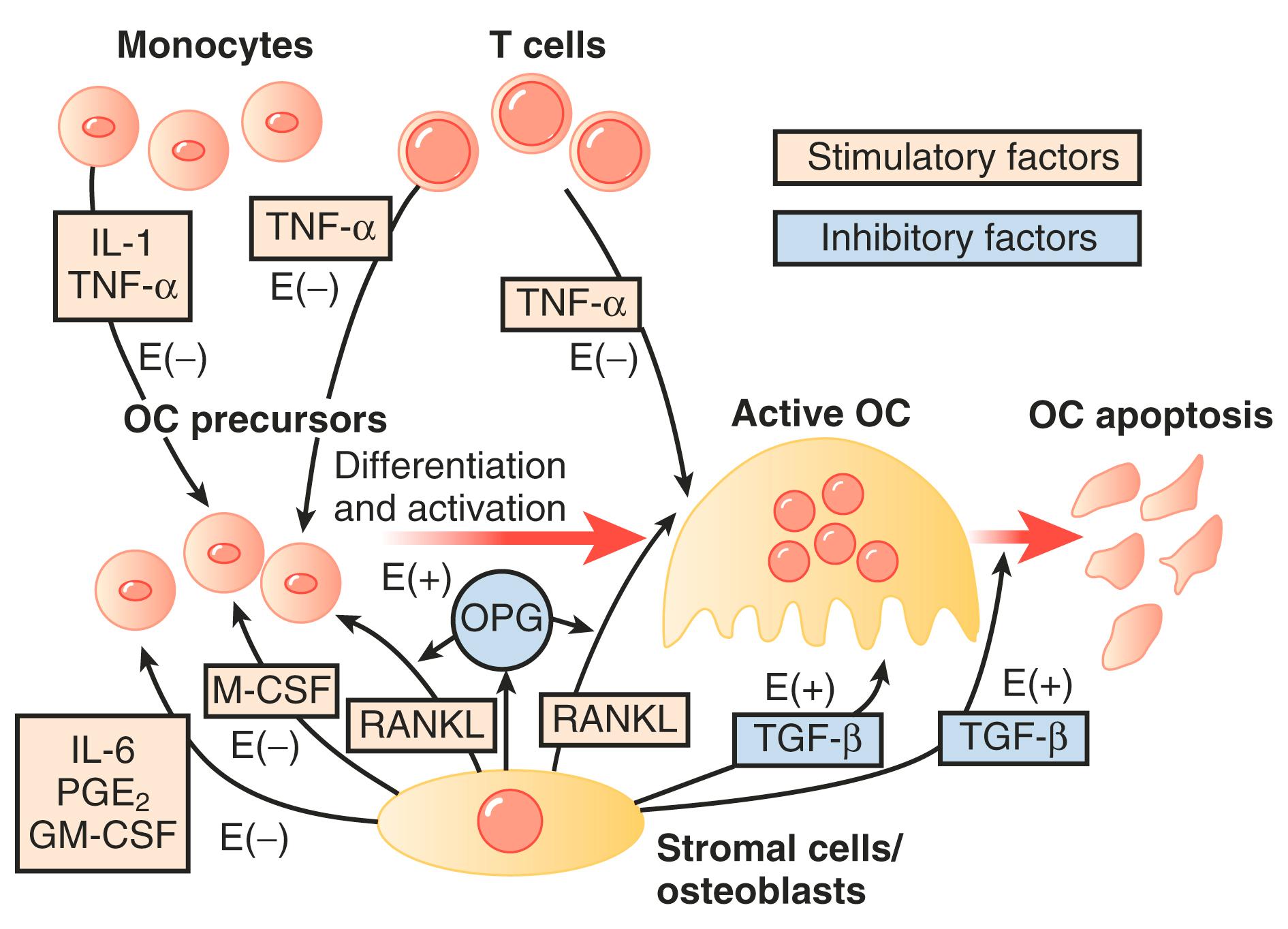
In women, Riggs has suggested that bone loss occurs in two phases. In the first phase, with declining estrogen at the onset of menopause there is an accelerated phase of bone loss; this loss is predominantly of cancellous or trabecula bone. Here 20% to 30% of cancellous bone and 5% to 10% of cortical bone can be lost in a short span of 4 to 8 years. Thereafter, a slower phase of loss (1%–2% per year) ensues where, proportionately, more cortical bone is lost. This phase is thought to be induced primarily by secondary hyperparathyroidism. The first phase is also accentuated by the decreased influence of stretching or mechanical factors, which generally promote bone homeostasis, as a result of estrogen deficiency.
Genetic influences on bone mass are more important for attainment of peak bone mass (heritable component, 50% to 70%) than for bone loss. Polymorphisms of the vitamin D receptor gene, TGFβ gene, and the Spl-binding site in the collagen type 1 AI gene have all been implicated as being important for bone mass.
While testosterone is important for bone formation and stimulation of bone mass, even in men estrogen action is of paramount importance. Bone mass was shown to increase in an aromatase-deficient man upon estrogen administration.
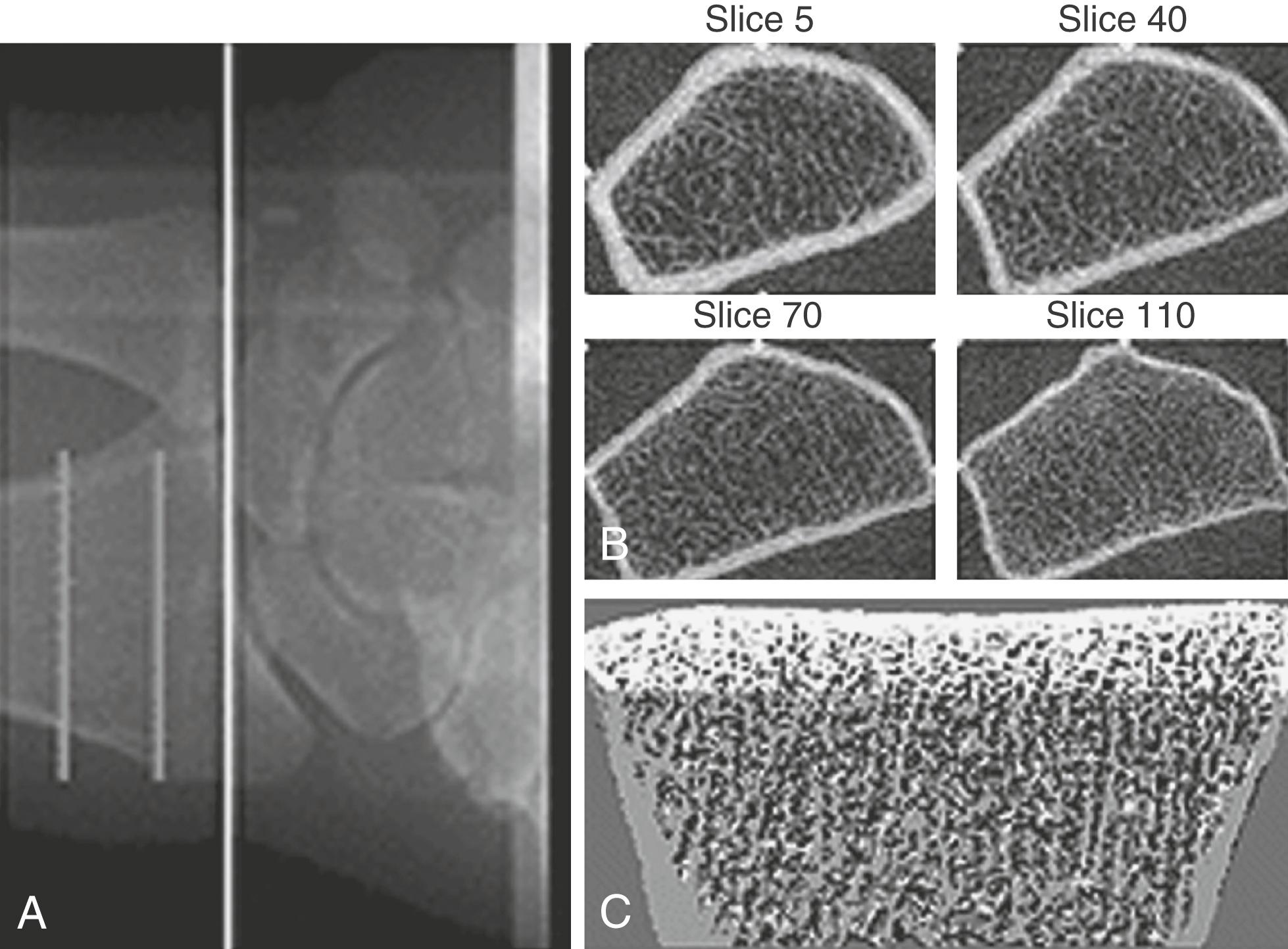
Bone mass can be detected by a variety of radiographic methods ( Table 14.2 ). Dual-energy x-ray absorptiometry scans have become the standard of care for detection of osteopenia and osteoporosis. By convention, the T score is used to reflect the number of standard deviations of bone loss from the peak bone mass of a young adult. Osteopenia is defined by a T score of −1 to −2.5 standard deviations; osteoporosis is defined as greater than 2.5 standard deviations. Since bone mass does not completely reflect bone strength, which is really what matters in terms of fracture risk, several approaches have been made to assess bone strength. An assessment of biochemical bone turnover (discussed later in the chapter), in addition to bone mass, is deemed important. One newer approach is to assess bone microarchitecture, a so-called virtual bone biopsy, by using a high-resolution (HR) peripheral (p) quantitative computed tomography ( Fig. 14.25 ). This technique has been used to assess bone strength and formation in boys and girls to assess sex differences in bone growth and strength, and the risk of fracture. Various biochemical assays are also available to assess bone resorption and formation in both blood and urine ( Table 14.3 ). At present, serum markers appear to be most useful for assessing changes with antiresorptive therapy. Biochemical assays can provide some functional information, which may be helpful in assessing bone strength, and can reflect changes in bone resorption/formation more rapidly than imaging studies. However, these assays do not correlate well with bone density measurements.
| Technique | Anatomical Site of Interest | Precision In Vivo (%) | Examination and Analysis Time (min) | Estimated Effective Dose Equivalent (μSv) |
|---|---|---|---|---|
| Conventional radiography | Spine, hip | NA | <5 | 2000 |
| Radiogrammetry | Hand | 1–3 | 5–10 | <1 |
| Radiographic absorptiometry | Hand | 1–2 | 5–10 | <1 |
| Single x-ray absorptiometry | Forearm, heel | 1–2 | 5–10 | <1 |
| Dual x-ray absorptiometry | Spine, hip, forearm, total body | 1–3 | 5–20 | 1–10 |
| Quantitative computed tomography | Spine, forearm, hip | 2–4 | 10–15 | 50–100 |
| Quantitative ultrasound | Heel, hand, lower leg | 1–3 | 5–10 | None |
| Marker | Specimen |
|---|---|
| Bone Resorption Markers | |
| Cross-linked N-telopeptide of type 1 collagen (NTX) | Urine, serum |
| Cross-linked C-telopeptide of type 1 collagen (CTX) | Urine (αα and ββ forms) |
| Serum (ββ form) | |
| MMP-generated telopeptide of type 1 collagen (ICTP or CTX-MMP) | Serum |
| Deoxypyridinoline, free, and peptide bound (fDPD, DPD) | Urine, serum |
| Pyridinoline, free, and peptide bound (fPYD, PYD) | Urine, serum |
| OHP | Urine |
| GylHyl | Urine, serum |
| HelP | Urine |
| Tartrate-resistant acid phosphatase | Serum, plasma |
| 5b Isoform specific for osteoclasts (TRACP 5b) | |
| Cath K | Urine, serum |
| uOC | Urine |
| Bone Formation Markers | |
| OC | Serum |
| Procollagen type 1 C-terminal propeptide (PICP) | Serum |
| Procollagen type 1 N-terminal propeptide (PINP) | Serum |
| Bone-specific alkaline phosphatase (bone ALP) | Serum |
When evaluating postmenopausal women for osteoporosis, it is important that metabolic diseases are ruled out; these may be the cause of accelerated osteoporosis as well as any history of fractures. Family history is important also here, as there are some genetic predispositions to the development of osteoporosis at a young age. A general medical workup should ensue with measurements of calcium, phosphorus, 20 hydroxy Vitamin D and selectively parathyroid hormone to rule out secondary osteoporosis.
Risk of falls is also important to assess, particularly in older women. Along with BMD, an assessment of fracture risk should be routinely carried out.
The World Health Organization (WHO) has produced guidelines for assessing an individual’s risk of osteoporosis based on history, anthropometry, and BMD. This risk assessment, called the Fracture Risk Assessment Tool, may be obtained at www.shef.ac.uk/FRAX . FRAX has now been used as part of the decision tree for the treatment of osteoporosis. Along with the BMD score suggesting normal BMD, osteopenia, or osteoporosis, suggestions for the need for treatment have been established when a 10-year risk of any fracture is ≥20% or a 10-year risk of hip fracture is ≥3%.
There are now many agents that can prevent osteoporosis. The use of estrogen will depend on whether or not there are other indications for estrogen treatment and on any possible contraindications. Estrogen has been shown to reduce the risk of osteoporosis and osteoporotic fractures. In the WHI study, hip fractures (and all other fractures) were reduced with conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA), and with CEE alone, which occurred in a nonosteoporotic population. Indeed, in a cohort of women who were followed after stopping hormones when the results of the WHI study were published, there was an increased risk of hip fracture and lower bone density compared to those women who continued therapy. ,
A dose equivalent to 0.625 mg of CEE was once thought to prevent osteoporosis, but we now know that lower doses (0.3 mg of CEE or equivalent) in combination with progestogens are able to prevent bone loss, , although there are no data on fractures. It is unclear whether or not the addition of progestogens by stimulating bone formation increases bone mass over that of estrogen alone. The androgenic activity of certain progestogens such as norethindrone acetate also has been suggested to play a role.
Selective estrogen receptor modulators (SERMs) , such as raloxifene, droloxifene, and tamoxifen, have all been shown to decrease bone resorption. Raloxifene has been shown to decrease vertebral fractures in a large prospective trial. Tibolone has also been shown to be an effective treatment for osteoporosis. Tibolone (not marketed in the United States) has SERM-like properties but is not specifically a SERM because it has mixed estrogenic, antiestrogenic, androgenic, and progestogenic properties due to its metabolites. The drug does not cause uterine or breast cell proliferation and is beneficial for vasomotor symptoms. It prevents osteoporosis and has been shown to be beneficial in the treatment of established osteoporosis. ,
Bisphosphonates have been shown to have a significant effect on the prevention and treatment of osteoporosis. With this class of agents (etidronate, alendronate, resedronate, ibandronate, and zoledronic acid), there is incorporation of the bisphosphonate with hydroxyapatite in bone, which increases bone mass. The skeletal half-life of bisphosphonates in bone can be as long as 10 years. Most data have been derived with alendronate, which prevents bone loss at a dosage of 5 mg daily (35 mg weekly). At a dosage of 10 mg daily (70 mg weekly), alendronate is an effective treatment for osteoporosis backed up by evidence that this treatment reduces vertebral and hip fractures. Ibandronate is available as a monthly pill (150 mg) and by injection (3 mg) every 3 months. It has primary efficiency for vertebral fracture protection. Zoledronic acid (5 mg) is available as an intravenous therapy with infusion over 15 minutes.
There has been some concern over this class of very powerful bone resorption agents causing fractures of the long bones such as the femur because of “brittle” bone. This only occurs with prolonged use of at least 7 years, and the incidence is in the range of 3.2 to 50 per 100,000 women. Osteonecrosis of the jaw has also been cited as a concern, but this mainly occurs in the presence of poor dental health and is rare, with an incidence in the range of 1 per 10,000 women. With long-term therapy, atrial fibrillation can also occur as an adverse event, although this too is rare. These adverse effects appear to be a “class” effect of bisphosphonates. Nevertheless, while all these findings are rare events, there are no substantial data for prolonged treatment of 10 or more years. For these and other reasons, bisphosphonates are not the drugs of choice in younger women prior to natural menopause and should not be used in women who wish to conceive.
Calcitonin, 50 IU subcutaneous injections daily or 200 IU intranasally, has been shown to inhibit bone resorption. Vertebral fractures have been shown to decrease , with calcitonin therapy. However, long-term effects have not been established.
Fluoride has been used for women with osteoporosis because it increases bone density. Currently, a lower dose (50 μg daily) of slow-release sodium fluoride does not seem to cause adverse effects (gastritis) and has efficacy in preventing vertebral fractures. ,
Denosumab is a monoclonal antibody targeting RANKL, which is secreted by osteoblasts and causes bone resorption (see Fig. 14.24 ). Thus it is an antiresorptive agent with significant potency. Denosumab 60 mg is administered subcutaneously every 6 months, and while it has a preventative role, it is usually considered to be a second-line treatment for difficult-to-treat cases. Denosumab has efficacy both at the vertebrae and the hip ; however, unlike the bisphosphonates, it is shorter acting and wears off quickly rather than being bound to the bone with a long half-life, such as in the case of bisphosphonates. It is also devoid of the other side effects of bisphosphonates (fractures, osteonecrosis, etc.) described above. Because of this profile and its benefit at the hip as well as the spine, denosumab has emerged as a popular therapy.
An inhibitor of cathepsin K, odanacatib, also has a significant effect on decreasing bone resorption and has been successful in clinical trials at vertebral and nonvertebral bone sites. The concern has been over stroke risk, and this agent has not been marketed in the United States.
Intermittent parathyroid hormone (PTH 1-34, teriparatide) is an agent that increases bone mass in women with osteoporosis. In a randomized trial lasting 3 years, average bone density increased in the hip and spine, with fewer fractures observed. This therapy is available in the United States at a standard dose of 20 μg/day injected subcutaneously for no longer than 18 months. It is expensive and is reserved for women who are difficult to treat and have a history of fractures.
Another agent, which has the ability to increase bone formation, is a monoclonal antibody against sclerosin. Sclerosin is an inhibitor of normal bone formation, which activates the Wnt signaling system. While this monoclonal antibody, romosozumab, has shown great efficacy in increasing bone formation, its cardiovascular risk profile has been an area of concern.
Adjunctive measures for the prevention of osteoporosis are calcium, vitamin D, and exercise. Calcium with vitamin D treatment have been shown to increase bone only in older individuals. These modalities alone are not thought to be effective for the treatment of osteoporosis. A woman’s total intake of elemental calcium should be 1200 to 1500 mg daily. If no agents are being used to inhibit resorption, 400 to 800 IU of vitamin D should also be ingested. Levels of serum 25 hydroxy vitamin D have been found to be abnormally low (<20 ng/mL) in a large population of women, particularly in geographic regions of less sunlight exposure. Exercise has been shown to be beneficial for building muscle and bone mass and for reducing falls. ,
Guidelines have also been set up for stopping therapy or instituting a “drug holiday.” This depends on the agent used, with modifiers being the assessment of BMD at 1 to 2 yearly intervals and the history of any fracture. For example, bisphosphonates are usually reassessed after 5 years of use, and teriparatides after 2 years.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here