Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors would like to thank Anne E. Becker and Sarah A. Kearney for their enormous contribution to this chapter in the 10th edition.
Eating disorders (EDs) are mental disorders characterized by disturbances in body image, weight control, and/or dietary patterns. In the Diagnostic and Statistical Manual of Mental Disorders , 5th edition (DSM-5), an updated and expanded section summarizes feeding and EDs that include: (1) anorexia nervosa (AN); (2) bulimia nervosa (BN); (3) binge-eating disorder (BED); (4) avoidant/restrictive food intake disorder (ARFID); (5) pica; (6) rumination disorder; (7) other specified feeding or eating disorder (OSFED); and (8) unspecified feeding or eating disorder (USFED). The focus of this chapter is on EDs seen in adults: AN, BN, and BED; other feeding and EDs, including pica, rumination disorder, and ARFID, are discussed only briefly. Although feeding and EDs are classified as mental disorders, their associated behaviors commonly result in and present with medical sequelae, many of which are GI. Because associated chronic undernutrition, overweight, and/or purging behaviors often result in medical complications that can be serious, chronic, and life threatening, individuals with feeding and EDs benefit from the ongoing care of a multidisciplinary treatment team. Indeed, AN and BN are among the mental disorders with the highest mortality. Reviews suggest that GI complications are among the most prevalent experienced by patients with EDs.
EDs have been described across diverse global settings, although epidemiologic data are best established for populations in North America and Europe. Most of the published epidemiologic data for feeding and EDs predate the revised diagnostic criteria published in the DSM-5. As expected, given the changes in DSM-5 that aimed to diminish the proportions of patients in residual categories, early studies suggest that the prevalence and incidence of AN, BN, and BED are higher, while proportional representation in the residual categories (previously ED not otherwise specified [NOS] and now OSFED and USFED) have decreased. Of note, few studies to date have investigated incidence and prevalence rates of ARFID, pica, or rumination using the DSM-5.
A recent systematic review drawing upon DSM-5 feeding and ED populations suggests that the prevalence rate for AN ranges from 1.7% to 3.6%, with a point prevalence of 0.67% to 1.2%. Although BN has traditionally been more common than AN, the paucity of studies drawing upon DSM-5 populations has yet to establish this conclusively. BN has most commonly been assessed using point prevalence. Two separate studies reported near identical point prevalence rates for BN of 0.6% in girls and women. A recent interview-based study revealed higher lifetime prevalence rates for BN compared with AN (2.6% vs. 0.8%, respectively). Point prevalence rates for BED have ranged from 0.62% to 3.6% (female subjects only and males and female subjects combined, respectively). Lifetime prevalence rates for OSFED are reported to be 0.3% in male subjects and 0.6% in female subjects, with a point prevalence rate of 0% in male subjects and 2.4% in a sample of male and female subjects. Lifetime prevalence rates for USFED range from 0.2% to 0.9% among male and female subjects.
Relatively high prevalence rates are also reported for specific symptoms associated with disordered eating. In 2017, 6% of school-going female adolescents in the US reported vomiting or laxative use; 5.9% reported taking diet pills, powders, or liquids without a doctor’s advice to lose weight or to prevent weight gain; and 17.4% reported fasting within the previous month to lose weight. EDs occur across ethnically and socioeconomically diverse populations, but each of the EDs is more common in women than in men. Historically, boys and men have accounted for less than 10% of individuals with AN, 10% of those with BN, and 40% of those with BED.
Studies have identified that approximately 14% of children and adolescents admitted to adolescent inpatient ED programs and as many as 22.5% of individuals in a pediatric ED day treatment program met the DSM-5 diagnosis for ARFID; patients were younger, had longer duration of illness, were more likely to be male compared with those who had AN or BN, and were commonly diagnosed with comorbid psychiatric and/or medical symptoms. Prevalence data on pica in the general population are unavailable and may vary widely in certain demographic strata. A recent systematic review of prevalence rates of DSM-5 feeding and EDs failed to identify any study that included pica or ARFID. Whereas pica appears to be uncommon in healthy children in the US, pica eating has been reported to be relatively more prevalent among US children treated for sickle cell disease, adults with iron deficiency, institutionalized individuals, some school-age populations in Africa, and in some populations of pregnant women (e.g., in Africa). The prevalence of rumination disorder is unknown, but it can occur in both children and adults.
Although incompletely understood, the cause of EDs is almost certainly multifactorial, with psychodevelopmental, sociocultural, and genetic contributions to risk. Exposure to risk factors for dieting appears to elevate risk for AN and BN, just as childhood exposure to negative comments about weight and shape elevate risk for BED. Body dissatisfaction in a social context in which thinness, self-efficacy, and control are valued may be an important means whereby dieting is initiated and disordered eating attitudes and behaviors ensue. Dietary restraint may precipitate a cycle of hunger, binge eating, and purging. Among numerous risk correlates, childhood GI complaints have been found associated with earlier age of onset and greater severity of BN in a retrospective study, and picky eating and digestive problems were found prospectively associated with AN in adolescence.
It has been suggested that physiologic vulnerabilities may increase risk for an ED. Neurobiological targets have been identified as possibly playing a role in the pathogenesis of AN, BN, and BED. For decades, researchers have studied the psychobiology of EDs and the neurophysiologic correlates and determinants of energy intake, hunger, and satiety. Findings highlight the multifactorial and phenotypically diverse nature of eating behavior. For example, energy intake is influenced by complex interactions among signaling molecules from peripheral systems (e.g., GI peptides, vagal stimulation) and CNS neuropeptides and neuroamines. As is true of the search for obesity treatments, it is unlikely that single mechanisms will become the basis of therapeutic interventions for EDs. However, the greater our understanding of the physiology of ingestive behavior, the more likely we are to establish integrated therapy models in the future. There is a vast amount of literature on this topic, and what follows is simply a brief overview of the more commonly investigated mechanisms.
Serotonin has long been a focus of attention for its possible role in disrupted satiety. There is substantial evidence that altered 5-hydroxytryptamine (5-HT, serotonin) functioning contributes to dysregulated appetite, mood, and impulse control in EDs and that such alteration persists after recovery from AN and BN, possibly reflecting premorbid vulnerability. There also is evidence that CCK levels are altered in ED populations. Findings for AN are inconsistent. Although there is some evidence that young women with AN have high levels of pre- and postprandial CCK that may impede treatment progress by contributing to postprandial nausea and vomiting, other reports have shown decreased CCK compared with controls. In patients with BN, there is consistent evidence for an impaired satiety response, characterized by a blunted postprandial CCK response as well as delayed gastric emptying. In contrast, individuals with BED and obesity do not differ in postprandial CCK responses from those with obesity but no BED. The relationships between CCK, binge eating, and BMI warrant further clarification.
Peptide tyrosine (PYY), the intestinally derived anorexigen that elicits satiety, appears to be dysregulated in individuals with AN and BN, but not in those with BED. Young women with AN have higher levels of PYY compared with controls, perhaps contributing to reduced food intake. In individuals with BN, expected elevations in PYY after meals are blunted, possibly playing a role in impaired satiety. A recent report found no differences between BED and non-BED groups in fasting levels and postprandial changes in PYY. Women with BN have been found to secrete abnormally low levels of the GI satiety peptides glucagon-like peptide 1 and pancreatic polypeptide, which is thought to be a consequence of the adaptation to large meals in the form of enlarged gastric capacity and reduced muscle tone in the gastric wall. Attenuated secretion of these GI satiety polypeptides may play a role in maintaining bulimic behavior.
The orexigenic peptide ghrelin is of interest in EDs because it is the only known GI hormone that stimulates appetite and promotes food intake. Ghrelin influences secretion of growth hormone, induces adiposity, and is implicated in signaling the hypothalamic nuclei involved in energy homeostasis. Gastric secretion of ghrelin is stimulated by a combination of neural (vagus nerve), mechanical (distension), chemical (osmolarity; caloric content and macronutrient composition of the meal) and hormonal (insulin) factors with unknown priority. Consistent findings in the literature examining ghrelin in patients with AN have shown that (1) circulating basal levels of ghrelin are elevated, a likely consequence of prolonged starvation ; (2) growth hormone and appetite responses to ghrelin are blunted, suggesting ghrelin resistance or altered ghrelin sensitivity ; and (3) ghrelin levels return to normal after partial weight recovery, suggesting a physiologic effect to compensate for lack of nutritional intake and energy stores. More recently, a randomized, double-blind, placebo-controlled trial using a ghrelin agonist in 22 outpatients with AN revealed that those treated with a ghrelin agonist demonstrated significantly decreased gastric emptying time, and a trend of greater weight gain after 4 weeks.
Plasma levels of ghrelin are normal or elevated in individuals with BN, which suggests that abnormal eating behaviors, including binge eating and purging, may influence ghrelin secretion ; there is a postprandial blunted response (i.e., reduced suppression of ghrelin) in these patients. The relationship between elevated ghrelin and binge eating in patients with BN requires further exploration. Investigations of ghrelin functioning in individuals with BED have reported lower circulating levels of pre- and postprandial ghrelin, possibly reflecting down-regulation in response to chronic overeating and smaller decreases in ghrelin after eating.
Leptin and adiponectin are hormonal signals associated with longer-term regulation of body fat stores. Leptin is also directly implicated in satiety through its binding to the ventral medial nucleus of the hypothalamus, an area termed the satiety center . Leptin and adiponectin are both altered in patients with EDs. A number of studies have found evidence for hyperadiponectinemia and hypoleptinemia in populations of underweight AN with reversal following restoration of weight ; increased adiponectin levels may act protectively to support energy homeostasis during food deprivation. Individuals with BN also exhibit decreased plasma levels of leptin and increased levels of total plasma-adiponectin which are inversely correlated with longer duration of illness and increased severity of symptoms. The mechanism of altered leptin functioning in BN is unclear because blunted postprandial leptin levels are not observed in individuals with BED.
There are other mechanisms of interest including neuropeptide Y, peptides glucagon-like peptide-2, orexins A and B, the endocannabinoids, resistin (adipose tissue-specific secretory factor), and brain-derived neurotrophic factor, but more research is necessary to elucidate their roles in the pathophysiology of EDs. One high priority for research is clarifying whether observed psychobiological abnormalities are antecedents or consequences of disturbed eating behavior that return to normal after recovery; this information could shed light on cause and possible treatment targets. Patients often report intense discomfort after eating as a reason they continue to restrict intake. The discomfort may be dismissed as perceptual or psychological in the absence of any medical findings to support the symptoms, but there may be disruptions in CNS or peripheral signals contributing to this and other reported symptoms.
AN and BN most commonly have their onset in adolescence, and BED usually manifests in the early 20s, but EDs can occur throughout most of the life span and appear to be increasing in frequency in middle-aged and older women. Diagnostic migration from one ED category to another is common. ARFID most commonly has its onset in the early years but can continue into adulthood. Pica has been described in both children and adults, but little is known about the courses of pica and rumination disorder.
Lifetime comorbidity of AN, BN, and BED with other psychiatric disorders has been reported as high at 56.2%, 94.5%, and 63.6%, respectively. Mortality associated with AN and BN combined is five times higher than expected and is one of the highest mortality rates among mental disorders. Some data support the chronicity of AN, reporting that slightly less than half of survivors with AN make a full recovery, with 60% attaining a normal weight and 47% regaining normal eating behavior; 34% improve but only achieve partial recovery, whereas 21% follow a chronic course. Other data suggest that recovery rates for AN may be more favorable than previously believed, with a large twin cohort study reporting a 5-year clinical recovery rate of 66.8%. In contrast, after a 5-year follow-up of 216 patients with BN and EDNOS, 74% and 78% of patients, respectively, were still in recovery. In a 6-year longitudinal study of patients with BED, 43% of individuals continued to be symptomatic. A recently published 22-year longitudinal study of patients with AN and BN demonstrated that the presence and persistence of binge eating and purging behaviors were poor prognostic indicators to overall recovery and that comorbidity with depression at initial assessment strongly predicted the continuation of AN at the 22-year mark.
In summary, despite decades of studies that have investigated potential treatment modalities for the EDs, up to 50% of treated individuals continue to be symptomatic in follow-up.
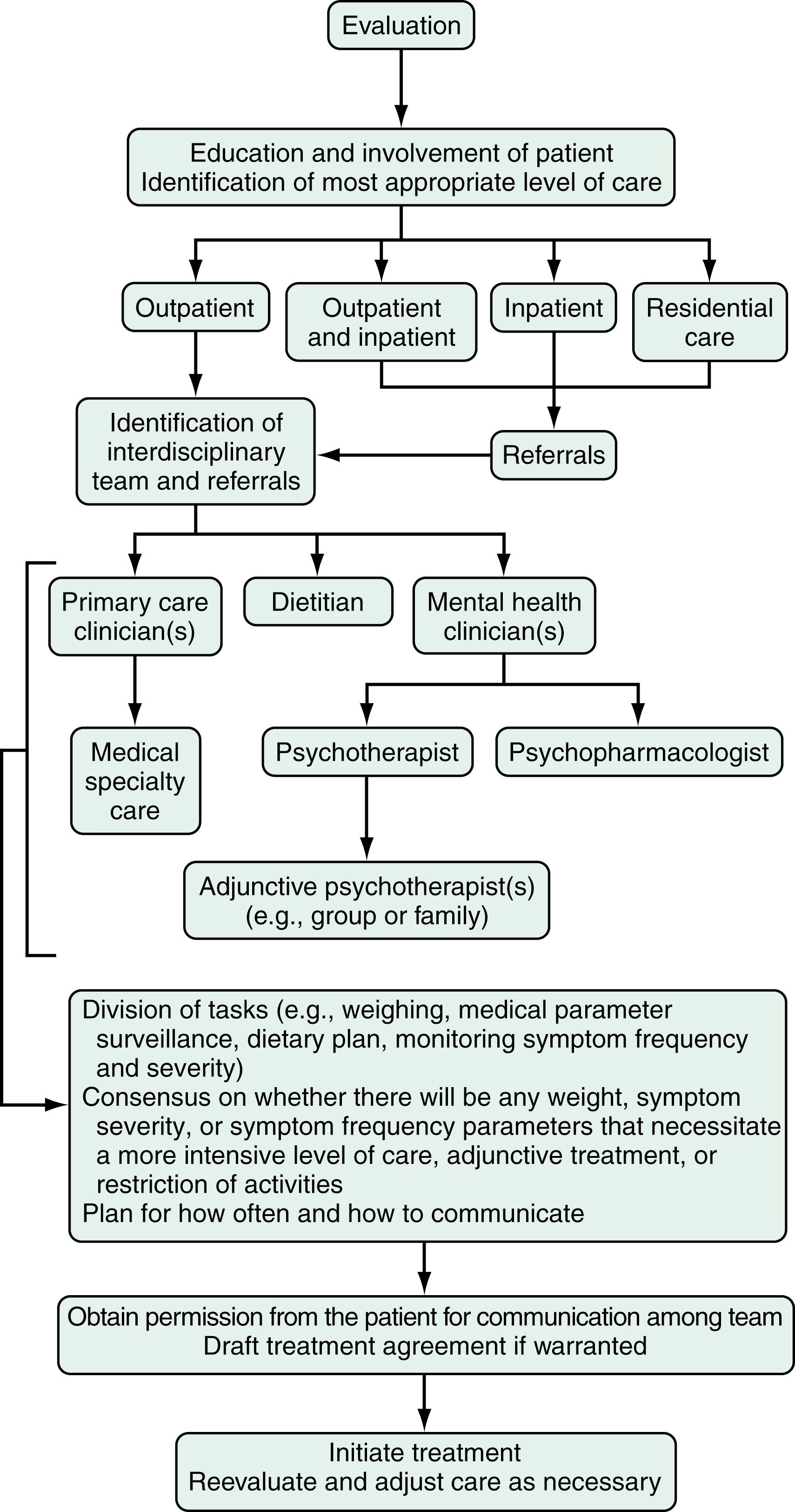
A substantial percentage of individuals with an ED in the US do not receive specific treatment for this problem. Despite clear diagnostic criteria for EDs, clinical detection is often problematic, and up to 50% of cases may go unrecognized in clinical settings. Moreover, individuals with EDs are often reluctant to disclose their symptoms. Although individuals with AN are underweight by definition, this can be easily missed in clinical settings. Even when noted on evaluation, the medical seriousness of low weight frequently is unappreciated. When an ED is suspected or confirmed, patients may decline or avoid medical or mental health care and a feature of AN can be denial of the medical seriousness of symptoms. Therefore, ascertainment of concerns about body shape, weight, fatness, and weight gain or loss can be especially challenging when patients are unable or unwilling to recognize or to disclose them. Given that many individuals with EDs initially present in primary care or medical subspecialty settings, recognition of clinical signs and symptoms across diverse health care settings will facilitate appropriate referrals and make diagnostic evaluation and treatment plans more efficient. One study has reported that individuals with BN are more likely to seek help for their GI complaints before seeking treatment for their ED. Familiarity with the diagnostic features and GI complications of eating disorders will help the clinician identify the most appropriate interventions, including the full spectrum of treatment resources available for a comprehensive treatment plan.
When an ED is suspected, a directed clinical interview about restrictive or binge eating and inappropriate compensatory measures to control weight ( Box 9.1 ) is essential in determining the scope and severity of symptoms that underlie specific GI complaints and pose medical risk. Accurate and timely diagnosis of EDs is challenging for several reasons. First, patients may be unreliable reporters of their history, and BN and BED may be present without any abnormal physical findings, as may pica and rumination disorder. In addition, some dietary modifications and exercise behaviors certainly are appropriate, and discerning pathologic behavior that is consistent with a clinically significant ED can be difficult. There is considerable overlap in symptoms among the EDs; diagnostic specificity, however, is critical to effective management.
Diuretic abuse
Laxative and/or enema abuse
Self-induced vomiting (including syrup of ipecac abuse)
Excessive physical activity
Fasting, skipping meals, restrictive-eating pattern
Inappropriate withholding or under-dosing of insulin (among individuals with diabetes mellitus)
Stimulant abuse (e.g., caffeine, ephedra, methylphenidate, cocaine)
Given the frequent reluctance of patients to recognize or to disclose symptoms of an ED, targeted history taking may be essential to making a prompt diagnosis. In some cases, an ED may not be suspected or the diagnosis confirmed until physical findings suggestive of purging are detected, a suggestive pattern is noticed in weight changes, and/or there is difficulty gaining weight notwithstanding appropriate nutritional treatment and exclusion of other potential causes for low weight.
AN is characterized by a significantly low weight (the weight that is less than minimally normal), fear of gaining weight (despite being thin), and a disturbance in the way body shape or weight is perceived (e.g., a denial of the medical seriousness of being underweight or feeling fat despite emaciation). It is not uncommon for an individual with AN to deny or minimize fear of weight gain at initial evaluation (and sometimes an apparent absence of this fear persists). Even if a patient does not admit or disclose an intense fear of weight gain, evidence of continuing behaviors that undermine weight gain (e.g., restrictive eating, purging, excessive exercising) may be used to establish this criterion. Individuals with AN typically restrict their food selections and caloric intake, but about half of those with AN also routinely binge eat and/or engage in inappropriate purging behaviors like self-induced vomiting or laxative use to prevent weight gain ( Box. 9.1 ). AN is further divided into 2 subtypes: restricting type (those who primarily control their weight through dieting, fasting, or exercising) and binge-eating/purging type (those who routinely purge calories to control weight and/or routinely binge eat). In middle-aged and older women new-onset AN may present in conjunction with difficulty making life transitions and fear of aging. The diagnosis of AN may be delayed when patients present to a GI specialty practice without disclosing their concerns and behaviors relating to weight. Presentation with GI complaints, even if related to real symptoms or disease, can sometimes prove to be a “red herring,” drawing attention away from and delaying diagnosis of an ED. One study of 20 consecutive patients who presented to a GI practice and ultimately were diagnosed with an ED found the diagnosis of an ED was delayed for an average of 13 months after presentation. Notably, all patients stated a desire to gain weight and denied attempts to lose weight via exercise, purging, or dietary restriction. Individuals with AN are not always able or willing to frame their difficulty maintaining a healthy weight as intentional, so diagnosis may initially be unsuspected and delayed.
The clinical hallmark of BN is recurrent binge eating accompanied by inappropriate compensatory behaviors to control weight or to purge calories consumed during a binge (see Box 9.1 ). On average, these behaviors must occur once each week for at least 3 months to meet diagnostic criteria. Also intrinsic to the diagnosis of BN is the excessive influence of weight and/or shape on self-image. Individuals with BN have poor self-image that is often anchored to their weight. It is not unusual for individuals with AN or BN to weigh themselves daily, even several times each day, and to experience fluctuations in self-esteem and mood based on the result.
By definition, binge eating is consumption of an unusually large amount of food during a “discrete period of time” (i.e., not overeating or “grazing” all day), accompanied by the feeling that the eating cannot be controlled. Many patients describe an emotional numbing during the period of eating; for some, this state appears to motivate the bingeing. Most clinicians are familiar with self-induced vomiting as the primary purging behavior, but individuals with BN may also use alternative or additional means to prevent weight gain, including abuse of laxatives and/or enemas, diuretics, stimulants (including methylphenidate, cocaine, over-the-counter “natural” supplements, and caffeine), under-dosing of insulin (for those with diabetes mellitus), fasting or restrictive eating, and excessive exercise (see Box 9.1 ). In adolescents with celiac disease, intentional consumption of gluten to promote weight loss has also been reported. Whereas most individuals with BN use compensatory behaviors that include purging, those who only use excessive physical activity or fasting are more challenging to identify. As with overeating and dieting, it is frequently difficult to determine the line between culturally normative and pathologic behavior with excessive exercise. Generally, clinical suspicion should be raised when an individual continues to exercise despite an injury or illness, or if he or she is exercising routinely in excess of what a coach is advising for the team.
It is recommended that clinicians ask about purging behaviors if an ED is suspected. Although it is not certain that a patient will respond candidly, individuals are more likely than not to eventually disclose information about symptoms when asked. Some patients report feeling relieved when clinicians pose such questions if they previously had not been able to discuss their symptoms. On occasion, however, patients report learning about techniques from clinicians’ questions, so it is advisable for clinicians to provide a psychoeducational context for the questions (e.g., by conveying serious physical consequences associated with the behavior) and to avoid introducing information about a dangerous behavior (e.g., under-dosing insulin), using appropriate discretion based upon the clinical context. Patients also benefit from learning that treatment is available and effective, and from feeling understood by their clinicians.
Whereas purging and other behaviors aimed at neutralizing or decreasing calorie intake and modifying weight can pose medical risks when chronic, some of them pose more immediate and potentially lethal consequences. Patients should be educated about these acute life-threatening risks, and steps should be taken to eradicate such behaviors immediately. For example, because of the serious neurotoxicity, cardiotoxicity, and risk of death associated with repeated ingestion of syrup of ipecac, its ongoing use is a clinical emergency and may require immediate hospitalization. Many patients are unaware of the serious risk associated with syrup of ipecac use. Similarly, ephedra, now banned in the US, poses risk of stroke or adverse cardiac events even in young adults. Some ephedra-free supplements marketed as weight-loss agents may also be pro-arrhythmic and pose medical risks. Although patients find it difficult to abstain from purging behaviors, they may be willing to substitute less immediately harmful behaviors while treatment is initiated.
BED is characterized by recurrent and persistent binge eating. To meet diagnostic criteria, binge episodes should occur at least weekly, on average, over a duration of 3 or more months. Unlike BN, BED is not associated with recurrent inappropriate compensatory behaviors to prevent weight gain. BED is distinguished from non-pathologic overeating by several possible associated symptoms including rapid eating, eating irrespective of hunger or satiety eating alone because of shame, and negative feelings after a binge. Apart from overweight or obesity, which is common in BED, BED patients frequently present without any specifically associated physical findings. Although in some cases binge eating associated with BED may cause or perpetuate weight gain, many with BED develop symptoms only after they have become overweight. Individuals with BED are frequently distressed enough about their symptoms to seek medical help, although they may present seeking a solution to their weight gain rather than their binge eating. A substantial percentage of patients who seek weight loss treatment will have comorbid BED. Therefore, medical subspecialists are likely to encounter these patients before they have been diagnosed with BED.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here