Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Iron is abundant in the Earth's soils and as such, it is ubiquitously present in life forms on this planet. Most species, including humans, require iron for numerous biological functions, yet iron in excess is toxic; as such, iron levels must be precisely managed. Iron is necessary for several critical physiological functions, including oxygen transport, regulation of cell growth and differentiation, energy production, and control of gene expression. Mammals have developed sophisticated regulatory mechanisms that govern iron absorption, storage, and recycling. Iron associates with many proteins and it mediates important functions, including, electron transfer reactions. Some iron-binding proteins have no known enzymatic function, but many others are enzymes, which contain iron as iron-sulfur clusters, heme moieties, or in other molecular configurations. Iron-binding proteins lacking enzymatic activity include those that transport oxygen (hemoglobin and myoglobin). Some iron-sulfur cluster-containing enzymes are involved in energy (i.e., ATP) production via their role in single-electron transfer reactions (e.g., in the cytochromes of the electron transport chain). Heme-containing proteins also participate in a variety of electron transfer reactions in association with various cofactors (e.g., cytochrome P450 complexes). Other proteins transiently bind iron and facilitate its movement across the plasma and intracellular membranes (e.g., divalent metal-ion transporter 1 [DMT1], ferroportin 1 [FPN1). The nonredundant roles of iron in these and other proteins exemplify the importance of iron in mammalian physiology.
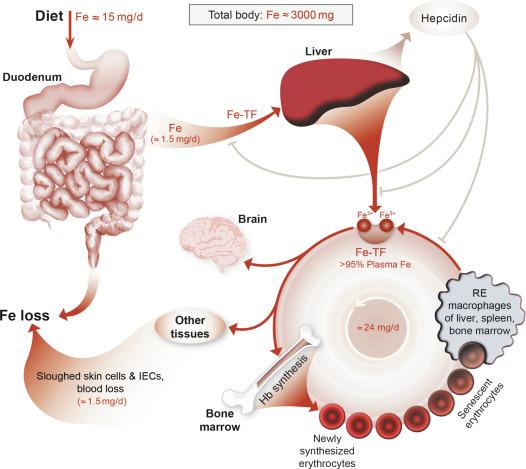
Overall body iron homeostasis is controlled by the peptide hormone hepcidin, which functions to decrease serum iron levels by inhibiting the absorption of dietary iron, and iron release from stores in reticuloendothelial (RE) macrophages of the spleen, liver and bone marrow, and hepatocytes. Hepcidin is induced by high body iron content, infection, and inflammation. Conversely, during iron deficiency and hypoxia and when erythropoietic demand increases, hepcidin production is very low, and additional regulatory mechanisms are invoked to increase intestinal iron absorption. Normally, absorption of dietary iron reflects body iron requirements; however, homeostatic dysregulation of iron absorption occurs in numerous disease states, with pathological consequences. Humans have not evolved an efficient iron excretory system, so the overall control of body iron content is ultimately determined by assimilation of dietary iron by the intestine. Absorption of dietary iron typically matches nonspecific losses, which occur via menstrual blood flows, desquamation of skin cells and exfoliation of GI epithelial cells, and in bile and urine. A normal human will absorb 25–50 g of dietary iron over their lifetime. In the adult males, this represents ~ 1 mg of iron/day, while adult females absorb 1.5–2 mg/day to compensate for iron losses associated with menstruation, pregnancy, and lactation. Furthermore, although some species can excrete greater quantities of iron than humans (e.g., rodents), the same basic regulatory schemes that have been described in man appear to operate in other mammals as well.
Physiological studies of iron absorption have been carried out for many decades, but it is only within the last 20 years that the application of molecular and genetic techniques has led to an understanding of basic mechanistic details regarding the transfer of iron across the intestinal epithelium. Anatomically, iron is absorbed predominantly by intestinal epithelial cells (IECs) in the duodenum and proximal jejunum. Absorption is restricted to the differentiated enterocytes on the upper half of the villus. Most dietary iron exists in the Fe 3 + , or ferric form, which is highly insoluble. Ferric iron must thus be reduced to the more soluble ferrous form (Fe 2 + ) before it can be efficiently absorbed. One possible brush-border membrane (BBM) ferrireductase is duodenal cytochrome b (DCYTB), although other candidate proteins have also been identified. Moreover, dietary (e.g., ascorbic acid) and endogenous (e.g., gastric acid) factors also promote reduction of ferric iron. Once reduced, ferrous iron is transported across the BBM via DMT1. The fate of iron within the enterocyte is determined by body iron status. If iron stores are replete, most absorbed iron will be stored within ferritin and later lost when enterocytes are exfoliated from the villus tip. However, if body iron stores are low, iron will be transported out of the cell across the basolateral membrane (BLM) via the iron exporter FPN1. The export (or transfer) process requires that iron be oxidized for binding to transferrin (TF) in the interstitial fluid and plasma, and this likely occurs via the iron oxidase (i.e., ferroxidase [FOX]) hephaestin (HEPH), which is expressed on the basolateral surface of enterocytes. Iron export from duodenal enterocytes is, in part, regulated by the liver-derived, peptide hormone hepcidin, which binds to FPN1 causing its internalization and degradation. Hepcidin expression in hepatocytes is regulated by physiological signals that are transduced to the HAMP gene (encoding hepcidin) by the hemochromatosis (HFE) protein, transferrin receptor 2 (TFR2) and hemojuvelin (HJV), among others. Mutations in the genes encoding these proteins lead to impaired hepcidin production, resulting in inappropriately increased intestinal iron absorption and consequent systemic iron loading, eventually resulting in tissue and organ damage and associated pathological phenotypes. Each of these steps in iron absorption and regulatory mechanisms will be considered in greater detail in subsequent sections.
Iron homeostasis in undifferentiated enterocytes of the intestinal crypts is quite different from what occurs in their mature counterparts. These immature epithelial cells are not specialized for vectorial iron (or nutrient) transport and act rather more like a generic cell. They are rapidly growing and dividing, so they have a high demand for iron, which they absorb as TF-bound iron from the serosal side, unlike mature (i.e., fully differentiated) enterocytes which get much of their iron from dietary components on the luminal side. Both transferrin receptor 1 (TFR1)-dependent and TFR1-independent diferric TF uptake have been demonstrated in cryptal enterocytes. As the cells differentiate and migrate upward along the villus, they take on an absorptive phenotype and subsequently lose the capacity to absorb iron from the circulation.
Disturbances in intestinal iron absorption can have significant clinical consequences. In humans, the most common pathological condition that perturbs iron absorption is hereditary hemochromatosis (HH). HH is a group of genetic diseases in which hepcidin production is impaired leading to dysregulated (and increased) intestinal iron absorption and iron release from stores, eventually resulting in tissue iron overload and associated damage. The most common form of HH results from mutations in the gene encoding HFE, but several less common forms have also been described. Pathological conditions associated with reduced iron absorption, though less common, are still important from a clinical perspective. Impaired iron absorption frequently occurs in patients with malabsorptive disorders, such as celic disease or inflammatory bowel disease (IBD), in individuals who have undergone gastric bypass surgery for morbid obesity, in those who take proton-pump inhibitors for chronic gastric reflux, and in older adults with achlorhydria. Iron-refractory anemias, resulting from impairments in iron absorption, have also been described, but to date, only one of these has been attributed to an intestinal iron transport molecule. These clinical disorders will be considered in greater detail below.
This chapter will describe the molecular details of intestinal iron absorption, a process not fully understood, but which is being actively investigated by a large number of groups around the world. The brief overview of the importance of iron as a nutrient and of the basics of the intestinal transport process presented above will be expanded upon in subsequent sections. Dietary and endogenous factors that affect the efficiency of intestinal iron absorption will be considered, along with physical and morphological attributes of the GI system that also regulate this process. The steps involved in transferring iron across the BBM, through the cytoplasm and out of the enterocyte for loading onto transferrin will be delineated in detail. How this complex process is altered during development, both pre- and postnatally, will be outlined. Moreover, systemic and local control of intestinal iron absorption will be considered, as well as how this process is either primarily or secondarily perturbed in various disease states.
Most iron is absorbed in the proximal small intestine, mainly by the duodenum and proximal jejunum. Small amounts of iron may also be absorbed from the stomach, ileum, and colon, but the relative contribution of these sites to overall iron absorption is probably small. The more efficient absorption in the proximal small bowel may be partly due to higher concentrations of luminal iron and a lower pH (which promotes iron reduction), but a number of studies suggest that it is an intrinsic property of the gut mucosa in this region. Recent studies demonstrating higher expression of iron transport proteins (e.g., DCYTB, DMT1, FPN1) in this region of the gut may provide a molecular explanation for these observations. At the level of the intestinal epithelium, iron absorption occurs through the differentiated enterocytes of the mid- and upper villus, rather than across the undifferentiated cells lower on the villus and in the crypts. It is these differentiated enterocytes that express the critical molecules of intestinal iron transit (e.g., DMT1, FPN1, HEPH). Finally, there is accumulating evidence that during the development of iron deficiency there is morphological adaptation of the intestinal mucosa to increase the effective absorptive area and upregulate the capacity of the intestine to absorb iron. For example, in hemolytic anemia, villus enterocytes from lower portions of the villus participate in absorption, during pregnancy villus size is increased, and during iron deficiency, absorption occurs in more distal gut segments and villus width and length are increased and mitosis increases in the intestinal crypts.
Iron is found in the diet in a wide variety of foods in two distinct forms, heme and nonheme (or inorganic) iron. Heme iron is derived from hemoglobin and myoglobin, which are found predominantly in meat products. Heme iron absorption is efficient and mostly unaffected by other dietary constituents. In contrast, nonheme iron, which derives from both plant and meat sources, is highly insoluble (as it exists mainly in the ferric form) and its bioavailability is affected by various dietary components. This is due to the fact that nonheme iron is not sequestered within a stable complex like heme iron, which makes it more reactive and free to interact with other molecules. Dietary and luminal factors, including gastric acid, ascorbic acid, and citrate, help keep inorganic iron in a more soluble, reduced state, and thus in the absorbable (ferrous) form. Decreased gastric juice production or decreased gastric acidity affect the reduction of insoluble ferric iron to soluble ferrous iron and thus may reduce the availability of absorbable iron. In addition to the positive effect of the low pH environment resulting from gastric HCl production, there also exists an acidic microclimate near the apical surface of enterocytes in the “unstirred” water layer. This is produced by the action of an apically expressed sodium‑hydrogen exchanger (NHE), notably NHE3, which exchanges extracellular Na + for intracellular H + . The resulting electrochemical H + gradient from outside to inside the cells serves as the driving force for the intestinal iron transporter, DMT1, which is a ferrous iron/proton cotransporter. This acidic microclimate thus likely has a positive influence on iron absorption. Another factor that has been shown to enhance iron absorption is the consumption of prebiotics, which are indigestible carbohydrates that are fermented by gut bacteria.
Furthermore, there also exists ample evidence that certain dietary factors, mainly derived from plant sources, negatively affect iron absorption. These include phytate, polyphenols, and tannins, which all tightly bind ferric or ferrous iron and decrease their bioavailability. Additional influences on iron absorption include chronic use of proton-pump inhibitors, which reduce gastric acid production and possibly iron bioavailability, alterations in intestinal motility, infection with Helicobacter pylori and mucosal pathologies such as celiac disease. Some of these are considered in more detail below.
As mentioned above, the proximal small intestine (duodenum and upper jejunum) is the major site of iron absorption. Given this limited area for absorption, variations in the rate that the digesta transit through the small intestine may contribute to altered intestinal iron uptake. Few studies, however, have measured iron absorption specifically relating to variable GI transit times. One such study utilized rats treated with metoclopramide (oral or I.M.) to reduce transit time or loperamide (orally) to increase transit time. Neither increasing or decreasing intestinal transit time altered the efficiency of Fe absorption, as assessed by whole-body retention of 59 Fe 7 days after a test meal, as compared to vehicle-treated, control rats. Moreover, conditions that decrease the available surface area for nutrient absorption (e.g., short gut syndrome, bariatric surgery), or hasten gastric emptying (infectious or inflammatory diarrhea) thus decreasing transit time, may prevent adequate levels of iron absorption. In addition to shortening transit time, some malabsorption syndromes induce marked inflammation (e.g., IBDs, celiac disease, etc.) and subsequent anemia. Anemia of inflammation (AI) is the result of decreased iron absorption and iron retention in the RE system triggered by proinflammatory cytokine-mediated upregulation of hepcidin expression (as discussed in subsequent sections). Thus, decreased transit time during chronic inflammation may exacerbate the impaired iron absorption that accompanies hepcidin-induced anemia.
Despite its decline in recent years, Helicobacter pylori ( H. pylori) infection remains prevalent worldwide. Multiple studies and metaanalyses have identified a distinct link between H. pylori infection and iron-deficiency anemia (IDA), suggesting that the bacteria may increase iron loss and impair iron absorption. Although the physiological mechanism(s) behind H. pylori -induced anemia remain to be elucidated in detail, it could relate to induction of hepcidin expression in infected individuals, perhaps even in gastric parietal cells. Another proposed mechanism by which H . pylori colonization may decrease iron absorption is through alteration of gastric pH. Specifically, H . pylori infection of the gastric mucosa causes changes in gastric physiology that can lead to atrophic gastritis. Subsequently, the loss of parietal cells due to atrophy causes hypo- or achlorhydria. Under physiological conditions, the acidic pH of the stomach promotes the reduction of ferric iron (insoluble form) to ferrous iron, the more soluble form transported by DMT1. An increase in gastric pH could thus potentially reduce the amount of absorbable ferrous iron. Furthermore, the reduction of ferric iron to ferrous iron is enhanced by dietary and tissue ascorbic acid. Indeed, studies have shown significantly decreased ascorbic acid levels in the mucosa of H. pylori -infected subjects when compared to uninfected individuals, which could potentially exacerbate iron malabsorption. Recent studies have also speculated that H. pylori competes with its host for available iron. Iron is an essential nutrient for bacterial growth and is required for bacteria to retain virulence. Thus, increased iron utilization by bacteria to support their growth may cause iron depletion in the host during H. pylori infection. The mechanism by which H. pylori acquires iron remains unknown, but in vitro studies suggest that the bacteria can disturb host cell polarity and disrupt intracellular iron homeostasis.
Iron is an essential mineral for the survival and growth of numerous cell types, including IECs, and also for commensal bacteria. Host intestinal cells and gut bacteria share a unique symbiotic relationship characterized by continuous cross-communication and subsequent mutually beneficial adaptive changes that ensure their survival. Given the complex physiology of the GI tract, this interconnectedness between host cells and gut bacteria could potentially affect a variable number of GI functions, including iron absorption. It is well established that dietary iron intake levels directly affect the composition of the gut microbiota ; however, the effects of the gut microbiota on iron absorption remain speculative. A recent study showed an 8- to 10-fold increase in intestinal DCYTB and DMT1 protein expression and a twofold reduction in FPN1 expression in the duodenum of germ-free (GF) mice, as compared to GF mice colonized with gut bacteria. Ferritin expression was strongly induced in the colon after bacteria were introduced into the GI tract. The authors suggested that the enterocytes of GF mice were depleted of iron and that upon bacterial colonization, the epithelial cells favor iron storage. Similar studies using germ-free models have shown that the gut microbiota affect many other host intestinal processes, including absorptive, immune, and secretory functions ; however, factors such as rate of colonization and the specific bacterial species present can significantly alter the resulting physiological response. Potential pathological alterations of the gut microbiota should be taken into consideration during iron supplementation or when managing iron-related pathologies, but further research is required to elucidate the mechanisms by which the gut microbiota may influence intestinal iron absorption.
Iron absorption occurs through the differentiated epithelial cells (enterocytes) of the mid and upper villus, and predominantly in the proximal part of the small intestine. The proteins involved in this process are listed and briefly described in Table 60.1 . The immature enterocytes of the intestinal crypts handle iron quite differently from their mature counterparts (as noted above). They are not absorptive in nature and take up a large amount of TF-bound iron from the blood, unlike mature enterocytes. As the cells mature and differentiate, they acquire their absorptive characteristics but lose the capacity to take up iron from the circulation. Iron metabolism in crypt cells will not be considered further here.
| Protein | Function |
|---|---|
| DCYTB a | Ferric iron reduction for absorption across BBM; other reductases may exist and could also play a role in this process |
| DMT1 | Cotransport of ferrous iron and H + into enterocytes; also transports manganese and possibly other divalent metal ions |
| FLVCR | Heme export across BLM into circulation |
| FPN1 | Ferrous iron exporter on basolateral surface of enterocytes; hepcidin receptor |
| Ferritin | Intracellular iron storage, including within enterocytes; dietary form of iron |
| HCP1 | Possible heme importer on apical surface of enterocytes |
| HEPH | Oxidation of exported iron for binding to transferrin and distribution to body cells and tissues in blood; expressed on basolateral surface of enterocytes |
| HO | Oxidation of heme molecule to release ferrous iron in endosomes |
| Hepcidin | Liver-derived, peptide hormone that inhibits intestinal iron export by promoting internalization and degradation of FPN1 |
| LTF | Iron-binding protein in breast milk; LTF receptor expressed on apical surface of enterocytes |
a DCYTB , duodenal cytochrome B; DMT1, divalent metal-ion transporter 1; FLVCR , feline leukemia virus, subgroup C, receptor; FPN1 , ferroportin 1; HCP1 , heme carrier protein 1; HEPH , hephaestin; HO , heme oxygenase; LTF , lactoferrin.
Iron movement across the enterocyte is usually considered in terms of three phases or steps ( Fig. 60.1 ): (1) iron uptake—the movement of iron from the intestinal lumen across the BBM and into the enterocyte; (2) an intracellular phase where iron is stored or utilized within the enterocyte or directed to the BLM for subsequent export; and (3) the export of iron across the BLM and into the interstitial fluids which bathe the serosal side of enterocytes, the so-called transfer step. The small intestine is able to utilize different forms of iron and it is the BBM proteins that must deal with this variety. The utilization of inorganic, or nonheme, iron (which is likely bound to low molecular weight organic acids) is particularly important and has been most extensively studied, but iron can also be delivered to enterocytes as heme, ferritin, or lactoferrin (as discussed later). Irrespective of the dietary source, it is likely that much of the iron taken up into enterocytes enters a common cytosolic “labile” iron pool and is subsequently exported via a common FPN1-mediated pathway.
Iron absorption is a rapid process. Following the administration of a radioactive dose of iron into the lumen of the proximal small intestine, radioactivity usually appears in the circulation within 15 s, and within minutes, 60%–80% of the total amount ultimately absorbed is transferred into the body. This initial phase of rapid iron uptake is followed by a slower rate of transfer that continues for 12–48 h. It is likely that this iron was initially retained within enterocytes in ferritin. Some iron stored in ferritin, however, never makes it into the circulation since it is lost when mucosal cells are exfoliated at the end of their lifespans. During iron deficiency, not only is the total amount of iron absorbed greater but also the fraction retained within the enterocyte is much smaller.
The quantity of iron absorbed by the intestine is related to the luminal iron concentration in a biphasic manner. At low iron concentrations, there is a direct relationship between the iron concentration and absorption, but with increasing amounts of luminal iron, proportionally smaller amounts of the nutrient are absorbed, that is, the process is saturable. This in turn reflects specific iron uptake processes. However, iron absorption never fully saturates, and with very high doses of iron, a linear relationship between the luminal iron concentration and the amount absorbed is again observed. These findings suggest that iron absorption is a saturable, carrier-mediated process at physiological intraluminal iron concentrations, but that a high-capacity, nonspecific process (possibly paracellular flux) is operating at higher concentrations. This same phenomenon has been described for the absorption of other essential minerals, notably calcium. Very large doses of oral iron can overcome feedback mechanisms, which normally limit iron absorption, likely reflecting the nonspecific component. The saturable process represents what we now recognize as the normal physiological DMT1/FPN1-mediated iron absorption pathway that will be described in detail below.
In the past, there was considerable debate over the issue of whether brush-border uptake or basolateral efflux was the rate-limiting step in iron absorption (summarized in Ref. ). Teleologically, it makes sense that the basolateral transfer step is rate limiting as the amount of iron crossing the BLM cannot exceed that crossing the BBM. The intestinal epithelium essentially acts as a buffer to control dietary iron assimilation. Iron can move across the BBM and into the enterocyte, but whether it proceeds any further depends on the export capacity of the BLM. Iron not absorbed immediately is stored within ferritin, but this iron can ultimately be utilized later if needed to meet metabolic demands. Although early kinetic and physiological investigations provided results that were somewhat conflicting, the consensus from these studies was that basolateral transfer was indeed rate limiting. In recent years, with improved understanding of the molecular basis of iron absorption and its regulation, strong support for the regulatory role of the basolateral transit of iron has been provided. This will be considered in greater detail below.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here