Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
LAS, MMH, and DFN are supported by NHMRC Australia grants 1079234, 1071153, and 1069757, respectively, and the research of TU and HE is supported by the Ministry of Education, Culture, Sports, Science, and Technology Science Research Funds Grant 2212005, Japan Society for the Promotion of Science Grant 25460279, and the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency. We thank Annette Bergner, Lauren Young and John Stephenson for the images shown in Fig. 11.1 .
The neural crest is a population of cells that emigrates from the dorsal neural tube around the time of neural tube closure. Neurons and glial cells of the enteric nervous system (ENS) arise from multiple sources of neural crest-derived cells.
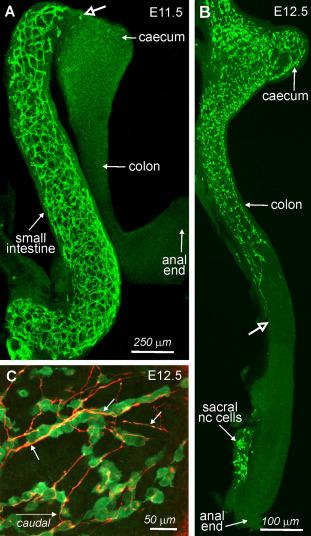
Surgical removal of premigratory neural crest cells from different levels of the neural axis of chick embryos and chick-quail chimera studies showed that the most important source of enteric neurons is “vagal” neural crest cells, which emigrate from the caudal hindbrain adjacent to somites 1–7. Vagal neural crest cells enter the foregut ( Fig. 11.1 ), and then migrate caudally within the gut mesenchyme ( Fig. 11.2 ). In mice, when vagal neural crest-derived cells enter the midgut, the midgut and hindgut are transiently closely apposed, and a subpopulation of cells leaves the midgut, takes a short-cut through the mesentery and enters the colon without passing through the caecum. These “transmesenteric” neural crest-derived cells are then the front-runners to colonize the colon.
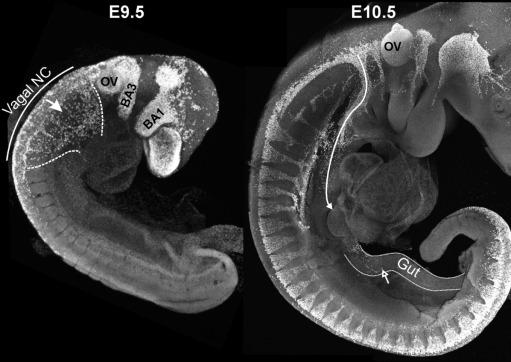
In human embryos, it takes around 3 weeks from when vagal enteric neural crest-derived cells (ENCCs) enter the foregut until they reach the anal end of the gastrointestinal tract , and in mice and chicks it takes 5 days.
Sacral level neural crest cells have been shown to give rise to some enteric neurons in mice, chick and quail. In mice, around 12% of all neural crest-derived cells in the distal hindgut are of sacral origin, while in the chick, sacral cells give rise to around 17% of neurons.
Studies in chick have shown that following surgical ablation of vagal neural crest cells, sacral crest cells cannot compensate for their loss, probably due to lower levels of expression of the receptor tyrosine kinase, RET, by sacral crest cells. In both mouse and chick, sacral neural crest cells aggregrate in the connective tissue adjacent to the hindgut, but then undergo a 2–3 day waiting period before they enter the hindgut ( Fig. 11.2 B). Although sacral neural crest cells do not enter the hindgut until after the hindgut has been colonized by vagal neural crest-derived cells, sacral crest cells do not require the presence of vagal neural crest cells to enter the hindgut, and the delay in the entry of sacral cells is due to the expression of inhibitory cues in the hindgut mesenchyme (see Section 11.2.10 ).
Sensory, visceromotor, and sympathetic neurons project their nerve fibers to the gut after the arrival of vagal ENCCs. Neural crest-derived Schwann cell precursors (SCPs) migrate along these extrinsic nerves (mesenteric nerves) and invade the gut. SCPs are derived from neural crest cells at all axial levels and associate with all nerves that project to peripheral tissues. Most SCPs in the mesenteric nerves of rodents differentiate into non-myelin-forming Schwann cells, which form bundles by surrounding several axons, and ensheath each axon in a pocket of cytoplasm without forming compact myelin. In contrast, enteric glial cells display distinct morphological features: (1) process extension over nerve fibers, (2) a partial sheath to nerve fibers, (3) long laminar processes between nerve processes and wrapping multi-axonal bundles. A fate-tracing study of SCPs revealed that a subset of SCPs generate enteric neurons postnatally, and contribute to a small proportion (< 5%) of submucosal neurons in the small intestine and < 20% of both myenteric and submucosal neurons in the large intestine. Given that SCPs are derived from all neural axial levels, SCP-derived enteric neurogenesis suggests the contribution of trunk neural crest to enteric neurons.
The embryonic gut grows considerably in length while it is being colonized by ENCCs. For example, in mice, the post-caecal gut increases in length about fivefold between when ENCC first enter the post-caecal gut and when ENCC reach the anal end of the gut at E14.5. ENCC therefore migrate further than any other neural crest cell population. Failure of ENCC to colonize the entire length of the gastrointestinal tract results in distal aganglionosis, which in humans is called Hirschsprung’s disease. Because the ENS is essential for propulsive gut motility, infants with Hirschsprung’s disease suffer from intractable constipation and develop a megacolon, which is treated by surgical removal of the affected bowel region.
After emigrating from the hindbrain neural tube, vagal neural crest cells migrate through paraxial tissues to the foregut. Retinoic acid is produced by paraxial tissues and induces the vagal cells to migrate in chains within the gut mesenchyme. ENCC chains migrate in close association with the neurites of caudally projecting, early enteric neurons.
While some vagal ENCCs must advance caudally towards the distal hindgut, a subpopulation of ENCCs must remain behind in each location to ensure that there are neurons present along the entire bowel. It had been assumed that the ENCCs that get left behind in each gut region had ceased migrating, however, time-lapse imaging studies have shown that the cells that remain behind continue to migrate for at least 24 h, but they migrate circumferentially, rather than caudally.
Sacral neural crest cells enter the hindgut along the axons of extrinsic pelvic plexus neurons and then migrate rostrally within the gut. Schwann cell precursors also enter the gut along the axons of extrinsic neurons.
The development of the ENS from neural crest-derived cells occurs as a series of overlapping processes including migration, proliferation, differentiation (neuronal and glial), neurotransmitter specification, axon growth and navigation, target selection, synapse formation and development of mature electrical properties. Importantly, migration, proliferation and neurogenesis occur concurrently and are linked (see Sections 11.2.1 and 11.2.2 ). A large number of molecular and cellular mechanisms have been shown to influence ENS development, but most of the mechanisms identified to date regulate the earlier events in ENS development-migration, survival, proliferation and neuronal differentiation-and little is known about the later events including neurotransmitter specification and synaptogenesis. The molecules involved in the development of the ENS are summarized in Table 11.1 , and the roles of some of these molecules and processes are also discussed in greater detail below and in several reviews.
| Role | References | |
|---|---|---|
| Molecules required by vagal neural crest cells before entering the gut | ||
| Geminin | Promotes survival of vagal NCCs by protecting against DNA damage | |
| Retinoic acid | Regulation of Ret expression; migration, chain-formation and foregut colonization | |
| Treacle (encoded by Tcof1 gene) | Survival of vagal NCCs | |
| Molecules expressed by gut mesenchyme or epithelium | ||
| GDNF | Survival, proliferation, differentiation and migration of ENCCs | |
| Endothelin-3 | Proliferation of undifferentiated ENCCs, inhibits neuronal differentiation, promotes migration | |
| NRTN (Neurturin) | Proliferation of undifferentiated ENCCs, promotes axon projections from excitatory motor neurons | |
| Neurotrophin-3 | Survival and differentiation of developing enteric neurons | |
| Sonic hedgehog | Proliferation of ENCCs, indirectly influence concentric patterning of neurons | |
| Gli3 | Migration of ENCCs | |
| Indian hedgehog | Survival of a subpopulation of ENCCs | |
| BMP2, BMP4 (bone morphogenetic proteins 2 and 4) | Influence ENCC migration and differentiation, ganglion formation, concentric patterning of neurons | |
| Netrin | Induce migration of subpopulation of ENCCs from myenteric region to submucosal region | |
| Semaphorin 3A | Negatively regulates the time of entry of extrinsic axons and sacral neural crest cells into the distal hindgut | |
| Retinaldehye dehydrognenases | Influence differentiation of ENCCs | |
| Heparin-binding EGF-like growth factor (HB-EGF) | Migration of ENCCs | |
| Molecules expressed by ENCCs (i) transcription factors | ||
| SOX10 | Regulates Ret , Phox2b and Ednrb ; survival of ENCCs and development of glia | |
| FOXD3 | Regulates Sox10 expression; maintains progenitors | |
| PHOX2B | Survival of ENCCs by regulating RET expression | |
| HAND2 | Terminal differentiation of subpopulation of enteric neurons | |
| ASCL1 (MASH1) | Survival of regional subpopulation of ENCCs (esophageal ENCCs) and in the development of some enteric neuron subtypes in the intestine | |
| PAX3 | Acts with SOX10 to activate Ret expression | |
| AP-2 family | In zebrafish, ap2α acts with foxd3 to regulate Sox10 expression | |
| ZEB2 (also called ZFHX1B or SIP1) | Specification of vagal neural crest cells. In cooperation with SOX10, regulates EDNRB expression | |
| HLX | Required for migration of ENCCs beyond stomach | |
| NR2F1 | Overexpression causes premature glial differentiation of ENCCs | |
| TashT | Regulates ENCC migration speed | |
| Molecules expressed by ENCCs (ii) cell surface receptors | ||
| RET | Signaling molecule for GDNF and NRTN | |
| GFRα1 | GPI-anchored receptor to which GDNF binds | |
| GFRα2 | GPI-anchored receptor to which NRTN binds | |
| Ednrb | Receptor for endothelin-3 | |
| Ntrk3 (also called TrkC) | Receptor for neurotrophin-3 | |
| ErbB3 | Development of enteric glia | |
| Molecules expressed by ENCCs (iii) other | ||
| Spry2 (also called Sprouty homolog 2) | Negative regulator of RET | |
| KIF26A | Negative regulator of RET | |
| L1-CAM | Influences ENCC migration and rate of neuronal differentiation | |
| NCAM | Influences clustering of developing enteric neurons | |
| β1-integrin (Itgb1) | Rregulates ENCC migration, ganglion geometry | |
| C3a and cadherin | Regulates ENCC cell adhesion and directionality of migration | |
| collagen VI | Influences ENCC migration speed | |
| tenascin-C | Influences ENCC migration | |
| Inosine 5′ monophosphate dehydrogenase 2 (IMPDH2) | Proliferation of vagal NCCs | |
| Notch | Regulates progenitor proliferation and glial development | |
| Kif1-binding protein | Promotes neurite formation | |
| Pten | Inhibits ENCC migration and proliferation | |
| Phactr4 | Influence directionality of ENCC migration via regulation of integrin signaling | |
| small GTPases | Regulates ENCC migration, proliferation and neurite extension | |
| Neuregulin | Survival of postnatal enteric neurons | |
| microRNAs | Neuronal differentiation | |
| Electrical activity/neurotransmitters | ||
| Sodium-dependent action potentials | Regulates rate of neuronal differentiation of some neuron subtypes | |
| Serotonin | Regulates neuronal differentiation | |
| Slc6a2 (also called NET, norepinephrine transporter) | Survival and/or differentiation of some subtypes of enteric neurons | |
| Nitric oxide | Influences ENCC differentiaton and motility in chick embryos | |
| Environmental factors | ||
| Microbiota | Signals from the microbiota regulate the initial colonization and homeostasis of the mucosal enteric glia | |
| Diet: Vitamin A/retinoic acid | Promotes ENCC migration | |
| Medicine: ibuprofen | Reduces speed of ENCC migration | |
| Medicine: mycophenolate | Impairs ENCC proliferation and migration | |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here