Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
During erythropoiesis the red cell membrane responds to erythropoietin and imports the billion or so iron atoms each red cell needs to complete hemoglobin synthesis. It sequesters the reductants required to protect hemoglobin and other cell proteins from corrosion by oxygen, and selectively retains other vital components such as organic phosphates, manufactured with precious adenosine triphosphate (ATP) energy, but it lets metabolic detritus escape. It even helps regulate metabolism by reversibly binding and inactivating many glycolytic enzymes. In the circulation it carefully balances cation and water concentrations so red cells do not shrivel or burst. Simultaneously, it exchanges tremendous numbers of bicarbonate and chloride anions (≈10 to 30 billion/second/red cell), which aids transfer of carbon dioxide from the tissues to the lungs. It also maintains a slippery exterior so that red cells do not adhere to endothelial cells or aggregate and clog capillaries. Finally, buttressed by the “membrane skeleton,” a protein scaffold that lines the inner membrane surface, the membrane achieves the critical combination of strength and flexibility needed to survive four months in the circulation. Failure of any of these, or numerous other, functions shortens red cell survival, a process termed hemolysis. In fact, all hemolysis is ultimately due to membrane failure. This chapter will focus on inherited disorders of red blood cell membranes, particularly hereditary spherocytosis and hereditary elliptocytosis, the two most common and best understood diseases.
Hereditary spherocytosis (HS) ( Table 16-1 ) is a common, inherited hemolytic anemia in which defects of spectrin or of proteins that attach spectrin to the membrane—ankyrin, protein 4.2, or band 3—lead to spheroidal, osmotically fragile cells that are selectively trapped in the spleen, resulting in a shortened red cell life span.
| Common Conditions |
| Hereditary spherocytosis |
| Immunohemolytic anemias (warm antibody type) |
| ABO hemolytic disease in neonates † |
| Uncommon to Rare Conditions |
| Hereditary stomatocytosis † |
| Hereditary cryohydrocytosis † |
| Clostridial sepsis |
| Hemolytic transfusion reactions |
| Severe burns and other red cell thermal injuries |
| Spider, bee and snake venoms † |
| Severe hypophosphatemia † |
| Acute red cell oxidant injury (e.g., glucose-6-phosphate dehydro-genase deficiency during hemolytic crisis, Wilson disease) † |
| Congenital dyserythropoietic anemia, type II † |
* Some spherocytes may be seen in other diseases where they are not the predominant red cell morphology (e.g., hereditary pyropoikilocytosis, Heinz body hemolytic anemias, severe liver disease, microangiopathic hemolysis, hexokinase deficiency).
† Spherocytes are the predominant morphology in only a subset of patients.
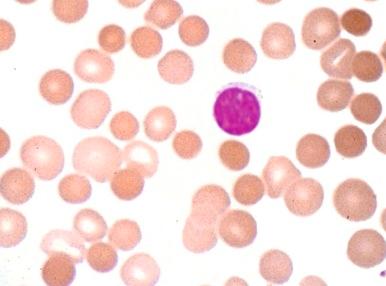
HS was first recognized more than 140 years ago by two Belgian physicians, Vanlair and Masius, who gave a remarkably thorough account of the disease. They described a woman who suffered from the clinical symptoms now known as hallmarks of HS—anemia, jaundice, splenomegaly, and recurrent abdominal pain. The authors noted that most of the woman's red cells were spherical ( Fig. 16-1 ) and hypothesized that a combination of splenic enlargement and liver atrophy led to their rapid destruction and the patient's anemia. They also noted that the patient's sister had suffered from an identical illness. In the 1890s, the British physicians Wilson and Stanley recognized the hereditary nature of the disease and were the first to note the characteristic pathologic appearance of the spleen engorged with red cells. Subsequently, a report by Minkowski in 1900, in the German literature, received wide attention, and many additional papers soon appeared, including Chauffard's classic description of osmotic fragility and reticulocytosis as hallmark laboratory features of the disease. The first successful splenectomy for HS was unintentionally performed by Wells in England 20 years before splenectomy became widely accepted and 3 years before Wilson's description of HS appeared. While operating on a jaundiced woman for a supposed uterine fibroid, Wells encountered and bravely removed an enormous spleen. The patient recovered and her jaundice disappeared. Forty years after the splenectomy performed by Wells, Dawson found the abnormal erythrocyte osmotic fragility during an examination of the woman and her son. The major clinical features of HS were defined by the 1920s, although nothing was known about the pathophysiology of the disease. Readers interested in more details about these and other aspects of the history of HS should consult the chapters by Dacie, Crosby, and Wintrobe in the wonderful book Blood, Pure and Eloquent. Recent reviews of HS are also available.

HS occurs in all racial and ethnic groups. It is particularly common in Europeans and their descendants in the New World. The classic estimate is that 1 person in 5000 is affected, but this is almost certainly an underestimate. Surveys of red cell osmotic fragility suggest that mild forms of the disease may be four or five times more common than that. In addition, testing of Swiss patients identified as having hyperchromic red cells on a routine complete blood count led to a diagnosis of HS in 1 of every 1700 individuals, most of whom were asymptomatic. So the prevalence in Europeans is probably closer to 1 in 1500 to 1 in 2000. The disease is also common in Japan and most other countries. Clinical experience suggests it is less common in African Americans and Southeast Asians.
HS exhibits both dominant and nondominant inheritance. About 75% of patients have typical autosomal dominant disease. Homozygotes for dominant HS are very rare, with only a handful of reported cases. The remaining 25% of HS patients show nondominant inheritance. In these patients, both parents are clinically normal but some of them exhibit subtle laboratory abnormalities that suggest a carrier state. The only available molecular survey suggests that the majority of nondominant HS patients have new dominant mutations, which tend to occur at CpG dinucleotides and are associated with small insertions or deletions. This study suggests that true recessive HS is quite rare, perhaps less than 5% of the total, but larger-scale studies are needed.
HS is caused by unique mutations in the membrane skeletal proteins ankyrin, β-spectrin, α-spectrin, band 3, and protein 4.2.
The typical clinical picture of HS combines evidence of hemolysis (anemia, jaundice, reticulocytosis, gallstones, and splenomegaly) with spherocytosis and a positive family history ( Table 16-2 ; Fig. 16-2 ). Mild, moderate, and severe forms of HS have been defined according to differences in various clinical and laboratory parameters under baseline conditions ( Table 16-3 ). Unfortunately, patients don't fit neatly into these categories: a person my be classified as “mild” by one criterion and “moderate” by another.
| Clinical Features |
| Pallor |
| Splenomegaly |
| Intermittent jaundice |
| From hemolysis |
| From biliary obstruction |
| Anemic crises: hemolytic, aplastic or megaloblastic |
| Inheritance |
| Dominant ≈ 75% |
| Nondominant ≈ 25% |
| Rare manifestations |
| Leg ulcers |
| Chronic dermatitis |
| Extramedullary hematopoietic tumors |
| Angioid streaks |
| Spinocerebellar degeneration |
| Myocardiopathy |
| Excellent response to splenectomy |
| Laboratory Features |
| Anemia |
| Reticulocytosis |
| Spherocytosis |
| Elevated MCHC and elevated percentage of hyperchromic or hyperdense cells |
| Increased RDW (red cell distribution width, a measure of poikilocytosis) |
| Abnormality in one or more “HS tests” |
| Decreased band 3 (decreased fluorescence of red cells labelled with eosin-5′-maleimide) |
| Increased osmotic fragility (especially in the incubated osmotic fragility test) |
| Abnormal acidified glycerol lysis time or Pink test |
| Abnormal cryohemolysis test |
| Characteristic osmotic gradient ektacytometry profile |
| Normal Coombs (direct antiglobulin) test |
| Decreased red cell spectrin, or spectrin and ankyrin, or band 3 and protein 4.2, or absent protein 4.2 and decreased CD47 |
| Normal | Mild Spherocytosis | Moderate Spherocytosis | Moderately Severe Spherocytosis | Severe Spherocytosis * | |
|---|---|---|---|---|---|
| Inheritance | — | Autosomal dominant | Autosomal dominant, de novo mutation | Autosomal dominant, de novo mutation | Autosomal recessive |
| Proportion of hereditary spherocytosis cases | — | ≈20% to 30% | ≈60% to 70% | ≈10% | <5% |
| Hemoglobin (g/dL) † | 11.5-16 ‡ | 10.5-15 | 8-12 | 6-8 | <6 |
| Reticulocytes (%) † | 0.5-1.5 | 1.5-6 | ≥6 | ≥10 | ≥10 |
| Bilirubin (mg/dL) † , ‖ | 0-1 | 0.5-2 | ≥2 | ≥2 | ≥3 |
| Peripheral smear † | Normal | Mild spherocytosis | Spherocytosis | Spherocytosis | Spherocytosis ± poikilocytosis |
| Osmotic fragility (fresh) | Normal | Normal or slightly increased | Increased | Increased | Greatly increased |
| Osmotic fragility (incubated) | Normal | Usually increased | Increased | Increased | Greatly increased |
| MCHC (g/dL) § | 32-36 | 34-37 | 34-38 | 35-39 | |
| RDW (%) § | 11-14 | 12-19 | 16-23 | 20-30 | |
| Hb/MCHC * | 0.38-0.41 | 0.35-0.40 | 0.29-0.33 | 0.18-0.28 | |
| Hb/RDW § | 0.95-1.05 | 0.7-1.0 | 0.48-0.74 | 0.16-0.35 | |
| Serum transferrin receptor (nmol/L) § | 18-25 | 30-65 | 80-125 | 100-150 | |
| Erythropoietin (mIU/mL) § | 7-16 | 9-30 | 25-90 | 30-300 | |
| Membrane protein patterns (SDS-PAGE) ¶ | — | “Normal” # Slight ↓ spectrin Slight ↓ spectrin and ankyrin Slight ↓ band 3 and 4.2 Absent protein 4.2 and ↓ CD47 |
↓ Spectrin ↓ Spectrin and ankyrin ↓ Band 3 and protein 4.2 Absent protein 4.2 and ↓ CD47 |
↓ Spectrin ↓ Spectrin and ankyrin ↓ Band 3 and protein 4.2 |
↓↓ Spectrin ↓↓ Spectrin and ankyrin ↓↓ Band 3 and protein 4.2 ** |
| Transfusions | — | No | Sometimes required in infancy or with aplastic crisis | Occasionally with crises | Regular * |
| Splenectomy | — | Rarely, partial splenectomy †† | Sometimes; consider partial splenectomy | Usually (6-9 years) | Yes (>3 years) |
* Patients with severe disease are transfusion dependent by definition. Values are in untransfused patients or at nadir before transfusion.
† Data modified from Eber SW, Armbrust R, Schröter W: Variable clinical severity of hereditary spherocytosis: relation to erythrocytic spectrin concentration, osmotic fragility and autohemolysis. J Pediatr 177:409–416, 1990.
§ Ranges shown encompass the majority of individuals in each category. From Rocha S, Costa E, Rocha-Pereira P, et al: Complementary markers for the clinical severity classification of hereditary spherocytosis in unsplenectomized patients. Blood Cells Mol Dis 46:166–170, 2011.
‖ Multiply by 17.1 to convert to µmol/L.
¶ Indicates common patterns observed on SDS gels. Decreased spectrin alone is seen in α-spectrin or β-spectrin defects. Decreased spectrin and ankyrin are observed with ankyrin defects. Decreased band 3 and protein 4.2 occur with band 3 defects. Absent protein 4.2 and decreased CD47 occur with protein 4.2 defects.
# Patients with mild spherocytosis who appear normal probably have small deficits (10% to 15%) that cannot be distinguished from normal findings on SDS gels.
** Rare patients with severe spherocytosis who are homozygous or compound heterozygous for band 3 defects.
†† Consider in adolescents and adults who require a cholecystectomy or have disfiguring chronic jaundice.
Initial assessment of a potential HS patient should include questions about neonatal or subsequent jaundice, anemia, splenomegaly, or gallbladder symptoms; transfusions; and a family history of these things and of splenectomies or cholecystectomies. The physical examination should seek signs of pallor, scleral icterus, and splenomegaly. Pediatricians should also examine the parents and siblings if they are present, particularly for splenomegaly, which is more evident in older patients. Scleral icterus is usually subtle and best perceived in daylight and at the edge of the conjunctivae ( Fig. 16-3 ). Similarly, a spleen that cannot be palpated in a supine patient can sometimes be felt when the patient is placed on his or her right side with knees bent.

HS typically presents in infancy or childhood, but it may present at any age. In children, anemia is the most common presenting complaint (50%), followed by splenomegaly, jaundice, or a positive family history. No comparable data exist for adults. The majority of patients with HS have incompletely compensated hemolysis and mild to moderate anemia. The anemia is often asymptomatic except for fatigue and mild pallor or, in children, nonspecific parental complaints such as irritability. Jaundice is seen at some time in about one half of patients, usually in association with viral infections. When jaundice is present it is acholuric (i.e., unconjugated [indirect] hyperbilirubinemia without detectable direct bilirubinuria). Palpable splenomegaly is detectable in about one half of infants and most (70% to 95%) older children and adults. Typically, the spleen is modestly enlarged (2 to 6 cm below the costal margin), but it may be massive. There is no proven correlation between the size of the spleen and the severity of HS, although, given the pathophysiology and the response of the disease to splenectomy, such a correlation probably exists.
Typical HS is associated with both dominant and nondominant inheritance. The nondominant disease tends to be more severe, but there is considerable overlap. Clinical severity and the response to splenectomy roughly parallel the degree of spectrin (or ankyrin) deficiency in patients with defects in either protein. Disease in such patients can vary from mild to severe. Patients who are heterozygotes for band 3 defects have typical, moderate HS and moderate band 3 deficiency (15% to 35%). Severe HS is rare in this population unless the patient carries two defective band 3 genes. Protein 4.2 deficiency is also associated with milder disease. Overall, patients with deficiency of spectrin or both spectrin and ankyrin have more severe disease than patients with band 3 or protein 4.2 deficiency, but the differences are not great because true “severe” HS is rare.
In general, members of the same family will have a similar clinical picture, but there are exceptions, presumably due to varying compensation by the normal allele in patients who are heterozygous for a mutant gene, or by the coinheritance of another deleterious allele. Such alleles should be sought in patients whose disease is much more severe than that of the rest of the family.
The parents of patients with “nondominant” HS are clinically asymptomatic and do not have anemia, splenomegaly, hyperbilirubinemia, or spherocytosis on peripheral blood smears. However, some have subtle laboratory signs of HS, including slight reticulocytosis (average, 2.1 ± 0.8%), diminished haptoglobin levels, slightly elevated osmotic fragility, or slightly shortened times in the acidified glycerol lysis test (AGLT). Screening of normal Norwegian and German blood donors with the osmotic fragility test or AGLT showed a 0.9% to 1.1% incidence of previously unsuspected “very mild” HS. Presumably, some of these individuals were silent carriers of recessive HS genes, whereas others just had mild dominant HS. Unfortunately, no systematic molecular survey of this group of patients has been conducted.
Red cell production and destruction are balanced or nearly balanced in 20% to 30% of patients with HS. These persons are considered to have “compensated hemolysis” and are usually asymptomatic. In some patients, the diagnosis may be difficult because hemolysis, splenomegaly, and spherocytosis are unusually mild. In this group of patients, reticulocyte counts are generally less than 6%, and only 60% of patients have obvious spherocytosis on peripheral blood smears. About 10% of HS patients have very few (≤2%) or no detectable spherocytes. In addition, red cell spectrin and ankyrin levels are typically more than 80% of normal. As noted, many of these patients have band 3 or protein 4.2 defects.
Hemolysis may become severe with illnesses that cause splenomegaly, such as infectious mononucleosis. Hemolysis may also be exacerbated by pregnancy or exercise, to the point where it may impair athletic performance in endurance sports. In many of these patients, HS is diagnosed during family studies or discovered when adult patients are diagnosed with splenomegaly or gallstones. Although mild HS is usually familial, it develops sporadically in families with more severe disease. Presumably this is due to the coinheritance of modifying genes, such as those affecting spectrin or ankyrin synthesis or splenic function.
It is unclear why patients with HS and “compensated hemolysis” (i.e., normal hemoglobin levels) continue to have erythroid hyperplasia. Erythroid stimulation should disappear once hemoglobin levels reach normal if hemoglobin functions normally, as it does in HS. That it does not is shown by the fact that erythropoietin is inappropriately elevated (up to eight times normal) in HS patients with compensated hemolysis and no anemia, and the fact that the serum transferrin receptor concentration, a measure of erythropoiesis, is elevated. The levels of 2,3-diphosphoglycerate (2,3-DPG) are reportedly low in hereditary spherocytes before splenectomy, which could explain it; however the P 50 of blood from HS patients is normal, which does not make sense. A more likely possibility is that the dehydrated HS red cells are rheologically impaired and do not adequately perfuse the juxtaglomerular renal vessels, where erythropoietin is produced, even when the hematocrit is normal. If this hypothesis is correct, then erythropoietin production should correlate inversely with red cell deformability in any disorder where red cells are dehydrated (e.g., sickle cell anemia, hereditary xerocytosis). Clinically, the high level of erythropoietin and persistent erythroid hyperplasia in patients with HS and a normal hemoglobin level indicate that physiologically the patients are still anemic. Indeed, it is possible that all HS patients are physiologically more anemic than their hemoglobin values suggest.
A small fraction of patients with HS (5% to 10%) have moderately severe to severe anemia. Patients with “moderately severe” disease typically have a hemoglobin value of 6 to 8 g/dL, a reticulocyte count of about 10%, a bilirubin level greater than 2 mg/dL, and 40% to 70% of the normal red cell spectrin content. The mean corpuscular hemoglobin concentration (MCHC) is high and the red cell distribution width (RDW) is very high (typically >20%) (see Table 16-3 ). Such patients are more susceptible to the dangers of hemolytic and aplastic crises (see later) and may occasionally need a transfusion. This category includes patients with both dominant and recessive HS and a variety of molecular defects in spectrin and ankyrin. Occasional patients with band 3 defects may fall into this category.
Patients with “severe” disease, by definition, have life-threatening anemia and are transfusion dependent. They often have recessive HS. Most of these patients probably have isolated, severe spectrin deficiency (<40% of normal) resulting from a defect in α-spectrin, but some have ankyrin defects or are homozygous or compound heterozygous for band 3 mutations. Patients with severe HS are also distinguished by red cell morphologic findings. They often have some irregularly contoured or budding spherocytes and bizarre poikilocytes, in addition to typical spherocytes. Such cells are rare before splenectomy in patients with less severe disease, although some may be seen after splenectomy. In addition to the risks of recurrent transfusions, patients with “severe” disease often suffer from an aplastic crisis and may develop growth retardation, delayed sexual maturation, or aspects of thalassemic facies.
In general, unsplenectomized patients with HS have no significant complications during pregnancy except for anemia, which worsens because of plasma volume expansion and sometimes because of increased hemolysis. Hemolytic crises during pregnancy requiring transfusion have been reported. Folic acid deficiency is also a risk. One group reported that 20% of patients with HS received transfusions during pregnancy, but in our experience, pregnant patients with HS rarely need transfusions.
HS often presents as jaundice in the first few days of life. Roughly one half of all patients with HS have a history of neonatal jaundice, and 91% of infants discovered to have HS in the first week of life are jaundiced (bilirubin level >10 mg/dL). In a French study of 402 jaundiced neonates requiring phototherapy, 1% were found to have HS. Hyperbilirubinemia usually appears in the first 2 days of life and bilirubin levels may rise rapidly, driven by the combination of hemolysis and the reduced capacity of the neonatal liver to conjugate bilirubin. Newborns with both HS and Gilbert syndrome, a common polymorphism in the promoter region of the uridine diphosphate–glucuronosyltransferase gene (UGT1A1) (see Chapter 4 ), develop neonatal jaundice more frequently and may develop severe hyperbilirubinemia. Kernicterus is a risk and exchange transfusion or treatment with barbiturates are sometimes necessary, but in most patients the jaundice is controlled with phototherapy.
Only 43% of neonates with HS are anemic at birth (hemoglobin level <15 g/dL), and severe anemia is rare, but those who present at birth tend to have a more severe course. Most have a normal hemoglobin level at birth, which then decreases sharply over the first 3 weeks of life, leading to a transient, severe anemia. In up to three fourths of HS infants, the anemia is severe enough to warrant transfusion or treatment with erythropoietin (see below). This anemia appears to be aggravated by an inability of the infant's bone marrow to mount an appropriate erythropoietic response to anemia and to the development of splenic function.
Hydrops fetalis has been reported in a few patients and is associated with homozygosity or compound heterozygosity for spectrin or band 3 defects. If hydrops fetalis is detected in utero, the fetus may require intrauterine transfusions. No instances of hydrops fetalis have been associated with ankyrin deficiency. Perhaps infants with ankyrin defects, similar to ankyrin-deficient nb/nb mice, are partially protected in utero by the expression of ankyrin-related proteins in embryonic and fetal erythroblasts.
The diagnosis of HS is sometimes more difficult in the neonatal period than later in life. Splenomegaly is uncommon—at most the spleen tip is palpable—and reticulocytosis is variable and usually not severe. Only 35% of affected neonates have a reticulocyte count greater than 10%. In addition, the haptoglobin level is not a reliable indicator of hemolysis during the first few months of life. An even greater problem is that 33% of neonates with HS do not have significant numbers of spherocytes on their peripheral blood smears. Moreover, because fetal red cells are more osmotically resistant than adult cells when fresh and more osmotically sensitive after incubation at 37°C for 24 hours, the osmotic fragility test occasionally gives false-positive (incubated) or false-negative (unincubated) results unless fetal controls are used. Fortunately, these results have been published, and they appear to reliably differentiate neonates with HS, particularly when the incubated osmotic fragility test is used. However, in our experience it is rarely necessary to use fetal controls. The standard osmotic fragility test usually suffices to make the diagnosis in conjunction with the blood smear, Coombs test, and other data. Tests such as the eosin-5′-maleimide–binding test, AGLT, Pink test, and cryohemolysis test and osmotic gradient ektacytometry (all discussed later) are more sensitive and specific than the osmotic fragility test in older children and adults, but they have not been systematically tested in the neonatal period. Studying the erythrocytes from the parents is also very useful in the diagnosis of HS, particularly if the infant has received a transfusion.
However, it is clear that many infants with HS are not detected in the neonatal period on clinical grounds alone, even infants with hyperbilirubinemia, probably because the disease is not considered or is not obvious. Ascertainment is greatly enhanced if all infants with a high MCHC and a high RDW are evaluated for HS.
As noted above, some infants with HS become progressively more anemic in the first month or two of life and require transfusion. Fortunately, the problem is transient, except in rare patients with severe HS, and usually remits after one or two transfusions. Almost all infants with HS will outgrow the need for transfusion by the end of the first year of life. If the child is otherwise well, we allow the hemoglobin level to fall to 5.5 to 6.5 g/dL before giving transfusions to try to stimulate the marrow and only raise the hemoglobin level to 9 to 11 g/dL after transfusion to avoid suppressing the desired marrow response.
Administration of recombinant human erythropoietin (rHuEpo) to infants with HS was beneficial in reducing blood transfusions in an uncontrolled, open-label study. In this study, 13 of 16 infants given weekly subcutaneous injections of rHuEpo at a dosage of 1000 IU/kg had increases in the absolute reticulocyte count and hemoglobin values. The infants were given the same dosage of rHuEpo weekly based on their initial weight (at a range of initial ages from 16 to 119 days), a weekly hemoglobin level of less than 8 g/dL, and an absolute reticulocyte count of 200 × 10 9 /L or less. Subsequent studies show similar effectiveness with different dosages and different frequencies. Therefore erythropoietin therapy in infants with HS has become an alternative to transfusion, although weekly erythropoietin injections can be challenging for parents. Also, erythropoietin is twice as expensive as a single transfusion and erythropoietin-treated infants don’t always avoid a transfusion. Further studies of recombinant erythropoietin therapy for neonatal anemia in HS are needed to determine the optimal time to initiate treatment, the optimal dosage, and the optimal duration of therapy.
Close observation of infants with HS is necessary to avoid complications of severe anemia. All infants with HS should have hemoglobin and reticulocyte measurements at least monthly during the first 6 months of life to avoid unnecessary side effects of severe anemia. The intervals may be increased thereafter, depending on the severity of anemia, but care must be taken to instruct parents on the signs and symptoms of an aplastic crisis.
The primary molecular defects in HS reside in membrane skeleton proteins, particularly the proteins whose vertical interactions connect the membrane skeleton to the lipid bilayer: spectrin, ankyrin, protein 4.2, and band 3 ( Fig. 16-4 ). Because each of these proteins has more than one vertical attachment (ankyrin to RhAG as well as band 3, protein 4.2 to CD47 as well as band 3, spectrin to phospholipids and to the band 3 complex near actin), missense mutations that only impair a single protein-protein interaction are not pathologic, whereas reductions in the amounts of the four proteins are. In contrast, missense mutations predominate in hereditary elliptocytosis, which is caused by diminished horizontal interactions between individual proteins that hold the membrane skeleton together, especially defects in spectrin self-association (see Fig. 16-4 ).
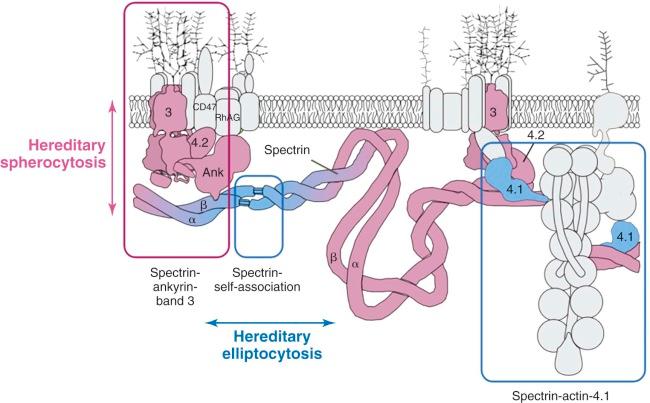
Four patterns predominate: isolated, partial spectrin deficiency, seen with defects in α-spectrin or β-spectrin; combined, partial spectrin and ankyrin deficiency, seen with primary ankyrin defects; combined, partial band 3 and protein 4.2 deficiency, seen with defects in band 3; and nearly complete loss of protein 4.2 and CD47, seen in primary defects of protein 4.2. In Europeans and Americans ankyrin and band 3 defects are most common, roughly 45% and 30%, respectively; β-spectrin defects account for perhaps 20%. Protein 4.2 and α-spectrin defects are both recessive and are rare. However, the pattern of membrane protein defects varies in different populations. In Japan, deficiencies of band 3 and protein 4.2 are the most common forms of HS. In Korean patients, ankyrin and spectrin defects are most common, but protein 4.2 deficiency occurs more often than in European or American patients.
Because most mutations are unique within each family and the mutation itself does not usually predict the phenotype, it is rarely clinically useful to determine the specific molecular defect in patients with HS. Readers who are interested in tables listing many of the mutations described to cause HS should consult lists in various chapters and reviews, including the previous edition of this text, or one of the two versions of the Human Gene Mutation Database: HGMD Professional, a subscription database that is available through many medical libraries, and the public version of HGMD, which is free but lags 3 years behind the subscription version.
For patients whose red cells are spectrin deficient, the degree of deficiency correlates well with the spheroidicity of HS red cells, the severity of hemolysis, and the response to splenectomy ( Fig. 16-5, A ). The mechanical properties of the cells, particularly their ability to withstand shear stress, also correlate with their spectrin content (see Fig. 16-5, B ). Microscopically, HS red cells with spectrin deficiency show fewer spectrin filaments interconnecting spectrin–actin–protein 4.1R junctional complexes, but overall skeletal architecture is preserved, except in the most severe forms of the disease.

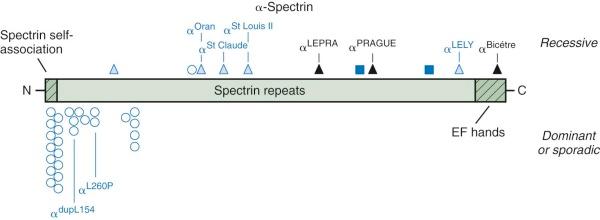
In humans, α-spectrin synthesis exceeds β-spectrin synthesis by about four to one. Heterozygotes for α-spectrin synthetic defects still produce enough normal α-spectrin chains to pair with all of the β chains that are made. Thus α-spectrin defects are only apparent in the homozygous or compound heterozygous state and exhibit recessive inheritance. Moreover, there may only be a limited spectrum of mutations that cause clinical disease. Production-defective or “thalassemia-type” mutants that only moderately affect expression, such as α-spectrin LELY (to be discussed under hereditary elliptocytosis), have no phenotype, even in the homozygous state. Based on the few reported cases, it appears that the output of the α-spectrin gene must be less than 25% of normal to cause disease and may be as low as 8% of normal and still be compatible with life. No homozygous null mutations have been reported, so it is possible they are lethal in the embryo.
To date, only 2% of all the mutations reported to cause HS involve α-spectrin ( Fig. 16-6 ). Wichterle et al reported a patient with severe HS who was a compound heterozygote for two different α-spectrin gene defects: in one allele, there was a splicing defect associated with an upstream intronic mutation, α LEPRA (low-expression allele Prague); in the other allele, there was a null mutation, α PRAGUE . The α LEPRA allele produces one-sixth as much correctly spliced α-spectrin mRNA as normal. It is a relatively common allele, estimated to be 5% among Caucasians, and is in linkage dysequilibrium with α Bughill , which is an innocent amino acid polymorphism (Ala970Asp). Delaunay reported a similar family combining α LEPRA /α Bughill on one chromosome with a double mutant α LELY /α Bicêtre on the other, where the latter mutation essentially abolished α-spectrin output. In both cases the combination of the severely impaired α LEPRA allele with a null mutation of α-spectrin in trans , leads to significant, spectrin-deficient spherocytic anemia. As expected, even in the most severe forms of α-spectrin–linked recessive HS, obligate heterozygotes are asymptomatic and have little or no spectrin deficiency.
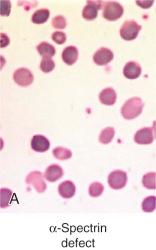
Less than 5% of patients with HS have primary defects in α-spectrin, but most of those patients have severe HS. Their red cells only contain 25% to 50% of the normal amount of both spectrin chains ( Table 16-4 ). Many are transfusion dependent, and even after splenectomy they may experience only a partial recovery. Blood smears are more bizarre than is typical for HS and show intense spherocytosis and irregular contracted cells ( Fig. 16-7, A ).
| <5% of hereditary spherocytosis |
| Autosomal recessive since four-fold more α-spectrin is made than β-spectrin |
| A low expression, “thalassemia-type” defect in α-spectrin is often paired with a second presumably null allele on the opposite chromosome (in trans ). The combination must reduce α-spectrin expression by at least 75% to impair αβ-spectrin assembly enough to cause HS. Homozygous null mutations may be lethal |
| Severe hemolysis and anemia, often transfusion dependent |
| 50%-75% spectrin deficiency in red cells |
| Red cell morphology not well defined but dense, irregular poikilocytes as well as spherocytes in some patients |
β-Spectrin synthesis limits formation of αβ-spectrin heterodimers, so even modest defects in the expression of β-spectrin are apparent in the heterozygous state and exhibit a dominant pattern of inheritance. Of the reported HS mutations 17% are in β-spectrin, a finding that fits with the general impression that β-spectrin defects account for 15% to 25% of cases. Some groups report a much higher proportion of HS patients with isolated spectrin deficiency when sodium dodecyl sulfate (SDS) gels are used for membrane protein analysis, but that may reflect problems with assessing ankyrin deficiency on SDS gels (see later). The characteristics of HS due to β-spectrin deficiency are listed in Table 16-5 .
| 15%-25% of hereditary spherocytosis cases |
| Almost all individual, dominant null mutations |
| Spectrin content decreased 15%-40% |
| Mild to moderately severe anemia |
| Blood smear with spherocytosis plus 5%-15% spiculated red cells in most patients |
| Spherocytosis plus elliptocytosis (i.e., spherocytic elliptocytosis) in some patients with C-terminal mutations that impair spectrin self-association and decrease spectrin content |
The described mutations in β-spectrin include initiation codon disruptions, frameshift and nonsense mutations, gene deletions and rearrangements, and splicing defects ( Fig. 16-8 ) . Most patients have defects that impair or abolish β-spectrin production from the affected allele. All of the β-spectrin is generated from the normal allele in trans , which compensates to varying degrees, so that the red cell spectrin content is only decreased by 15% to 40% instead of the expected 50%. This suggests that β-spectrin is also synthesized in excess, though much less so than α-spectrin. The degree of compensation probably accounts for much of the variation in clinical severity among patients with β-spectrin HS.
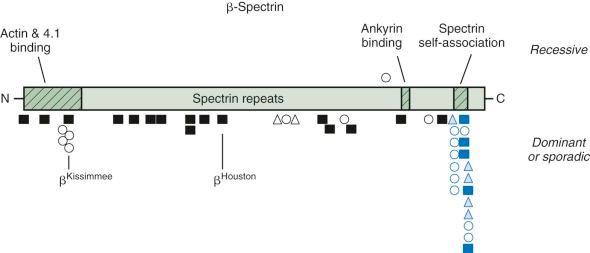
Patients with deletions of the whole β-spectrin locus, or with gross rearrangements, also have autism and learning disabilities, indicating that genes close to β-spectrin on chromosome 14 are involved in cognition. There are HS patients with potentially instructive missense mutations, including a group clustered in the actin and protein 4.1R binding domain (spectrins Atlanta, Kissimmee, and Oakland ) and in the spectrin repeat domain (spectrins Columbus, Birmingham, and Sao Paulo ). It would be interesting to know whether these amino acid substitutions cause HS because they impair a specific function or whether the missense mutations simply destabilize β-spectrin and act like null mutations. In the case of spectrin Kissimmee (Trp202Arg), which is the only mutation where function was examined, both things were true. The mutant protein lacked the ability to bind protein 4.1R and, as a consequence, bound poorly to actin, but it was also unstable and decreased in amount.
All of the reported defects of β-spectrin are limited to single families with the exception of spectrin Houston , a null mutation that has been found in several unrelated kindreds.
Patients with HS caused by β-spectrin mutations have hemolysis and anemia that vary from mild to moderately severe (see Table 16-5 ). Interestingly, in HS patients in whom peripheral blood morphologic characteristics have been described, those with β-spectrin mutations have had a small population (5% to 15%) of acanthocytes or spiculated spherocytes (spheroechinocytes) as well as spherocytes (see Fig. 16-7, B ).
Spectrin heterodimers are only stable when bound to the membrane, and ankyrin, the high-affinity binding site, is normally present in limiting amounts. As a result, ankyrin defects are typically expressed as a dominant defect, although recessive mutations have been described. Ankyrin mutations are estimated to account for 30% to 60% of HS cases in Northern European populations and 5% to 10% of HS cases in Japan. Of all the known HS mutations, 35% are Ank1 defects. The characteristics of HS due to ankyrin defects are listed in Table 16-6 .
| 30%-60% of hereditary spherocytosis in the United States and Europe; 5%-10% in Japan |
| Autosomal dominant and autosomal recessive |
| Dominant mutations are mostly null mutations and are nearly all unique |
| Recessive mutations are missense and promoter defects: − 108T→C promoter mutation is common |
| Both spectrin and ankyrin are equally decreased by 15%-50% |
| Mild to moderately severe anemia. Occasionally causes severe disease |
| Blood smear shows spherocytosis without other abnormal morphology |
A role of ankyrin in HS was initially suggested by two patients with an atypical, unusually severe form of HS characterized by bizarre-shaped microspherocytes and red cell spectrin and ankyrin levels that were only 50% of normal. Ankyrin synthesis was half normal; spectrin synthesis was normal, but only 50% of the synthesized spectrin attached to the membrane, presumably owing to the lack of ankyrin sites. These results were the initial indication that ankyrin deficiency or dysfunction leads to concomitant spectrin deficiency in HS.
Additional studies showed that ankyrin deficiency is common in typical, dominant HS. When measured by radioimmunoassay, red cell membrane spectrin and ankyrin levels were less than the normal range in a large fraction of American kindreds, and the two proteins were proportionally decreased in nearly all the deficient kindreds. Similar data have been obtained in multiple populations.
Genetic analyses supported the hypothesis that a defect of the ankyrin gene could cause HS. Initially, studies of atypical patients with HS and karyotypic abnormalities, including translocations and interstitial deletions, defined a locus for HS at chromosomal segments 8p11.2-21.1. After the ankyrin gene was cloned, it was localized to this same region, 8p11.2. Fluorescence-based in situ hybridization of metaphase spreads from a patient with HS and an interstitial deletion of chromosome 8p11.1-p21 provided direct evidence that one copy of the erythrocyte ankyrin gene was deleted. Ankyrin content in the patient's red blood cell membranes was reduced by 50%. Gene mapping then linked ankyrin to HS in a large family with typical dominant HS. Further studies showed that ankyrin mutations are common in HS and that many HS patients express only one of their two ankyrin alleles.
Ankyrin mutations occur in both dominant and nondominant HS. Frameshift and nonsense mutations, which abolish the normal ankyrin product, are particularly common in dominant HS ( Fig. 16-9 ). They produce mild to moderately severe hemolytic anemia, depending on the output of the remaining normal ankyrin gene and, perhaps, other modifier genes. For example, ankyrin Marburg and ankyrin Einbeck are frameshift mutations that occur in the NH 2 -terminal (membrane) domain. Neither produces a detectable product in mature red cells. They would be expected to have a similar phenotype, but patients with ankyrin Einbeck have very mild disease and normal ankyrin levels, whereas patients with ankyrin Marburg have moderate to severe HS and moderate ankyrin deficiency (64% of normal). Understanding such phenotypic variations is one of the critical issues in HS research.
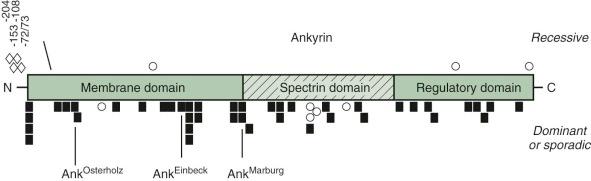
The most common ankyrin variant in patients with recessive HS is a T→C nucleotide substitution in the ankyrin gene promoter −108 bp from the translation start site. The allele occurs in 29% of German HS patients and 3% of normal individuals. It is usually in cis with a G→A nucleotide substitution 153 bp from the start site and impairs ankyrin synthesis in transgenic animals. A −72/73 dinucleotide deletion in the ankyrin promoter also impairs expression by disrupting the recognition site for the TATA-binding protein and interfering with transcription initiation. These “thalassemia-like” promoter mutations are silent in obligate heterozygotes; thus patients with nondominant HS must have a second mutation. The promoter mutations aggravate ankyrin mutations if they are inherited in trans , on the “normal” allele, particularly the −108 mutation, which is relatively common. For example, in a family with ankyrin Osterholtz , which is a null mutation, the daughter, who has a combination of ankyrin Osterholtz /ankyrin −72/73 , has more severe disease than her mother, who only carries ankyrin Osterholtz .
Patients with gene deletions involving the ankyrin locus at 8p11.2 often have features both of HS and Kallmann syndrome (hypogonadotrophic hypogonadism, type 2, due to FGFR1 defects) because the two loci are less than 4 Mb apart. Most of these patients also have psychomotor developmental delay, resulting from loss of another still undefined gene in the region.
Peripheral blood smears demonstrate only prominent spherocytosis (see Fig. 16-7, C ). Hemolysis can be mild to severe, and the patients with recessive mutations typically have a more severe clinical course. Almost all dominant ankyrin mutations are private; that is, each individual kindred has a unique mutation. There is one exception, ankyrin Florianopolis , a frameshift mutation that has been identified in HS patients from three unrelated kindreds. Differences in a linked ankyrin gene polymorphism show that the three ankyrin Florianopolis alleles are different and arose independently.
Band 3 deficiency is present in approximately 25% to 45% of European and American patients with dominant HS, and band 3 defects account for 37% of the known HS mutations. Erythrocyte membranes from these patients are equally deficient in band 3 and protein 4.2, which requires band 3 to bind to the red cell membrane. The characteristics of HS due to band 3 deficiency are listed in Table 16-7 .
| 25%-45% of hereditary spherocytosis |
| Individual, dominant functionally null mutations. No common defects |
| 15%-40% decrease in band 3 and a similar decrease in protein 4.2 |
| Mild to moderate hemolysis and anemia |
| Spherocytosis with a small number of characteristic button mushroom-shaped or “pincered” cells in most patients |
Defects in band 3 produce a variety of red cell phenotypes ( Fig. 16-10 ), including stomatocytosis, cryohydrocytosis, Southeast Asian ovalocytosis (SAO), and acanthocytosis, as well as HS. These are discussed in later sections of the chapter. The HS mutations are spread throughout the band 3 gene, occurring in both the cytoplasmic and membrane-spanning domains. They include nonsense mutations, frameshift mutations, partial gene duplications, other missense mutations, and splicing defects. Many of the mutations cause messenger RNA (mRNA) instability or a misfolded protein that is degraded or not inserted into the membrane. Conserved arginine residues are frequent sites of missense mutations in the transmembrane domain of the band 3 gene. The affected arginine residues are all located in the same position on the inside edge of membrane-spanning segments and are believed to interact with negatively charged phospholipids in the inner bilayer and help orient the segments. As predicted, in vitro studies show that these arginine substitutions, as well as several other HS-associated band 3 missense mutations, exhibit defective cellular trafficking from the endoplasmic reticulum to the plasma membrane, perhaps due to misfolding. Determination of the structure of the cytoplasmic domain of band 3 and detailed studies of the topography of the transmembrane domain are beginning to provide insights into the biologic significance of the missense mutations.
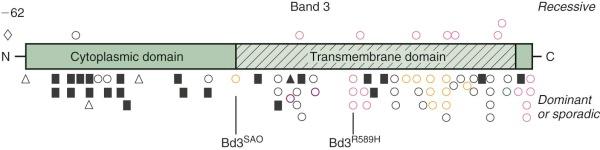
Similar to the other molecular defects that cause HS, band 3 mutations are unique familial defects with no common mutations among affected groups.
Clinically, most patients with band 3 mutations have mild to moderate hemolysis and anemia. The band 3 protein is reduced by 15% to 35% in HS red cells. On average, band 3 defects are somewhat milder than ankyrin or β-spectrin defects, although there is considerable overlap in severity. Patients with HS and band 3 deficiencies have a small number (≤1%) of button mushroom–shaped or “pincered” erythrocytes in addition to spherocytes on peripheral blood smears (see Fig. 16-7, D ). This morphologic characteristic is not observed in the other membrane protein defects.
Distal renal tubular acidosis (dRTA) is a disorder characterized by the inability of the kidney to acidify the urine below pH 5.5 in the presence of systemic metabolic acidosis. It is caused by failure of H + secretion in the distal nephron. Patients present with various combinations of failure to thrive, hyperchloremic metabolic acidosis, hypokalemia, weakness, metabolic bone disease, and kidney stones. Because a shortened form of band 3 lacking the first 65 amino acids is expressed in the intercalated cells of the kidney cortical collecting ducts where its anion exchange capability is coupled to H + excretion, defects in band 3 would be expected to produce dRTA, and in fact a number of recessive and dominant missense mutations within the membrane domain of band 3 have been identified in dRTA patients (see Fig. 16-10 ). The dominant defects tend to cluster in the middle of the membrane domain (Arg589His has been described in multiple kindreds) or at the C-terminus (see Fig. 16-10 ). The recessive mutations are localized throughout the membrane domain. The dominant mutations seem to be more prevalent in Europe, whereas the recessive mutations are more prevalent in Southeast Asia, but this may be because SAO (band 3 SAO or band 3 Δ400-408 ) is very common in that part of the world (discussed later) and causes dRTA when it is coupled with a recessive dRTA allele.
Surprisingly, few patients with HS due to band 3 defects have dRTA. There are a few exceptions in patients who are homozygous or compound heterozygous for band 3 defects ; however, the majority of HS patients with mutations in band 3 do not have dRTA. Conversely, few patients with band 3 defects causing dRTA have hemolysis or spherocytosis. In general, the dRTA mutations do not impair anion exchange. They cause band 3 to either be retained in the endoplasmic reticulum or Golgi apparatus, or to be mistrafficked to the wrong membrane surface in polarized cells. Most of the transport and trafficking studies have been done in oocytes, Madin-Darby canine kidney (MDCK) cells, or other epithelial cells and not in erythroid cell lines, so it is not possible to test whether band 3 trafficking is aberrant in kidney cells but not in red cells. This would explain why the dRTA mutants do not cause HS, and there is some evidence supporting the idea. Another possibility is that dominant dRTA results from the association of mutant band 3 and wild-type band 3 into heterooligomers in the kidney, but not in erythroid cells, leading to intracellular retention or mistargeting of both proteins in the kidney and normal expression in red cells.
Carbonic anhydrase II (CAII) binds to the C-terminus of band 3 and aids in chloride-bicarbonate transport. Patients who lack CAII develop dRTA, osteopetrosis, and neurologic disease. This raises the possibility that some C-terminal band 3 dRTA mutations (see Fig. 16-10 ) may cause dRTA through loss of CAII function instead of, or in addition to, mistrafficking of band 3.
HS caused by protein 4.2 deficiency is most common in Japan, although a few patients have been described in other populations. Overall, protein 4.2 mutations account for 9% of the published HS molecular defects. The characteristics of this form of HS are summarized in Table 16-8 .
| <5% of hereditary spherocytosis in the United States and Europe; 45%-50% of hereditary spherocytosis in Japan |
| Autosomal recessive |
| 95%-100% deficiency of protein 4.2, decreased amount of CD47 |
| 4.2 Nippon is common in Japan |
| Blood smear with variable red cell morphology: spherocytes, acanthocytes, ovalostomatocytes, normal discocytes. Spherocytes may not be the predominant morphology |
Multiple protein 4.2 mutations have been described ( Fig. 16-11 ). The most common one is 4.2 Nippon , in which the red cells contain only a small quantity of a 74/72-kD doublet of protein 4.2 instead of the usual abundant 72-kD species, because of an Ala142→Thr mutation that affects the processing of the protein 4.2 mRNA. Protein 4.2 Nippon –deficient membranes lose 70% of their ankyrin with low ionic strength extraction, and the ankyrin loss is blocked by preincubation of the membranes with purified 4.2, which suggests that protein 4.2 stabilizes ankyrin on the membrane. This hypothesis is supported by the observations that the amount of protein 4.2 is low in the red cell membranes of ankyrin-deficient nb/nb mice and in HS patients who lack one ankyrin gene. In addition, red cells from patients homozygous for 4.2 Nippon are fragile and have heat-sensitive skeletons, clumped intramembranous particles, and increased lateral mobility of band 3. A weakening of ankyrin–band 3 binding due to loss of protein 4.2 has also been observed. However, ankyrin lability is not evident in all patients with protein 4.2 deficiency. Patients whose red cells lack protein 4.2 also lack CD47. CD47 connects the band3–ankyrin–4.2 complex to the Rh complex of which CD47 is a part. Loss of CD47 may also be important because CD47 is recognized by an inhibitory receptor on macrophages that blocks phagocytosis.

Finally, partial erythrocyte protein 4.2 deficiency has also been associated with mutations of band 3. Presumably, these mutations affect sites of band 3–protein 4.2 interactions.
Clinically, patients with HS due to protein 4.2 deficiency have mild to moderate HS. The peripheral smear sometimes contains typical spherocytes, but it may also contain ovalocytes, stomatocytes, or spiculated cells and relatively few spherocytes (see Fig. 16-7, E ). For this reason the diagnosis of HS may be missed in patients with protein 4.2 deficiency. The mechanism behind this unique and variable red cell morphologic picture is currently unknown.
The primary membrane lesions in HS all involve vertical interactions between the skeleton and the bilayer and fit the theory that HS is caused by local disconnection of the skeleton from the bilayer and its integral membrane proteins, followed by vesiculation of the unsupported surface components. This, in turn, leads to progressive reduction in membrane surface area and to a shape called a spherocyte, although it usually ranges between a thickened discocyte and a spherostomatocyte.
Biomechanical measurements show that HS membranes are fragile. The force required to fragment the membrane is diminished and is proportional to the density of spectrin on the membrane. Membrane elasticity and bending stiffness are also reduced and are proportional to spectrin density. In addition, HS red cells lose membrane more readily than normal red cells when metabolically deprived or when their ghosts are subjected to conditions facilitating vesiculation. This has not been shown to occur in metabolically healthy HS spherocytes, perhaps because it occurs slowly (1% to 2% per day) under such conditions. However, massive vesiculation is evident in mice and zebrafish with severe spherocytic hemolytic anemias ( Fig. 16-12 ).

Because budding red cells are rarely observed in typical blood smears from patients with HS, the postulated vesiculation may either involve microscopic vesicles or occur in the reticuloendothelial system. Vesiculation may also occur in the bone marrow of patients with HS because similar decreases in the red cell surface area have been found in HS reticulocytes and in mature red blood cells. When membrane vesicles are induced in normal red cells, they originate at the tips of spicules, where the lipid bilayer uncouples from the underlying skeleton (see Fig. 15-29 ). The vesicles are small (≈100 nm) and devoid of hemoglobin and skeletal proteins and would be invisible by light microscopy. Tiny (50- to 80-nm) bumps have been detected by atomic force microscopy, on the surface of red cells obtained from patients with HS whose red cells are actively hemolyzing ( Fig. 16-13 ). The bumps could be microvesicles, although this needs to be proven. They are less than the length of a spectrin molecule (100 nm) and are not present on red cells from splenectomized patients.

The observation that spectrin or spectrin/ankyrin deficiencies are common in HS has led to the suggestion that they are the primary cause of spherocytosis. According to this hypothesis ( Fig. 16-14 , hypothesis 1), interactions of spectrin with bilayer lipids or proteins are required to stabilize the membrane. Spectrin-deficient areas would tend to bud off, leading to spherocytosis. However, this conjecture does not explain how spherocytes develop in patients whose red cells are deficient in band 3 but have normal amounts of spectrin.
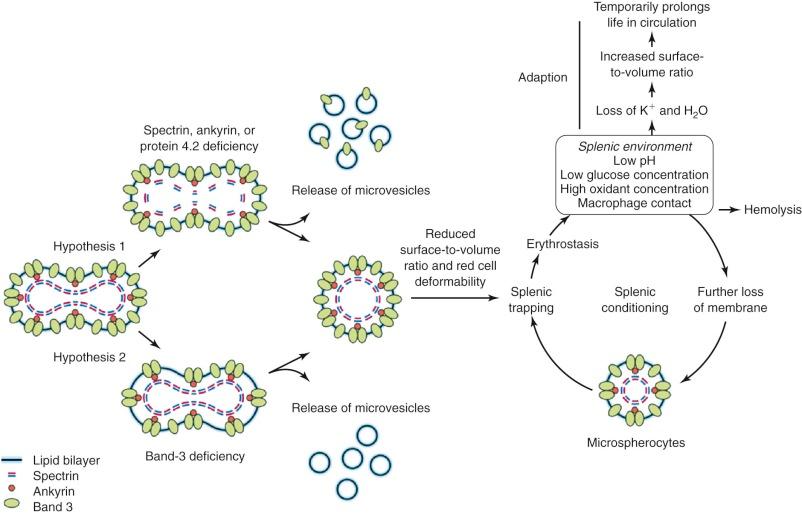
An alternate hypothesis argues that the bilayer is stabilized by interactions between lipids and the abundant band 3 molecules (see Fig. 16-14 , hypothesis 2). Each band 3 molecule contains about 12 hydrophobic transmembrane helices, many of which must interact with lipids. These interactions presumably spread beyond the first layer of lipids and influence the mobility of lipids in successive layers. In deficient red cells the area between band 3 molecules would increase, on average, and the stabilizing effect would diminish. Transient fluctuations in the local density of band 3 could aggravate this situation and allow unsupported lipids to be lost, resulting in spherocytosis. This hypothesis is supported by targeted disruption of the band 3 gene in mice. Erythrocytes from these mice lose massive amounts of membrane surface despite a normal membrane skeleton. This concept is also consistent with early studies of intramembrane particle aggregation, which leads to particle-free domains, as discussed in Chapter 15 . These domains are unstable, giving rise to surface blebs that are subsequently released from the cells as vesicles.
It is most likely that both of these hypotheses operate to a different degree depending on the membrane skeletal protein which is deficient—hypothesis 1 dominating in spectrin and ankyrin disorders and perhaps in protein 4.2 deficiency, and hypothesis 2 dominating in band 3 defects. Hypothesis 1 predicts that released microvesicles will contain band 3 and other skeleton-linked integral membrane proteins; hypothesis 2 predicts such vesicles will contain mostly lipids. This should be testable but has not yet been examined.
Hereditary spherocytes hemolyze because of the rheologic consequences of their decreased surface-to-volume ratio. The red cell membrane is very flexible, but it can only expand its surface area about 3% before rupturing. Thus erythrocytes become less and less deformable as surface area is lost. Clever experiments show that the spleen begins to sense and restrain red cells that have lost as little as 2% to 3% of their membrane surface and that loss of more than 18% of the red cell surface leads to complete splenic sequestration. This corresponds to the 15% to 20% loss of membrane lipids and, presumably, membrane surface in HS red cells. For HS red cells, poor deformability is only a hindrance in the spleen, because the cells have a nearly normal life span after splenectomy.
In the spleen most of the arterial blood empties directly into the splenic cords, a tortuous maze of interconnecting narrow passages formed by reticular cells and lined with phagocytes (see discussion of splenic structure and function in Chapter 23 ). Histologically, this is an “open” circulation, but most of the blood that enters the cords normally travels by fairly direct (i.e., functionally closed) pathways. If passage through these channels is impeded, red cells are diverted deeper into the labyrinthine portions of the cords, where blood flow is slow and the cells may be detained for minutes to hours. Whichever route is taken, to reenter the circulation, red cells must squeeze through spaces between the endothelial cells that form the walls of the venous sinuses ( Fig. 16-15 ). Even when maximally distended, these narrow slits are always much smaller than red cells, which are greatly distorted during their passage. Experiments have shown that spherocytes are selectively sequestered at this cordal-sinus juncture. As a consequence, spleens from patients with HS have massively congested cords and relatively empty sinuses. In electron micrographs, few spherocytes are seen in transit through the sinus wall, which contrasts to the situation in normal spleens, where cells in transit are readily found.

During detention in the spleen, HS red cells undergo additional damage marked by further loss of surface area and an increase in cell density. Many of these “conditioned” red cells escape the hostile environment of the spleen and reenter the circulation. In unsplenectomized patients with HS, two populations of spherocytes are detectable: a minor population of hyperchromic “microspherocytes” that form the “tail” of very fragile cells in the unincubated osmotic fragility test and a major population that may be only slightly more spheroidal than normal.
By 1913, it was known that HS red cells obtained from the splenic vein were more osmotically fragile than those in the peripheral circulation. However, the significance of this finding was not clear until the classic studies in the 1950s by Emerson and Young, who showed that osmotically fragile microspherocytes are concentrated in and emanate from the splenic pulp ( Fig. 16-16 ). After splenectomy, spherocytosis persists, but the tail of hyperfragile red cells is no longer evident on osmotic fragility testing. These and other data led to the conclusion that the spleen detains and “conditions” circulating HS red cells in a way that increases their spheroidicity and hastens their demise. The kinetics of this process were beautifully illustrated in vivo by Griggs, who found that a cohort of 59 Fe-labeled HS red cells gradually shifted from the major, less fragile population to the more fragile, conditioned population 7 to 11 days after entering the circulation. Although most conditioned HS red cells that escape the spleen are probably recaptured and destroyed, the damage incurred is sufficient to permit extrasplenic recognition and removal, because conditioned spherocytes, isolated from the spleen and reinfused postoperatively, are rapidly eliminated.

The mechanism of splenic conditioning is less certain. It is difficult to obtain accurate information about the cordal environment, but the existing data suggest it is metabolically inhospitable. Crowded red cells must compete for limited supplies of glucose in acidic surroundings (pH 6.8 to 7.2) where glycolysis is inhibited. The acidic environment induces Cl − and water entry and cell swelling but also stimulates the K + ,Cl − cotransporter, which produces a net loss of K + and water from the cells. The adverse effects of the cordal environment are further compounded by the presence of oxidant-producing macrophages. It has also been suggested that methylation of erythrocyte membrane proteins could contribute to the splenic conditioning of spectrin-deficient HS red cells. Hence, the spherocyte, detained in the splenic cords because of its surface deficiency, is stressed by erythrostasis in a metabolically threatening environment.
The spherocyte is particularly vulnerable to erythrostasis. This is the basis of the well-known autohemolysis test. During prolonged sterile incubation in the absence of supplemental glucose, red cells undergo a series of changes that culminate in hemolysis. The sequence of the changes is the same for HS and normal red cells; however, because HS cells are abnormally leaky and bear unstable membranes, their degeneration is accelerated.
HS red cells are initially jeopardized, because their membrane permeability to Na + is mildly increased. Their propensity to accumulate Na + and water is normally balanced by increased Na + pumping; however, the increased dependence on glycolysis is detrimental in erythrostasis, where substrate is limited. HS red cells exhaust serum glucose and become ATP depleted more rapidly than normal red cells. As ATP levels fall, ATP-dependent Na + ,K + and Ca 2+ pumps fail, and the cells gain Na + and water and swell. Later, when the Ca 2+ -dependent K + (Gárdos) pathway is activated, K + loss predominates and the cells lose water and shrink. The Na + gain is accelerated in HS red cells but is insufficient by itself to induce hemolysis. However, HS red cells are doubly jeopardized. As noted earlier, they are inherently unstable and fragment excessively during metabolic depletion. Membrane lipids (and probably integral membrane proteins) are lost at more than twice the normal rate. At first this surface loss is balanced by cell dehydration, but eventually (within 30 to 48 hours) membrane loss predominates, the cells exceed their critical hemolytic volume, and autohemolysis ensues.
Calculations indicate that the average normal red cell passes through the splenic cords about 14,000 times during its lifetime and has an average transit time of 30 to 40 seconds, surprisingly close to measured transient times in normal human spleens in vivo. The calculated residence time of the average HS red cell in the splenic cords is much longer, perhaps as long as 15 to 150 minutes, but still far short of the time required for metabolic depletion to occur. This conclusion is supported by direct analysis of splenic red cells. HS red cells obtained from the splenic pulp and containing 80% to 100% conditioned cells are moderately cation depleted and show changes in adenosine diphosphate (ADP) and 2,3-DPG concentrations consistent with metabolism in an acidic environment, but their ATP levels are normal. Others have reported similar results.
The data suggest that splenic conditioning is caused by mechanisms other than ATP depletion. For example, K + loss and membrane instability may be exacerbated by the high concentrations of acids and oxidants that must exist in a spleen filled with activated macrophages lunching on trapped HS red cells. In vitro, oxidants from activated phagocytes can diffuse across the membranes of bystander red cells and damage intracellular proteins within minutes. Red cells moving through the rapid transit pathways in the spleen might escape damage, but those caught in cordal traffic would be vulnerable. Oxidants, even in relatively low concentrations, cause selective K + loss by a variety of mechanisms and also damage membrane skeletal proteins. Finally, there is some evidence that HS red cells may be abnormally sensitive to oxidants. When exposed to peroxides they undergo remarkable blebbing and, presumably, vesiculation. If a similar process occurs in the spleen, it could be responsible for the excessive surface loss observed in conditioned cells.
The possibility that macrophages may directly condition HS red cells should also be considered. It is well known that spherocytosis often results from the interaction of immunoglobulin G (IgG)–coated red cells with macrophages, but HS red cells do not have abnormal levels of surface IgG. Macrophages also bear receptors for oxidized lipids (scavenger receptor) and phosphatidylserine, but there is no evidence at present that HS red cells expose the relevant ligands. The involvement of macrophages is supported by observations that large doses of corticosteroids markedly ameliorate HS in unsplenectomized patients. The effects are similar to those produced by splenectomy. It is well known that similar doses of corticosteroids inhibit splenic processing and destruction of IgG- or C3b-coated red cells in patients with immunohemolytic anemias, probably by suppressing macrophage-induced red cell sphering and phagocytosis. Electron microscopy shows that splenic erythrophagocytosis is common in HS, particularly in the splenic cords. In addition, phagocytes expressed from the cords of patients with HS contain bits of ghostlike “debris,” presumably resulting from membrane fragmentation.
It has been known for many years that HS red cells intrinsically leak more Na + and K + ions than normal cells. All the Na + transmembrane movements are increased, including passive diffusion, Na + ,K + -ATPase pump, Na + ,K + ,2Cl − cotransport, and Na + , Li + countertransport. The excessive Na + influx activates the Na + ,K + -ATPase pump, and the accelerated pumping, in turn, increases ATP turnover and glycolysis. At one time, it was believed that this modest Na + leak was responsible for the hemolysis of hereditary spherocytes, particularly those cells trapped in the unfavorable metabolic environment of the spleen, but it now is clear that this is incorrect, because the magnitude of the Na + flux does not correlate with the extent of hemolysis in HS.
The dehydration of HS red cells, as reflected in their high MCHC, is likely to be caused, at least in part, by the adverse environment of the spleen. This assumption has been made on finding that spherocytes from surgically removed spleens are the most dehydrated. The pathways causing HS red cell dehydration have not been clearly defined. One likely candidate is the Gárdos channel, a Ca 2+ -activated K + channel, which is known to contribute to erythrocyte dehydration in sickle cell anemia and thalassemia. One study showed that the Gárdos channel is functionally upregulated in a form of mouse HS caused by lack of erythrocyte protein 4.1R. When these mice were treated with clotrimazole, a known Gárdos channel blocker, hemolysis and anemia worsened, indicating that red cell dehydration protected the hereditary spherocytes, presumably by preserving their surface-to-volume ratio and therefore their flexibility in the face of surface loss.
Another possible cause of red cell dehydration in HS is increased K + ,Cl − cotransport, which is activated by acid pH. HS red cells, particularly from unsplenectomized subjects, have a low intracellular pH, reflecting the low pH of the splenic environment. The K + ,Cl − cotransport pathway is also activated by oxidation, which is likely to be caused by splenic macrophages.
Hyperactivity of the Na + ,K + pump, triggered by increased intracellular Na + , can dehydrate red cells directly, because three Na + ions are extruded in exchange for only two K + ions. The loss of monovalent cations is accompanied by water.
A fourth possible cause is the recently described mechanosensitive Piezo1 channel, which responds to membrane stretch and is thought to be involved in homeostatic adjustments to changes in volume. It is defective in hereditary xerocytosis, a disease characterized by red cell dehydration.
Whichever transport pathways cause HS red cells to lose K + and water, it is likely that the dehydration is a form of “adaption” ( Fig. 16-14 ) that prolongs the life of the HS red cell. How much it does so is unknown. Polymorphisms in cation channels like Piezo1 and calcium ATPase 4, in the globin loci, in major membrane skeletal proteins, and in genes like TAF3 , DNAJA4 , WDR61 and HBS1L-MYB are associated with variation in human MCHC. It will be interesting to see if these genes affect the severity of HS.
Most of the classic laboratory features of HS are those found in other forms of hemolytic anemia, such as reticulocytosis, erythroid hyperplasia of the bone marrow, indirect hyperbilirubinemia, and increased fecal urobilinogens. The plasma hemoglobin level is often normal, and the haptoglobin value is only variably reduced, because most of the hemoglobin that is released when HS red cells are destroyed is catabolized at the site of destruction, so-called extravascular hemolysis. The spherocytic morphology combined with laboratory tests that measure a loss of surface area, such as the osmotic fragility test or eosin-5′-maleimide–binding test, distinguish HS from most other forms of hemolytic anemia. Membrane protein analysis and molecular genetic testing are only performed in research laboratories, as these tests are expensive and not very useful clinically. This is likely to change in the near future, though, as the cost of whole-exome and even whole-genome sequencing is falling very rapidly.
Spherocytes (see Fig. 16-2 ) are the hallmark of HS. They are dense, round, and hyperchromic; lack central pallor; and have a decreased mean cell diameter. They are always evident in blood smears from patients with moderate or moderately severe HS but are obvious in only 25% to 35% of patients with mild HS. Hereditary spherocytes are technically misnamed, as they range in shape from thickened discocytes to spherostomatocytes when examined under the scanning electron microscope.
In patients with HS, spherocytes and microspherocytes are the predominant abnormal cells on the peripheral blood smear (other than polychromatophils). However, there are morphologic variations that occur with some of the specific membrane defects in HS (see Fig. 16-7 ). Patients with ankyrin defects, the most common subgroup, have typical smears (see Fig. 16-7, C ), but many patients with β-spectrin defects also have a subpopulation of acanthocytes or hyperchromic echinocytes (see Fig. 16-7, B ) and most patients with band 3–deficient HS also have a small number of button mushroom–shaped red cells on their smears (see Fig. 16-7, D ). In severe HS, caused by homozygous or compound heterozygous α-spectrin mutations or by severe ankyrin defects, misshapen spherocytes, spiculated red cells, and bizarre poikilocytes may be seen and even dominate the blood smear (see Fig. 16-7, A ). Red cell morphologic findings are variable in protein 4.2 deficiency. Some patients have typical spherocytosis, but acanthocytes, echinocytes, ovalostomatocytes, and other poikilocytes have been observed in others (see Fig. 16-7, E ). Finally, some patients with truncated β-spectrin chains have spherocytic elliptocytosis (see later).
In contrast to red cells in IgG-mediated autoimmune hemolytic anemias, where spherocytosis also dominates the blood picture, HS red cells lose most of their surface area in the bone marrow instead of in the circulation. Nucleated red cells are uncommon in blood smears, except in the most severe forms of HS. Howell-Jolly bodies are also uncommon before splenectomy (4% of patients) and suggest reticuloendothelial blockade.
Most patients have mild to moderate HS with mild anemia (hemoglobin level of 9 to 12 g/dL) or no anemia at all (so-called compensated hemolysis). In moderate to severe HS, the hemoglobin concentration ranges from 6 to 9 g/dL. In patients with the most severe disease, the hemoglobin concentration may drop to as low as 4 to 5 g/dL. The reticulocyte count is elevated without a comparable increase in the immature reticulocyte fraction.
The MCHC of HS red cells is increased due to relative cellular dehydration. The average MCHC exceeds the upper limit of normal (36%) in about one half of patients with HS, but all patients have some dehydrated cells. The Technicon H1 blood counter and its successors, which use the principle of flow cytometry (i.e., dual-angle laser light scattering), measure the mean corpuscular volume (MCV) and MCHC directly and provide histograms of both parameters. There is a right shift of the MCHC histogram in HS with an increased (>4%) population of hyperdense cells (MCHC > 41 g/dL) due to red cell dehydration, and a broadening of the distribution of MCVs, due to the mixture of microspherocytes and reticulocytes ( Fig. 16-17 ). This excess proportion of hyperdense cells is accurate enough to identify nearly all patients with HS and is one of the easiest and most accurate ways to diagnose the disease when one member of a family is already known to have HS.
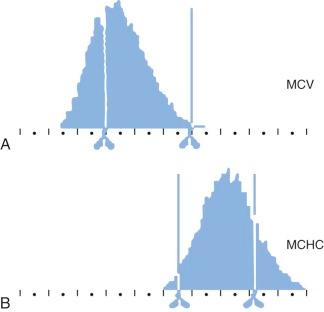
The MCV usually falls within the lower normal range in HS, but it is low in about 8% of adults and 16% of children, especially those with more severe HS. It is rare for the MCV to be less than 70 fL unless there is concomitant thalassemia or iron deficiency. However, the MCV is low relative to the age of the cells (reticulocytes have a high MCV) in all patients with HS, reflecting the dehydrated state of HS red cells. The reticulocyte MCV is also low, which contrasts with IgG-mediated autoimmune hemolytic anemia, where spherocytes are present but the reticulocyte indices are normal.
The RDW of HS erythrocytes is also very high, particularly in patients with more severe disease (see Table 16-3 ).
Various studies show that the MCHC or the percentage of hyperdense cells and the high RDW discriminate HS patients from normal individuals, though less so following splenectomy. An MCHC greater than 35 g/dL has a sensitivity of 70% and a specificity of 86% in diagnosing HS; an RDW greater than 14 has 85% sensitivity and 97% specificity. Combining the MCHC with the erythrocyte distribution width leads to a specificity approaching 100%. The RDW, MCHC, hemoglobin : MCHC ratio, and hemoglobin : RDW ratio all correlate with the severity of disease (see Table 16-3 ).
Osmotic fragility testing (see Fig. 16-16 ) is performed by suspending red cells in increasingly hypotonic buffered NaCl. In hypotonic solutions, normal erythrocytes increase their volume by swelling until they become spherical and burst, releasing hemoglobin into the supernatant. Cells that begin with a decreased surface-to-volume ratio, like spherocytes or stomatocytes, reach the spherical limit at a higher NaCl concentration than normal cells and are termed osmotically fragile. Freshly drawn red cells from approximately one fourth of individuals with HS will have a normal or nearly normal osmotic fragility curve, approximating the number of HS patients with few spherocytes seen on their peripheral blood smears. However, after incubation at 37°C for 24 hours, HS red cells lose membrane surface area more readily than normal red cells, because their membranes are leaky and unstable. This incubation accentuates the defect in HS erythrocytes and brings out the defect of osmotic fragility. When the spleen is present, a subpopulation of very fragile erythrocytes that have been conditioned by the spleen form the “tail” of the osmotic fragility curve. This tail disappears after splenectomy.
The osmotic fragility test detects as abnormal (i.e., osmotically sensitive) any red cells with a decreased surface-to-volume ratio. This includes red cells in hereditary stomatocytosis (HSt), hereditary pyropoikilocytosis (HPP), autoimmune hemolytic anemia, and, sometimes, congenital dyserythropoietic anemia, type II (CDAII). Red cells with a large surface-to-volume ratio, whether owing to an increase in membrane surface, like target cells, or to cellular dehydration, like hereditary xerocytes, have decreased osmotic fragility (i.e., are osmotically resistant).
The incubated osmotic fragility test is thought to be more sensitive than the unincubated osmotic fragility test, although this is not well proven. For a long time the osmotic fragility test, particularly the incubated test, was the gold standard test for diagnosing HS, but recent comparisons show that other tests are more sensitive and specific.
The glycerol lysis test was developed as an alternative to the osmotic fragility test and is widely used in Europe. In this test, red cells are incubated in glycerol-sodium phosphate–buffered hypotonic saline solution. The glycerol slows the entry of water into the cells, so that the time for the cells to lyse is prolonged and can be measured accurately. The glycerol lysis time is shortened for HS red cells because of the reduced surface-to-volume ratio.
The original glycerol lysis test lacked sensitivity and specificity, but the acidified glycerol lysis test (AGLT), and especially its incubated variant, have a high sensitivity (82% to 98%) and specificity (91% to 95%). Another variation achieves 100% sensitivity and specificity by changing the concentration of the sodium phosphate buffer from 5.3 to 9.3 mmol/L. This eliminates the need for preincubation. The modified test is easy to perform and requires very little blood, though accurate control of pH is critical. The simplicity of this test allows it to be used for rapid screening of a large number of blood samples.
Another adaptation of the original glycerol lysis test is the Pink test . Modifications of the test have made it adaptable to microsize samples (e.g., fingerstick blood samples). However, direct comparisons suggest that it is less specific than the osmotic fragility test or incubated AGLT.
The AGLT, like the osmotic fragility test, is often abnormal in patients with autoimmune hemolytic anemia, but it is also sometimes abnormal in a variety of other conditions, including CDAII, paroxysmal nocturnal hemoglobinuria, hereditary elliptocytosis, myelodysplastic syndromes, mechanical hemolysis, and chronic renal failure, and in pregnancy.
This method is based on the fact that HS red cells are particularly sensitive to cooling at 0°C in hypertonic solutions, which causes the cells to lyse and release hemoglobin. The molecular basis for this sensitivity is not understood, which is a disadvantage, but the test is reported to have a high sensitivity (90% to 100%) with one exception (79%), and to have good specificity (94% to 100%). It is very easy to perform, requires very little blood (a capillary blood sample is sufficient ), and is compatible with the common blood specimens anticoagulated with ethylenediaminetetraacetic acid (EDTA). These qualities make it a good test for large-scale screening. The hypertonic cryohemolysis test is not widely used but deserves more attention.
The autohemolysis of erythrocytes after 48 hours at 37°C is normally less than 5% in the absence of glucose or less than 1% in the presence of glucose. Autohemolysis of spherocytes increases to 15% to 45% in the absence of glucose. In HS, the degree of autohemolysis is reduced by the addition of glucose, whereas in red cell glycolytic disorders and acquired disorders such as immune-mediated anemias, the degree of autohemolysis is not reduced. Although this differentiation may be occasionally helpful, the autohemolysis test is time consuming and cumbersome; gives variable results, with a sensitivity of only 70% to 80% and a specificity of 80% to 90% ; and is rarely performed.
When red blood cells are treated with the fluorescent probe eosin-5′-maleimide (EMA), 80% of the fluorophore binds to Lys430 on the outside surface of band 3. The other 20% binds to three proteins in the Rh complex: the Rh blood group proteins, RhAG, and CD47. Thus the measurement of EMA fluorescence detects loss of membrane surface if that loss includes band 3 or the Rh complex. The EMA test is usually done with a flow cytometer so the fluorescence of individual cells is quantified. Because membrane vesicles containing band 3 are believed to be lost in HS due to spectrin and ankyrin deficiency (see Fig. 16-14 ) and CD47 and, perhaps, band 3 molecules are lost in HS due to protein 4.2 deficiency, the EMA test detects all the different forms of HS, not just HS due to primary band 3 deficiency. The test is both highly sensitive (92% to 100%) and highly specific (94% to 100%) for HS. The only exception is one study that reported only 70% specificity. The test requires very little blood, does not require preincubation, takes little time to perform, is not affected by storage of red cells for a week, and is probably the single best test for diagnosing HS at the present time. It is recommended by the General Haematology Task Force of the British Committee for Standards in Haematology, and when combined with the AGLT achieved 100% sensitivity in one recent comparison of tests for HS. In particular, the test is normal in autoimmune hemolytic anemias, which give false-positive results in the osmotic fragility test, AGLT, and cryohemolysis test. The EMA test is abnormal in disorders where membrane surface is decreased or band 3 is abnormal, such as HPP, SAO, cryohydrocytosis, congenital dyserythropoietic anemia, type II, and Rh null disease. However, these are all rare disorders and are fairly easy to distinguish by red cell morphologic findings and other criteria.
The ektacytometer is a laser diffraction viscometer, a device that measures how much red cells are deformed by shear stress. The device can be used to measure membrane mechanical strength by determining the shear stress needed to fragment isolated red cell membranes, or it can be used to assess red cell surface area, surface-to-volume ratio, and cellular hydration by measuring red cell deformation as a function of osmolarity at a constant shear stress. The latter method is termed osmotic gradient ektacytometry and is the gold standard method for diagnosing HS ( Fig. 16-18 ). One study demonstrated a 100% detection rate of HS using osmotic gradient ektacytometry and only a 66% detection rate of HS using the osmotic fragility test in the same population. Unfortunately, only a few laboratories in the world are equipped with an ektacytometer and the instrument is no longer commercially available.

Specialized tests are occasionally used to study patients with complicated disease or those for whom additional information is desired. Useful tests for these purposes include structural and functional studies of erythrocyte membrane proteins, such as protein quantitation, limited tryptic digestion of spectrin, membrane protein synthesis and assembly, and ion transport studies.
Membrane protein concentrations are usually assessed using SDS gels. Individual stained bands are quantified by densitometry or by eluting the dye from an excised band and measuring its concentration spectrophotometrically. This technique is satisfactory for detecting spectrin deficiency (expressed as a spectrin-to–band 3 ratio), although it is not as accurate as a radioimmunoassay or enzyme-linked immunoassay. With densitometry, spectrin and ankyrin deficiencies may be underestimated because they are normalized to band 3, which is partially lost along with membrane lipids as spherocytes circulate. As a result, the spectrin-to–band 3 ratio is lower after splenectomy. This is even more true for ankyrin, which is present in smaller amounts, migrates close to β-spectrin on SDS gels, and is elevated in reticulocytes. It is likely that patients with ankyrin defects, who have combined spectrin and ankyrin deficiency, are often misdiagnosed as having pure spectrin deficiency on SDS gels. SDS gels are satisfactory for detecting band 3 deficiency (elevated spectrin-to–band 3 ratio) or protein 4.2 deficiency, and they are useful in combination with antibody staining (Western blots) for detecting mutant proteins of altered size.
Moreover, SDS gels do not always identify the abnormal protein in HS, because missense mutations may cause functional defects rather than quantitative defects and because protein deficiencies may be as small as 10%. Therefore several other approaches have been described in an attempt to define specific molecular defects. In families with multiple affected members, linkage analysis has been performed to include or exclude the candidate genes. In addition, suspected proteins can be sequenced using direct nucleotide sequence analysis of polymerase chain reaction (PCR)–amplified complementary DNA (cDNA) or genomic DNA. Currently, no commercial laboratories identify the molecular defects in HS membrane proteins, and these analyses are performed only by a few research laboratories. However, with the rapidly falling costs of both whole-exome and whole-genome sequencing it is likely that DNA sequencing may become the most cost-effective way to diagnose HS (and many other inherited diseases) in the near future. Because HS membrane protein mutations are mostly restricted to specific families and do not predict the severity of the disease, in most cases it is not clinically important to know the exact molecular defect. More detailed studies, sometimes including DNA sequencing, should be considered in patients where the diagnosis is unclear after standard testing and in patients whose HS is more severe than in other family members, possibly due to coinheritance of another deleterious allele.
In general, HS is easily diagnosed if there are typical laboratory findings of spherocytosis, Coombs negative hemolysis, increased osmotic fragility, high MCHC, high RDW, and a positive family history. However, HS must be differentiated from other causes of spherocytosis, and there are several situations in which diagnosis can be difficult. Other causes of spherocytosis are listed in Table 16-1 .
In the neonatal period it may be challenging to differentiate HS from ABO incompatibility. Anemia, hyperbilirubinemia, circulating microspherocytes, and altered osmotic fragility are found in both conditions, and results of the direct Coombs test are sometimes negative in ABO incompatibility. However, in most infants with ABO incompatibility and significant hemolysis, anti-A (or anti-B) antibodies can be eluted from the red cells, and free anti-A (or anti-B) IgG antibodies can be detected in the infant's serum. The combination of HS and ABO incompatibility, though rare, is particularly severe.
Occasionally, patients with immunohemolytic anemias and spherocytosis also have so few antibody molecules attached to their red cells that results of the direct Coombs test are negative. In these rare cases, the differentiation of the disease from HS is possible only with the use of radioactive antiglobulin reagents, the so-called super–Coombs test.
Spherocytosis is also seen in Heinz body hemolytic anemias during an acute hemolytic crisis and occasionally in the steady state. However, in this situation, bite cells and/or blister cells and various dense, irregular cells are always present on the peripheral blood smear, and Heinz bodies can often be detected in red cells supravitally stained with methyl violet.
Diagnostic difficulties may also arise in HS patients who are seen during an aplastic crisis (see below). Early in the crisis, the acute nature of the symptoms may suggest an acquired process and the absence of reticulocytes may divert the physician from a diagnosis of hemolytic anemia. Later, as marrow function returns, physicians may occasionally be misled by the properties of the emerging young HS red cells, which are less spherocytic and osmotically fragile than usual.
In a preliminary report, Mariani et al noted that 13% of cases referred to their reference laboratory with a provisional diagnosis of HS were found to have CDAII, a disorder featuring underglycosylated band 3 and prominent binuclearity of late erythroblasts that is caused by defects in the secretory pathway gene SEC23B (see Chapter 7 ). Although CDAII patients typically display a variety of morphologic findings, a subset have prominent spherocytosis or elliptocytosis.
As discussed later in this chapter, patients with disorders of cation permeability, especially hereditary stomatocytosis (HSt) and hereditary xerocytosis (HX), may be misdiagnosed as having HS because they have a dominant hemolytic anemia with an abnormal osmotic fragility test. Care must be taken to exclude these disorders in any HS patient who is to be treated with splenectomy because there is a high risk of thromboembolic complications following splenectomy in patients with HSt and HX.
HS may be camouflaged by disorders that increase the surface-to-volume ratio of the red cells, such as iron deficiency, vitamin B 12 or folate deficiency, obstructive jaundice, β-thalassemia, or sickle cell–hemoglobin C disease (hemoglobin SC disease). In these difficult cases, additional tools, such as red cell membrane protein analysis, or exome or genome sequencing may be necessary to confirm the diagnosis of HS.
Iron deficiency corrects the abnormal shape, fragility, and high MCHC of hereditary spherocytes but does not improve their life span. Megaloblastic anemia can mask the morphologic characteristics of HS. Osmotic fragility is also improved. The masking effect is observed in both vitamin B 12 and folate deficiencies and is rapidly reversed after correction of the nutritional deficit.
When obstructive jaundice develops, spherocytosis and osmotic fragility transiently improve and hemolysis abates. This is due to the expansion of red cell membrane surface area that follows the increased uptake of cholesterol and phospholipids from the abnormal plasma lipoproteins that are released by the obstructed liver. In normal cells, this increase in surface area leads to the formation of target cells, but in spherocytes, it leads to the appearance of discocytes. For example, we have seen a 6-year-old child who developed jaundice and symptoms of biliary obstruction. She had a palpable spleen tip, evidence of mild compensated hemolysis (hemoglobin concentration of 14 g/dL; reticulocyte count of 3.3%), a normal peripheral blood smear, and normal results for an osmotic fragility test (fresh and incubated cells). Abdominal radiographic studies showed calcified stones in the gallbladder and common bile duct. After cholecystectomy and relief of the partial biliary obstruction, the child's hemolysis worsened (reticulocyte count of 10.8%) and she developed anemia (hemoglobin concentration of 10.2 g/dL), spherocytosis, and abnormal results on an incubated osmotic fragility test. She subsequently underwent splenectomy with clinical improvement.
The coexistence of HS and β-thalassemia trait has been described in a few case reports and has been reported to worsen, ameliorate, or have no effect on the clinical status of the patient. In a large French family with independently segregating HS and β-thalassemia trait, patients with both traits had signs of both diseases: small, hemoglobin A 2 -rich, osmotically fragile cells and some spherocytes on peripheral blood smears. However, the HS phenotype in these patients was less severe than that of family members with HS but without β-thalassemia trait.
Coinheritance of HS and hemoglobin SC disease may exacerbate the hemoglobinopathy. A 15-year-old male with both diseases was much more anemic than his siblings with hemoglobin SC disease and experienced five splenic sequestration crises. However, the two diseases may also disguise each other morphologically. Only a few spherocytes or target cells were evident on the boy's blood smear, and the surface-to-volume ratio of his red cells (and probably their osmotic fragility) was normal. This is likely due to the balancing effects of HS (loss of surface area) and hemoglobin SC (loss of cell volume) on red cells.
Sickle trait may also be worsened by HS. Yang and colleagues reported two patients with the combination who suffered multiple splenic sequestration crises. Splenic infarcts also occur. On the other hand, spontaneous regression of HS, presumably due to development of a hyposplenic state, has also been observed in two family members who had HS and sickle cell trait.
Most patients with HS have reasonably well-compensated hemolysis and are rarely symptomatic. These patients only seek medical attention when complications occur. Complications are generally related to chronic hemolysis and anemia.
The formation of bilirubinate gallstones is one of the most common complications of HS and is a major impetus for splenectomy in many patients. Instances of gallstones occurring in infancy have been reported, but most appear in adolescents and young adults. In a retrospective study of 152 consecutive patients with HS, conducted before the development of ultrasonography, gallstones were detected in only 5% of children younger than age 10 years who were adequately examined and in approximately 50% of HS patients between 10 and 30 years of age ( Fig. 16-19 ). More recent data are similar; about 15% of patients less than 18 years old develop gallstones. The increase in the incidence of gallstones after age 30 years parallels the incidence in the general population, which suggests that cholelithiasis owing to HS is primarily manifested in the second and third decades.

The incidence of gallstones in HS patients is related to the ability of the liver to metabolize increased amounts of bilirubin. A common polymorphism in the promoter of the uridine diphosphate-glucuronyl transferase gene ( UGT-1A ) causes decreased enzyme production and, in the homozygous form, causes Gilbert syndrome. Coinheritance of Gilbert syndrome with HS increases the risk for cholelithiasis. One retrospective study of 103 children with HS showed that the rate of gallstone formation was 2.2 times higher in those patients with a homozygous mutation in the UGT-1A gene than in those with a heterozygous mutation and 4.5 times higher than in healthy patients. HS patients with a homozygous mutation in the UGT-1A gene also develop gallstones at a younger age. Therefore analysis of this allele is useful in predicting the risk of cholelithiasis in HS.
Because the pigment stones typical of HS are easily detected by ultrasonography, all patients with HS should have ultrasound examinations about every 5 years if the spleen is intact and just prior to splenectomy. Unfortunately, longitudinal prospective studies using modern techniques (i.e., liver and biliary tree ultrasonography) are not available, making the true incidence and natural history of gallstones in this population unknown. One recent retrospective study showed that patients with HS (or other hemolytic anemias) were much more likely to develop symptomatic disease (i.e., pain, emesis, obstructive jaundice) than children with gallstones for other reasons. In that study, 68% of HS patients with gallstones had symptoms and all of them required cholecystectomy. This coincides with earlier reports. The most common complication was biliary obstruction, followed by acute cholecystitis. However, studies that include large numbers of patients with mild HS show a much lower incidence of symptomatic gallbladder disease.
More accurate, longitudinal studies are needed to determine the optimal treatment of patients with mild to moderate HS and cholelithiasis, particularly asymptomatic cholelithiasis that is detected on routine screening. Only about 15% to 20% of adults with asymptomatic stones (all types) develop symptoms over a prolonged period, but the retrospective data above suggest the complication rate may be much higher for bilirubinate stones. Surgery is indicated for recurrent symptoms or signs of biliary obstruction. It is unclear whether children who are undergoing cholecystectomy for symptomatic cholelithiasis should also have a splenectomy or partial splenectomy if it is not otherwise indicated (see later). Most authorities recommend it, arguing that the risk of stones persists, presumably in the remaining biliary radicals or cystic duct stump. However, cholelithiasis after cholecystectomy for typical gallbladder disease is rare unless a gallbladder remnant remains. Presumably the risk would be higher in someone with continuing hemolysis, but it is not clear how much higher.
If an HS patient requires a splenectomy, an ultrasound study of the gallbladder is indicated prior to surgery to assess the need for concomitant cholecystectomy. Cholecystotomy (removal of stones but not the gallbladder) is an option in children with silent stones because the risk of new stones forming is markedly reduced after splenectomy. For this reason, prophylactic cholecystectomy at the time of splenectomy solely to prevent the formation of gallstones is not indicated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here