Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
At no other time in the life of a patient is the physician confronted with as many diagnostic considerations in the interpretation of apparent disturbances of the erythrocyte as during the neonatal period. Neonatal erythrocytes are fundamentally different from those of older children and adults, varying in size, shape, globin composition, oxygen transport, membrane characteristics, cellular metabolism, and life span. Because of these differences, evaluation of a neonate with a suspected erythrocyte disorder mandates a thorough understanding of developmental erythropoiesis (reviewed in Chapter 1 ), appreciation of the differences between neonatal and adult red blood cells (RBCs), understanding of perinatal conditions influencing the fetus and newborn, and knowledge of inherited and acquired disorders of the erythrocyte that manifest in the neonate.
Early in embryogenesis, the size and volume of erythrocytes are significantly greater than that of neonatal and adult erythrocytes, with the mean corpuscular volume (MCV) ranging from 150 to 180 fl and cellular diameter ranging from 20 to 25 µ. Erythrocyte size and volume decrease throughout gestation to values of MCV between 108 to 118 fl and diameter between 8 to 10 µ at term ( Table 2-1 ). After birth, erythrocytes continue to decrease in size and volume, with values similar to those of adults by 1 year of age.
| Gestational Age (wk) | Hematocrit (%) * | Hemoglobin (g/dL) | MCV (fl) | Reticulocytes (%) |
|---|---|---|---|---|
| 18-20 † | 36 ± 3 | 11.5 ± 0.8 | 134 ± 9 | NR |
| 21-22 † | 38 ± 3 | 12.3 ± 0.9 | 130 ± 6 | NR |
| 22-23 † | 38 ± 1 | 12.4 ± 0.9 | 125 ± 1 | NR |
| 24-25 | 63 ± 4 | 19.4 ± 1.5 | 135 ± 0 | 6.0 ± 0.5 |
| 26-27 | 62 ± 8 | 19.0 ± 2.5 | 132 ± 14 | 9.6 ± 3.2 |
| 28-29 | 60 ± 7 | 19.3 ± 1.8 | 131 ± 14 | 7.5 ± 2.5 |
| 30-31 | 60 ± 8 | 19.1 ± 2.2 | 127 ± 13 | 5.8 ± 2.0 |
| 32-33 | 60 ± 8 | 18.5 ± 2.0 | 123 ± 16 | 5.0 ± 1.9 |
| 34-35 | 61 ± 7 | 19.6 ± 2.1 | 122 ± 10 | 3.9 ± 1.6 |
| 36-37 | 64 ± 7 | 19.2 ± 1.7 | 121 ± 12 | 4.2 ± 1.8 |
| Term | 61 ± 7 | 19.3 ± 2.2 | 119 ± 9 | 3.2 ± 1.4 |
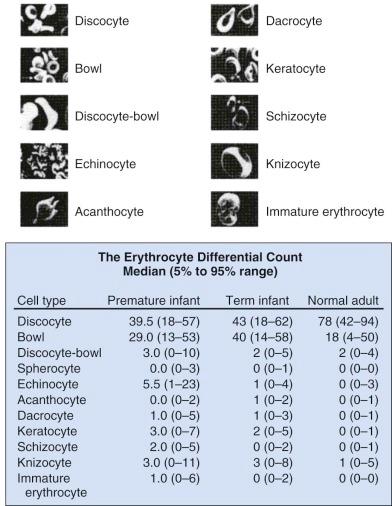
Marked variability exists in the shape of fetal and neonatal erythrocytes. Irregularly shaped erythrocytes are found in larger numbers on the peripheral blood smears of hematologically normal term neonates than on those of adults ( Fig. 2-1 ). Acanthocytes, target cells, and immature erythroid cells with various membrane projections are frequently seen. These variations have been attributed to poor or absent splenic function in neonates. Decreased splenic function is also associated with membrane surface “pits,” the site of formation of endocytic vacuoles, attributed to immaturity of the neonatal macrophage pitting process. Nearly half of erythrocytes from preterm infants and a quarter of erythrocytes from term infants have surface pits compared with approximately 2% of normal adult erythrocytes. Erythrocyte deformability is determined primarily by three factors: the surface/volume relationship of the cell, cytoplasmic viscosity, and intrinsic membrane rigidity. Cellular deformability influences several processes, including whole blood viscosity, which in turn affects peripheral vascular resistance and cardiac workload, flow in the peripheral circulation, and the life span of the erythrocyte. As erythrocytes age, neonatal cells lose more volume, have a higher mean corpuscular hemoglobin concentration, and become less deformable than adult RBCs, with decreased deformability leading to increased splenic sequestration. Together these properties lead to accelerated membrane loss and decreased erythrocyte life span.
Fetal and neonatal erythrocyte membranes demonstrate additional characteristics that differ from those of adult cells ( Box 2-1 ). Fetal and neonatal erythrocytes contain more phospholipid and cholesterol per cell, resulting in a larger surface to volume ratio and rendering them slightly more osmotically resistant. This observation has practical clinical importance, because when osmotic fragility testing is used in a neonate to diagnose hereditary spherocytosis (HS), a normative neonatal osmotic fragility curve should be used rather than the standard adult curve. The surface charge of neonatal erythrocytes is more negative than that of adult cells because of a higher sialic acid content. This increased negative charge contributes to the decreased erythrocyte sedimentation rate observed in newborns. Neonatal erythrocytes contain increased amounts of total and membrane-associated myosin compared with adult cells. Membrane acetylcholinesterase is decreased. Permeability to monovalent cations is increased, and less sodium-potassium adenosine triphosphatase activity is required for monovalent cation removal.
Less osmotically fragile
Differences in surface antigen expression
A and B antigens diminished
Lewis system diminished
I antigen very diminished (or absent): i antigen present
Possible decreased structural stability as a result of increased levels of unbound 2,3-diphosphoglycerate
Membranes less deformable
Red cells of different shapes with a greater predominance of stomatocytes than adult cells
Increased variability of the smear with multiple morphologic changes
Red cell membrane pits (as a result of decreased splenic function)
The expression of various antigens and surface receptors vary between neonatal and adult erythrocytes. The major RBC antigens—Rh, MN, Kell, and Duffy—are well developed in early intrauterine life. Other membrane-associated antigens—Lutheran, ABO, I, and XgA—are incompletely developed at birth. Other antigens, such as the Lewis antigen, are absent at birth. Neonatal erythrocytes express higher levels of several membrane-associated proteins, such as the insulin and insulinlike growth factor receptors, while expressing fewer digoxin receptors than adult erythrocytes.
The i antigen is a commonly used marker of fetal erythropoiesis. Differences in the glycans attached to the external surface of band 3, the anion exchanger, account for this developmental marker. In fetal cells the chain is arranged in an unbranched, linear fashion (i antigen with i reactivity), whereas the chain is arranged in a branched manner in adult cells (I antigen with I reactivity). The i antigen predominates in fetal and neonatal cells, gradually decreasing in a reciprocal relationship with the I antigen, which gradually increases, until approximately 18 months of age. Adult erythrocytes express minimal to no i antigen.
The synthesis and composition of embryonic, fetal, and neonatal globins are described in Chapter 19 . Depending on gestational age, approximately 70% to 90% of the hemoglobin in the erythrocyte of the fetus and neonate is fetal hemoglobin (HbF). HbF is more soluble in strong phosphate buffers and is resistant to acid denaturation. This latter characteristic is the biochemical basis of the Kleihauer-Betke test (discussed later).
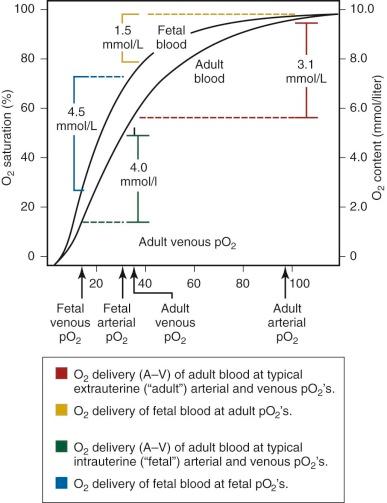
An important functional difference between HbF and adult hemoglobin (HbA) results from the ability of HbA, but not HbF, to interact with phosphorylated compounds, particularly adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (2,3-DPG). The interaction of HbA with 2,3-DPG leads to a marked decrease in oxygen affinity. In contrast, HbF has no or minimal interaction with these organic phosphates, leading to a relatively higher oxygen affinity of fetal blood, with a leftward shift of the oxygen dissociation curve ( Table 2-2 ; Fig. 2-2 ) compared with adult blood. In utero, this characteristic is of significant benefit to the fetus because it facilitates placental oxygen exchange from maternal blood to fetal erythrocytes.
| ARTERIAL OXYGEN SATURATION (%) † | ||||
|---|---|---|---|---|
| P 50 (mm Hg) | 95 | 90 | 85 | 80 |
| 20 ‡ | 10.0 | 13.0 | 20.0 | >25.0 |
| 23 | 7.3 | 9.0 | 11.0 | 15.5 |
| 25 | 6.2 | 7.3 | 8.8 | 11.0 |
| 27 § | 5.3 | 6.3 | 7.3 | 8.9 |
* Assumes a cardiac output of 250 mL/kg/min and an oxygen consumption of 6.5 mL/kg/min.
† The lower the arterial oxygen saturation, the less resting pulmonary function (e.g., bronchopulmonary dysplasia).
Fetal and neonatal erythrocytes have a number of metabolic differences compared with adult cells ( Box 2-2 ). Glucose and ATP production are increased, probably representing compensatory activity to maintain normal intracellular homeostasis by energy-dependent ion transport. Several glycolytic enzymes demonstrate increased activity, but this increase may reflect a larger percentage of younger RBCs in the neonatal circulation compared with the adult circulation rather than a characteristic specific to neonatal erythrocytes. Activities of other enzymes, including carbonic anhydrase and methemoglobin reductase, are reduced.
Glucose consumption increased
Galactose more completely used as substrate under normal circumstances and for methemoglobin reduction *
* Appears to be a unique characteristic of the newborn's erythrocytes and not merely a function of the presence of young red blood cells.
Decreased activity of sorbitol pathway *
Decreased triokinase activity *
Increased activity of hexokinase, phosphoglucose isomerase, * aldolase, glyceraldehyde-3-phosphate dehydrogenase, * phosphoglycerate kinase, * phosphoglycerate mutase, enolase, pyruvate kinase, lactate dehydrogenase, glucose-6-phosphate dehydrogenase, 6-phosphogluconic dehydrogenase, galactokinase, and galactose-1-phosphate uridyl-transferase
Decreased activity of phosphofructokinase *
Distribution of hexokinase isoenzymes differs from that of adults *
Increased activity of glutamic oxaloacetic transaminase and glutathione reductase
Decreased activity of NADP-dependent methemoglobin reductase catalase, * glutathione peroxidase, carbonic anhydrase, * adenylate kinase, * and glutathione synthetase *
Presence of alpha-glycerol-3-phosphate dehydrogenase
Increased potassium efflux and greater degrees of hemolysis during short periods of storage
More rapid assumption of altered morphologic forms during brief incubation *
Decreased ouabain-sensitive ATPase *
Decreased potassium influx *
Decreased permeability to glycerol and thiourea *
Decreased membrane filterability *
Increased sphingomyelin, decreased lecithin content of stromal phospholipids
Decreased content of linoleic acid *
Increase in lipid phosphorus and cholesterol per cell
Greater affinity for glucose *
Increased methemoglobin content *
Increased affinity of hemoglobin for oxygen *
Glutathione instability *
Increased tendency for Heinz body formation in the presence of oxidant compounds *
ATP, Adenosine triphosphate; ATPase, adenosine triphosphatase; NADP, nicotinamide adenine dinucleotide phosphate.
One important characteristic of the neonatal erythrocyte is increased susceptibility to oxidant-induced damage, leading to glutathione instability, Heinz body formation, and methemoglobinemia. Alterations in the pentose phosphate pathway, decreased glutathione peroxidase, diminished oxidant capacity of neonatal plasma, variations in superoxide dismutase levels, and decreased membrane sulfhydryl groups have all been implicated in the susceptibility of the neonatal erythrocyte to oxidative stress. This increased susceptibility to oxidative injury may lead to hemolysis when severe hypoxia and acidosis is present in the fetus and newborn.
The life span of the erythrocyte in a term neonate is between 60 and 90 days, with preterm infants demonstrating even shorter life spans, from 35 to 50 days. This shortened life span has been attributed to several properties specific to neonatal erythrocytes, including increased membrane deformability, more rapid loss of membrane surface area, increased susceptibility of membrane proteins and lipids to peroxidation, and a rapid decrease in intracellular enzyme activity and ATP content. When neonatal erythrocytes are transfused into adults, they exhibit a shortened life span that is attributed to the intrinsic differences between neonatal and adult erythrocytes. When adult erythrocytes are transfused into a neonate, they exhibit a normal life span.
A number of factors influence hematologic values in the neonate, including many of the developmental changes previously listed. Interpretation of laboratory data in the neonate requires consideration of a number of factors, because a value that is normal for one infant may not be normal for another infant. The gestational and chronologic age of the infant, the infant's sex, timing of cord clamping, and the site of blood sampling are important factors to be considered.
Hemoglobin concentration rises gradually throughout gestation and peaks shortly after birth. The mean hemoglobin at 10 weeks' gestation is 9 g/dL, increasing to 11 to 12 g/dL by approximately 23 weeks and to 13 to 14 g/dL by 30 weeks' gestation (see Table 2-1 ). Hemoglobin concentration is relatively stable the last 6 to 8 weeks of gestation, with a mean concentration 16 to 17 g/dL at term. Hemoglobin may increase by 1 to 2 g/dL at birth as a result of placental transfusion. Decreased plasma volume leads to a peak in hemoglobin between 2 to 6 hours of life, with levels stabilizing by 8 to 12 hours of age.
Erythropoiesis decreases at birth, leading to a gradual decrease in hemoglobin concentration during the next several weeks of life ( Table 2-3 ). The nadir of hemoglobin concentration is reached at 8 weeks of life, with levels of approximately 11 g/dL in healthy term infants before erythropoiesis increases and the hemoglobin rises. This process is called physiologic anemia (discussed later).
| Value | Cord Blood | Day 1 | Day 3 | Day 7 | Day 14 |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 16.8 | 18.4 | 17.8 | 17.0 | 16.8 |
| Hematocrit (%) | 53.0 | 58.0 | 55. | 54.0 | 52.0 |
| Red cells (mm 3 ) | 5.25 | 5.8 | 5.6 | 5.2 | 5.1 |
| MCV (fl) | 107 | 108 | 99 | 98.0 | 96.0 |
| MCH (pg) | 34 | 35 | 33 | 32.5 | 31.5 |
| MCHC (g/dL) | 31.7 | 32.5 | 33 | 33 | 33 |
| Reticulocytes | 3-7 | 3-7 | 1-3 | 0-1 | 0-1 |
| Nucleated RBCs (/mm 3 ) | 500 | 200 | 0-5 | 0 | 0 |
| Platelets (1000's mm 3 ) | 290 | 192 | 213 | 248 | 252 |
* During the first 2 weeks of life, a venous hemoglobin value below 13.0 g/dL or a capillary hemoglobin value below 14.5 g/dL should be regarded as anemic.
The timing of cord clamping significantly influences hemoglobin values in the newborn (discussed later). Around the time of birth, placental blood is rapidly transferred to the infant. Approximately a quarter of this transfusion occurs within 15 seconds of birth and half occurs by the end of the first minute after birth. Delayed cord clamping (DCC) typically increases the infant's blood volume by 30%, because the placental vessels contain between 75 to 125 mL of blood at birth. Holding the infant above the level of the placenta prevents placental transfusion and may even lead to neonatal transfusion into the placenta, resulting in neonatal anemia.
Significant variation in hemoglobin levels may exist, depending on the site of blood sampling. Hemoglobin levels from capillary blood samples typically are higher than those obtained from indwelling venous or arterial catheters. Capillary samples may lead to overestimation of the hemoglobin, particularly when the samples are obtained from a poorly perfused extremity. The largest differences between capillary and venous hematocrits exist in very preterm infants, particularly those with acidosis, hypotension, and anemia. In infants younger than 30 weeks' gestation, capillary hematocrit values are 20% higher than venous hematocrit values, compared with capillary values that are 12% higher than venous hematocrit levels at term. “Arterializing” a capillary sample by prewarming the extremity may improve the correlation between capillary and venous hematocrit levels. Finally, values of samples from similar sources, such as arterial, venous, and capillary samples, correlate independent of the site of sampling. For example, umbilical, radial, and femoral arterial hemoglobin levels show little variation from sample to sample.
Reticulocyte counts and the absolute reticulocyte count are elevated at birth, with values from 4% to 7% and 200,000 to 400,000/µL in term infants and 6% to 12% and 400,000 to 550,000/mL in preterm infants, respectively. In healthy infants, the reticulocyte count falls over the first few days of life to levels of 0 to 1% by day 4 of life (see Table 2-3 ). A reticulocyte count of 0 on day 1 of life indicates that there is erythrocyte under production (discussed later).
The average volume of circulating erythrocytes is estimated by the MCV. During pregnancy an inverse relationship exists between gestational age and MCV, with MCV values decreasing throughout gestation to 108 to 118 fl at term (see Table 2-1 ). When an MCV less than 95 fl is observed in a term neonate, α-thalassemia trait or iron deficiency should be considered.
Similar to the MCV, the mean corpuscular hemoglobin is higher in preterm and term neonates, ranging from 33 to 41 pg/cell compared with 27 to 31 pg/cell in adults.
The mean corpuscular hemoglobin concentration does not vary between neonates and adults. Although neonatal erythrocytes are larger and contain more hemoglobin, the hemoglobin in the erythrocyte is not more concentrated.
As previously noted, significant variations in neonatal erythrocyte morphology are commonly seen on peripheral blood smears, particularly in preterm infants, without intrinsic abnormalities of the erythrocyte. These variations include discocytes, bowls, echinocytes, and keratocytes (see Fig. 2-1 ).
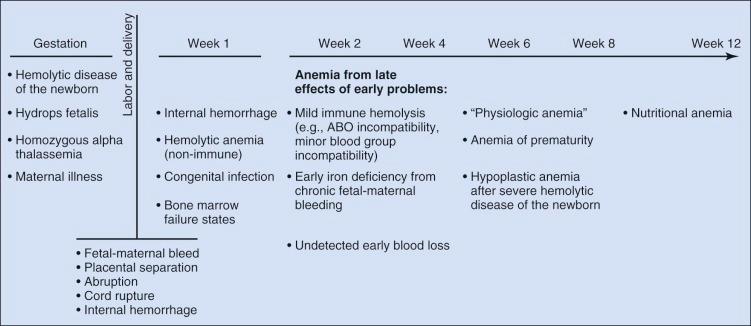
Determining the cause of neonatal anemia can present a significant challenge. The differential diagnosis is extensive and includes not only many of the causes of anemia seen in older patients but also many causes unique to the fetus and newborn that are associated with pregnancy, labor, and delivery ( Fig. 2-3 ). Knowledge of these disorders and an understanding of the associated pathophysiology provide the appropriate context for the evaluation, diagnosis, and treatment of neonatal anemia. This chapter reviews anemia by etiology using the broad classifications of hemorrhage, hemolysis, and inadequate erythrocyte production.
Fetal blood loss prior to birth is usually via the transplacental route, including fetomaternal hemorrhage (FMH), placental abruption, placenta previa, vasa previa, and twin-twin transfusion ( Box 2-3 ). Fetal blood loss, for instance due to intracranial hemorrhage in a thrombocytopenic infant with platelet isoimmunization or in a fetus after traumatic amniocentesis or cordocentesis, is less common.
Transplacental Hemorrhage
Fetomaternal hemorrhage
Abruptio placentae
Placenta previa
Vasa previa
Velamentous insertion of umbilical cord
Twin-twin transfusion
Traumatic Fetal Hemorrhage
Maternal trauma
Amniocentesis
Cordocentesis
Placental Lesions
Chorioangioma
Hematoma
Hemangioma
Choriocarcinoma
Other Causes of Fetal Hemorrhage
Intracranial bleeding
Gastrointestinal bleeding
Placenta
Abnormalities of placentation previously listed
Surgical laceration, such as incision of the anterior placenta or cord at delivery
Umbilical Vessels
Rupture of normal cord
Rupture of abnormal cord
Aneurysm
Varix
Cyst
Funisitis with weakened vessels
Short cord
Cord entrapment, for example, by forceps
Tight nuchal cord
Occult cord prolapse
Intrapartum Neonatal Trauma
Cranial hemorrhage including subgaleal, subarachnoid, and intraventricular hemorrhage
Cephalohematoma
Splenic rupture
Adrenal hemorrhage
Liver laceration
Retroperitoneal hemorrhage
Intracranial bleeding in the VLBW infant
Gastric or intestinal ulceration
Hemorrhage from vascular malformation
Coagulopathy
Iatrogenic causes
Phlebotomy
Tracheal mucosal tear during endotracheal intubation
Posterior pharyngeal tears during laryngoscopy or OGT placement
Vessel perforation during umbilical catheterization
Intercostal vessel laceration during thoracostomy tube placement
Gastric rupture from overdistention or OGT placement
Pulmonary hemorrhage during ventilation
Bladder mucosal tear during catheterization
Surgical wounds
OGT, Orogastric tube; VLBW, very low birth weight.
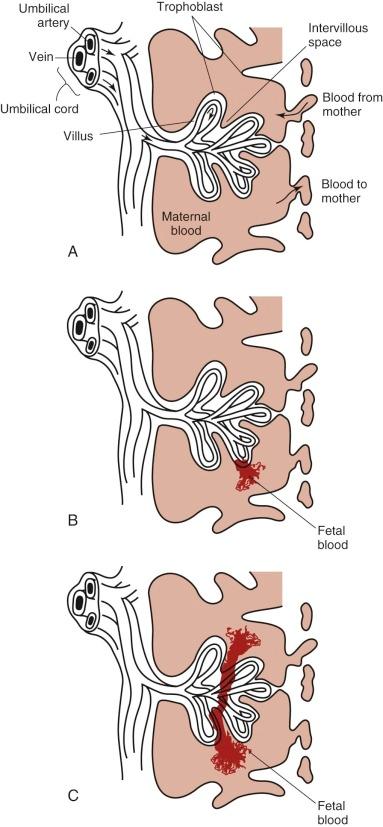
FMH is the passage of fetal blood into the maternal circulation before or during delivery. Antenatal FMH is associated with variable effects on the fetus and neonate, ranging from none to neurologic injury, hydrops fetalis, and in utero demise of the fetus or, in the neonate, congestive heart failure (CHF), hypovolemic shock, persistent pulmonary hypertension, neurologic injury, and death. The structure of the fetal-placental unit sets up a large pressure gradient from the umbilical artery to the intervillous space. The maternal intervillous space is separated from the pulsatile fetal circulation by a thin, two-cell membrane, with the constant possibility for rupture of the trophoblastic-endothelial juncture and contamination of the maternal intervillous space with fetal blood ( Fig. 2-4, A ). Given this very fragile mechanical relationship, it is surprising that significant FMH does not occur more frequently. Most spontaneous FMH occurs during the third trimester ( Fig. 2-4, B ), most commonly during labor and delivery, although hemorrhage has been reported as early as 3 to 4 months' gestation ( Fig. 2-4, C ). Approximately 99% of deliveries are associated with FMH of less than 15 mL. However, 3 in 1000 deliveries are associated with FMH greater than or equal to 30 mL, and 1 in 2000 pregnancies is associated with an FMH greater than or equal to 100 mL.
Fetal RBCs can be detected in the maternal circulation in a number of ways. The most frequently used test is microscopic demonstration of fetal RBCs in the maternal circulation with use of the Kleihauer-Betke staining procedure. With this method, HbA is denatured and eluted from smears of adult RBCs with an acid solution, leaving behind largely empty RBC membranes. Because HbF resists denaturation and elution, fetal cells stain darkly. Simple observation of the acid-treated maternal blood smear can provide a qualitative picture. The actual amount of fetal blood lost is estimated using the following formula: 2400 × the ratio of fetal to maternal cells = 1 mL of fetal blood. Using this equation, it has been shown that most deliveries result in less than 3 mL of fetal blood in the maternal circulation, and only 0.3% have a fetomaternal transfusion of 10 mL or more. However, the volume of fetal blood in the maternal circulation does not reflect the timing or acuity of the hemorrhage. Anti-HbF–based flow cytometry is an alternative method for detecting and quantifying cells that contain HbF in the maternal circulation. Conditions that elevate maternal HbF production (e.g., thalassemia minor, sickle cell disease, hereditary persistence of HbF, and pregnancy-induced maternal HbF production) confound these analyses. ABO blood group incompatibility can complicate interpretation of testing for FMH because removal of fetal cells from the maternal circulation by maternal anti-A or anti-B antibodies may lead to a false-negative result.
Placental abruption—that is, separation of the placenta from the uterine wall—occurs in approximately 1% of pregnancies. Abruption is more common in pregnancies complicated by chronic or pregnancy-induced hypertension. The classic triad of placental abruption is vaginal bleeding, uterine contractions, and an irritable, tender uterus. If bleeding occurs in a retroplacental location, recognition and treatment may be delayed. Fetal hypoxemia may develop as a result of decreased placenta surface area and may be worsened by fetal hemorrhage accompanying the maternal bleeding. If fetal hemorrhage is significant, severe anemia, hypovolemia, and heart failure may rapidly develop, leading to in utero fetal demise. Placental abruption is typically an acute event. However, fetal blood loss may occur gradually when the area of placental separation is small with a low-grade, chronic hemorrhage. At birth, these neonates exhibit anemia with compensation of varying degree, marked reticulocytosis, and prominent normoblastosis. In severe cases, iron deficiency may occur.
Placenta previa—that is, implantation of the placenta in a low-lying position in advance of the fetal presenting part, usually overlying part or all of the cervical os—occurs in approximately 1 in 250 births. The classic presentation is acute onset of painless vaginal bleeding, with a peak incidence around 34 weeks' gestation. When placenta previa persists to the third trimester, a cesarean section is indicated. Fetal blood loss of varying amounts occurs in approximately 50% of cases. Signs of fetal anemia include intrauterine growth retardation, abnormal biophysical testing, and tachycardia. Vaginal bleeding may be intermittent and recurrent or massive and acute. In the former situation, the neonate presents with a partially or completely compensated anemia and marked reticulocytosis. In the latter situation, the neonate may present with acute hypovolemic shock with risk of death. Up to 10% of neonates born after the occurrence of placenta previa exhibit significant anemia, making it the most common placental anomaly that causes neonatal anemia.
Vasa previa occurs when fetal vessels exiting the placental end of the umbilical cord travel across the fetal membranes and then return to the fetal placental surface. If the fetal vessels are near the cervical os, they may tear when membranes rupture or during labor, when progressive cervical dilatation occurs. In both situations, laceration of the fetal vessels may lead to rapid exsanguination and death of the fetus. Acute onset of vaginal bleeding (usually at the initiation of labor or during the progression of labor) with acute fetal tachycardia is a classic presentation of vasa previa. Prompt recognition of this condition, delivery, and resuscitation of the anemic infant are indicated to prevent peripartum death.
In cases of velamentous insertion of the cord, which occurs in approximately 1% of pregnancies, the umbilical vessels are unprotected and may tear spontaneously or during labor. Rarely the unprotected umbilical vessel crosses the cervical os, combining a velamentous insertion with vasa previa, with a high potential for tearing when the membranes rupture. Velamentous insertion is more common in twins and with low-lying placentas. The presentation of painless vaginal bleeding followed by signs of fetal distress is similar to vasa previa. The incidence of fetal loss is 1% to 2%.
Monochorionic twin pregnancies are at high risk for an adverse outcome because of vascular anastomoses that create shared fetal circulations. This situation may lead to complications, including twin-twin transfusion syndrome (TTTS), the twin anemia polycythemia sequence, the twin reversed arterial perfusion sequence, and monoamniotic twinning. TTTS complicates 8% to 15% of twin gestations with monochorionic diamniotic placentation, with the unbalanced transfer of blood from one fetus to the other via vascular anastomoses in the placenta. Classically, the donor twin is small and anemic and the recipient is large and plethoric. Fetal demise may occur from exsanguination of the donor or circulatory overload in the recipient. This condition may be dynamic with varying consequences to the twin fetuses, because the vascular anastomoses may vary in both size and number, as well as proceed in opposite directions. Antenatal factors that may predict outcome include the presence of hydrops fetalis and gestational age. Perinatal therapies have included serial amnioreduction, fetoscopic laser occlusion of communicating placental anastomoses, maternal digoxin therapy, and selective feticide.
At birth, both infants may be critically ill. The anemic donor twin may experience CHF and respiratory failure, whereas the plethoric recipient twin may require treatment for polycythemia hyperviscosity syndrome (PHS) and its complications, including thrombosis, hyperbilirubinemia, and, rarely, disseminated intravascular coagulation (DIC). In the classic presentation of TTTS in which the donor twin has been chronically hemorrhaging into the recipient, the donor anemic twin has marked reticulocytosis and normoblastosis, whereas the plethoric, polycythemic infant weighs greater than or equal to 20% more with a hemoglobin greater than or equal to 5 g/dL higher than its sibling. Acute transfusions, which typically occur closer to the time of delivery, do not lead to significant differences in neonatal weights, and there is an equal chance of either twin being the donor. Despite advances in pregnancy management, mortality in fetuses with TTTS remains high. The risk of long-term neurodevelopmental morbidity in survivors is significant.
Fetal bleeding has been described after multiple trauma and after blunt abdominal trauma to the pregnant mother. It has also been described after traumatic amniocentesis or cordocentesis and after external cephalic version.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here