Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lung cancers commonly have structural chromosome aberrations and aneuploidy, with many of them associated with carcinogenesis.
Gene amplification is a common mechanism of oncogenic activation in nonsmall cell lung cancer (NSCLC) involving genes such as MYC , EGFR , ERBB 2, MET , PIK3CA, and FGFR1. Gene amplification is also associated with resistance to drugs, for instance, to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors when the T790M EGFR allele or the MET gene is amplified.
More recently, genes such as ALK , ROS1 , RET , and NTRK1 were found to be activated in NSCLC by fusions with gene partners subjected to constitutive transcription or carrying specific domains inducing phosphorylation.
Novel therapeutics have been developed to target those specific molecular drivers, and several drugs have succeeded in substantially improving survival and quality of life of patients carrying such molecular changes.
NSCLC tumor profiling is achieved by numerous technologies focusing on different levels such as DNA (e.g., sequencing, fluorescence in situ hybridization [FISH]), RNA (e.g., reverse transcription-polymerase chain reaction), and protein (e.g., immunohistochemistry [IHC]). Technologies using in situ (FISH, IHC) and extraction (PCR based, sequencing) platforms have distinct advantages and limitations.
Single tests and test panels are available, with the latter being most effective due to the low incidence of the rearrangements in the overall NSCLC population, the lower cost per gene tested, and the scarcity of tumor tissue in patients with advanced stage disease. Molecular drivers have been detected more commonly in lung adenocarcinomas than in squamous cell carcinomas (SCCs) but a strong effort is ongoing to better define potential therapeutic targets in SCC. Very little is known regarding markers for therapy in small cell lung cancer.
Lung cancer is a group of diseases displaying a high level of genomic instability and complex molecular changes. This chapter reviews the impact of molecular events detectable by cytogenetic techniques, such as instability, at the DNA and chromosome levels in lung cancer patients. In addition, the chapter also examines two of the major molecular mechanisms leading to the nonviral activation of oncogenes: gene amplification and gene fusion. Overexpression of proteins in conditions in which they are normally absent is commonly driven by an increase in gene copy number or the release of a specific gene from the ligand binding control, thus having its active domain under the control of the promoter or of the active domain of a constitutively activated gene. Amplification of genes in lung cancer was discovered in the mid-1980s for v-myc avian myelocytomatosis viral oncogene homolog ( MYC ) and Kirsten rat sarcoma viral oncogene homolog ( KRAS ). Conversely, fusion proteins are a much newer phenomenon in lung cancer. Despite being common and well-known in leukemia and lymphoma as causal factors and targets for therapy, gene fusions were not described in lung cancer research before the start of the 21st century. However, with the progress of genomic technology, the number of activated gene fusions discovered mainly in NSCLC has increased rapidly, as will be detailed here.
Accumulation of multiple genetic abnormalities is known to be associated with lung cancer initiation and progression. Genetic instability, which may cause these abnormalities, is a general term that refers to both chromosomal instability (CIN) and microsatellite instability (MSI). Instability involving whole or partial regions of chromosomes (CIN) includes deletion, duplication, insertion, and translocation. CIN may induce loss of heterozygosity (LOH) of tumor suppressor genes or DNA repair genes when deletions occur, and amplification of oncogenes by multiple duplications of focal chromosome regions. Consequently, compelling evidence has supported the role of CIN in the pathogenesis of lung cancer. In addition to alterations at the chromosomal level, instability at the nucleotide level, frequently referred to as MSI, is usually connected to mismatch repair (MMR) defects. MSI may cause missense mutations that facilitate the inactivation of tumor suppressor genes, such as p53 , which may contribute to the development and progression of lung cancer. Both phenomena—CIN and MSI—obviously contribute to the phenotype instability and versatility of cancer cells. Therefore an understanding of the molecular mechanisms leading to genetic instability holds promise for the development of novel therapeutic strategies in lung cancer.
Microsatellites, also known as simple sequence repeats, are tandem repeats of short (fewer than 10 bp) DNA sequences, which are useful markers for genetic mapping and LOH of defined chromosomal loci. The most common microsatellite in humans is a dinucleotide repeat of CA, which occurs tens of thousands of times across the genome. Although the length of these microsatellites is highly variable from person to person, each individual has microsatellites of a set length. MSI, a hallmark of genetic instability, generally occurs because of abnormalities of the MMR genes, such as hMSH2 and hMLH1 , impairing the correction of errors that spontaneously occur during DNA replication. The loss of MMR function renders tumor cells susceptible to the acquisition of somatic mutations throughout the genome, and microsatellites are particularly susceptible to mutations in the absence of MMR. MSI was initially identified in colorectal cancer and was immediately clinically significant because of its association with hereditary nonpolyposis colon cancer (HNPCC). In HNPCC, the MSI of MMR genes due to germline alterations is an essential molecular basis of its development. By contrast, in lung cancer, CIN plays a more important role in carcinogenesis, as homozygous and heterozygous deletions of certain chromosomal loci or amplification of oncogenes frequently occur, as will be described.
There are conflicting data on the relevance of MSI in lung cancer. The frequency of MSI has been reported to range from 0% to 69% in NSCLC and from 0% to 76% in small cell lung cancer (SCLC). Interestingly, several studies of NSCLC have demonstrated a higher frequency of MSI in tetranucleotide-repeating regions than in traditional mononucleotide-repeating or dinucleotide-repeating regions, and the term “elevated microsatellite alterations at selected tetranucleotide” (EMAST) has been proposed to designate the phenomenon. Furthermore, EMAST was reported to be associated with SCC with lymph node metastasis. The molecular mechanisms leading to EMAST, distinct from traditional MSI, were not associated with defects in MMR, but it was suggested that p53 alterations may be involved.
Most cancer cells possess an abnormal number of chromosomes, often in the triploid or tetraploid range. In addition to the altered number of chromosomes, cancer cells commonly have structural chromosome aberrations, such as inversions, deletions, duplications, and translocations. Aneuploidy, defined as numerical and structural abnormalities of chromosomes, commonly results from CIN. Aneuploidy and CIN can mediate the evolution of cancer cell populations under selection pressure and are associated with poor prognosis and distinctive histopathologic features in many tumors. CIN plays an important role in lung carcinogenesis by accelerating homozygous and heterozygous deletions of tumor suppressor genes and effectively amplifying oncogenes. Therefore a better understanding of the causes and effects of aneuploidy and CIN may lead to new therapeutic venues for solid malignancies, including lung cancer.
Early studies have shown that lung cancer frequently exhibits marked LOH as a result of CIN when genome-wide or specific regions such as chromosomes 12p, 14q, and 17q were investigated. Moreover, LOH at 3p loci containing genes associated with antioxidant defenses (e.g., glutathione peroxidase I) is not only associated with the development of lung cancer but also with higher responsiveness to DNA damaging agents (e.g., radiation).
Multiple mechanisms during cell cycle progression have been implicated in the advent of CIN and aneuploidy in lung cancer. These include failure at the mitotic checkpoint, mutations and amplifications in the kinetochore (protein structure on chromatids where the spindle fibers attach during cell division) and centrosome components, and mutations in DNA repair genes. The mitotic checkpoint, also called the spindle assembly checkpoint, is activated when the kinetochore is not attached to the spindle, lacks microtubules, or has poor or inadequate tension, thereby deregulating metaphase–anaphase progression. Loss-of-function mutations, or reduced gene expression of the mitotic checkpoint genes (mitotic arrest deficient-like 1 [ MAD1 / MAD2 ] and mitotic checkpoint serine/threonine kinase [ BUB1 , BUBR1 ]), lead to chromosomal missegregation and contribute to aneuploidy. Given that loss of mutations in mitotic checkpoint genes were rarely detected in lung cancers (less than 3%) in the recent comprehensive genome-wide sequencing data collection, development of lung cancer and CIN is more closely related to their dysfunction due to phosphorylation or cytoplasmic location. Interestingly, a study in MAD2 +/– p53 +/– and MAD1 +/– MAD2 +/– p53 +/– mice suggested a cooperative role of MAD1 / MAD2 and p53 genes in generating increased aneuploidy and tumorigenesis. Furthermore, the mitotic checkpoint has also been linked to DNA-damage response, and a defective mitotic checkpoint confers cancer cells’ resistance to certain DNA-damaging anticancer drugs. The centrosomes are thought to maintain genomic stability through the establishment of bipolar spindles during cell division, ensuring equal segregation of replicated chromosomes to two daughter cells. STK15, encoding aurora kinase A (AURKA), is amplified and overexpressed in diverse types of human tumors, leading to centrosome amplification, CIN, and tumorigenesis. Aurora kinases are serine/threonine kinases that function as key regulators of the mitosis process. Their dysfunction interferes with cell cycle checkpoints and allows genetically aberrant cells to enter mitosis and undergo cell division. Overexpression of aurora kinases can lead to aneuploidy, resulting in the failure to maintain chromosomal integrity.
In one study, AURKA was highly overexpressed in 50% of NSCLC, and its overexpression was significantly upregulated in tumor samples compared with matched lung tissue ( p < 0.01), suggesting a role as a tumor marker. Moreover, AURKA was principally upregulated in moderately and poorly differentiated lung cancers, as well as in SCCs and adenocarcinomas, compared with the noninvasive bronchioloalveolar subtype. The frequency of AURKA amplification in NSCLC ranges from 1% to 6% and seems to be more common in lung adenocarcinomas than in lung SCCs. In comparison, aurora kinase B (AURKB) plays a less clear role in tumorigenesis. However, many studies now support an association between AURKB and malignant transformation, with the involvement of additional factors. Although AURKB overexpression alone did not transform rodent fibroblast cells, increased kinase activity did facilitate Harvey rat sarcoma viral oncogene homolog ( HRAS )-induced transformation, which led to the production of aneuploid cells. In an IHC analysis of 160 NSCLC samples, 78% of tumors were found to overexpress AURKB, and its overexpression was also associated with adverse tumor features and poor prognosis in lung adenocarcinomas. Contrary to AURKA, the overexpression of which is associated with gene amplification, AURKB overexpression was associated with aberrant transcriptional regulation in primary lung carcinoma.
Therefore overexpression and amplification of aurora kinases have been associated with neoplastic transformation, serving as attractive targets for cancer therapy. A growing number of inhibitors of aurora kinases have been developed and, at the time of publication, were being evaluated in clinical trials to assess the therapeutic potential of aurora-based targeted therapy. These inhibitors include AMG900 (Amgen, Thousand Oaks, CA, USA), AT9283 (Astex Therapeutics, Dublin, CA, USA), AZD1152 (Astra Zeneca, London, UK), and PF03814735 and BI811283 (Boehringer-Ingelheim, Ridgefield, CT, USA). Some of these drugs have selective activity against one aurora kinase subtype, whereas others exhibit pan-inhibitory effects.
In addition to mitotic checkpoint proteins and centrosome components, CIN may be caused by defects in the DNA double-strand break repair genes—ataxia telangiectasia mutated ( ATM ), BRCA1 , BRCA2 , x-ray repair complementing defective repair in Chinese hamster cells (double-strand-break rejoining; XRCC5 )—or DNA-damage response. Interestingly, the chromosomal regions at 2q33–35 and 13q12.3, which included loci encoding the XRCC5 and BRCA2 genes, showed a high frequency of LOH in NSCLC. More recently, it was reported that low messenger RNA and protein expressions in BRCA1 / BRCA2 and XRCC5 genes occur in lung adenocarcinoma and SCC and that promoter hypermethylation is the predominant mechanism in deregulation of these genes. Given that BRCA1/BRCA2 proteins are central to p53-dependent elimination of tetraploid or aneuploid (often preceded by tetraploid state) cells, it is not surprising that these proteins are frequently inactivated or downregulated in NSCLC, synergizing with p53 inactivation to establish an atmosphere of tolerance for a nondiploid state. Although unrepaired or incorrectly repaired DNA lesions may give rise to cancer-initiating mutations, one way to efficiently tackle cancer is to take advantage of such biologic differences between cancer and normal cells and exploit the defects of tumor-associated DNA-damage response in smart therapeutic strategies.
Gene amplification refers to the expansion of gene copy number in a restricted region of a chromosome arm. It is prevalent in some tumors and is often associated with overexpression of the amplified gene, causing cancer cells to grow or become resistant to anticancer drugs. Often, although not necessarily, gene amplification is seen as karyotypic abnormalities including the extrachromosomal, acentric structure known as double minutes and the homogeneously staining regions ( Fig. 10.1 ). High-throughput genomic analyses of thousands of cancer specimens showed that the majority (approximately 75%) of the gene amplifications were focal in nature (50 kb to 300 kb) and targeted primarily oncogenes, encoding signaling proteins crucial for cellular proliferation and survival. This finding strongly supports the notion that gene amplification promotes tumor formation, tumor maintenance, and drug resistance. The preponderance of focal amplification targeting oncogenes contrasts sharply with large genomic deletions, which are mostly passenger mutations with only a few exceptions, such as cyclin-dependent kinase inhibitor 2A/B ( CDKN2A/B ), retinoblastoma 1 ( RB1 ), and FAT atypical cadherin 1 ( FAT1 ) tumor suppressor genes. The contributing factors to CIN, including common chromosomal fragile sites, errors in DNA replication, and telomere dysfunction, are causally linked to amplification and large genomic deletions.
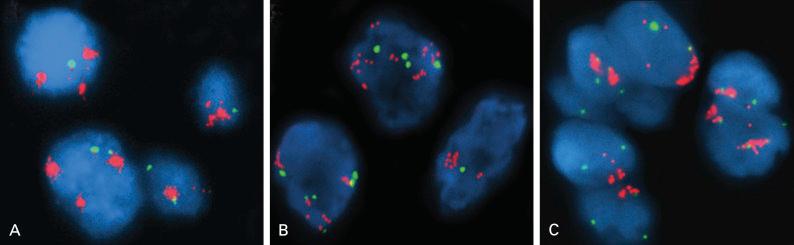
Gene amplification is a common mechanism of oncogenic activation in NSCLC and, on a whole genome scale, has a strong effect on the level of protein expression. Given the potential role of gene amplification in lung tumorigenesis and tumor progression, this event is commonly associated with unique clinicopathologic features and aggressive tumor behavior. Not surprisingly, amplifications of the EGFR, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 ( ERBB2 ), met proto-oncogene ( MET ), MYC , and fibroblast growth factor receptor 1 ( FGFR1 ) genes have been reported to be significantly associated with poor prognosis in NSCLC (as will be discussed). In addition, amplification has been identified as a mechanism of resistance to therapy.
Because the tumor can become dependent on overexpression of the oncogenes for its survival and proliferation, amplifications of oncogenes usually define unique subsets of lung cancer and support their use as therapeutic targets. As best illustrated by the example of the success of trastuzumab in ERBB2-amplified breast cancer, amplification of specific oncogenes may provide diagnostic utility based on their impact on therapeutic response and patient outcome. In lung cancer, however, although the results of several studies have clearly demonstrated the clinical usefulness of testing for EGFR mutations and anaplastic lymphoma kinase (ALK) fusions to guide treatment and improve patient outcomes, selection of therapy based on gene amplification is yet to be approved.
This chapter focuses on the most relevant examples of gene amplifications that have shown diagnostic usefulness because of prognostic and predictive values.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here