Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intraoperative radiotherapy (IORT) is the delivery of radiation during surgery ( ). The rationale is straightforward: escalating radiation dose may enhance local tumor control. In many clinical situations, the dose delivered by external-beam radiation techniques is limited by tolerance of surrounding normal tissues. To overcome this, intraoperative irradiation has been employed as a technique facilitating tumor dose escalation. In the era of precision medicine, IORT is a technical component of radiation dose delivery that optimizes risk-adapted strategies in local cancer therapy, offering a highly individualized approach to improve the therapeutic index. Recent Phase III trials have explored IORT, testing noninferiority or equivalence in patients with early breast cancer.
This chapter reviews the rationale and treatment strategies of intraoperative electron radiotherapy (IOERT), intraoperative high-dose-rate brachytherapy (HDR-IORT), and orthovoltage techniques with surgery. These strategies frequently integrate external-beam radiotherapy (EBRT) and chemotherapy.
The use of IORT was first employed almost 100 years ago. The contemporary approach to IORT was initiated in the 1960s by Abe et al. in Japan. These investigators advocated resection (where possible) with large, single-dose radiotherapy (25-40 Gy using cobalt-60). In the mid- to late 1970s, many institutions in the United States adopted IORT as a treatment approach, primarily as a radiation boost component, using linear accelerator (LINAC)-based electron treatment in the operating room, including Howard University, Massachusetts General Hospital (MGH), the Mayo Clinic, and the National Cancer Institute (NCI). In recent years, National Comprehensive Cancer Network guidelines have included IORT as a treatment option in soft-tissue sarcomas, resected pancreatic cancer, oligo-recurrent intraabdominal disease, T4 rectal cancer, and accelerated partial breast irradiation breast cancer candidates. At present, there are about 90 centers in at least 16 countries worldwide with active IORT programs.
IORT has the potential to improve local control and the therapeutic ratio in many tumor sites by reducing the volume of the irradiation “boost” field by direct tumor/tumor bed visualization and conformal treatment, exclusion of part or all of dose-limiting normal structures by operative mobilization, direct shielding, or varying electron beam energy, and allowing the delivery of high-dose irradiation by the preceding methods.
Although early investigators studied this modality separately in the treatment of resected and unresectable malignancies, current approaches frequently employ this technique in combination with fractionated EBRT (with or without concomitant chemotherapy) and resection. The rationale is that EBRT fields encompass the primary tumor and surrounding tissues harboring potential microscopic disease. In contrast to a large single fraction of irradiation, fractionated radiation (EBRT) is radiobiologically advantageous in promoting tumor control while minimizing late normal tissue injury.
Shrinking-field techniques permit dose escalation. This approach is used in many malignancies—including head and neck cancers, breast cancer, and cervical cancer—with excellent local control and acceptable morbidity to dose-limiting normal tissues. These “boost” fields can be delivered in a multitude of ways, including interstitial and intracavitary techniques as well as superficial electrons. For selected intraabdominal, pelvic, thoracic, and other malignancies, IORT is a technique for localized dose escalation while optimizing normal tissue protection.
When EBRT is fractionated, there is a preferential therapeutic advantage for normal tissues relative to tumor as defined by the 4Rs of classical radiobiology (normal tissue repair, tumor reoxygenation, cell-cycle redistribution, and normal tissue repopulation). With a single large fraction of radiotherapy, these advantages are lost. In addition, large doses per fraction may result in increased risk of late effects. There is evidence that small-vessel injury caused by large doses per fraction may contribute to late effects, and ischemic complications are dose dependent. Furthermore, tumor response to single and fractionated radiotherapy depends on the percentage of hypoxic cells within a tumor. This differential sensitivity between hypoxic and well-oxygenated cells increases with increasing dose.
Using alpha/beta calculations (α/β), biologically equivalent doses to a fractionated EBRT course using 2 Gy per fraction for varying IORT doses have been estimated ( Table 22.1 ). As shown, there are disadvantages from a late-effects standpoint with IORT. However, many of these disadvantages are mitigated by exclusion of nontarget tissues from the radiation field by direct inspection, mobilization, and shielding. When combined with EBRT and resection, IORT doses of 10 to 20 Gy provide local control for most solid tumors, especially in the setting of microscopic residual disease. When combined with EBRT and surgery, there is little reason to exceed IORT doses of 10 to 20 Gy. Late normal-tissue complications are often the limiting sequelae of IORT administration; careful planning and administration with techniques designed to reduce dose to nontarget tissues is of paramount importance. Experimental animal data and clinical studies have documented the tolerance of normal tissues to IOERT, EBRT, or both modalities combined, with detailed description of incidence and characteristics of reported observations and toxic events expected in the clinical practice scenario.
| IORT Dose | 10 Gy | 15 Gy | 20 Gy |
|---|---|---|---|
| Normal tissue (acute) | 20 Gy | 37 Gy | 60 Gy |
| (α/β = 7) | |||
| Tumor | 17 Gy | 31 Gy | 50 Gy |
| (α/β = 10) | |||
| Normal tissue (late) | 30 Gy | 65 Gy | 120 Gy |
| (α/β = 2) |
For any treatment, a patient is incurable if local control of the tumor is not achieved. If conventional treatment methods of EBRT, chemotherapy, and surgery provided high local control rates, IORT as a component of treatment would be unnecessary. Single-dose irradiation with precise radiotherapy techniques has also emerged as a valid alternative in patients with metastatic disease or as a potentially cost-effective technique for patients with tumors in early stages and with a favorable prognosis. Although local control rates are satisfactory in many tumor sites using conventional techniques, local failure is problematic in other sites, including abdominal and pelvic malignancies. Treatment of these areas employing standard EBRT techniques is limited by normal tissue tolerance. Examples of such sites are discussed herein.
EBRT with 5-fluorouracil (5-FU)–based chemotherapy employed in the treatment of unresectable pancreatic cancer results in a doubling of median survival compared with surgical bypass/stenting alone (3-6 months vs. 9-13 months) and an increase in 2-year survival from between 0% to 5% and 10% to 20%. Unfortunately, these techniques result in poor local control rates (20%-30%). The use of IORT has been evaluated in patients with both resectable and unresectable pancreatic cancer.
When surgery is used as the primary treatment modality for retroperitoneal sarcomas, local failure has been reported to range from 40% to 90%. Despite the addition of EBRT to surgery, local failure rates are 40% to 80%. This is in contrast to extremity sarcomas, where local control rates approach 90%. Because of the limited tolerance of surrounding normal tissue (small intestine, stomach, liver, kidney, and spinal cord), EBRT doses are limited. A randomized NCI trial evaluating IORT in retroperitoneal sarcomas demonstrated that patients receiving IORT with EBRT experienced significantly improved local control and less small-bowel toxicity versus patients treated with EBRT alone (80% in-field relapse; discussed later).
In patients with locally advanced (T4) or locally recurrent colon and rectal cancers, local control is difficult to achieve despite multimodality therapy. Studies from Princess Margaret Hospital and the Mayo Clinic report local failure rates of 90% or greater in evaluable patients treated with EBRT with or without systemic therapy. In patients who are radiation naïve, the general approach in locally advanced tumors is preoperative EBRT combined with 5-FU-based chemotherapy, potentially further systemic therapy, followed by resection. Despite this, local recurrence occurs in 30% to 70% of patients.
For patients with cervical cancer, paraaortic nodal metastases are common. Despite the presence of these “distant” metastases, approximately 15% to 20% of patients are cured by radical radiotherapy techniques employing EBRT doses of 55 to 60 Gy. However, high complication rates have been reported with these doses and techniques. As in rectal cancer, patients with recurrent cervical cancer in the pelvis or paraaortic region have a poor long-term prognosis, with 5-year overall survival (OS) rates ranging from 5% to 30%. These patients have often been previously irradiated and retreatment with meaningful doses of EBRT is usually not feasible given normal tissue tolerance. When patients have paraaortic or locally recurrent disease, administration of IORT is a feasible method to escalate dose and enhance local control.
Active follow-up of patients with cancer initially treated for cure has identified new entities, including oligometastatic or oligorecurrent disease still amenable to salvage treatment by combining surgical and radiotherapy components. Analyses of patient cohorts, including varying cancer primary sites and histological subtypes, have reported local control rates of greater than 80% and 5-year survivals of 35% in patients with extrapelvic, oligotopic disease. In the case of intrapelvic gynecological oligorecurrences, salvage therapy with extended surgery and IOERT has demonstrated a 10-year locoregional control of 58%, with improved results if EBRT was integrated into this approach.
In both animal and human models, the probability of local control of a tumor by radiation is generally proportional to the total dose administered. The dose of radiation to control a tumor locally depends on several factors, including tumor type, clonogen number, and tumor microenvironment. Thus, a given radiation dose may be able to control a small tumor with high probability and acceptable morbidity; however, that same dose may be insufficient against disease of larger volume. Clinical experience has generated a body of data correlating local control by tumor type and radiation dose. Figure 22.1 summarizes in vivo data for a variety of irradiated human tumors of varying sizes and types.
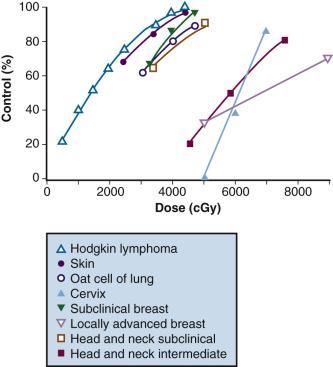
Fletcher examined local control probability as a function of radiation dose for patients undergoing treatment for breast carcinoma and squamous cell carcinoma of the upper aerodigestive tract ( eTable 22.1 ). For patients with breast cancer, control of subclinical disease was approximately 60% to 70% with 30 to 35 Gy, 85% with 40 Gy, and 95% with 45 to 50 Gy. For larger/palpable tumors, EBRT doses of 46 Gy, 59 Gy, and 76 to 90 Gy result in a local control probability of 20%, 35% to 50%, and 70% to 80%, respectively. Dose-response data is summarized for patients with squamous cell carcinomas of the upper aerodigestive tract in eTable 22.1 . These data suggest that marked improvements in local control can be achieved by escalating radiation doses.
| Dose (Gy) | Tumor Control Probability |
|---|---|
| Squamous Cell Carcinoma: Upper Aerodigestive Tract | |
| 50 a | > 90% subclinical |
| ~ 60% T1 lesions of nasopharynx | |
| ~ 50% 1-cm to 3-cm neck nodes | |
| 60 a | ~ 90% T1 lesions of pharynx and larynx |
| ~ 50% T3 and T4 lesions of tonsillar fossa | |
| ~ 90% 1-cm to 3-cm neck nodes | |
| ~ 70% 3-cm to 5-cm neck nodes | |
| 70 a | ~ 90% T2 lesions of tonsillar fossa and supraglottic larynx |
| ~ 80% T3 and T4 lesions of tonsillar fossa | |
| Adenocarcinoma of the Breast | |
| 50 a | > 90% subclinical |
| 60 a | 90% clinically positive axillary nodes, 2.5-3 cm |
| 70 a | 65% 2-3 cm primary |
| 70-80 (8-9 wk) | 30% >5 cm primary |
| 80-90 (8-10 wk) | 56% >5 cm primary |
| 80-100 (10-12 wk) | 75% 5-15 cm primary |
The chief limitation of EBRT to control macroscopic disease in the abdomen and pelvis is normal tissue tolerance. Normal organs such as the stomach, small bowel, and kidney have tolerance levels well below the radiation doses required to eradicate most abdominal and pelvic malignancies. Exceeding these, EBRT doses result in prohibitive risk of late normal-tissue damage ( eTable 22.2 ). Because of this, “conventional” tolerable doses of EBRT from 45 to 55 Gy using 1.8 to 2 Gy per fraction are not curative in most abdominal and pelvic malignancies, with resultant local persistence/local recurrence of disease common in patients treated with radiotherapy alone. This often results in tumor-related morbidity and mortality, such as bowel obstruction and perforation, ureteral obstruction, and neuropathy.
| Organ | Injury at 5 yr | Doses (in Gy) a | Volume or Length | |
|---|---|---|---|---|
| TD 5/5 | TD 50/5 | |||
| Esophagus | Ulcer, stricture | 60-65 | 75 | 75 cm 3 |
| Stomach | Ulcer, perforation | 45-50 | 55 | 100 cm 3 |
| Intestine (small) | Ulcer, stricture | 45-50 | 55 | 100 cm 3 |
| Colon | Ulcer, stricture | 55-60 | 75 | 100 cm 3 |
| Rectum | Ulcer, stricture | 55-60 | 75 | 100 cm 3 |
| Anus | Ulcer, stricture | 60-65 | ≥75 | — |
| Pancreas | Secretory functions | — | — | — |
| Liver | Liver failure, ascites | 35 | 45 | Whole |
| Biliary ducts | Stricture, obstruction | 50 | 70 b | — |
a Data based on supervoltage (6/18 MV), 9 Gy/wk (5 × 1.8).
b External beam radiation to 50.4 Gy ( ![]() weeks) plus 20 Gy at 1-cm radius with iridium 192.
weeks) plus 20 Gy at 1-cm radius with iridium 192.
Although local control is enhanced with increasing doses of radiation, tumor dose-response curves with EBRT closely resemble normal-tissue complication curves. Therefore, efforts to improve local control through escalating EBRT doses may also result in treatment-related complications ( Fig. 22.2 ). In an R1 (microscopic residual) resection, EBRT doses of 60 Gy or higher using conventional fractionation schemes are necessary to achieve a high probability of local control. In an R2 (gross residual) resection, even higher doses are usually required. Such doses often exceed normal-tissue tolerance (see eTable 22.2 ).
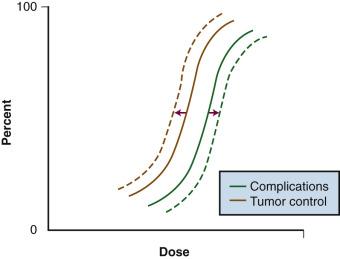
Because of the risks associated with dose escalation beyond normal-tissue tolerance, an attractive alternative in patients with locally advanced malignancies is to deliver moderate doses of EBRT (i.e., at or below accepted tolerance of surrounding normal tissue). A typical course would range from 45 to 50 Gy at 1.8 to 2 Gy per fraction, followed by surgical exploration. After resection, IORT would be performed, avoiding or minimizing irradiation of surrounding organs by shielding or mobilization. With this approach, an increase in local control with decreased risk of normal-tissue complications (relative to an EBRT-only approach) can be achieved (see Fig. 22.2 —local control curve shifts to left with IORT; complication curve shifts to right with increasing EBRT doses).
The concept of shrinking-field irradiation, otherwise known as administering “boost” treatments, has been used for decades by radiation oncologists. This strategy entails treating larger fields encompassing the primary/recurrent tumor along with locoregional lymph node basins and other tissues at risk for subclinical disease. These larger fields receive a dose sufficient to control microscopic disease yet respect normal organ tolerance (often 45-50 Gy using 1.8-2 Gy per fraction). Fields are then reduced to encompass gross disease with smaller margins, excluding dose-limiting normal tissues. An additional 20 to 35 Gy may then be administered to these fields using either EBRT or brachytherapy techniques, bringing the cumulative dose to 65 to 80 Gy. These approaches are employed in many tumor sites, including gynecological and head and neck cancers, with excellent long-term outcomes and local control with relatively low and acceptable morbidity levels. The concept of administering IORT in conjunction with EBRT is a logical application of this approach.
Preclinical data suggests that the incidence of distant metastases is related to both tumor size and the development of locally recurrent disease in multiple spontaneous tumor systems. In fibrosarcoma and squamous cell carcinoma cell lines in rodent models, Ramsay et al. reported increased rates of distant metastases in tumors measuring 12 mm versus 6 mm, as well as recurrent versus primary tumors. Additionally, Suit et al. showed that in mouse mammary tumors treated with single-dose irradiation, increasing rates of local failure were associated with increasing rates of distant metastases. Specifically, the incidence of metastatic disease was 31% (16 of 52) of mice with local control, 50% (9 of 18) in those with local relapse salvaged by resection, and 80% (12 of 15) in mice with local relapse in whom salvage was not attempted. Similar high rates of metastases associated with local failure have been observed in human malignancies, including cervix, prostate, head and neck, and breast cancers. These and other data suggest that metastases may arise from locally recurrent disease.
Candidates for IORT should be evaluated by the treating surgeon and radiation oncologist in a multidisciplinary setting. This allows for joint decisions regarding the appropriateness of IORT and whether further studies that may influence IORT and EBRT planning are appropriate. Additionally, joint decisions can be made defining the optimal sequencing of surgery/IORT and EBRT. Informed consent should be obtained from both specialties, specifically with regard to potential risks, benefits, and side effects of proposed treatments. Criteria for the appropriate selection of patients for IORT generally include the following:
Surgery alone will result in a high probability of incomplete resection (microscopic or gross residual disease) and resultant high probability of failure within the tumor bed. Potential candidates must be appropriate for surgical attempts at gross total resection. IORT administration should be performed at the time of a planned operation.
There is no evidence of distant metastases. Rare exceptions include resectable single-organ metastasis, slow progression of systemic disease, excellent chemotherapy options, and patients with oligometastatic disease with slow systemic progression and high probability of symptomatic local failure.
EBRT doses required for high probability of local control following subtotal or no resection exceed normal-tissue tolerance (total doses required for eradication in this setting: 60-70 Gy for microscopic disease and 70-90 Gy for gross disease at 1.8-2 Gy per fraction).
Surgical displacement or shielding of dose-limiting structures or organs can be accomplished during IORT administration, allowing for acceptable risks of immediate and late effects. Theoretically, EBRT in conjunction with IORT should result in an improved therapeutic ratio between disease eradication and normal-tissue complications.
Pretreatment patient evaluation in patients eligible for IORT should include a thorough history and physical examination, with attention to palpable disease and its relationship to anatomically immobile normal structures. Examples include pelvic disease and its relationship to the pelvic sidewall, presacral space, prostate, or vagina. Computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound may aid in identifying adherence to structures (e.g., bony pelvis and large vessels) that may be surgically unresectable for cure. Examination under anesthesia may be helpful in some situations, including locally advanced gynecological and rectal cancers. Routine blood work—including complete blood count (CBC), liver function tests, renal function tests, and tumor-specific serum test (e.g., carcinoembryonic antigen, CA19-9)—should be obtained when appropriate. Patients should be evaluated clinically and radiographically for evidence of distant spread. Positron emission tomography (PET), preferably in conjunction with CT, may facilitate defining local disease extent as well as unsuspected distant metastases. Evaluation of distant metastases is particularly important in the recurrent setting in which concurrent distant failure is common. Biopsy confirmation of disease should usually be obtained before proceeding with resection.
For patients with localized malignancy, the goal of curative oncological surgery is an R0 (margin-negative) resection. Because of the locally advanced and infiltrative nature of many primary tumors (including colorectal, gynecological, upper gastrointestinal [GI] malignancies, sarcomas, among others) and locally recurrent malignancies, surgery may be compromised with close margins or microscopic/gross residual tumors. For patients with locally advanced tumors, preoperative EBRT to doses of 45 to 50 Gy using 1.8 to 2 Gy fractions (with or without chemotherapy) followed by laparotomy, resection, and IORT offers theoretical and clinical advantages over resection and IORT followed by EBRT. These are listed as follows:
By postponing surgical resection until after preoperative therapy is completed, patients with disease that is rapidly progressive may avoid an unnecessary surgical procedure with associated morbidity.
Preoperative therapy may allow for tumor downstaging and facilitate resection with curative intent.
Preoperative therapy may reduce the risk of tumor seeding/dissemination at resection.
Preoperative therapy allows delivery of treatment to disease with an intact vasculature, potentially improving the delivery of chemotherapy and improving oxygen delivery for EBRT.
The morbidity and delayed recovery time associated with extensive surgical procedures may prevent the timely delivery of postoperative therapy in a high percentage of patients.
The role of preoperative versus postoperative therapy has been evaluated in rectal cancer. A large German randomized trial demonstrated that patients undergoing neoadjuvant irradiation and chemotherapy experienced significantly improved local control and less toxicity than patients receiving postoperative irradiation and chemotherapy.
Techniques combining EBRT and IORT have been fairly uniform in the United States and Europe. In previously untreated patients, EBRT doses of 45 to 54 Gy have been employed, delivering 1.8 to 2 Gy per fraction, 5 days per week, over a period of 5 to 6 weeks. Because of the anatomic location of pelvic and abdominal malignancies, high-energy (> 10 MV) photons delivered via LINACs using multifield, shaped techniques are generally appropriate. CT-, PET-, or MRI-based treatment planning permits accurate definition of the target volume. For extrapelvic, unresected, or residual disease following resection, radiation doses of 40 to 45 Gy delivered at 1.8 to 2 Gy per fraction with a 3- to 5-cm margin accounting for microscopic extension and target mobility are sometimes used. Treatments are generally delivered through multifield techniques aided by three-dimensional (3D) or IMRT-based treatment planning. Reduced-field—or “boost”—techniques are often used to bring the total dose to 45 to 54 Gy as dictated by tolerance of surrounding normal tissue (see previous discussion). Concurrent chemotherapy administration during EBRT varies by tumor site. For patients with gastrointestinal malignancies, concurrent 5-FU-based regimens (plus cisplatin or mitomycin C for squamous cell histologies) are frequently implemented, and for patients with gynecological cancers, concurrent cisplatin is frequently given. In carefully selected patients who have received prior irradiation, moderate preoperative EBRT doses of 30 to 36 Gy at 1.8 to 2.0 Gy per fraction (often with concurrent chemotherapy) may be safely employed if all of the previously irradiated small bowel can be excluded.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here