Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Critical care, including postoperative care, of the neurologically ill patient involves application of the general critical care principles of management with an added focus on prevention and treatment of secondary brain injury. Systemic and neurologic monitoring is typically required and the knowledge and implementation of emergent, timely intervention is paramount. Neurologic injuries, including traumatic brain injuries (TBI), may incur complications or sequelae , which result from the primary (initial) injury or a secondary (subsequent) injury. The lesion sustained at the time of impact, described as the primary injury, results from the high-energy acceleration or deceleration of the brain within the cranial vault. The secondary injury manifests as a result of a more complex process induced by alterations in cerebral blood flow (CBF), inflammation, hypermetabolism, and tissue necrosis. Sequelae of head injury often correlate with the severity of head injury. The Glasgow Coma Scale (GCS) is a commonly used tool for quantifying the severity of head injury. The GCS score ranges from 3 to 15. Scores of 9 through 15 indicate mild-to-moderate head injury, and a score of 8 or less indicates severe injury. Multisystem sequelae of severe head injury include airway obstruction, respiratory dysfunction, cardiovascular dysfunction, fat embolism syndrome, hematologic abnormalities, neuromuscular dysfunction, metabolic abnormalities, electrolyte imbalances, gastrointestinal abnormalities, immunologic abnormalities, endocrine abnormalities, infectious complications, secondary brain injury, and cerebral hyperperfusion syndrome (CHS). Independent predictors of poor outcome include hypotension, hypoxia, hypoglycemia, hyperthermia, hypocapnia, and intracranial hypertension.
Spontaneous respirations are controlled by neural centers in the pons (pontine respiratory group [PRG]) and medulla oblongata (dorsal respiratory group [DRG] and ventral respiratory group [VRG]). These centers send impulses down the spinal cord to the motor neurons supplying the respiratory muscles. Efferent impulses from the DRG travel primarily to the inspiratory muscles whereas those from the VRG travel mainly to the expiratory muscles, some inspiratory muscles, as well as muscles of the tongue, pharynx, and larynx. On either side of the medulla between the nucleus ambiguus and the lateral reticular nucleus lie a group of synaptically coupled neurons, which form a complex called the pre-Botzinger complex (pre-BOTC). This pre-BOTC is responsible for the initiation of the rhythmic respirations and is, therefore, also known as the pacemaker of respirations. Chemoreceptors in the medulla respond primarily to changes in pCO 2 and, to a lesser extent, to changes in pH and pO 2 by altering respiratory patterns to compensate for imbalances. These compensatory responses are affected in brain injury leading to hypoxia, hypercapnia, or both. In patients with mild-to-moderate brain injury, the primary response is that of hyperventilation and hypocapnia, which in turn reduces cerebral blood flow causing a relative decrease in the ICP. In contrast, patients with severe brain injury are prone to develop significant hypopnea or even apnea causing a sudden, severe increase in pCO 2 levels. Hypercarbia can cause cerebral vasodilatation, which in turn leads to an increase in the ICP.
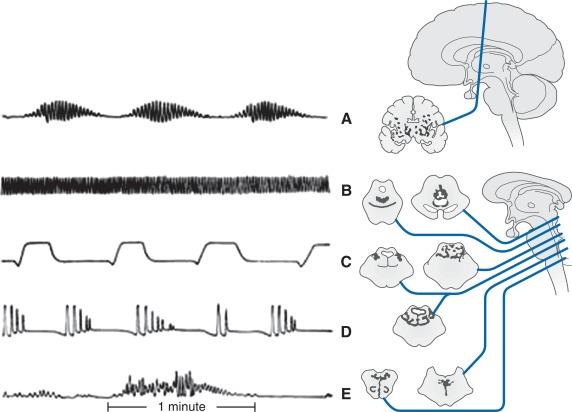
Alterations in respiratory patterns are common in patients with brain injury. These include the following:
Cheyne–Stokes pattern : Cheyne–Stokes is a common pattern of cyclical breathing seen in patients with neurologic injuries. It is characterized by repeated crescendo-decrescendo patterns of breathing and includes a period of apnea at the end of each cycle. The level of consciousness also follows this pattern where the patient becomes increasingly alert to nearly awake during the crescendo phase and possibly vocalizing at the peak of this phase. A gradual slowing of respirations follows a waning of consciousness until the patient is completely apneic and unarousable. This pattern of breathing is associated with deep cerebral hemispheric lesions (stroke, TBI, brain tumor), severe metabolic derangements and central chemosensitivity to changes in CO 2 and O 2 tensions. High pCO 2 leads to excessive compensatory hyperventilation, in turn causing decreased pCO 2, which causes apnea, restarting the cycle.
Biot’s (ataxic) pattern : Ataxic breathing refers to an irregular pattern of both rate and rhythm of breathing. It occurs with lesions in the dorsomedial medulla and may be accompanied by hypersensitivity to respiratory depressants and is considered to be a preterminal event.
Apneustic pattern : Pontine injury can result in a long, gasping inspiration followed by an insufficient length of expiration.
Cluster pattern : Cluster breathing is associated with an irregular frequency and amplitude followed by apneic episodes of varying duration. It can occur due to upper medullary and lower pontine lesions, anoxic encephalopathy, Shy–Drager syndrome as well as subarachnoid, cerebellar and brainstem hemorrhagic lesions.
Central hyperventilation pattern : Patients during early periods of brainstem herniation may cause an increase in the rate and depth of respirations resulting in respiratory alkalosis. This must be differentiated from Kussmaul’s respiration, which is described below.
Kussmaul’s pattern : Initially, acidosis prompts rapid, shallow breathing. As acidosis worsens, Kussmaul’s breathing develops, characterized by deep, labored, and gasping breaths. This compensatory overbreathing can result in reduced carbon dioxide tension and reduced cerebral blood flow ( Fig. 23.1 ).
Respiratory complications can be separated into upper respiratory and lower respiratory tract complications.
Upper respiratory tract (airway) complications are a leading cause of morbidity and mortality in brain-injured patients. In addition, traumatic injuries may cause significant bone injuries such as fractures and displacements that can subsequently lead to bone fragments being embedded in the soft tissues of the airway. Airway dysfunction and edema are relatively common especially following TBI. The patients may lose sensorimotor functional integrity of the airway leading to an increased incidence of aspiration pneumonia. Cranial nerve injuries may further render the patient susceptible to pulmonary aspiration syndrome. Airway protection is ideally managed with endotracheal intubation in patients with extensive brain injury. Injuries involving the cervical spine and/or facial structures may require emergent control of airway via a cricothyrotomy or tracheostomy. Airway edema, secondary to direct trauma or in the postoperative setting, is assessed by performing the “leak test”. The lack of detection of the characteristic audible leak upon deflation of the endotracheal tube cuff signifies the presence of significant tracheal edema. Airway edema is a major problem in the pediatric age group due to the inherent narrowing of the subglottic portion of the airway along with smaller diameter of the airways. Nonetheless, it is always prudent to maintain a secure airway in suspected or documented cases involving edema of the airway until one can establish resolution of this edema. Therapeutic modalities include parenteral steroids, inhaled racemic epinephrine and upright positioning. A helium/oxygen mixture can be used to treat dyspnea due to airway edema following extubation. This mixture generates less resistance than atmospheric air when passing through the narrow airways of the lungs, and thus requires less effort by a patient to breathe in and out of the lungs ( Fig. 23.2 ).

Lower respiratory complications may occur as a result of pulmonary edema, pneumonia, acute lung injury or acute respiratory distress syndrome, or due to physical trauma. Pulmonary edema secondary to neurologic injury and pulmonary thromboembolism causes respiratory dysfunction and cardiovascular compromise (discussed later).
It is known that up to 24% of TBI patients on mechanical ventilation develop ventilator-associated pneumonia (VAP). In TBI, the risk of developing pneumonia increases by 7% per day of mechanical ventilation. Nosocomial pneumonia in mechanically ventilated patients increases ICU and hospital length of stay, days on mechanical ventilation, morbidity, and mortality. Treatment involves initiation of empiric antibiotic therapy to cover Gram-positive organisms, including MRSA, and Gram-negative bacteria, including pseudomonas. The antibiotic therapy can be modified once results of cultures sent prior to initiation of antibiotics are available. VAP management should include modification of risk factors such as daily awakening trials, head of bed elevation greater than 30 degrees, deep tracheal suctioning, early extubation, early initiation of enteral nutrition, and restriction of gastric acid suppressants. Antibiotics must be continued until resolution of the infectious process is reached, which may take anywhere between 8 and 21 days. Radiologic resolution of the process may take from weeks to months and, therefor,e therapy is not dependent upon X-ray.
Acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) may occur as a result of secondary brain injury, direct trauma, infection or blood product transfusion. The pathophysiology involved in both of these conditions is similar and, in fact, may progress from ALI to ARDS. ALI/ARDS are both forms of noncardiogenic pulmonary edema, which lead to respiratory failure and hypoxemia. Abnormalities in gas exchange can be exacerbated by simultaneous injury to the respiratory control centers in the brainstem. In patients with severe TBI, the combination of lung pathology and dysfunctional respiratory drive and mechanics can lead to significant hypoxemia and hypercarbia. Mechanical ventilation of the lungs, which is necessary for maintenance of adequate gas exchange, has been shown to decrease further tissue damage.
Some common ventilatory modes utilized to ventilate and oxygenate these patients include volume-cycled assist control ventilation/continuous mandatory ventilation (ACV or CMV), which involves delivery of similar volumes of breath per cycle. This is usually the default mode for most patients receiving mechanical ventilation. Alternatively, pressure control ventilation (PCV) is the mode of choice in cases involving poor lung compliance, such as ARDS, where the high peak inspiratory pressures in volume cycled ventilation result in lower delivered tidal volumes. The parameters of ventilation must be adjusted to achieve adequate oxygenation and carbon dioxide elimination. Synchronized intermittent mandatory ventilation mode (SIMV) enables ventilator–patient synchrony, that is, coordination of patient and machine initiated breaths. Pressure support ventilation (PSV) is usually utilized along with SIMV to permit better support of the patient initiated breaths. Both these modes are used in conjunction to assist in weaning patients from mechanical ventilation.
Advanced modes, if available, may also be used to assist patients who are on mechanical ventilation. These include airway pressure release ventilation (APRV), pressure regulated volume control (PRVC), volume assured pressure support (VAPS), neurally adjusted ventilatory assist (NAVA), proportional assist ventilation (PAV), adaptive support ventilation, volume support ventilation, high frequency oscillatory ventilation (HFOV), and high frequency jet ventilation (HFJV). The common theme amongst these modes of ventilation is the aim of ensuring adequate oxygenation and ventilation, preventing further additional lung injury, minimizing patient ventilator dyssynchrony, and enabling faster weaning from mechanical ventilation.
Positive end expiratory pressure (PEEP) is a therapeutic modality that is used concomitantly with the abovementioned modes of ventilation. It refers to the pressure in the airways at the end of passive expiration. PEEP is used commonly to improve oxygenation by recruiting and stabilizing lung units that participate in gas exchange. There is conflicting evidence in the literature regarding use of PEEP in patients with brain injury. It has been postulated that application of PEEP may lead to an increase in the intracranial pressure as a result of increased venous pressure and decreasing cerebral venous return, thereby causing a decrease in the cerebral perfusion pressure. However, the jugular veins are Starling resistors, and in the head elevated position, there is a limited correlation between intrathoracic and intracranial pressure. In any event, the benefits of the administration of low-to-moderate amounts of PEEP, by improving oxygenation, outweigh the potential risks.
Direct trauma to the chest may lead to fracture(s) of the rib cage causing a flail chest, pulmonary contusions, pneumothorax, hemothorax, chylothorax or a combination of any of these conditions. In addition, it may also cause cardiovascular injuries, which have a very high mortality rate. Management of these conditions is usually surgical in nature and involves operative repair, thoracocentesis and/or thoracostomy, oxygen supplementation with or without mechanical ventilation.
NPE is an acute and life-threatening complication that may occur secondary to CNS injury, e.g., trauma, hemorrhage, infection, inflammation, space occupying lesions, ischemic events, or post neurosurgical states. NPE can develop in patients with autoimmune neurologic conditions, such as multiple sclerosis and Guillain–Barré syndrome. The incidence of NPE in patients with traumatic brain injuries can be as high as 20%.
It has been postulated that sudden, rapid and intense elevation of ICP leads to an activation of the sympathetic nervous system causing a catecholamine surge. The exact sources of this surge, labeled “NPE trigger zones,” have been identified in the hypothalamus and the medulla. This surge in catecholamines leads to an arterial vasoconstriction and increased systemic vascular resistance (SVR), as well as venoconstriction resulting in increased preload and cardiac output. The influx of large volumes of blood into the pulmonary arterial circulation, in combination with pulmonary venous constriction, results in increased pulmonary capillary pressure, swelling and subsequent destruction of the capillary and alveolar walls, and leakage of fluid and cells into the interstitium and intra-alveolar space ( Fig. 23.3 ).

Respiratory distress or failure in a patient with acute and severe CNS injury is common. NPE is a diagnosis of exclusion where one must rule out all other causes of noncardiogenic edema, especially aspiration pneumonia, and volume overload. Two types of NPE have been described. The first and more common one is the early form where symptoms develop within minutes to hours following the neurologic injury. The second type of NPE, also known as the delayed form, is not as common and develops 12–24 hours following the injury. In either of these cases, the patient becomes acutely dyspneic and hypoxemic within a span of minutes and exhibits clinical signs of pulmonary edema, such as pink, frothy sputum, and bilateral rales. Radiographic imaging revealing bilateral fluffy pulmonary infiltrates corroborates the clinical suspicion of pulmonary edema.
Management of NPE involves treatment of the primary neurologic condition in order to minimize sympathetic discharges causing the lung injury. The following diagnostic criteria have been suggested by Davison et al. to establish a diagnosis of NPE in the group of patients who may benefit from sympathetic blockade: (1) bilateral infiltrates; (2) PaO 2 :FiO 2 < 200; (3) no evidence of left atrial hypertension; (4) presence of severe CNS injury, which could lead to an increased ICP; and (5) absence of other causes of respiratory distress or ARDS (e.g., aspiration, massive blood transfusion, sepsis). Mechanical ventilation is usually required in cases involving NPE and involves utilization of moderate levels of PEEP (up to 15 cm H 2 O) to improve oxygenation. ,
Patients with neurologic injuries including spinal cord injuries have a high risk of pulmonary embolism and deep vein thrombosis. This is especially true in patients with associated multiple trauma and fractures. Management of these injuries involves immobilization of the affected areas, which poses a significant risk for development of deep vein thrombosis and subsequent pulmonary embolism. Therefore, use of sequential compression devices (SCD) is highly recommended, particularly when the risk of developing an intracranial bleed following the initial injury precludes the utilization of pharmacologic prophylaxis for prevention of these conditions.
Most neurologic injuries, with the exception of the mild variety, are associated with autonomic dysfunction. Initial responses to neurologic injuries include an increase in heart rate, blood pressure and cardiac output as a compensatory phenomenon. However, these compensatory mechanisms become exhausted once the duration of injury becomes more prolonged or if there are associated severe secondary injuries. This leads to a significant alteration in the systemic hemodynamics that in turn triggers the release of greater amounts of catecholamines into the circulation. There is evidence to show that the extent of catecholamines is directly proportional to the severity of the brain injury and to patient outcomes. Excessive catecholamine release, mainly from the hypothalamus, is the likely cause of myocardial damage seen on autopsy in patients with severe TBIs. However, it has been argued that the levels of circulating catecholamines in the blood may be less important than intracardiac noradrenergic neuronal activity in myocardial damage and necrosis. Troponin I levels correlate with the extent of left ventricular dysfunction and severity of neurologic injury.
Cardiac dysrhythmias are a significant complication of neurologic injuries. Autonomic dysregulation (dysautonomia), electrolyte imbalances and hypoxemia are some risk factors that may precipitate these dysrhythmias. Dysautonomia uncouples the relationship between heart rate and sympathetic nervous system regulation. It may last up to 3 months following injury and is directly proportional to the severity of neurologic injury. , Arrhythmias are frequent following TBI with tachyarrhythmias being the most common and up to 95% of patients who suffer brain injuries (traumatic and atraumatic hemorrhagic) manifest electrocardiographic changes within the first 48 hours. Sinus and supraventricular tachycardias, prolonged qT c intervals (> 440 msec), increased P wave amplitude, shortened P–R intervals, nonspecific ST segment and T wave alterations are some of the common presentations of such arrhythmias , and are associated with an increased mortality in patients particularly when they persist for a prolonged period of time. The supraventricular and nodal arrhythmias, though associated with a 50% mortality rate, are postulated to be the result of terminal events than being the cause of high mortality. However, there is no significant correlation between the type of neurologic injury and specific ECG changes. In the pediatric population with TBI, there is ample evidence to suggest a high incidence of electrolyte abnormalities, especially hypokalemia, which has been linked to the high incidence of cardiac conduction abnormalities. This is associated with an increased incidence of longer qT c intervals and greater qT c dispersion, but there is no evidence that links these findings to a higher mortality rate in children.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here