Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Recognition and treatment of acute pain is a vital component of pediatric medicine. , Adequate pain control is necessary to prevent adverse neurohormonal and developmental changes in response to painful stimuli. Fortunately, advances in pharmacologic therapy and regional anesthesia techniques have helped expand the scope of pediatric acute pain management. In addition, the establishment of pediatric acute pain services has played an important role in ensuring timely and consistent care of children. ,
The study of pain in neonates has been a significant focus in the field of neuroscience. Nociceptive pathways are well developed even at birth, resulting in hormonal, metabolic, cardiorespiratory, emotional, and behavioral changes in response to painful stimuli. A study of brain perfusion in response to pain has demonstrated significant changes in perfusion with noxious stimuli versus non-noxious stimuli. Newborn rats appear to have a significant proliferation of A and C fibers with a pattern of hyperalgesia developing at sites exposed to painful stimuli. Analogously, human neonates exposed to repeated heel sticks develop cutaneous hyperalgesia, which can be reversed with topical local analgesia. Therefore it is a misconception that pain control is less important in neonates and small children because they cannot form memories of painful experiences or communicate discomfort. Adequate analgesia can limit the immediate and long term effects of noxious stimuli, and treatment of pain in young patients should receive the same level of attention as in adults.
Reliable assessment of pain level is essential for effective management. Unfortunately, pediatric patients may be too young, developmentally immature, or unwilling to adequately communicate their pain level. Assessment of acute pain in such patients often relies on observer reports, whereas older children may use self-report measures (see Table 28.1 ). Observational pain assessment tools rely on the interpretation of pain related activity such as body movements, facial expression, and vocalizations; physiologic changes such as heart rate and oxygen saturation; and the child’s behavioral state. These measures have been designed to assess procedural pain (e.g. premature infant pain profile [PIPP], neonatal facial coding system [NFCS] ) or postoperative pain (e.g. Children’s Hospital of Eastern Ontario pain scale [CHEOPS], toddler-preschooler postoperative pain scale [TPPPS]). The FLACC scale (faces, legs, activity, cry, consolability) is a tool that can be used for all ages, , while the revised FLACC (rFLACC) scale incorporates additional observable behaviors to improve validity in children with cognitive impairment (see Table 28.2 ). The rFLACCs ease of use and validation in patients with a wide range of ages and cognitive levels make it a practical choice for assessment of patients who cannot self-report. Observational assessment tools have limits in their specificity, particularly when including physiologic parameters that can vary because of conditions not associated with pain. Despite their intrinsic limits, these scales have been shown to have construct validity and internal and interrater reliability. ,
| Age Group | Measure | Type of Measurement | Type of Pain |
| Neonates and infants | Premature infant pain profile (PIPP) (preterm and full-term neonates) | Behavioral, physiologic, gestational age | Procedural |
| Neonatal facial coding system (NFCS) (preterm and full-term neonates, infants ≤18 months) | Behavioral | Procedural | |
| COMFORT scale (zero to three years) | Behavioral, physiologic | Procedural, postoperative | |
| rFLACC scale (two months to seven years) | Behavioral | Postoperative | |
| Toddlers and preschoolers | Faces scales , | Self-report | Procedural, postoperative |
| Oucher (≥ three years) | Self-report | Procedural | |
| Poker chip tool (four to eight years) | Self-report | Procedural | |
| Toddler-preschooler postoperative pain scale (TPPPS) (one to five years) | Behavioral | Postoperative | |
| Children’s Hospital of Eastern Ontario pain scale (CHEOPS) (one to seven years) | Behavioral | Postoperative | |
| Children’s and infants’ postoperative pain scale (CHIPPS) (zero to four years) | Behavioral, physiologic, alertness, calmness | Postoperative | |
| rFLACC scale (two months to seven years) | Behavioral | Postoperative | |
| School-age children and adolescents | Colored analog scale (CAS) (≥ five years) | Self-report | Procedural, recurrent, chronic |
| Visual analog scale (VAS) (≥ five years) , | Self-report | Procedural, recurrent, chronic | |
| Faces pain scale | Self-report | Procedural, recurrent, chronic | |
| Non-communicating children, children with cognitive impairment | Non-communicating children’s pain checklist- postoperative version (NCCPC-PV), non-communicating children’s pain checklist-R (NCCPC-R) , | Behavioral | Procedural, postoperative injury, pain related to a chronic medical condition |
| VAS | Self-report | Procedural | |
| rFLACC scale , | Behavioral | Postoperative |
| Categories | Scoring 0 | Scoring 1 | Scoring 2 |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested, appears sad or worried | Frequent to constant frown, clenched jaw, quivering chin, distressed-looking face; expression of fright or panic |
| Legs | Normal position or relaxed, usual tone and motion to limbs | Uneasy, restless, tense, occasional tremors | Kicking or legs drawn up, marked increase in spasticity , constant tremors or jerking |
| Activity | Lying quietly, normal position, moves easily; regular rhythmic respirations | Squirming, shifting back and forth, tense, tense, or guarded movements; mildly agitated (e.g. head back and forth, aggression); shallow, splinting respirations, intermittent sighs | Arched, rigid, or jerking, severe agitation; head banging; shivering (not rigors); breath holding, gasping or sharp intake of breaths, severe splinting |
| Cry | No cry (awake or asleep) | Moans or whimpers, occasional complaint, occasional verbal outburst or grunt | Crying steadily, screams or sobs, frequent complaints, repeated outbursts, constant grunting |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, being talked to; distractible | Difficult to console or comfort, pushing away caregiver , resisting care or comfort measures |
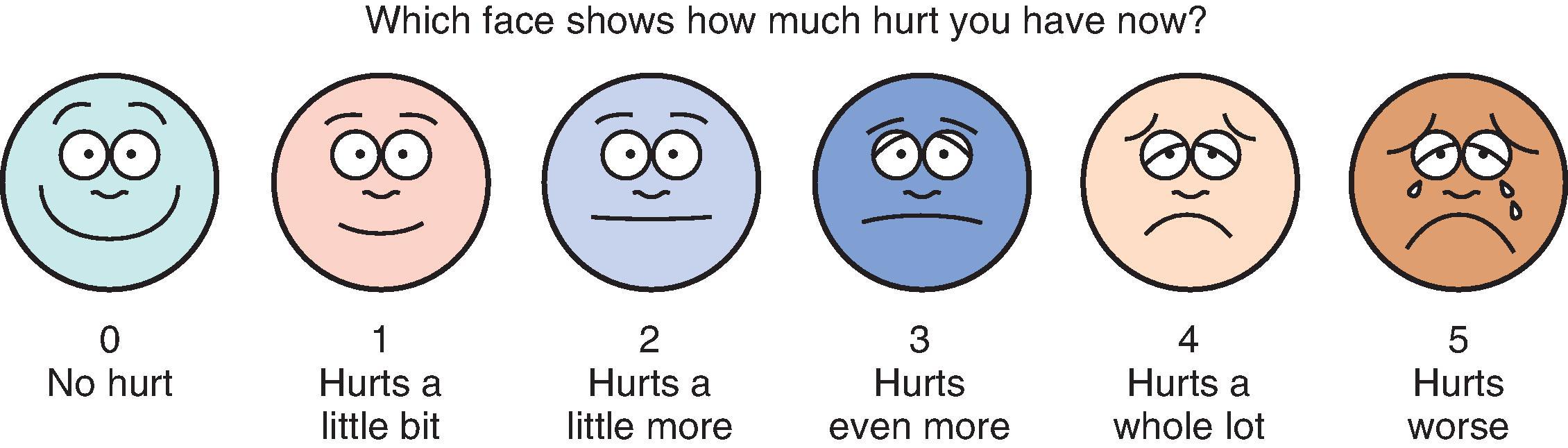
Developmentally appropriate children five years and older can typically provide self-reports on one of several validated visual analog (e.g. colored analog scale [CAS] ) or faces scales (e.g. faces pain scale-revised [FPS-R], , Oucher ) ( Fig. 28.1 ). McGrath and Hillier developed a separate facial affective scale designed to measure pain affect, as distinct from pain intensity. Interestingly, the faces scales anchored with a smiling face produce higher pain ratings than do those anchored with a neutral face. Discordance between an observer’s ratings of a child’s pain and the child’s self-report is well described. , Therefore the child’s self-reported pain level should be considered the “gold standard” whenever it can reliably be obtained.
The majority of pediatric pain assessment measures that have been developed focus on acute, procedure-related pain. , Alterations in the behavioral and sensory aspects of pain that can habituate when pain becomes chronic may not be captured by these measurement scales. A systematic evaluation of chronic pain in children is beyond the scope of this chapter (see Chapter 42).
Management of pain through nonmedical techniques (e.g. environmental and behavioral strategies) has proved effective in modulating pain, both independently and in conjunction with pharmacologic interventions in children. Cognitive behavior therapy (e.g. relaxation, problem solving, cognitive coping skills) and distraction techniques such as deep breathing, cartoon videos, party blowers, and hypnosis have strong empirical support for their efficacy in easing procedure-related pain in children. Distraction methods are hypothesized to work by engaging children and redirecting their attention away from the pain, thereby reducing perceived pain intensity and inhibiting the neural activity that underlies pain perception. Complementary and alternative medicine techniques such as acupuncture have also been described as potential treatments of acute pain in children. ,
Recent studies have identified preoperative risk factors for worse postoperative pain, including patient behaviors such as catastrophizing and psychological and somatic symptoms such as depression and fatigue. Additional research is needed to determine whether psychological and other nonpharmacologic interventions may be beneficial for this pain-vulnerable group of children.
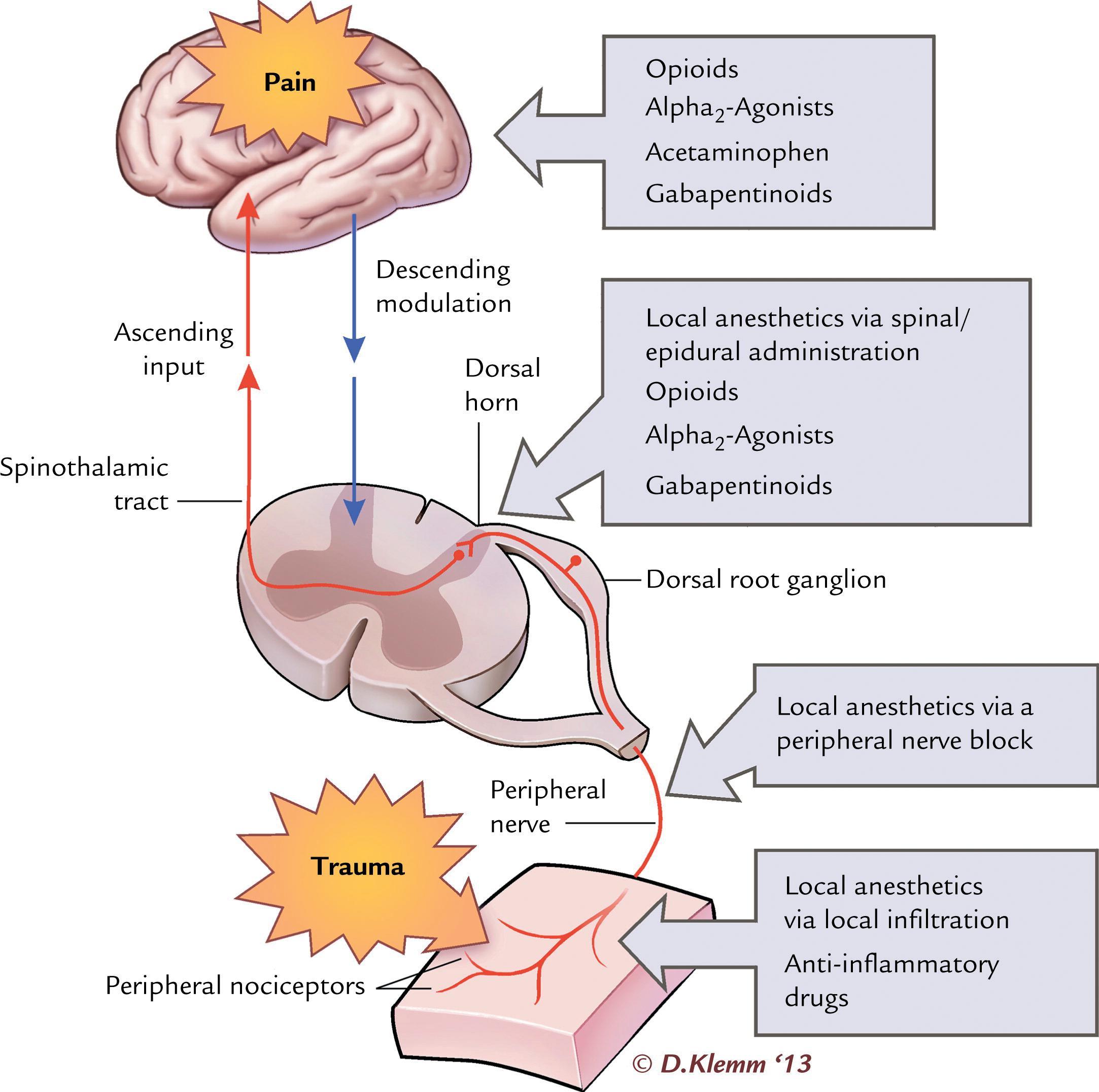
Acute pain in infants and children may result from several causes, including surgery, trauma, sickle cell vaso-occlusive episodes, and oncologic processes and treatments. Non-opioid analgesics can provide adequate control of mild pain with minimal side effects. For moderate to severe pain, a multimodal strategy that incorporates non-opioid analgesics, opioids, and regional anesthesia is preferred. Multimodal analgesia involves multiple agents working synergistically at different biologic locations along the pain pathway ( Fig. 28.2 ). This allows improved pain control with reduced side effects (sedation, respiratory depression, nausea, pruritis, ileus) that can occur with the higher doses required when opioids are used alone for treatment of severe pain. , Patient factors such as comorbidities, developmental level, opioid use history, and ability to take oral medications must be considered along with pain cause, severity, location, and expected duration when developing an optimal analgesia plan.
Oral administration of glucose and sucrose can provide mild analgesia, as opioid peptides in the ventral striatum and cingulate gyrus may play a role in regulating positive responses to energy-rich food sources. , A Cochrane database review suggested that sucrose may be effective in reducing procedural pain in neonates. Doses in the range of 0.01–0.1 g can be used to reduce procedural pain in infants younger than six months.
Acetaminophen is commonly used in children to reduce or eliminate pain from a variety of conditions. It can be administered via the oral, rectal, and intravenous routes. There is no evidence to suggest the superior analgesic effect of one route over another when appropriately dosed, and the oral route is the most cost effective. The rectal and intravenous routes are used for patients unable to take oral medications, as is often the case in the perioperative period. Higher rectal doses (i.e. 30–40 mg/kg loading dose followed by 15–20 mg/kg maintenance doses at 4–6 h intervals in children over two years old) are required to produce therapeutic serum concentrations in the majority of patients, and there is large interindividual pharmacokinetic variability. , Intravenous acetaminophen has more predictable bioavailability and achieves maximum concentration more rapidly than rectal dosing, and therefore may be the preferred non-oral route for administration to hospital inpatients.
While rare, hepatotoxicity is a dose-dependent risk with acetaminophen use. Compared to older children, neonates exhibit decreased acetaminophen clearance because of renal and hepatic immaturity. Doses should be reduced, especially in preterm infants. Another potential source of overdose is inadvertent administration from multiple sources, including over-the-counter cold remedies and fixed-dose opioid combinations, both of which often contain acetaminophen. All medications being taken by a patient, including those available without a prescription, should be carefully reviewed before recommending acetaminophen ( Table 28.3 ).
| Medication | Dose | Dosing Interval (h) | Maximum Daily Dose (mg/kg) | Maximum Daily Dose (mg) |
| Acetaminophen * (oral) | 10–15 mg/kg | 4–6 | 75 | 3000 |
| Acetaminophen * (IV) | 15 mg/kg | 6 | 75 | 3000 |
| Acetaminophen * (rectal) | 30–40 mg/kg as loading dose, 15–20 mg/kg maintenance | 6 | 75 | 3000 |
| Ibuprofen ^ | 5–10 mg/kg | 6–8 | 40 | 2400 |
| Ketorolac (IV) ^ , # | 0.5 mg/kg | 6 | 2 | 120 |
| Celecoxib (oral) | 10–25 kg: 50 mg >25 kg: 100 mg |
12 | 400 | |
| Gabapentin (oral) | <50 kg: 15 mg/kg loading dose, 5 mg/kg maintenance >50 kg: 300–600 mg |
8 | ||
| Pregabalin (oral) | <50 kg: no available evidence >50 kg: 50 mg |
8–12 | ||
| Ketamine (infusion) | 0.1–0.2 mg/kg/h | Continuous | ||
| Diazepam (oral, IV) | 0.05–0.1 mg/kg | 6–8 |
* Dose should be decreased in preterm and term neonates.
^ Use with caution in neonates <6 months old.
# Avoid use in neonates <37 weeks post gestational age or 21 days postnatal age.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used in children and can be administered via oral, intravenous, or intramuscular routes. NSAIDs are an effective treatment for various pain states and have been shown to be more effective than opioids for mild traumatic musculoskeletal pain in children. When used in the postoperative setting, NSAIDs can produce analgesia equivalent to a dose of opioid, and their use with or without additional opioids has been associated with less nausea, vomiting, respiratory depression, and other adverse events. , , Ketorolac is commonly used in children and can be administered via intravenous or intramuscular routes.
The clinical significance of the effects of NSAIDs on platelet function remains controversial, which has led to its avoidance by some for procedures with a significant risk of postoperative bleeding. Known side effects, including bleeding, renal toxicity, and gastritis, are more likely to occur with prolonged administration and in the presence of coexisting disease. Adverse events are more common in neonates; NSAIDs should be used cautiously in patients less than six months of age and avoided in patients less than 21 days old or 37 weeks post gestational age. , Evidence from animal models has caused concern that NSAIDs may interfere with osteogenesis. However, studies of children receiving a short course of NSAIDs, including ketorolac, after spinal fusion or operative fracture repair do not appear to be at increased risk for bone nonunion. , Aspirin, a unique NSAID that irreversibly inhibits cyclooxygenase (COX)-1 and COX-2, should not be routinely used as an analgesic in patients under the age of 18 years because of the risk of Reye’s syndrome, a serious acute encephalopathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here