Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews the pharmacology of vasopressors and inotropes used commonly in acute care settings and comparable new drugs with promising clinical potential. It focuses on the pharmacodynamic properties of the drugs to a greater degree than their pharmacokinetic properties because most of these drugs have short half-lives, are administered by continuous infusion, and are titrated to clinical effect.
Relying on landmark studies from the past as well as recent findings, this chapter seeks to build the scientific foundation on which the clinical use of these agents is based. Because their application to human pharmacology is unreliable, data derived exclusively from animal studies are not considered. Even when only human data are considered, the effects of vasopressors and inotropes vary substantially because of patient factors. Clinicians know that when treating patients experiencing severe hypotension or cardiac failure, the effects of vasopressors and cardiotonic drugs depend on many associated factors, including acid-base status, temperature, blood volume, and concomitant drug administration.
Vasoactive drugs have an extensive history and have been in clinical use for millennia. The early identification and isolation of vasoactive substances was based on extraction from plants and endocrine glands. For instance, ephedrine has been in clinical use as a diaphoretic and circulatory stimulant for more than 5000 years as the active component of the Chinese drug ma huang. Until the drug was finally isolated in 1887, it was extracted from the plant Ephedra sinica .
Similarly, foxglove had been in use for hundreds of years; William Withering published his historic book An Account of the Foxglove, and Some of Its Medical Uses in 1785. This text detailed Withering's work with extracts of the plant Digitalis purpurea and described effects and side effects of the drugs now known as digoxin and digitoxin .
In the late 17th century, it was recognized that “an extract of the suprarenal glands caused contraction of the arteries and led to an increase in the beat of the auricles and ventricles,” and that an extract of the pituitary gland possessed vasopressor activity. These substances would eventually be named epinephrine and vasopressin .
While early medicinal chemistry work focused on developing progressively purer isolates of the active substances from natural sources, it eventually shifted to synthesizing drugs chemically. Dopamine was first synthesized in 1910 by Barger and Ewins, who immediately recognized its potency as a vasopressor. Vasopressin was the first polypeptide hormone successfully synthesized, for which du Vigneaud won the Nobel Prize in Chemistry in 1955. Work by von Euler confirmed that norepinephrine helped mediate the activity of the sympathetic nervous system and contributed to his 1970 Nobel Prize.
In the modern era, attempts were made to develop drugs with specific characteristics. Dobutamine was synthesized in the early 1970s for the specific purpose of providing a high level of inotropy without the vasodilatory limitations of isoproterenol. Similarly, milrinone was developed in the early 1980s as an alternative to amrinone but without the high incidence of fever and thrombocytopenia that limited the use of amrinone.
The development of novel vasopressors and inotropes continues to this day. Levosimendan, for instance, entered clinical use in Europe as recently as 2000 and numerous drugs are currently under investigation around the world.
Many of the drugs in this chapter share structural similarities that affect their pharmacologic actions, although a few are chemically unrelated ( Fig. 25.1 ). Many sympathomimetics are derived from the parent compound β-phenylethylamine. Many of these drugs are also referred to as catecholamines due to the presence of hydroxyl substitutions on carbons 3 and 4 of the benzene ring of β-phenylethylamine. The most basic example of a catecholamine is dopamine, which is the 3,4-hydroxyl substituted form of βphenylethylamine. It is the metabolic precursor to both norepinephrine and epinephrine as the substrate for dopamine β-hydroxylase. The addition of an N-substitution increases the activity at β-adrenergic receptors. Norepinephrine, like epinephrine, is derived from β-phenylethylamine, but the lack of N-substitution decreases its activity at the β receptors. The impact of the degree of amino substitution on β-receptor activity is further reflected in the structures of isoproterenol and dobutamine. Both of these drugs have bulky side chains and as such have a high degree of β specificity.
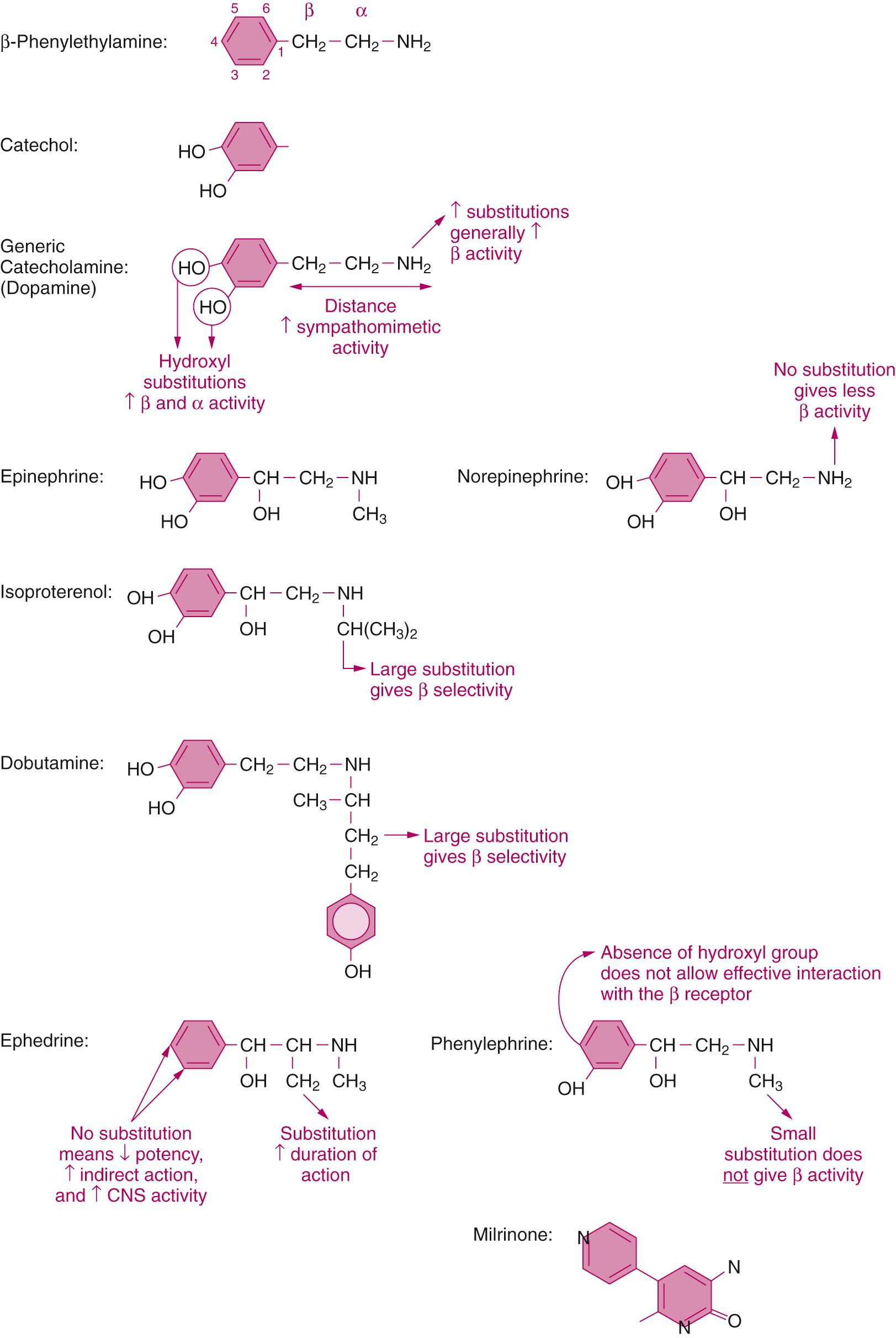
Phenylephrine and ephedrine are not considered catecholamines, in that they are not hydroxylated on both the 3 and 4 carbons of their benzene ring (phenylephrine has a single substitution and ephedrine has none). This lack of hydroxylation prevents phenylephrine from effectively binding the β receptor despite the N-methyl substitution. Ephedrine's lack of hydroxylation substantially decreases its ability to stimulate directly adrenergic receptors. The presence of a methyl group on the α-carbon of ephedrine blocks oxidation by monoamine oxidase and prolongs its action.
Milrinone, vasopressin, and levosimendan neither share structural similarities with the sympathomimetic drugs discussed nor with one another. Milrinone is a bipyridine methyl carbononitrile derivative of amrinone. Vasopressin, as a nonapeptide hormone, consists of a sequence of nine amino acids (Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly), whereas levosimendan is a pyridazone-dinitrile derivative.
Although the drugs discussed in this chapter are applied in similar clinical settings, they do not all share a pharmacologic class or mechanism. They are perhaps best classified and considered based on their mechanism for increasing inotropy and vasoconstriction (see Chapter 23 ). Although the drugs that increase inotropy do so by different mechanisms, the common endpoint is positively influencing the interaction of the calcium ion (Ca 2+ ) with actin and myosin in the cardiac myocyte ( Fig. 25.2 ). Each of the β agonists, phosphodiesterase inhibitors, cardiac glycosides, and calcium sensitizers accomplishes this in a different way.
![Fig. 25.2, Mechanisms of action of selected positive inotropes indicating where the agents act in a cardiomyocyte. Ultimately cytosolic calcium ion (Ca 2+ ) and its interaction with the actin-myosin complex cause myocyte contraction. The β agonists and phosphodiesterase inhibitors accomplish this by increasing the activity of protein kinase A (PKA). The calcium sensitizers act directly by increasing Ca 2+ affinity for troponin C at the actin-myosin complex. Digitalis compounds inhibit sodium-potassium adenosine triphosphatase (Na + ,K + -ATPase [Na + pump]) indirectly increasing intracellular Ca 2+ . AC, Adenylyl cyclase; AMP, adenosine monophosphate; BAR, β-adrenergic receptor; cAMP, cyclic adenosine monophosphate; Gs, G stimulating α subunit; PDE, phosphodiesterase; PDE-I, phosphodiesterase inhibitor; PKA, protein kinase A; SR, sarcoplasmic reticulum. Fig. 25.2, Mechanisms of action of selected positive inotropes indicating where the agents act in a cardiomyocyte. Ultimately cytosolic calcium ion (Ca 2+ ) and its interaction with the actin-myosin complex cause myocyte contraction. The β agonists and phosphodiesterase inhibitors accomplish this by increasing the activity of protein kinase A (PKA). The calcium sensitizers act directly by increasing Ca 2+ affinity for troponin C at the actin-myosin complex. Digitalis compounds inhibit sodium-potassium adenosine triphosphatase (Na + ,K + -ATPase [Na + pump]) indirectly increasing intracellular Ca 2+ . AC, Adenylyl cyclase; AMP, adenosine monophosphate; BAR, β-adrenergic receptor; cAMP, cyclic adenosine monophosphate; Gs, G stimulating α subunit; PDE, phosphodiesterase; PDE-I, phosphodiesterase inhibitor; PKA, protein kinase A; SR, sarcoplasmic reticulum.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/VasopressorsandInotropes/1_3s20B9780323481106000259.jpg)
Drugs that act on the β 1 receptor (such as epinephrine, dobutamine, dopamine, isoproterenol, and, to a lesser extent, ephedrine and norepinephrine) begin by stimulating the receptor on the cardiac myocyte sarcolemma with subsequent activation of the G s protein. This protein activates adenylyl cyclase and enhances the formation of cyclic adenosine monophosphate (cAMP), which activates protein kinase A, thereby phosphorylating and increasing the open probability of voltage-gated Ca 2+ channels. These channels allow Ca 2+ influx to increase cytosolic Ca 2+ concentration, which activates the coupling of actin and myosin in the myocyte. Protein kinase A also activates a Ca 2+ -adenosine triphosphatase (ATPase) on the sarcoplasmic reticulum, leading to increased Ca 2+ uptake in diastole and improved lusitropic function.
The inotropic effects of phosphodiesterase inhibitors (e.g., milrinone), like those of the adrenergic agonists, are mediated by cAMP. Unlike adrenergic agonists that increase cAMP by stimulating adenylyl cyclase, milrinone inhibits the breakdown of cAMP by phosphodiesterase type III (PDE3). Increased cAMP enhances Ca 2+ release from the sarcoplasmic reticulum and increases the force generated by actin-myosin. The vasodilatory action of milrinone is also cAMP mediated. In vascular smooth muscle, cAMP inhibits myosin light chain kinase, the enzyme responsible for phosphorylating myosin light chains and causing smooth muscle contraction. Inhibition of PDE3 increases cAMP, thereby promoting vascular smooth muscle relaxation.
Digoxin increases cytosolic Ca 2+ by inhibiting the action of a sodium-potassium adenosine triphosphatase (Na + ,K + -ATPase) on the cell membrane of cardiac myocytes. This leads to an increase in cytosolic sodium ion (Na + ), thereby decreasing the activity of Na + -Ca 2+ exchange and indirectly resulting in an increase in intracellular Ca 2+ available to interact with actin and myosin.
Levosimendan, referred to as a calcium sensitizer , has a mechanism that is fundamentally different from the other inotropes discussed herein. Rather than increasing the content of intracellular Ca 2+ , it acts to modulate the interaction of Ca 2+ . It first binds the N-terminal lobe of cardiac troponin C (TnC), thereby stabilizing the Ca 2+ -bound form of the protein. This prolongs the systolic interaction between actin and myosin and increases the force of contraction. Because binding of levosimendan to TnC is dependent on the cytosolic Ca 2+ concentration, it occurs almost exclusively during systole, leaving diastolic function relatively unaffected. Importantly, unlike other drugs discussed in this chapter, the increased inotropy is achieved without an increase in myocardial oxygen demand.
The majority of drugs discussed in this chapter exert their vasoconstrictive actions via α 1 receptors in the vasculature; the exception is vasopressin that acts on the V1 receptor. Stimulation of α 1 or V1 receptors on vascular smooth muscle act (via separate G proteins) to stimulate phospholipase C (PLC), which hydrolyzes phosphatidylinositol bisphosphate (PIP 2 ) to generate inositol trisphosphate (IP 3 ) and diacylglycerol (DAG). IP 3 increases Ca 2+ release from the sarcoplasmic reticulum, while DAG activates protein kinase C to increase Ca 2+ influx via voltage-gated Ca 2+ channels. This increase in cytosolic Ca 2+ increases vascular smooth muscle tone.
Vasopressors and inotropes generally have short half-lives and are rapidly metabolized, are administered by continuous infusion, and are titrated to clinical effect. This means that for practical purposes these drugs are pharmacokinetic equals; thus pharmacokinetic factors do not typically play an important role in rational drug selection of a specific inotrope or vasopressor. In general, these drugs exert their effects with an ongoing infusion; the effects rapidly decrease once the infusion is terminated. Levosimendan is a notable exception to this general rule.
The catecholamine class of drugs, which includes epinephrine, norepinephrine, dopamine, dobutamine, and isoproterenol, are all rapidly inactivated by methylation of a hydroxyl group of the catechol structure by catechol- O -methyltransferase (COMT). In addition, monoamine oxidase (MAO) catalyzes oxidative deamination of this group of compounds (with the exception of dobutamine). Approximately 25% of dopamine is converted to norepinephrine in adrenergic nerve terminals; these nerve terminals also take up norepinephrine. Even though phenylephrine is not a catecholamine, it is nonetheless metabolized by MAO. Ephedrine and milrinone largely resist metabolism and are excreted in the urine, whereas vasopressin is metabolized by specific vasopressinases in the liver and kidney.
Levosimendan is unique in this group of drugs, in that it is metabolized to active compounds that are eliminated slowly. This results in clinical effects for up to a week after discontinuation of an infusion.
The pharmacodynamic profile of specific inotropes and vasopressors is a function of their relative receptor activities and mechanisms; an overview of receptor activities and physiologic effects is presented in Table 25.1 . Adrenergic receptors have traditionally been divided into α and β, and have been subdivided into α 1 , α 2 , β 1 , β 2 , and β 3 . Further subtyping has been performed, and several genetic variations have been described (see “Pharmacogenetics”).
| Drug | α Receptor | β 1 Receptor | β 2 Receptor | Cardiac Output | Heart Rate | SVR | MAP | PVR |
|---|---|---|---|---|---|---|---|---|
| Epinephrine | ++ | ++ | ++ | ↑ | ↑ | ↑ | ↑ | 0 |
| Isoproterenol | 0 | +++ | +++ | ↑ | ↑ | ↓ | ↓ | 0 |
| Norepinephrine | +++ | ++ | 0 | 0 | 0 | ↑ | ↑ | ↑ |
| Dopamine | ++ | ++ | 0 | ↑ | ↑ | ↑ | ↑ | 0 |
| Dobutamine | 0 | +++ | + | ↑ | ↑ | ↓ | ↓ | ↓ |
| Milrinone | 0 | 0 | 0 | ↑ | 0 | ↓ | ↓ | ↓ |
| Phenylephrine | +++ | 0 | 0 | 0 | ↓ | ↑ | ↑ | ↑ |
| Vasopressin | 0 | 0 | 0 | 0 | 0 | ↑ | ↑ | 0 |
| Ephedrine | + | + | + | ↑ | ↑ | ↑ | ↑ | 0 |
| Levosimendan | 0 | 0 | 0 | ↑ | 0 | ↓ | ↓ | ↓ |
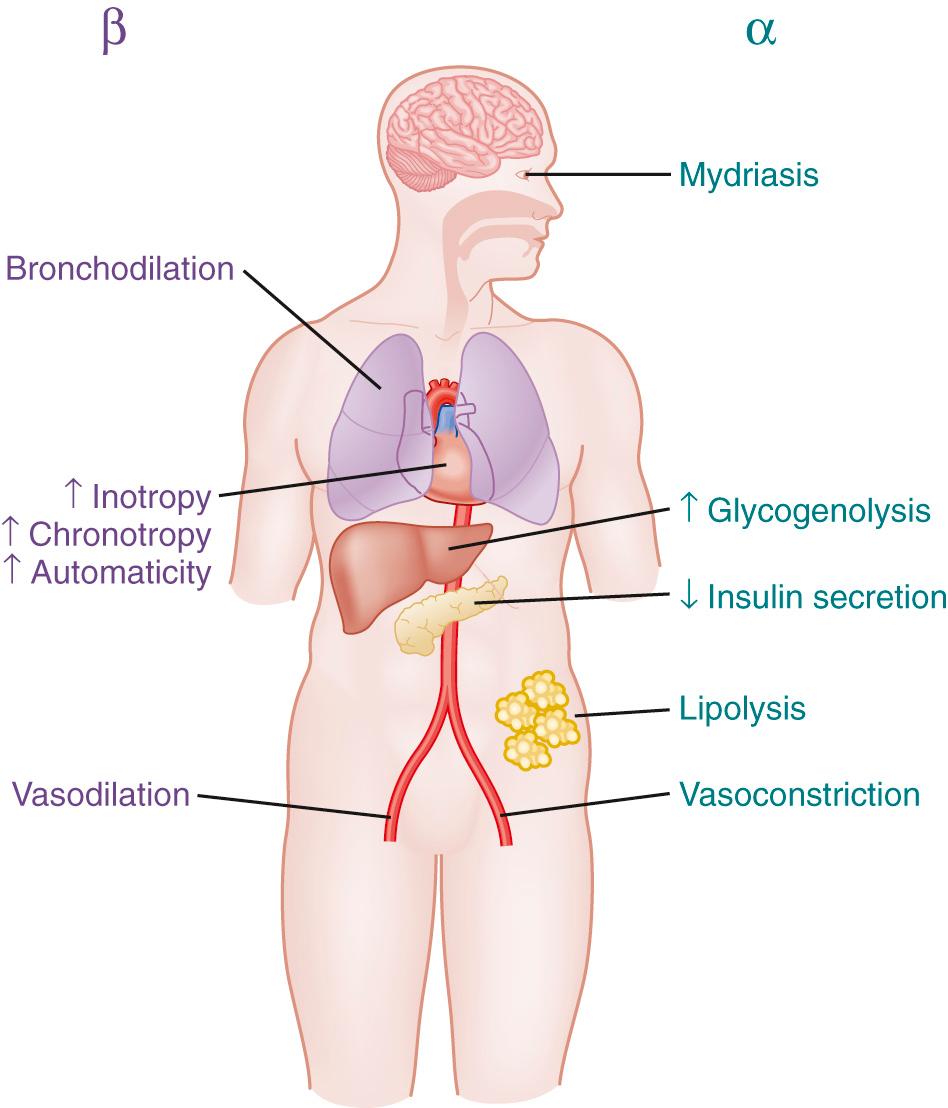
The predominant location of α 1 receptors is on peripheral vasculature; stimulation results in vasoconstriction of the skin, muscles, and renal and mesenteric vasculature. There is some contribution of peripheral α 2 receptors to vasoconstriction, but agonism of α 2 receptors is not a major characteristic of drugs discussed here (for pharmacology of α 2 agonists, see Chapter 10 ).
β 1 Receptors are primarily located in the heart, where their stimulation results in increased inotropy, chronotropy, and lusitropy. β 2 Receptors are widely distributed through the vasculature. Stimulation in peripheral vasculature results in dilation of muscular, splanchnic, and renal vessels. Bronchial smooth muscle has a high concentration of β 2 receptors, the activation of which causes bronchodilation. Additional effects include stimulation of glycogenolysis in the liver and a slowing of peristalsis. The β 3 receptor has been known for years to exist in adipose tissue, where its stimulation results in lipolysis. Its existence in the heart has been more recently recognized, and its role in normal physiology and disease, as well as the pharmacologic implications, is still being investigated. Current thinking suggests that β 3 -receptor agonism in the heart causes a decrease in inotropy.
There is a potential interaction between monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants (TCAs) and several inotropes and vasopressors ( Table 25.2 ). Because MAO contributes to the metabolism of norepinephrine and TCAs inhibit its reuptake, patients taking either type of drug can have an exaggerated hypertensive response to norepinephrine, drugs that enhance norepinephrine release (ephedrine and dopamine), and drugs that are metabolized by MAO. This adverse pharmacokinetic drug interaction can have important implications in the perioperative and intensive care settings.
| Drug | Drug of Choice | Bolus Dose | Infusion Dose | Relevant Drug Interactions |
|---|---|---|---|---|
| Epinephrine | Anaphylaxis; cardiac arrest | 5–10 µg, up to 1 mg for cardiac arrest | 0.02–0.3 µg/kg per minute | β-blockers, MAOIs, proarrhythmic medications |
| Isoproterenol | Refractory bradycardia | No bolus dosing | 0.01–0.2 µg/kg/min | No co-infusion with alkaline medications |
| Norepinephrine | Septic shock | No bolus dosing | 0.05–0.5 µ/kg per minute | MAOIs, TCAs |
| Dopamine | Septic shock with systolic dysfunction | No bolus dosing | 1–20 µg/kg per minute | MAOIs, TCAs, butyrophenones, phenothiazines, phenytoin |
| Dobutamine | Stress echocardiography | No bolus dosing | 2–20 µg/kg per minute | Co-administration with alkaline solutions can decrease activity |
| Milrinone | Weaning from cardiopulmonary bypass | Loading dose: 20–50 µg/kg over 10 min | 0.2–0.75 µg/kg per minute | Can precipitate with furosemide |
| Phenylephrine | Mild hypotension from general or regional anesthesia | 50–200 µg | 20–200 µg/min | MAOIs, TCAs |
| Vasopressin | Post–cardiopulmonary bypass vasoplegia | 0.5–2 units for mild hypotension, 20 units | 0.01–0.04 µ/min | Carbamazepine, TCAs, norepinephrine, lithium, heparin |
| Ephedrine | Mild hypotension from general or regional anesthesia | 5–10 mg | No infusion dosing | MAOIs, TCAs |
| Levosimendan | Unclear at this time | Loading dose: 12 µg/kg over 10 min | 0.05–0.2 µg/kg per minute | None yet identified |
Although basic knowledge of the α- and β-adrenergic receptors forms the foundation for understanding the pharmacology of inotropes and vasopressors, recent research has unveiled considerable genetic complexity in the receptors. There are at least nine distinct receptor subtypes (three subtypes of each α 1 , α 2 , and β) that are expressed in a variety of tissues. Many of these receptor subtypes also have well-described genetic variants. For example, 12 single-nucleotide polymorphisms (SNPs) have been identified in the β 1 receptor and 19 in the β 2 receptor. These are simple variations in the genetic code, but it is believed that they translate into clinically significant phenotypes. Polymorphisms have also been identified in the α 1 and α 2 receptors. There appears to be an association between some of these genotypes and the development of hypertension and heart failure.
The majority of research on the impact of adrenergic receptor genetic variation has focused on its implications on the development and treatment of cardiovascular disease, as well as on the clinical outcomes after certain cardiac diagnoses (e.g., myocardial infarction). Little work has focused on the effects of vasopressors and inotropes in these different genotypes. It is reasonable to expect, however, that clinically significant differences seen in the response to receptor antagonists (e.g., β blockers) might also be observed for receptor agonists. Indeed, a polymorphism in the β 1 receptor affects the response to dobutamine, with a significantly greater heart rate and inotropic response. Even though much work remains to be done in this area, it is likely that at least some of the large interindividual variability seen in the response to these drugs is a function of genetic variation.
Epinephrine is a naturally occurring sympathomimetic with nonselective adrenergic agonist activity. It is synthesized, stored, and released by the chromaffin cells of the adrenal medulla in response to physiologic stress. It binds to α, β 1 (the predominant β receptor in the heart), and β 2 (the predominant ββ receptor in the lungs and vasculature) receptors. Action at the β 3 receptor is not currently a target of clinical application of epinephrine.
Epinephrine is the drug of choice in two extreme clinical conditions: anaphylactic shock and cardiac arrest ( Fig. 25.3 ). In anaphylaxis, α-receptor–mediated vasoconstriction of small arterioles and precapillary sphincters increases mean arterial pressure (MAP) and decreases mucosal edema. Its β-receptor–mediated effects cause bronchodilation and stabilization of mast cells. The latter decreases the release of histamine, tryptase, and other inflammatory mediators that perpetuate the pathophysiology of anaphylaxis. In cardiac arrest, epinephrine is given in large doses (1 mg every 3–5 minutes) to increase MAP, thereby increasing cerebral perfusion pressure during chest compressions. The value and safety of its β-receptor–mediated effects during cardiac arrest are controversial because they increase myocardial oxygen consumption. However, studies demonstrate better survival with epinephrine than without it.
Other indications for epinephrine take advantage of specific subsets of its nonselective adrenergic agonism profile. Epinephrine is used to treat asthma (β 2 -mediated bronchodilation), severe hypotension associated with bradycardia (β 1 -mediated chronotropy) and/or low cardiac output (β 1 -mediated inotropy), and to prolong the effects of local anesthetics (α-mediated vasoconstriction). Low doses of epinephrine (0.02–0.05 µg/kg per minute) are used to increase depressed cardiac output after cardiopulmonary bypass; other catecholamines and inotropes have similar effects, but none has proven superior to epinephrine in terms of patient outcome. Epinephrine has also been studied as an alternative to other vasopressors in the treatment of vasodilatory shock from sepsis, even though the data do not yet support its use as a first-line therapy.
Epinephrine's effects are route, time, and dose dependent. At low doses (0.01–0.05 µg/kg per minute), the β-receptor effects of epinephrine predominate, while at higher doses, α effects predominate (see Table 25.1 ). An intravenous bolus of epinephrine (5–15 µg) causes an initial increase in heart rate, systolic blood pressure, and systemic vascular resistance (SVR, from stimulation of α and β receptors), and a subsequent decrease in systolic and diastolic blood pressure and vascular resistance (from continued stimulation of β receptors with peripheral vasodilation.)
In healthy subjects, increasing rates of epinephrine infusion (0.01–0.2 µg/kg per minute) progressively increase heart rate and systemic blood pressure. In general, at progressively higher continuous infusion rates, heart rate, blood pressure, SVR, and cardiac output increase while pulmonary artery pressure, central venous pressure, and pulmonary artery occlusion pressure remain unchanged.
Mast cell stabilization and bronchodilation via stimulation of β 2 receptors are the two most important nonhemodynamic, therapeutic effects of epinephrine. Epinephrine has numerous other nonhemodynamic effects that are potentially adverse. At doses typically administered for vasopressor and/or inotropic effects, these potentially adverse effects include the following:
Hyperglycemia—due to increased liver glycogenolysis, reduced tissue uptake of glucose, and inhibition of pancreatic secretion of insulin
Hypokalemia—due to increased uptake of K+ in skeletal muscle secondary to stimulation of β 2 receptors. Infusion of epinephrine at a rate of 0.1 µg/kg per minute reduces plasma K + concentration by about 0.8 mEq/L.
Lactic acidosis—in theory due to inhibition of pyruvate dehydrogenase, causing pyruvate to be shunted to lactate. Although the mechanism is not fully elucidated, epinephrine infusion results in lactic acidosis even in the absence of tissue hypoxia and may not necessarily signify a poor prognosis.
Myocardial ischemia—due to hypertension, tachycardia, and increased inotropy that increase myocardial oxygen demand.
Epinephrine is administered by continuous infusion, bolus, infiltration, or inhalation. Usual intravenous infusion doses are 0.02 to 0.3 µg/kg per minutes. Intravenous bolus doses range from 5 to 10 µg for moderate hypotension (MAP 40–60 mm Hg) unresponsive to other vasopressors up to 1 mg as recommended by the American Heart Association guidelines for cardiac arrest. The usual intramuscular dose is 0.3 mg administered into the lateral thigh (vastus lateralis), which produces significantly higher plasma concentrations than administration into the deltoid or subcutaneously. Subcutaneous administration results in delayed absorption and lower peak plasma concentrations than other routes. This is generally reserved for treatment of severe asthma in doses of 0.3 to 0.5 mg for adults or 0.01 mg/kg for children, when inhaled selective β 2 agonists cannot be administered. In addition, epinephrine can be administered via an endotracheal tube during cardiac arrest if other routes are not available; the recommended dose is double the intravenous dose diluted with 10 mL of normal saline solution. Epinephrine is not effective orally owing to rapid metabolism and does not cross the blood-brain barrier in sufficient amounts to directly affect the central nervous system.
Epinephrine should not be used in patients with acute cocaine intoxication because of the potential for exacerbation of myocardial ischemia and stroke. In patients with dynamic obstructions to ventricular outflow (e.g., tetralogy of Fallot and hypertrophic obstructive cardiomyopathy), epinephrine can worsen outflow obstruction and lower cardiac output. Administration of epinephrine with a β blocker can lead to significant α-receptor stimulation without opposing β-receptor–mediated vasodilation, which can result in severe vasoconstriction, hypertension, and heart failure. Care should also be taken when administering epinephrine with medications that predispose the heart to arrhythmia, particularly digitalis and halothane.
Isoproterenol was approved by the U.S. Food and Drug Administration (FDA) in 1947 and was used initially to treat asthma. Interestingly, it was the first drug for which the FDA required a package insert beginning in 1968. As the isopropyl derivative of norepinephrine, isoproterenol is a synthetic sympathomimetic with nonselective β-adrenergic activity.
Stimulation of cardiac β 1 receptors by isoproterenol increases heart rate, inotropy, and lusitropy, resulting in an increase in cardiac output and systolic blood pressure. Stimulation of β 2 receptors results in vasodilation of the muscle, kidney, skin, and splanchnic circulations, thereby decreasing total peripheral vascular resistance and mean and diastolic blood pressure. The decrease in systemic blood pressure combined with increases in myocardial contractility and heart rate can precipitate myocardial ischemia in patients with significant coronary artery disease. At higher doses, palpitations, headache, and flushing can occur.
Isoproterenol was used initially via inhaler to treat asthma and bronchospasm but has been replaced by β 2 -selective bronchodilators. Currently isoproterenol is indicated in hemodynamically significant bradycardia until cardiac pacing can be established. In prior years, it was used immediately following cardiac transplantation to enhance inotropy and chronotropy without concomitantly increasing systematic vascular resistance. Currently other drugs are used in this setting more commonly (e.g., epinephrine, milrinone, and vasopressin with cardiac pacing as necessary). Isoproterenol is also being used during electrophysiology procedures to stimulate the underlying arrhythmia for better mapping and increases the likelihood of a successful ablation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here