Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A thorough understanding of the basic principles of cardiovascular physiology is essential for effective and safe patient management in the perioperative period. This information provides a theoretical rationale for the use of drugs, intravenous infusions, and other therapeutic measures to maintain and optimize vital organ function.
The primary role of the circulation is to provide sufficient blood flow to satisfy the metabolic demands of body tissues. However, the circulation has additional functions, not considered here, including return of carbon dioxide to the lungs and other metabolic end products to the kidneys, supply of nutrients absorbed from the gastrointestinal tract to the tissues, regulation of body temperature, and distribution of hormones and other agents that regulate cellular function.
Tissue blood flow depends on activity of both the heart and blood vessels ( Fig. 24.1 ); arterial pressure, a major determinant of tissue blood flow, is the product of cardiac output and total peripheral resistance, while local vascular resistance, the other major determinant of tissue blood flow, is a function of local vasomotor tone. The complex interplay of the relationships summarized in Fig. 24.1 is the primary focus of this chapter.
The atria are thin-walled structures that are similar in size and dimension on the right and left sides of the heart. However, the left and right ventricles (LV and RV) are quite different; the LV has an ellipsoidal shape and is thick-walled, whereas the RV has a thin wall and is crescent-shaped (owing to the concave free wall opposite the convex interventricular septum) approximating a pyramid with a triangular base longitudinally. The mass of the LV is approximately six times that of the RV, which reflects their respective pressure loads (systemic vascular resistance is 10 times the pulmonary vascular resistance) and hence, the RV performs ![]() stroke work compared with the LV. Because of its thin wall, acute increases in afterload are poorly tolerated by the RV. A high degree of interaction and interdependence exists between the ventricles because of their shared interventricular septum and the restraining influence of the surrounding pericardium (except after cardiac surgery). The shift of the interventricular septum secondary to an increase in volume or pressure load of one ventricle impairs the filling of the other ventricle by reducing chamber compliance and diastolic filling.
stroke work compared with the LV. Because of its thin wall, acute increases in afterload are poorly tolerated by the RV. A high degree of interaction and interdependence exists between the ventricles because of their shared interventricular septum and the restraining influence of the surrounding pericardium (except after cardiac surgery). The shift of the interventricular septum secondary to an increase in volume or pressure load of one ventricle impairs the filling of the other ventricle by reducing chamber compliance and diastolic filling.
At the cellular level, the heart consists primarily of cells that contract (myocytes) or conduct impulses (Purkinje fibers). The myocardium proper is composed of individual myocytes linked by specialized gap junctions to form a functional syncytium that allows rapid conduction of electrical charge, and intercalated discs that modulate force transmission during contraction. Cardiac cells display five basic characteristics: excitability (bathmotropy), conductivity (dromotropy), rhythmicity (chronotropy), contractility (inotropy), and relaxation (lusitropy).
Cardiac output is the volume of blood pumped to body tissues per minute; it is equal to the product of heart rate and stroke volume ( Fig. 24.2 ). Normal values for cardiac output are 5 to 6 L/min in a 70-kg man, with a heart rate of 80 beats/min and a stroke volume of 60 to 90 mL/beat. Cardiac index is a normalized value for cardiac output based on body surface area, normally 2.5 to 3.5 L/min/m 2 . Cardiac output varies in proportion to work requirement and oxygen demand.
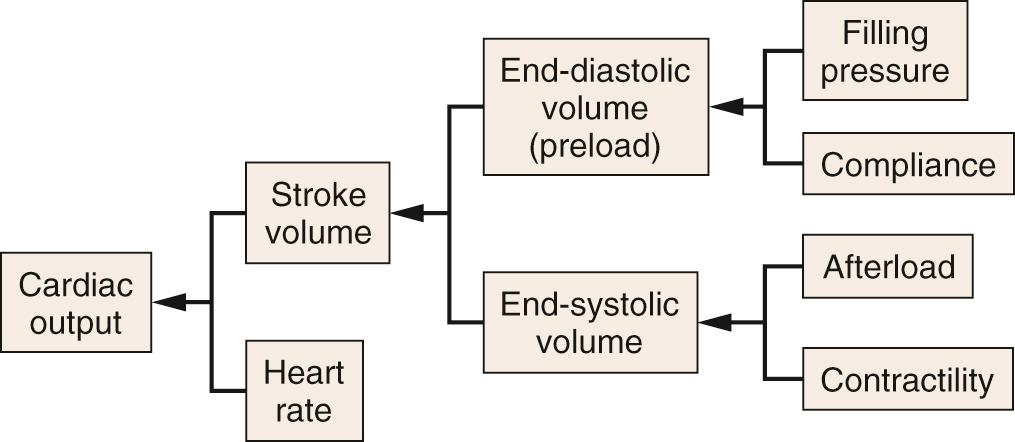
Heart rate is normally determined by rhythmic spontaneous depolarizations of pacemaker cells in the sinoatrial (SA) node. The rate of these depolarizations is modulated by the autonomic nervous system. Sympathetic stimulation increases activity, whereas parasympathetic stimulation (vagus nerve) decreases activity of the SA node.
Stroke volume is the difference between end-diastolic volume and end-systolic volume (see Fig. 24.2 ). It can be influenced by changes in end-diastolic volume (Starling's law), myocardial contractility, and afterload (see later text). The sarcomere is the basic functional unit of the myocardium ( Fig. 24.3A ). The ultrastructural arrangement of the thick (myosin) and thin (actin) myofilaments within the sarcomere and their interaction explain much of the mechanical behavior of cardiac muscle. Cardiac muscle contraction is initiated by an increase in intracellular calcium ions (Ca 2+ ), which results in the formation of cross-bridges between adjacent actin and myosin filaments. This process draws thin myofilaments and the Z-lines toward the center of the sarcomere and is the fundamental mechanism for myocardial contraction.
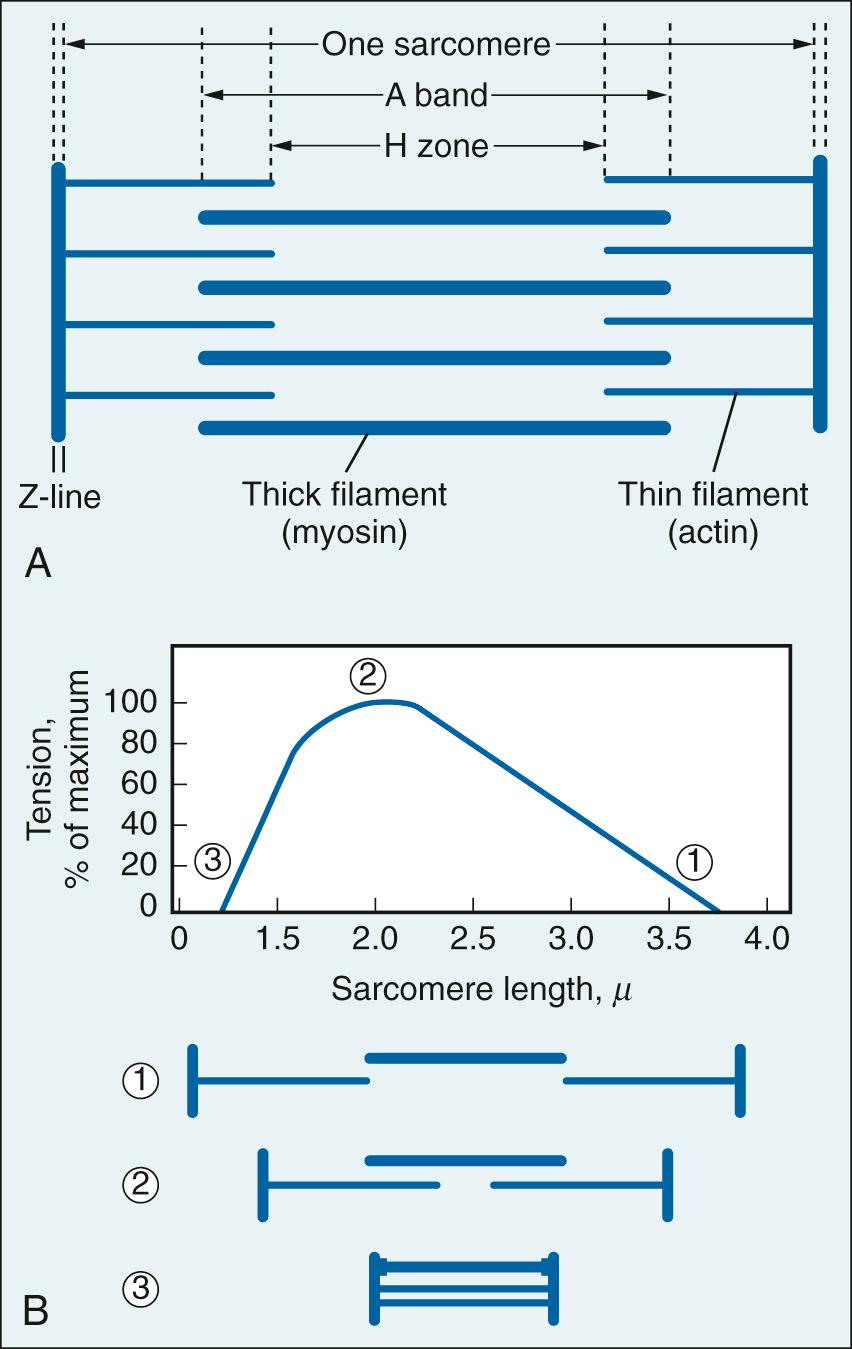
The relation between resting sarcomere length and developed tension was originally defined in isolated skeletal muscle fibers (see Fig. 24.3B ). Developed tension is a direct function of the number of cross-bridges pulling in parallel, and thus on the amount of overlap between thin and thick filaments prior to activation. Subsequent work extended this behavior to cardiac muscle. The length-tension relation provides the basis for Starling's law of the heart: the strength of contraction of the intact heart is proportional to the initial length of the cardiac muscle fiber—that is, end-diastolic volume (preload). This can be demonstrated using a cardiac function curve, which is a plot of ventricular performance (i.e., stroke volume) as a function of ventricular end-diastolic volume or an index thereof, such as ventricular end-diastolic pressure ( Fig. 24.4A ).
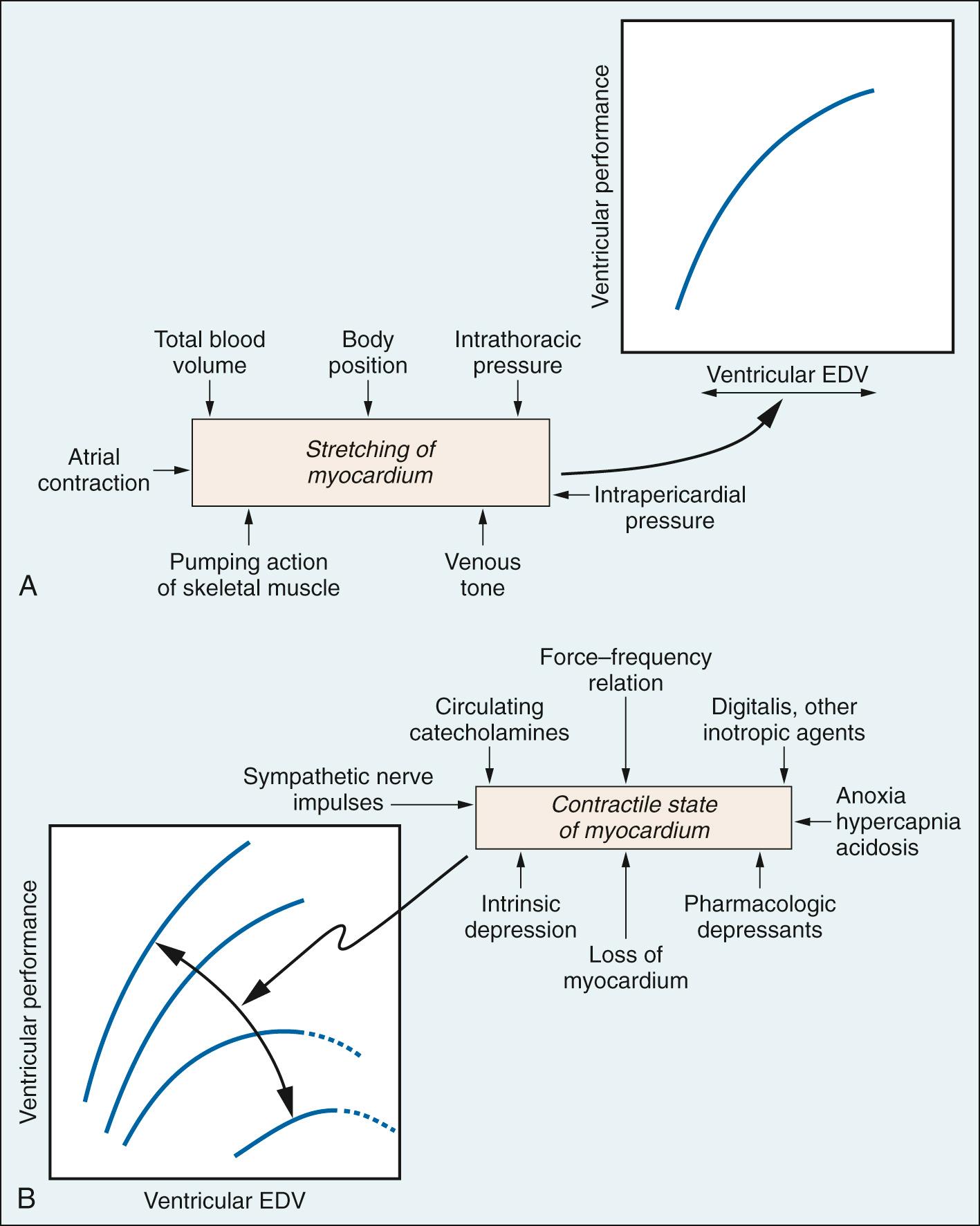
In vivo cardiac muscle fibers are stretched by venous filling pressure. Normally, the volume in the ventricle before contraction (preload) sets the sarcomere to a suboptimal length; thus the active tension that can be developed is not maximal. Increases in end-diastolic volume owing to enhanced venous return cause increases in sarcomere length, resulting in a proportional rise in force development and ventricular stroke work. Active tension development levels off as the sarcomere is increased to the optimal length. Beyond this point, overstretching of the sarcomere is accompanied by a decline in tension development. Clinicians traditionally have equated filling pressure with preload, which assumes that the relationship between end-diastolic pressure and end-diastolic volume (i.e., the compliance or distensibility) is constant. However, a fibrotic heart, hypertrophied heart, or aging heart has reduced compliance.
Venous return, and thus cardiac filling, is augmented by conditions associated with reduced systemic vascular resistance. These include the opening of arteriovenous fistulae or conditions that mimic it, such as fever (marked dilation of cutaneous beds), pregnancy, and exercise (see Fig. 24.4A ).
Rapid, large reductions in total blood volume reduce venous return. At any given total blood volume, venous return is a function of the distribution of blood between the intrathoracic and extrathoracic compartments. For example, assumption of an upright posture, because of gravity, tends to increase extrathoracic volume at the expense of intrathoracic volume, thus reducing venous return. Elevation of intrathoracic pressure, as occurs during positive-pressure ventilation or pneumothorax, has a similar effect.
Sympathetic nerve stimulation produces systemic venoconstriction, which augments cardiac filling, whereas drugs that interfere with adrenergic nerve function, such as ganglionic blockers or drugs that act directly to relax venous smooth muscle such as nitrates, have the opposite effect. Extravascular compression of veins by contracting muscle increases venous return during exercise.
Atrial contraction normally contributes less than 20% to LV diastolic filling in young healthy adults. It becomes more important at rapid heart rates “where the time available for passive ventricular filling is limited” and in patients with decreased LV compliance “where the atrial contraction contribution approaches 50% of the LV diastolic filling.”
Increases in pericardial pressure, as in cardiac tamponade, limit ventricular filling and stroke volume.
Contractility relates to the ability of the myocardium to perform mechanical work (i.e., to generate force and shorten), independently of changes in preload or afterload with heart rate fixed. Contractility can be illustrated graphically using a family of cardiac function curves (see Fig. 24.4B ). Changes in contractility can augment cardiac performance (positive inotropic effect) or depress it (negative inotropic effect). Movement of an entire curve upward (more work for a given preload) or downward (less work for a given preload) signifies a positive or negative inotropic effect, respectively. Positive inotropic factors include circulating catecholamines and the cardiac sympathetic nerves, whereas negative inotropic factors include severe anoxia, acidosis, and the volatile anesthetics.
Afterload is defined as the force opposing fiber shortening during ventricular ejection. It is not synonymous with systemic arterial pressure, vasomotor tone, or vascular resistance. Instead, it should be thought of as the tension or stress in the ventricular wall during ejection. In accordance with the law of Laplace, afterload is directly related to intraventricular pressure and size and inversely related to wall thickness. Because of changing size, pressure, and wall thickness, afterload varies continuously during ventricular ejection. Thus it is difficult to quantify with precision. Despite the widespread use of aortic pressure for the LV and pulmonary artery pressure for the RV as indices of afterload in vivo, this approach should not be considered quantitative.
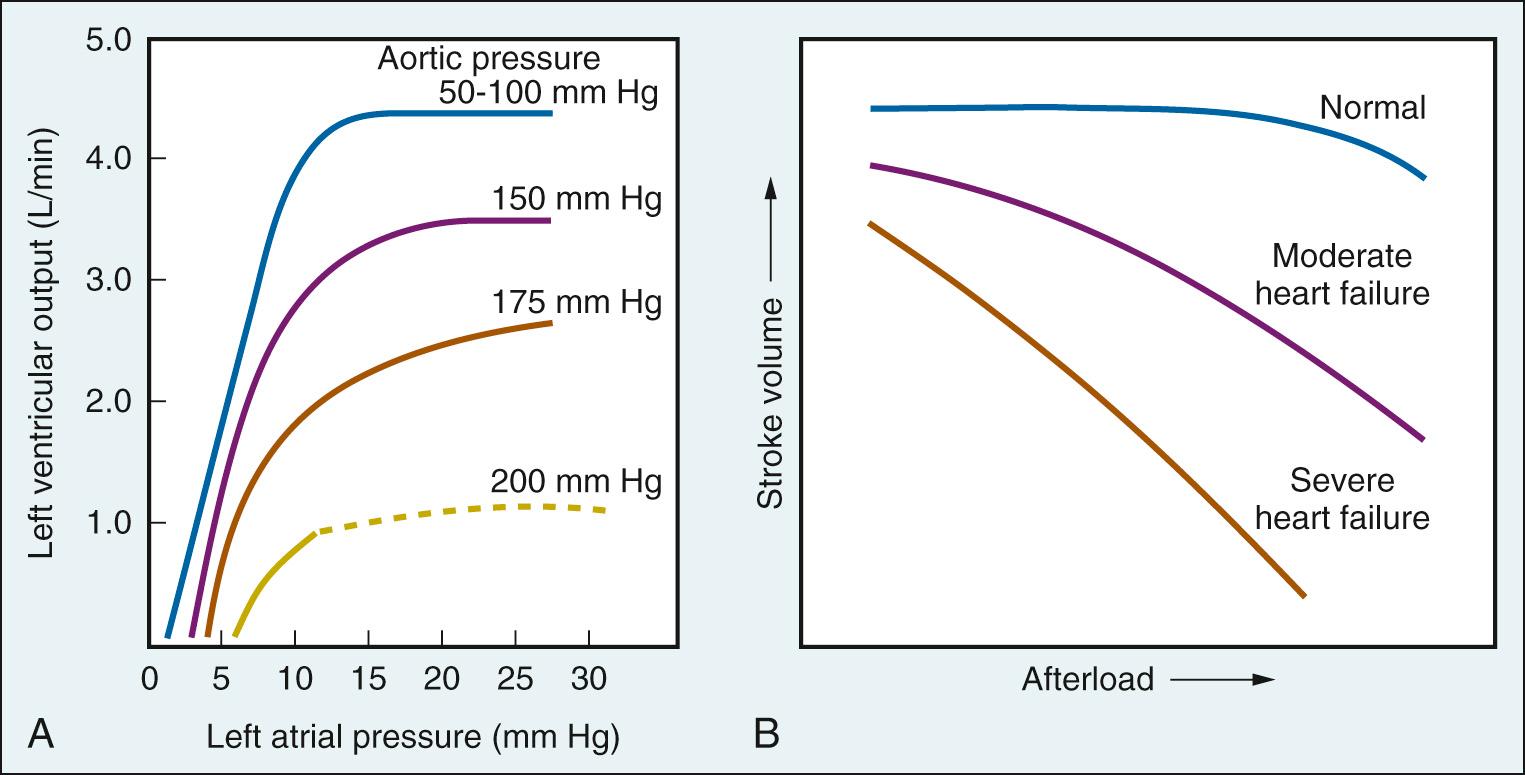
In isolated heart preparations, in which preload, inotropic state, and heart rate are controlled, increases in afterload cause reductions in left ventricular output (i.e., stroke volume; Fig. 24.5A ). In the intact circulation, this impairment in cardiac performance can be avoided by compensatory increases in contractility and/or venous return (preload). As demonstrated in Fig. 24.5B , there is no change in stroke volume when normal hearts are exposed to increased afterload (outflow resistance), but a decrease in stroke volume when failing hearts pump against similar conditions.
The events of the cardiac cycle are shown on Fig. 24.6 . The salient points are as follows:
Atrial systole begins after the P wave of the electrocardiogram; ventricular systole begins near the end of the R wave and ends just after the T wave.
When ventricular pressure exceeds aorta pressure, the aortic valve opens, and ventricular ejection begins (at point O in Fig. 24.6 ).
The amount of blood ejected by the ventricle (stroke volume) is typically approximately 65% of end-diastolic volume (ejection fraction).
Most ventricular filling occurs prior to atrial systole.
Events on the right side of the circulation are similar to those on the left side but are somewhat asynchronous. Right atrial systole precedes left atrial systole, and contraction of the RV typically begins after that of the LV. However, because pulmonary arterial pressure is less than aortic pressure, RV ejection precedes LV ejection.
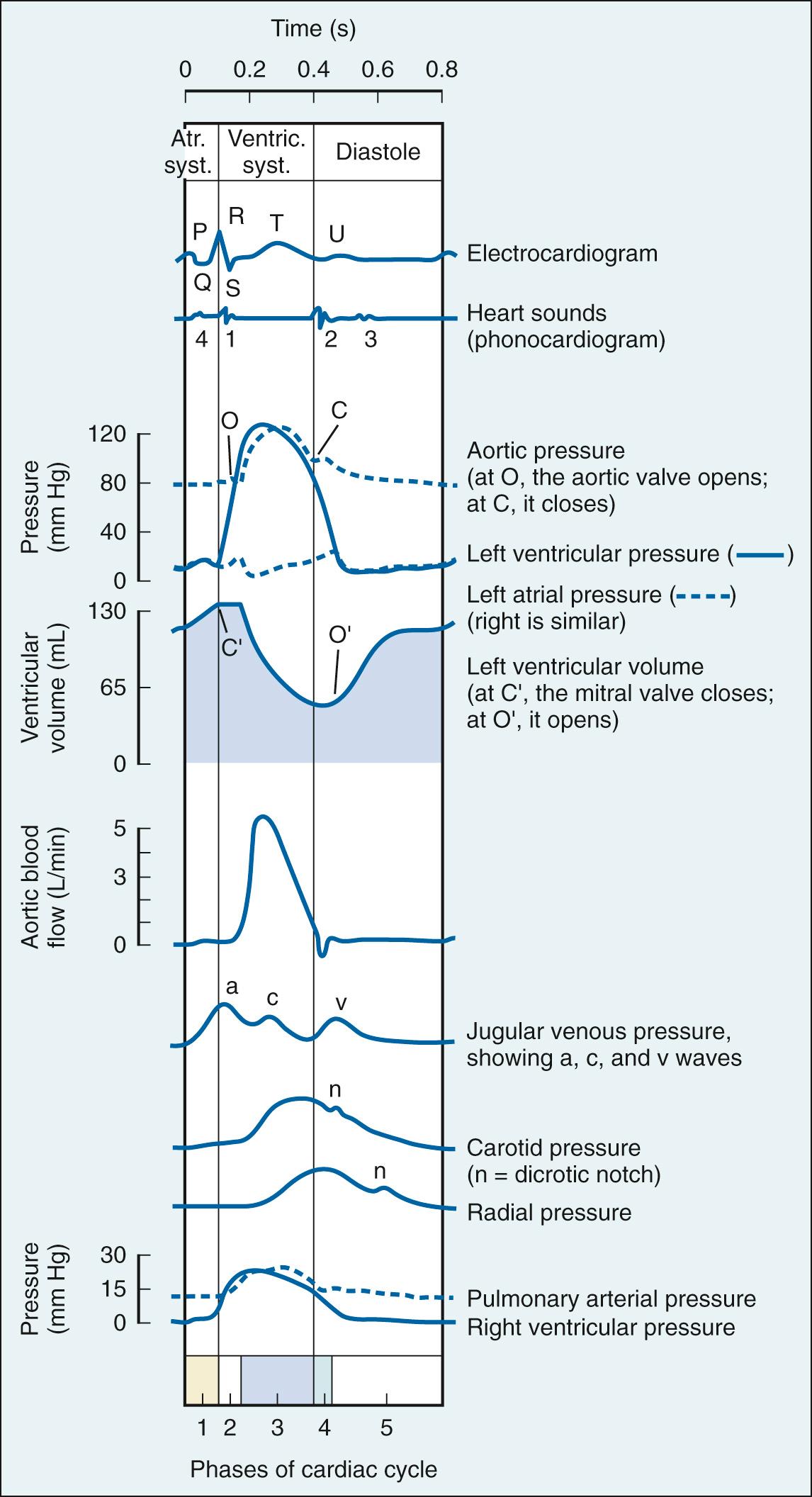
Ventricular diastole is defined as the period in the cardiac cycle from the end of ejection until the onset of ventricular tension development of the succeeding beat and is composed of four phases: isovolumic relaxation, early rapid filling, diastasis, and atrial systole. Ventricular lusitropy is defined as the active relaxation of the ventricles that requires expenditure of energy by ventricular myocytes, which coincides with the isovolumic relaxation phase. This active process can be quantified most precisely as a time constant of the isovolumic pressure decline (the Greek letter tau, τ), calculated as the mono-exponential decline of pressure during the isovolumic phase of the cycle. A prolonged τ indicates impaired relaxation, and thus impaired ventricular filling. This occurs in chronic pathologic conditions, such as hypertrophy and cardiomyopathy, and in acute processes, such as ischemia and administration of negative inotropic drugs. In contrast, a short τ indicates enhanced relaxation and ventricular filling, which can occur following administration of positive inotropic drugs.
The phase of rapid early filling follows isovolumic relaxation and begins when ventricular pressure falls below atrial pressure. During this period, elastic recoil of the myocardium in combination with continued relaxation create an atrial/ventricular pressure gradient (sometimes characterized as suction) that greatly facilitates ventricular filling. As the atrial/ventricular pressure gradient diminishes, the phase of diastasis (slow ventricular filling) begins, continuing until atrial systole. Early rapid filling normally accounts for 80% to 85% of ventricular end-diastolic volume; diastasis and atrial systole provide approximately 3% to 5% and 15% to 25%, respectively. Multiple processes can alter diastolic filling dynamics, most notably ectopy arising from the atrioventricular node and ventricular pacing (no atrial systole), and reductions in ventricular compliance, such as concentric hypertrophy, ventricular interaction, and pericardial constraint.
The pattern of contraction differs for the LV and RV. The LV contracts in a relatively homogeneous fashion with both the short and long axes shortening simultaneously. In contrast, the RV, which normally develops a pressure only 20% of that in the LV, contracts sequentially from the inflow tract to the outflow tract. The mechanical significance of this sequential pattern of contraction is unclear, but the process appears to reflect the different embryology of the inflow and outflow tracts, and is altered by sympathetic stimulation, positive inotropic drugs, autonomic blockade, and volatile anesthetics.
The thermodilution method was first described by George Neil Stewart at the end of the 19th century when he injected a bolus of sodium chloride solution into a central vein, which was detected in a contralateral femoral artery. The change in concentration of the sampled blood was proportional to the cardiac output. Later Hamilton et al. modified this method and promoted the concept of an explicit time-concentration curve. Cardiac output was calculated as the amount of indicator injected/area of dilution curves. Indocyanine green was the conventional indicator used until the 1970s.
Introduction of the flow-directed pulmonary artery catheter (PAC) in the late 1960s allowed measurement of both cardiac output using a Stewart-Hamilton thermodilution curve and pulmonary capillary wedge pressure, which is a reflection of LV preload. The measurement of cardiac output entails injection of an indicator (usually 10 mL of 0.9% sodium chloride), with a known temperature, into a central vein and detection of the change in blood temperature downstream at the tip of the PAC. This approach made it possible for clinicians to construct ventricular function curves at the bedside and to use this information to guide treatment. Its validity requires several conditions :
Pressure from the left atrium (which is on average equal to LV end-diastolic pressure) must be reflected back through the pulmonary circulation; thus the tip of the pulmonary artery catheter must be wedged in a small pulmonary vessel so blood cannot flow beyond it. In addition, the mitral valve should not have significant stenosis or regurgitation.
Changes in the relationship between ventricular volume and pressure during diastole (compliance) must be taken into account. A stiff or noncompliant ventricle, as evidenced by impaired diastolic filling and exaggerated pressure increase, erroneously implies an increased preload, even though muscle stretch may be unaltered or even reduced. A stiff ventricle is characteristic of a variety of pathologic states, including myocardial ischemia, healing or healed myocardial infarction, myocardial hypertrophy, and constrictive pericarditis.
Afterload and heart rate must be constant when multiple cardiac function curves are used to assess changes in myocardial contractility, as reflected in stroke volume at a given preload.
The tricuspid valve cannot have significant regurgitation because this causes variation in the transit time of the indicator, yielding both underestimates and overestimates of cardiac output.
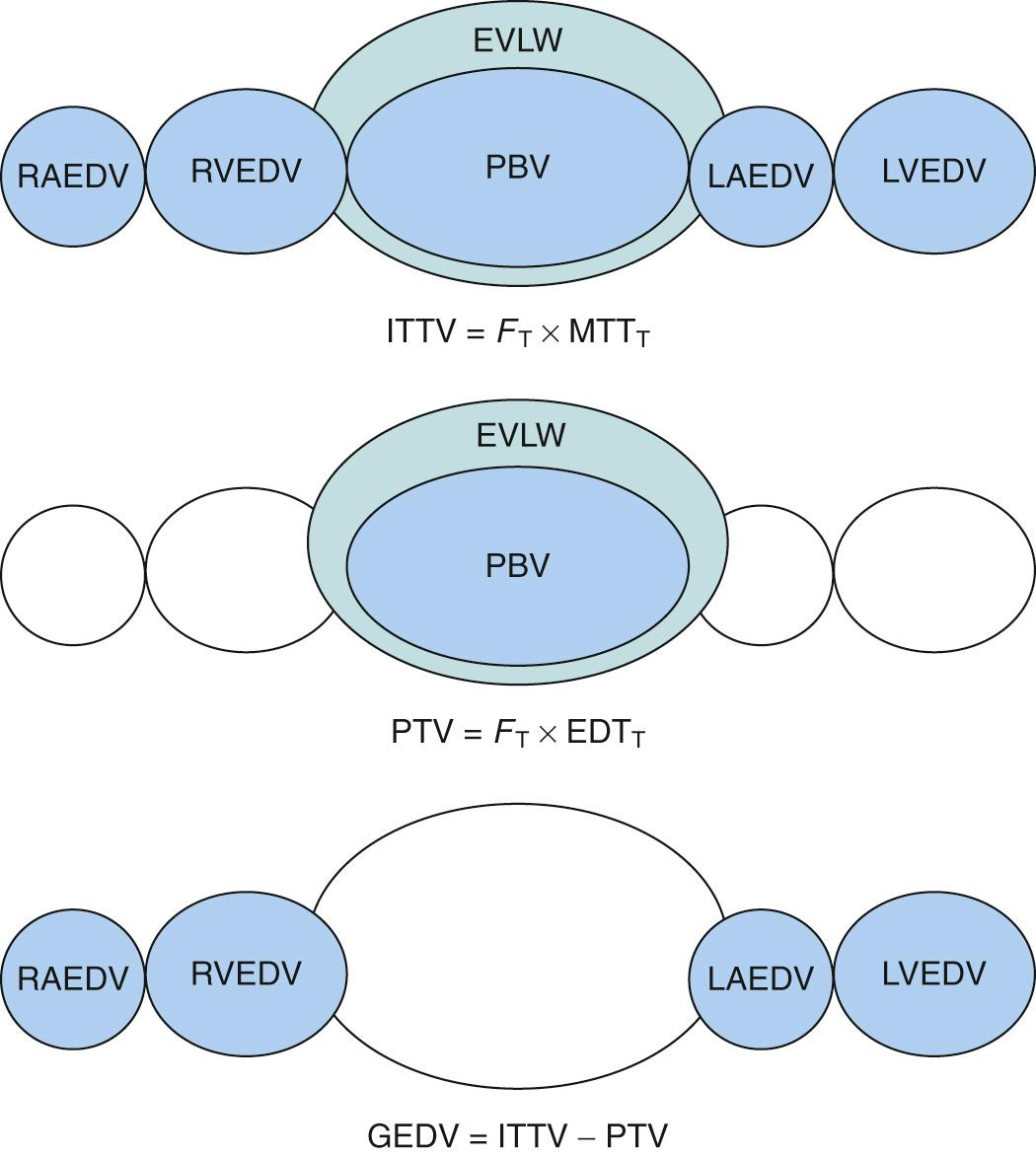
This method also applies to the Stewart-Hamilton principle to calculate cardiac output. However, in this case, cold saline solution is injected into a central vein and the change in temperature is detected with thermistor-tipped catheter placed in a central artery, commonly the femoral artery or the axillary artery. This method differs from that using a PAC in that it measures LV rather than RV cardiac output. The transpulmonary thermodilution method allows the calculation of additional indices of cardiac function such as the following:
Global end-diastolic volume (GEDV). GEDV is the volume of blood present in the four chambers of the heart. This parameter provides a better estimate for preload than pulmonary artery occlusion pressure because it is an actual volume rather than a pressure surrogate. Thus the use of GEDV avoids erroneous conclusions based on high LV filling pressure in the stiff noncompliant ventricle.
Extravascular lung water. The transpulmonary thermodilution method allows the calculation of pulmonary blood volume and thus an estimation of extravascular lung volume ( Fig. 24.7 ). The latter findings provide an early index of pulmonary edema and fluid overload in critically ill patients.
One of the most common and useful indices of contractility is the maximal rate of change of the ventricular pressure pulse, so-called dP/dt max. Historically, a high-fidelity catheter was inserted into the LV to obtain these measurements. Currently the use of echocardiography allows the determination of dP/dt max noninvasively. Interventions that acutely augment myocardial contractility, such as exercise and catecholamines, increase this index. Values in the normal LV are approximately 1000 mm Hg/sec, whereas those in the RV average only 400 mm Hg/sec. This difference has been attributed to the fact that peak dP/dt usually occurs at the instant of the opening of the semilunar valves and that developed pressure is substantially higher in the LV compared with the RV, and not to a difference in contractile state between ventricles. Disadvantages of dP/dt max as an index of contractility include distortion by wall properties and valvular dysfunction, and dependence on loading conditions and heart rate. To correct for load dependency, the ratio of dP/dt to developed pressure or to a standard pressure has been used.
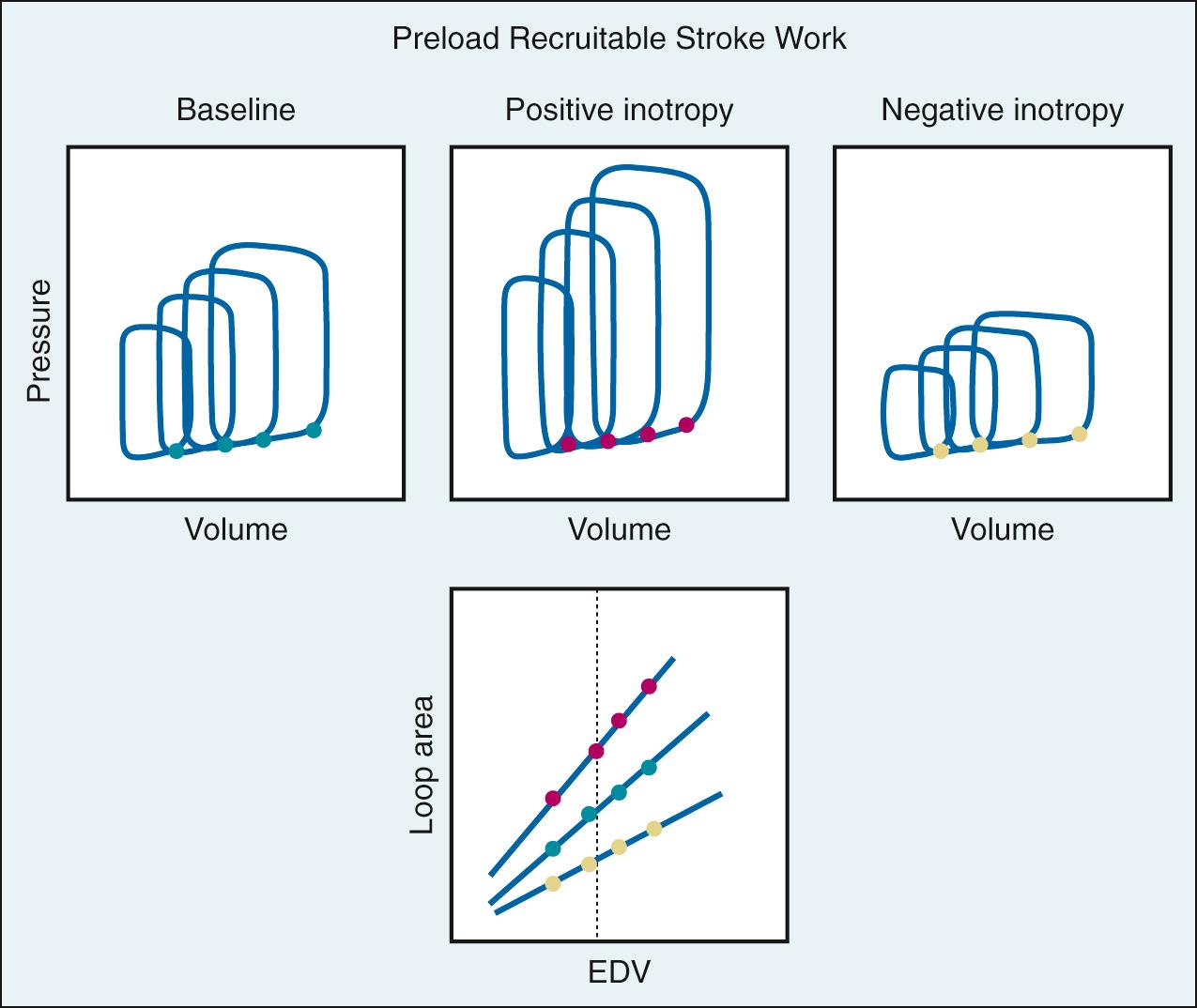
The most frequently used clinical index of global contractile function is ejection fraction. Both invasive and noninvasive techniques have been used to determine ejection fraction from image-based volume measurements (echocardiography, angiography, magnetic resonance imaging, positron emission tomography scanning) or indicator dilution techniques. Although the ejection fraction provides useful information about systolic pump performance, it is heavily influenced by afterload. Such load dependence is common to virtually all indices of contractility based on ejection phase parameters. Another ejection phase index of contractility is the preload recruitable stroke work (PRSW) relationship. To measure PRSW, venous return is decreased by inferior vena cava occlusion, resulting in a progressive reduction in ventricular end-diastolic volume. The area of the pressure-volume loop (which represents external work) is plotted for each beat as a function of end-diastolic volume ( Fig. 24.8 ). The slope of this relationship defines the work the ventricle can perform for a given preload and is a reflection of contractility; increased slope indicates increased contractility, and a fall in slope indicates decreased contractility. An advantage of PRSW is that it provides an index of overall ventricular performance, combining both systolic and diastolic components; however, it is not practical in the clinical environment.
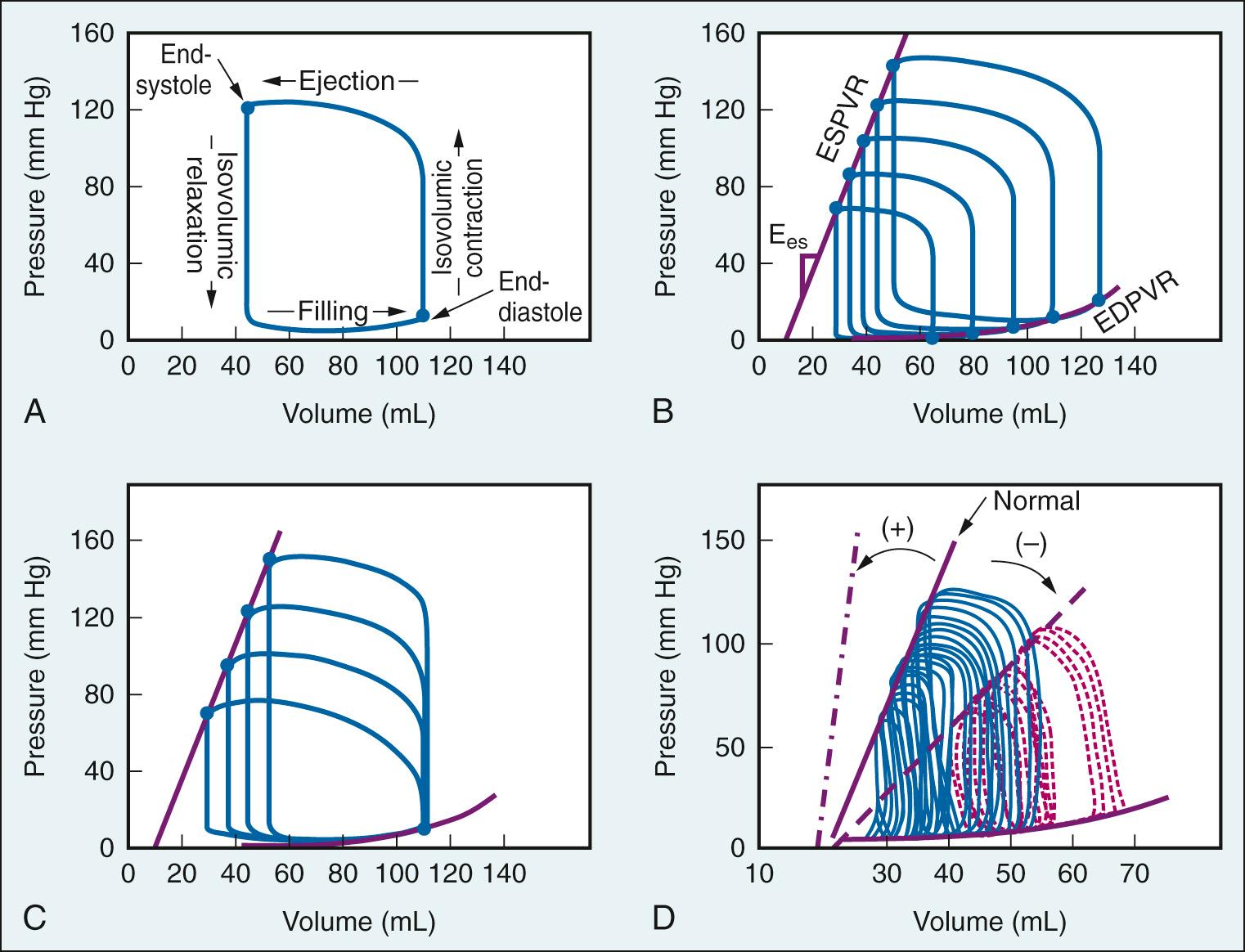
In 1898 Otto Frank, using data from studies in isolated frog ventricles, presented the cycle of ventricular contraction as a loop defined by pressure (P) in the vertical axis and volume (V) in the horizontal axis. Because of difficulties in measuring ventricular volume in vivo, research on pressure-volume relationships proceeded slowly during the first two-thirds of the 20th century. With the development of the isolated blood-perfused canine heart preparation, echocardiography, and ventriculography for studies in humans, there was a resurgence of activity in the 1970s and 1980s. The use of pressure-volume analysis has been established as a powerful method to characterize ventricular pump properties throughout the cardiac cycle independent of loading conditions. However, the method is highly sophisticated, laborious, and requires specialized equipment and training.
The hemodynamic changes during a single cardiac cycle are displayed by plotting instantaneous ventricular pressure versus volume ( Fig. 24.9A ). Under steady-state conditions, this loop is repeated with each contraction. For a given cardiac cycle, there is a pressure-volume point that coincides with end-diastole (at the lower end of the loop). The increase in ventricular pressure during this period of filling, which ends when the mitral valve closes, reflects the compliance of the ventricular wall. During isovolumic contraction, pressure increases steeply while volume remains constant. Ventricular pressure rises to a level in excess of aortic pressure, the aortic valve opens, and blood is ejected. Ventricular ejection (systole) continues until ventricular pressure falls below aortic pressure and the aortic valve closes. The upper left-hand corner of the loop coincides with end-systole. The period of isovolumic relaxation follows, which is characterized by a sharp decrease in pressure and no change in volume. The mitral valve then opens, thus completing one cardiac cycle. The area within the pressure-volume loop represents the internal work of the ventricle, whereas external work is determined by the product of stroke volume and aortic pressure. An intervention that acutely changes loading conditions but has no effect on contractility, such as transient caval occlusion to reduce preload (see Fig. 24.9B ) or administration of phenylephrine to increase afterload (see Fig. 24.9C ), generates a family of loops. The end-systolic points in the series of loops conform to a linear pressure-volume relationship, which defines the end-systolic pressure-volume relationship (ESPVR). End-systolic elastance (Ees) defines the slope of ESPVR and is a load-independent index of contractility. The diastolic pressure-volume points define a nonlinear end-diastolic relationship (EDPVR). With a constant contractile state and afterload, the progressive reduction in preload causes the loops to shift toward lower volumes at end-systole and end-diastole, resulting in a decrease in stroke volume (see Fig. 24.9B ). A selective increase in afterload causes narrowing and elongation of the loops, which results in a decrease in stroke volume (see Fig. 24.9C ). The ESPVR responds to acute changes in myocardial contractility; an increase in its slope, Ees, indicates a positive inotropic intervention and a decrease in Ees indicates a negative inotropic intervention (see Fig. 24.9D ).
The pressure-volume characteristics for the RV differ markedly from those of the LV ( Fig. 24.10 ). Although clear in the pressure-volume loop of the LV, end-systole is not well defined in the normal RV. Owing primarily to the sequential pattern of right ventricular free wall contraction, the low resistance of the pulmonary vascular bed, and the fact that blood ejected from the RV has inertia, the peak pressure within the RV normally occurs very early in systole and blood continues to leave via the pulmonic valve for an extended period. Consequently, the pressure-volume loop for the RV has a more triangular shape, with only a brief period of isovolumic relaxation. The prolonged low-pressure emptying of the RV renders this chamber very sensitive to changes in afterload. Because the RV does not demonstrate a defined end-systolic point, Ees analysis is not readily applicable, and PRSW is considered far superior for assessment of contractility. With pulmonary hypertension, the pressure-volume loop in the RV is not triangular but resembles that of the LV, largely because of a change in both the magnitude and timing of peak RV pressure.
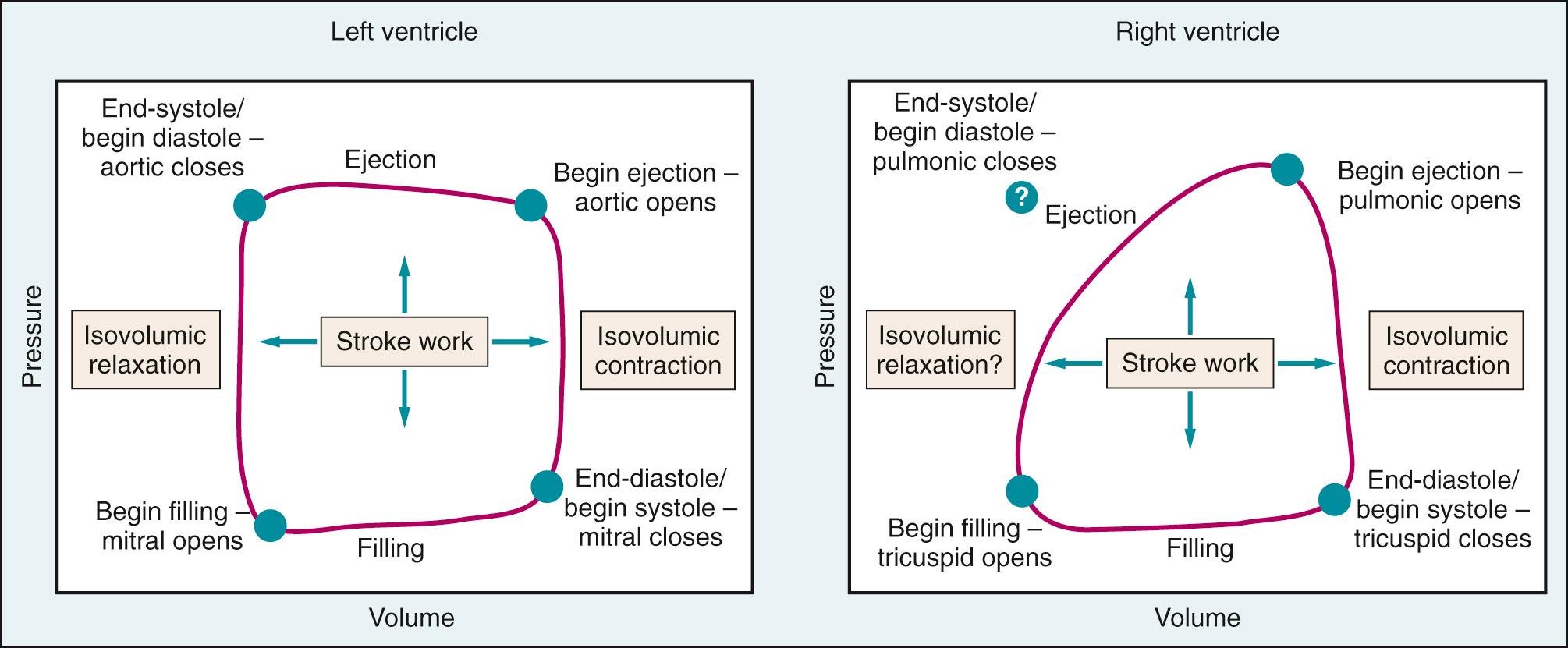
Fig. 24.11 compares the pressure changes as blood flows through the series-coupled components of the systemic and pulmonary circulations, from the large arteries to the arterioles, capillaries, and veins. The normal pulmonary circulation is a low-pressure, low-resistance circuit that accommodates the entire output of the RV. This results in a smaller workload for the RV. Although pressure in the ventricles falls nearly to zero during diastole, pressure is maintained in the large arteries. This is possible because a portion of the energy released by cardiac contraction during systole is stored in the distensible large arteries (the Windkessel effect). During diastole, the elastic recoil of the vessels converts this potential energy into forward blood flow, which ensures that capillary flow is continuous throughout the cardiac cycle. The most severe drop in pressure occurs in the arterioles; hence they are often termed resistance vessels. The diameter of arterioles is regulated by contractile activity of vascular smooth muscle. Variations in arteriolar diameter are an important determinant of local capillary blood flow and hydrostatic pressure. The summed effect of all the systemic resistance vessels—that is, the total peripheral resistance—is a major determinant, along with cardiac output, of arterial pressure, and thus of the driving force for tissue blood flow (see Fig. 24.1 ).
Blood flow (F) is a function of arteriovenous pressure gradient (Pa − Pv) and local vascular resistance (R), according to the equation:
This is analogous to Ohm's law in an electrical circuit. Because arterial and venous blood pressures are normally well maintained within narrow limits by homeostatic mechanisms, tissue blood flow usually varies inversely as a function of vascular resistance.
Poiseuille performed studies that yielded an equation describing resistance to flow in a straight, rigid tube of length (l) and radius (r):
where η is the viscosity. Of note is that flow resistance varies inversely with tube radius raised to the fourth power. Thus small changes in tube radius cause large changes in resistance.
Because the length of blood vessels is relatively fixed, geometric changes in blood flow resistance occur by variations in vessel radius. These adjustments are primarily the result of contraction or relaxation of the smooth muscle investing the arterioles, which are the principal site of vascular resistance. However, in certain vascular beds (e.g., the left ventricular myocardium), extravascular compressive forces also play a role (see later text). Chemical factors, which are linked to the metabolic activity of the tissue (e.g., carbon dioxide) modulate vascular resistance so that blood flow (and oxygen delivery) is commensurate with local oxygen demands.
Viscosity is the internal friction resulting from intermolecular forces operating within a flowing liquid. The term internal friction emphasizes that as a fluid moves within a tube, laminae in the fluid slip on one another and move at different speeds. The movement produces a velocity gradient in a direction perpendicular to the wall of the tube termed the shear rate . Shear rate shows a direct correlation to rate of blood flow. Viscosity is defined as the factor of proportionality relating shear stress and shear rate for the fluid.
Newton assumed that viscosity was a constant property of a particular fluid and independent of shear rate. Fluids that demonstrate this behavior are termed Newtonian . The units of viscosity are dynes per second per square centimeter, or poise .
The viscosity of blood varies as a direct function of hematocrit ( Fig. 24.12A ); the greater the hematocrit, the more friction there is between successive layers. Plasma is a Newtonian fluid, even at high protein concentrations. However, because blood consists of erythrocytes suspended in plasma, it does not behave as a homogeneous Newtonian fluid; the viscosity of blood increases sharply with reductions in shear rate (see Fig. 24.12A ). This non-Newtonian behavior of blood has been attributed to changes in the behavior of erythrocytes at low flow rates. At low flow, erythrocytes lose their axial position in the stream of blood, lose their ellipsoidal shape, form aggregates, and adhere to the endothelial walls of microvessels. The tendency toward erythrocyte aggregation appears dependent on the plasma concentration of large proteins such as fibrinogen that form cell-to-cell bridges. Fig. 24.12B , demonstrates that non-Newtonian behavior is localized on the venous side of the circulation because of its lower shear rates, but that this behavior can be blunted or abolished by hemodilution.
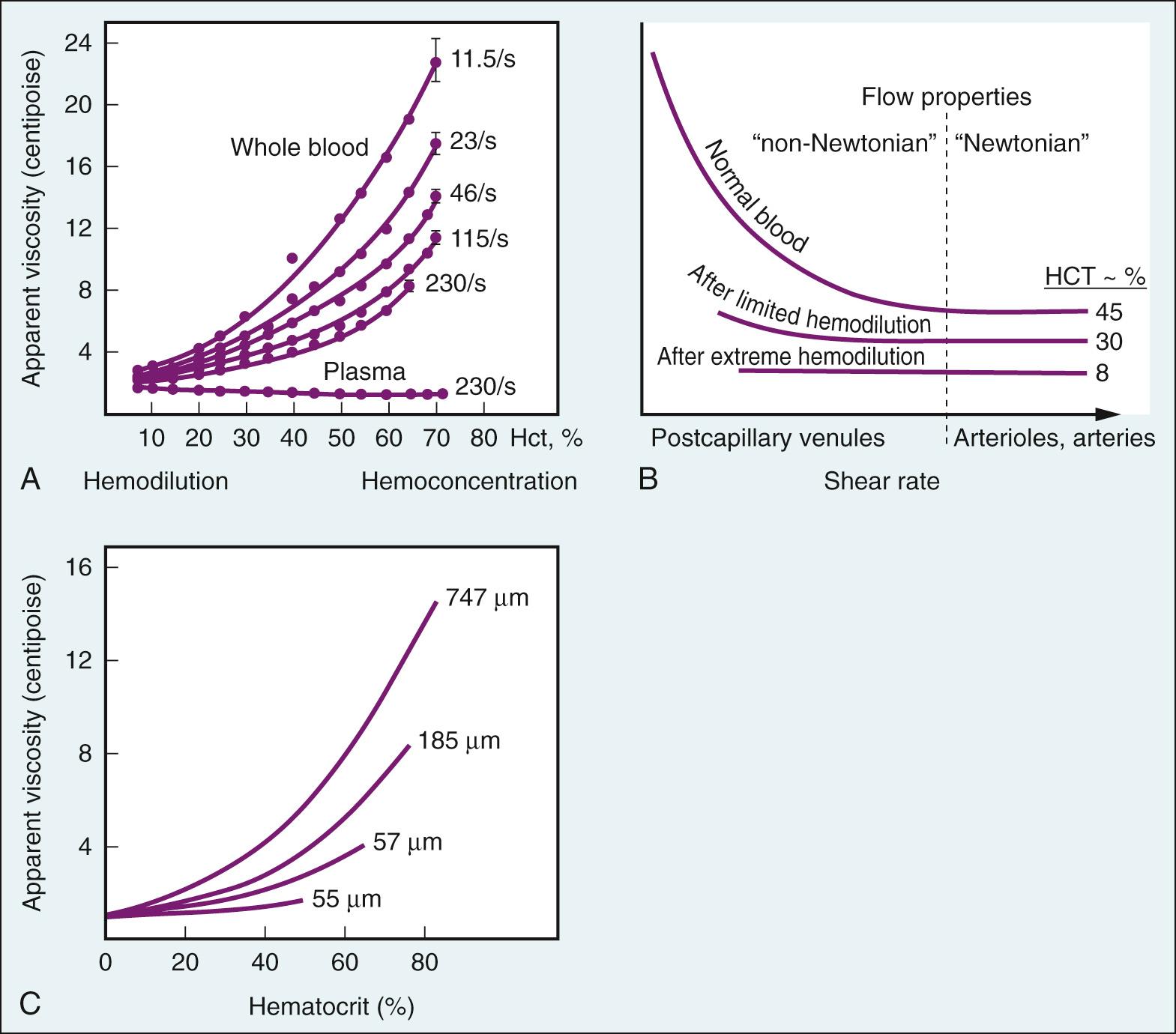
The tendency for increased hematocrit to increase blood viscosity is attenuated when blood flows through tubes of capillary diameter (see Fig. 24.12C ). This is because erythrocytes are normally very deformable, and with a diameter similar to that of the capillary, they can squeeze through the vessel lumen in single file with minimal extra force required. Thus the rate at which erythrocytes pass through the capillary has little influence on blood viscosity there.
Blood viscosity varies inversely with temperature. This is important during hypothermic cardiopulmonary bypass. After circulatory arrest, the shear stress required to reinitiate flow and to break up red cell aggregates is higher. Additional rheologic benefit may be gained by a further decrease in hematocrit.
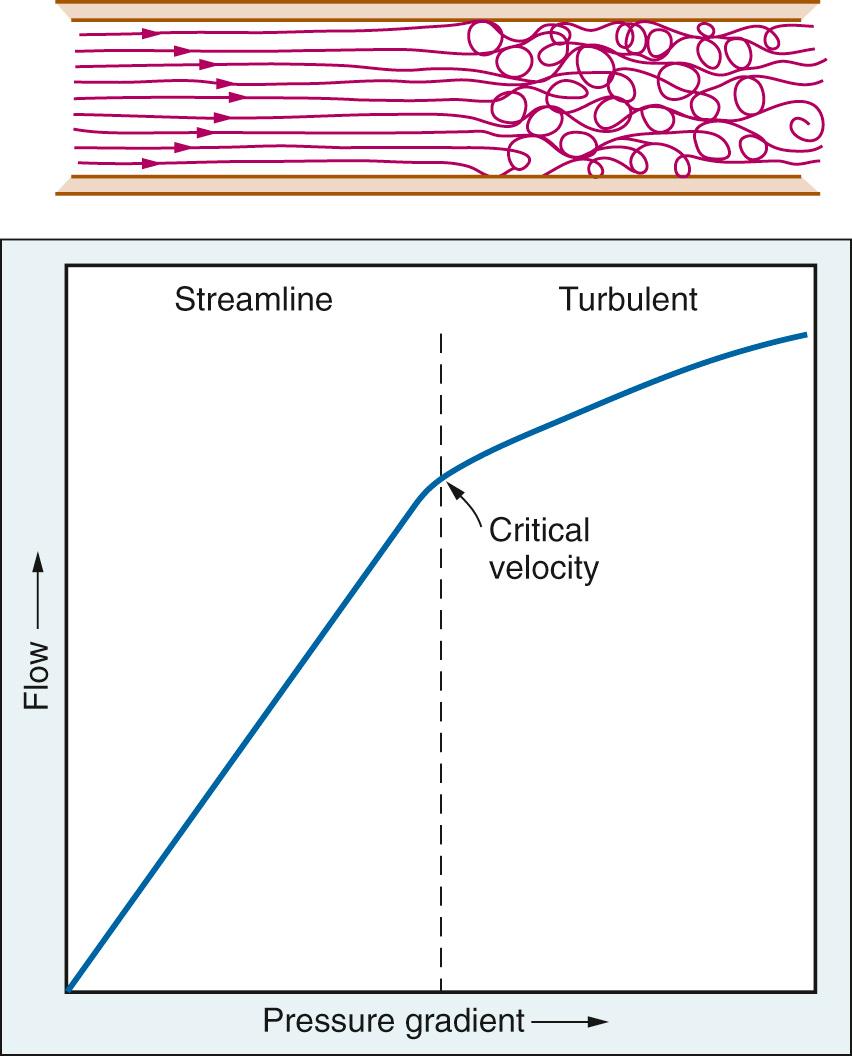
A principal condition of Poiseuille's law is that flow be laminar. Above a critical flow rate, the laminae break down into eddies that move in all directions. Such flow is said to be turbulent ( Fig. 24.13 ). The tendency for turbulence is given by the Reynolds number (Re):
where v is linear velocity, D is diameter, δ is density, and η is viscosity. Re is dimensionless because it is the ratio of inertial and cohesive forces. Inertial forces tend to disrupt the stream, whereas cohesive forces tend to maintain it. In long straight tubes, turbulence occurs when Re exceeds a value of approximately 2000. However, the critical Re is much less because of pulsatile flow patterns and complicated vascular geometries. When flow is turbulent, a greater portion of total fluid energy is dissipated as heat and vibration; thus the pressure drop is greater than predicted from the Poiseuille equation (see Fig. 24.13 ). The vibrations associated with turbulent flow can often be heard by auscultation as a murmur.
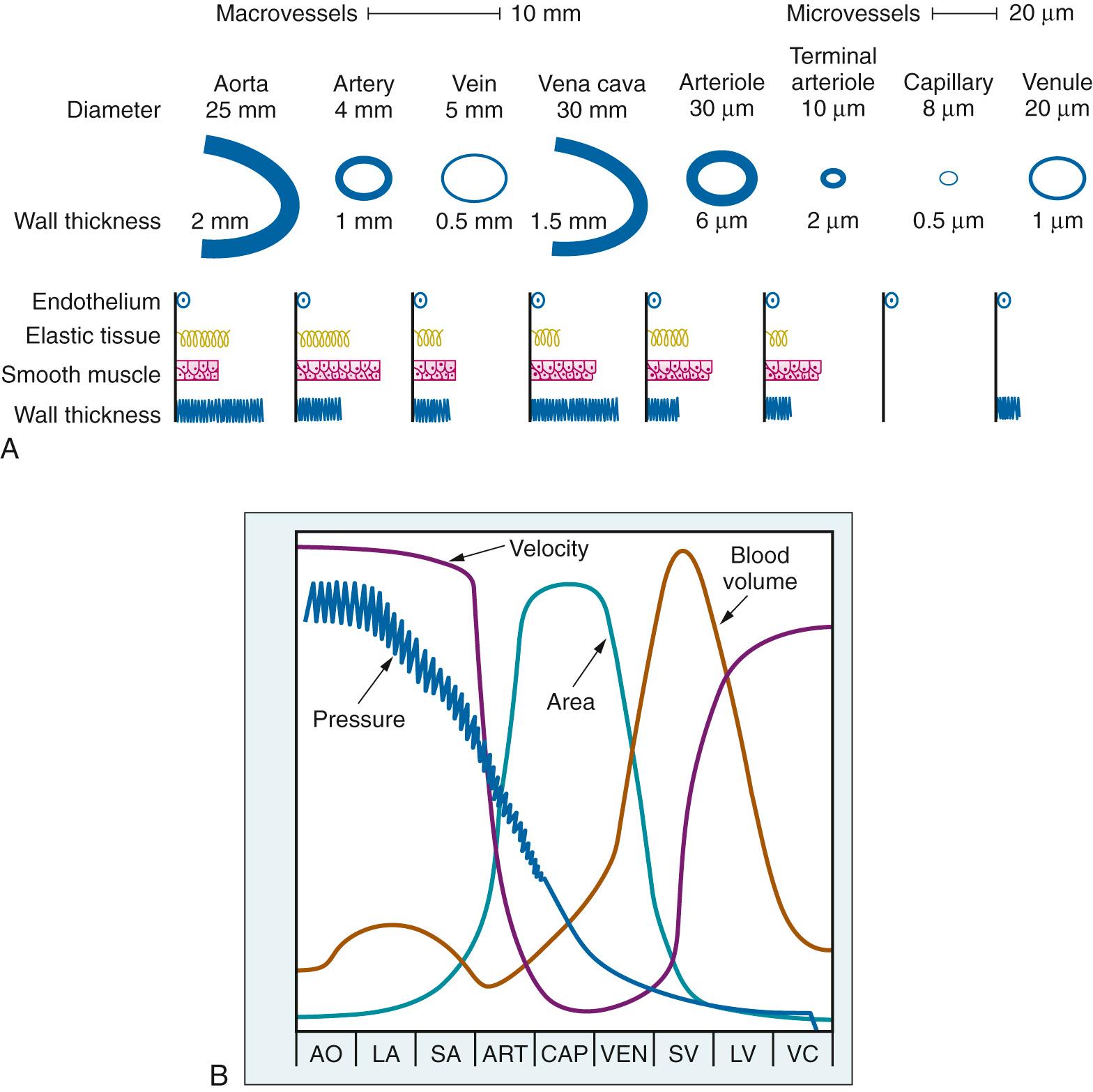
The various vessel types have structural and geometric features ( Fig. 24.14A ) that determine their functional characteristics within the circulation ( Fig. 24.14B ). The large conduit arteries are predominantly elastic structures, which allows them to convert intermittent cardiac output into continuous peripheral flow. Because the cross-sectional area of these vessels is small, the velocity of flow in them is high. The resistance to flow in the arteries is small, and thus the pressure drop is also small. The arterioles and the terminal arterioles have significant smooth muscle in their walls, which permits active changes in vascular diameter and modulation of local vascular resistance and blood flow. Capillaries have a very large aggregate cross-sectional area (which decreases flow velocity) and a thin wall, both factors favoring blood-tissue gas exchange. The veins and venules have the greatest volume, which makes an appropriate site for blood storage.
On the basis of his findings in the dog hind limb, in 1896 Ernest Starling presented a model to describe fluid exchange between the capillary and the interstitial space. The model proposed that the capillary wall is highly permeable to water and to almost all plasma solutes except plasma proteins, and that it acts like a porous filter through which protein-free fluid moves by bulk flow under the influence of a hydrostatic pressure gradients. Transcapillary filtration is defined as follows:
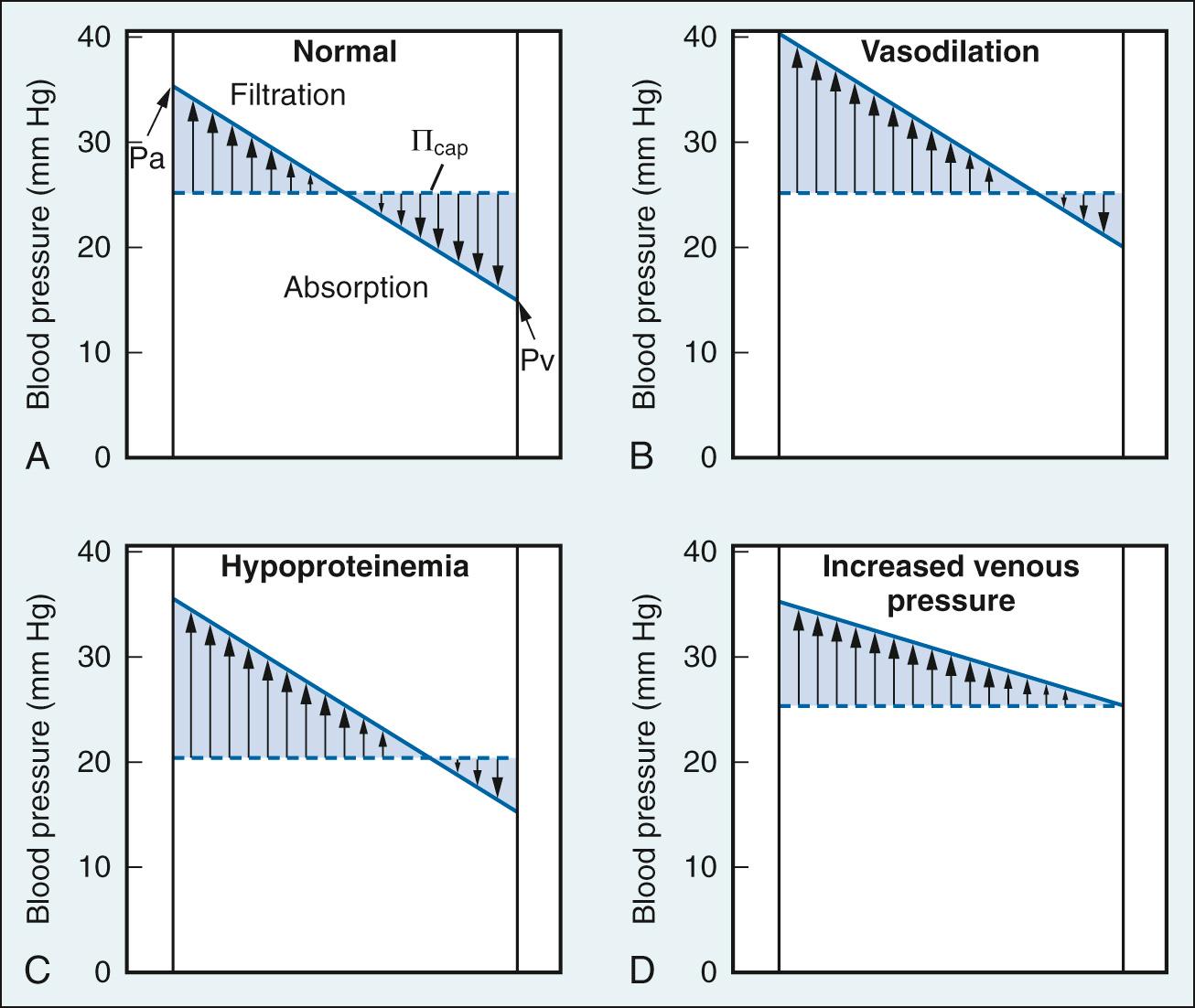
where C F is the capillary filtration coefficient; P cap is the capillary hydrostatic pressure; P IF is interstitial fluid hydrostatic pressure; Π IF is interstitial fluid oncotic pressure; and Π cap is capillary oncotic pressure. P cap and Π IF are forces of filtration. P cap is determined by arterial pressure, venous pressure, and the ratio of postcapillary to precapillary resistance. Elevations of arterial pressure, venous pressure, or venous resistance/arterial resistance produce elevations of P cap . P cap is approximately 35 mm Hg at the arterial end of the capillaries and approximately 15 mm Hg at the venous end. Π IF is due to plasma proteins that have passed through the capillary wall and is normally very low compared with P cap . Thus P cap is normally the major force for filtration. P IF and Π cap are forces favoring absorption. P IF is determined by the volume of fluid and the distensibility of the interstitial space, and is normally nearly equal to zero. Π cap is due to plasma proteins (predominantly albumin) and is approximately 25 mm Hg. Π p is normally the major force for absorption. The direction and magnitude of capillary bulk flow is essentially a function of the ratio of P cap to Π cap .
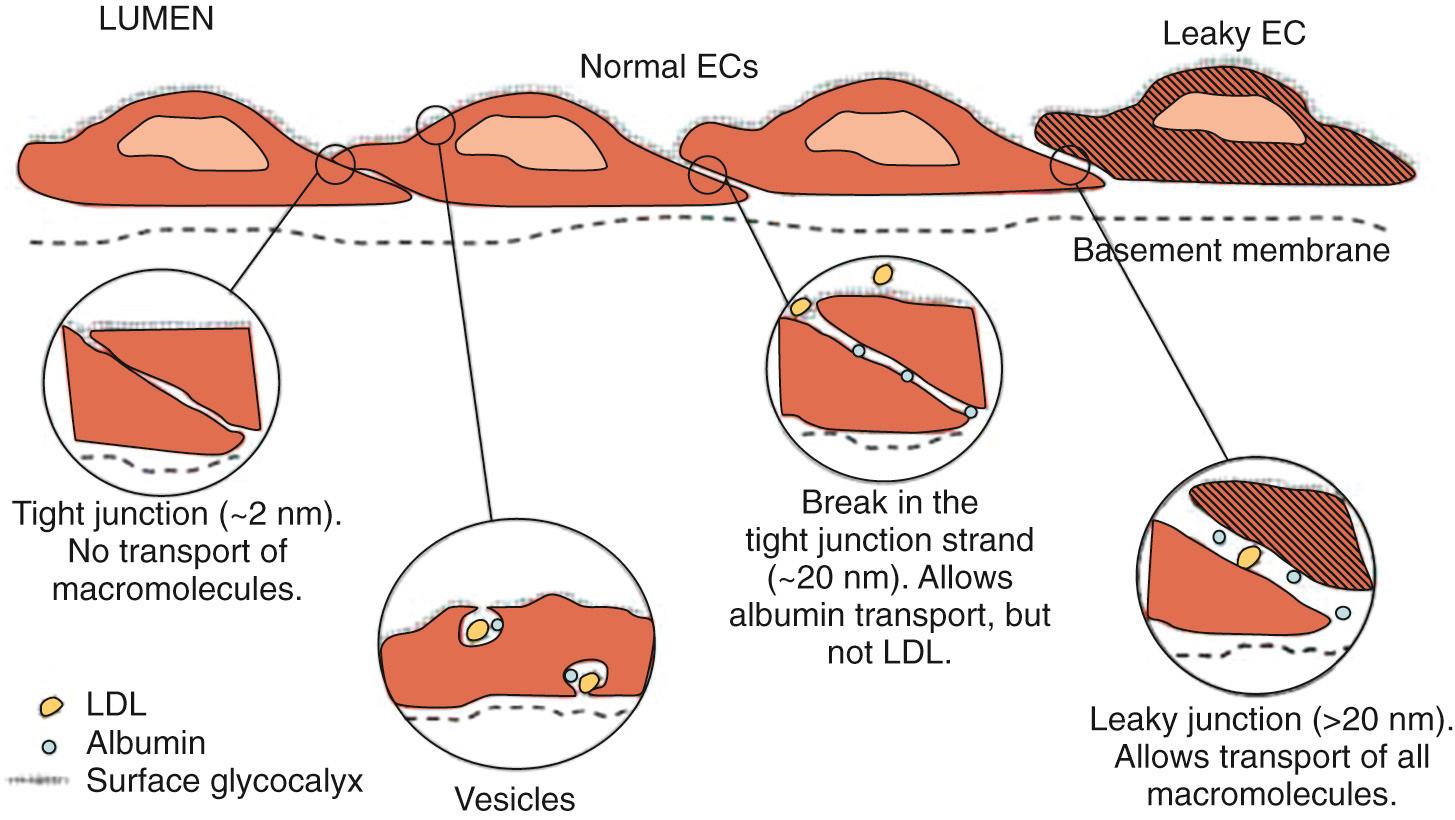
Fluid transport across capillary endothelium takes place through several pathways that include tight junctions, breaks in tight junctions, vesicular transport, and leaky junctions ( Fig. 24.15 ). The tight junctions allow transport of small water-soluble solutes (<2 nm in diameter) and are sealed by proteins linked to the cytoskeleton. Breaks in the tight junctions allow transport of larger water-soluble solutes up to 20 nm in diameter. Vesicular transport carries molecules up to 80 nm in diameter. Finally, leaky junctions associated with cell death allow transport of solutes up to 1330 nm in diameter. Filtered fluid that reaches the extravascular spaces is returned to the circulatory system via the lymphatic network. Under normal conditions ( Fig. 24.16A ), filtration dominates at the arterial end of the capillary and absorption at the venous end because of the gradient of hydrostatic pressure; there is a small net filtration, which is compensated by lymph flow. Edema is a condition of excess accumulation of fluid in the interstitial space and occurs when net filtration exceeds drainage via the lymphatics. Edema can be caused by (1) increased capillary pressure, (2) decreased plasma protein concentration, (3) accumulation of osmotically active substances in the interstitial space, (4) increased capillary permeability, or (5) inadequate lymph flow. Conditions resulting in edema are depicted in Fig. 24.16B.D .
The pulmonary circulation has distinctive features that affect fluid exchange. It has the ability to accommodate substantial changes in cardiac output without an increase in pulmonary capillary pressure. Under normal conditions, some of the pulmonary capillaries are either closed or open with no blood flow. When cardiac output increases, pulmonary capillary recruitment and distention reduce vascular resistance, which tends to normalize pulmonary capillary pressure. In an animal study, these mechanisms allowed a doubling of the left atrial pressure before a significant increase in pulmonary capillary permeability occurred. If these adaptive pulmonary mechanisms are overwhelmed, fluid begins to accumulate in the interstitial space and must be cleared by the lymphatic system. Lymph flow can increase 5- to 10-fold in response to chronic elevations in interstitial pressure in the lung. The rise in interstitial volume in response to an increase in pulmonary hydrostatic pressure is limited by the low compliance of the interstitial compartment. However, this protective mechanism is short-lived because of fragmentation within the proteoglycan skeleton of the interstitial matrix. The alveolar epithelial cells represent the last line of defense against pulmonary edema. An enhanced clearance of alveolar fluid via the epithelial sodium channels (ENaCs) can produce a rapid resolution of alveolar edema. ENaCs are stimulated by β-adrenergic agonists and inhibited by endothelin-1.
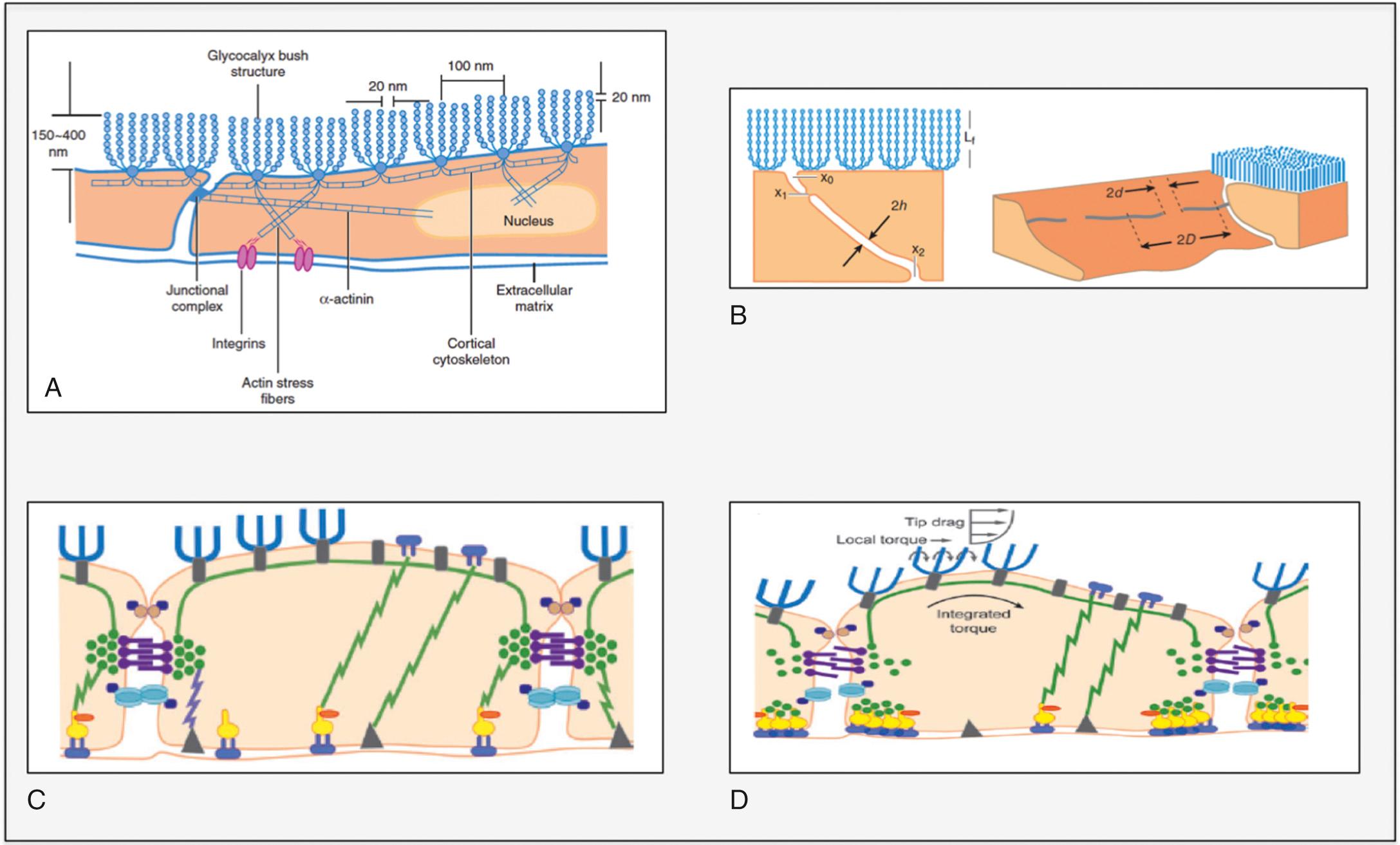
The traditional Starling model of transcapillary exchange has been revised and updated to include a role for the glycocalyx in the semipermeable layer of the capillary endothelium. The glycocalyx is a complex network of negatively charged glycosaminoglycans (GAGs), proteins and glycolipids. The most prominent GAGs are heparan sulfate, chondroitin sulfate, and hyaluronic acid ( Fig. 24.17A ). Recent studies suggest that the glycocalyx is a major determinant of capillary permeability, acting as a molecular sieve limiting water and protein flux across the cell-cell junction. Breakdown of the glycocalyx is associated with increased vascular permeability owing to loss of the junctional barrier and an opening of the intracellular junction ( Fig. 24.17B ). The glycocalyx also functions as a mechanotransducer that can sense increases in hydrostatic pressure ( Fig. 24.17C and D ). Heparan sulfate plays an important role in the transmission of this fluid shear stress through stimulation of endothelial nitric oxide synthesis. Recent evidence suggests that downstream microvessels are not in a continual state of fluid absorption, as described by the traditional Starling model, and that maintenance of tissue fluid balance is more critically dependent on lymphatic function.
The salient features of the major cardiovascular reflexes are presented in Table 24.1 .
| Reflex | Receptors and Location | Afferent Limb | Efferent Limb and Response |
|---|---|---|---|
| Arterial baroreceptor | Stretch receptors in vessel wall of carotid sinus and aortic arch respond to changes in arterial blood pressure | Fibers in glossopharyngeal and vagus nerves to medulla | Homeostatic control of arterial blood pressure via changes in cardiac output and systemic vascular resistance mediated by the autonomic nervous system |
| Bezold-Jarisch | Mechanical and chemosensitive receptors in ventricular walls | Nonmyelinated vagal C-fibers to medulla | Inhibition of sympathetic outflow resulting in bradycardia, peripheral vasodilation, and hypotension |
| Bainbridge | Stretch receptors at junction of the vena cava and right atrium and at junction of the pulmonary vein and left atrium respond to changes in volume in central thoracic compartment | Fibers in vagus nerve to medulla | Inhibition of vagal outflow and enhancement of sympathetic outflow to sinoatrial node causing tachycardia |
Arterial blood pressure is maintained within narrow limits by a negative feedback system called the arterial baroreceptor reflex. The major components of this system are shown in ( Fig. 24.18A ): (1) an afferent limb composed of baroreceptors in the carotid artery and aortic arch and their respective afferent nerves, the glossopharyngeal and vagus nerves; (2) cardiovascular centers in the medulla that receive and integrate sensory information; and (3) an efferent limb composed of sympathetic nerves to the heart and blood vessels and the parasympathetic (vagus) nerve to the heart. Fig. 24.18B presents the neural relationships of the arterial baroreceptor reflex. Baroreceptors are stimulated by stretch of the vessel wall by increased transluminal pressure. Impulses originating in the baroreceptors tonically inhibit discharge of sympathetic nerves to the heart and blood vessels, and tonically facilitate discharge of the vagus nerve to the heart. A rise in arterial pressure reduces baroreceptor afferent activity, resulting in further inhibition of the sympathetic and facilitation of parasympathetic output. This produces vasodilation, venodilation, and reductions in stroke volume, heart rate, and cardiac output, which combine to normalize arterial pressure. A decrease in arterial pressure has opposite effects. The cardiovascular centers in the medulla are also under the influence of neural influences arising from the arterial chemoreceptors, hypothalamus, and cerebral cortex, and of local changes in the partial pressure of carbon dioxide (P co 2 ) and the partial pressure of oxygen (P o 2 ).
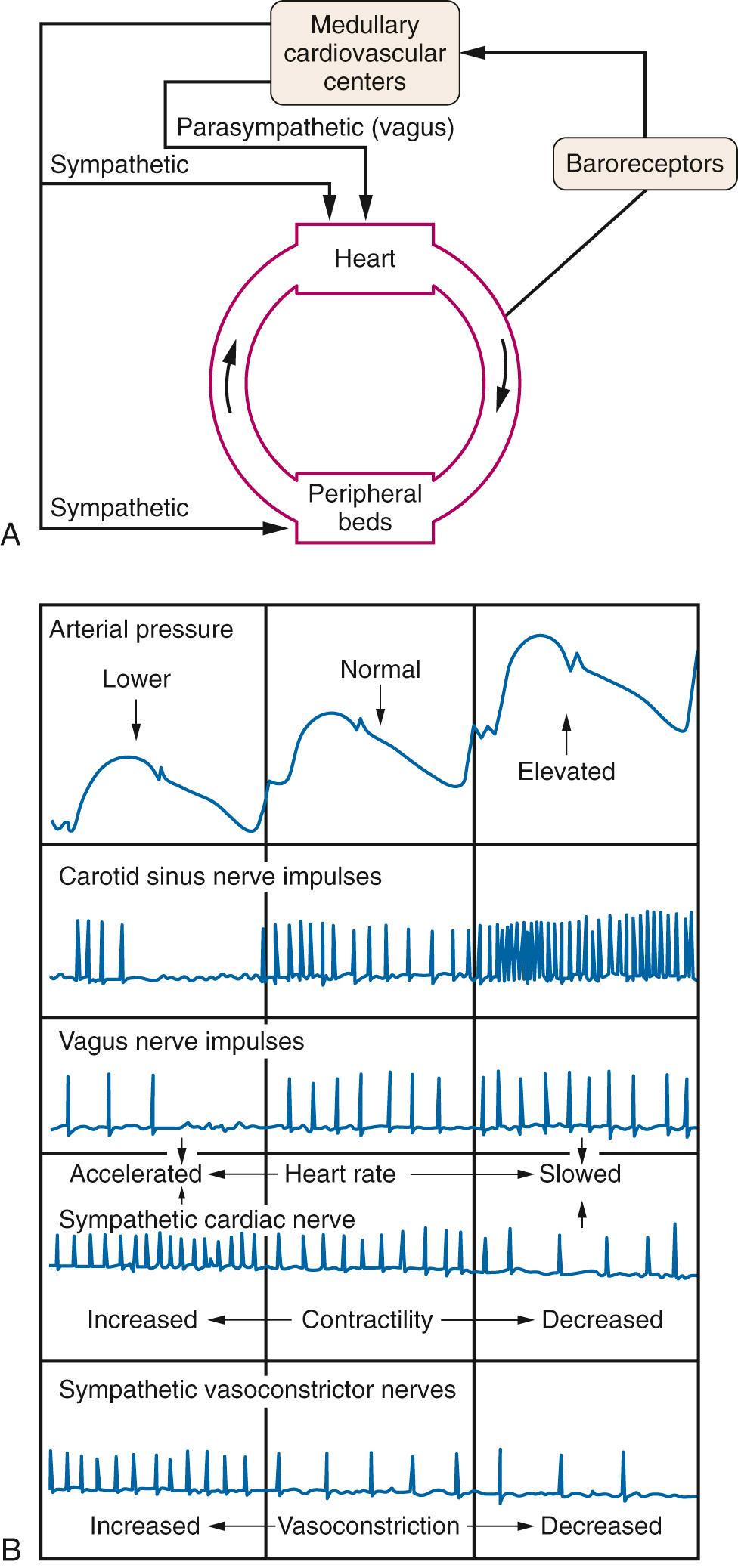
The Bezold-Jarisch reflex is a triad of responses (bradycardia, hypotension, and apnea) first observed following injection of Veratrum plant alkaloids in animals by von Bezold and Hirt in 1867. Seventy years later, Jarisch and Richter demonstrated that the receptor area was in the heart (not the great vessels), the afferent pathway was in the vagus nerve, and the efferent pathway involved inhibition of sympathetic outflow to peripheral vessels and increased activity in the vagus nerve to the heart. The ventricular receptors underlying the Bezold-Jarisch reflex are nonencapsulated terminals of unmyelinated vagal C-fiber afferents in the walls of the ventricles. Although Veratrum alkaloids are not normally present in animals, physiologic factors, including mechanical stimulation, can trigger the Bezold-Jarisch reflex. The literature contains reports of “paradoxical” bradycardia during severe hemorrhage in humans. Studies in a rabbit model demonstrated that this response is mediated by the ventricular receptors and by the ability of the Bezold-Jarisch reflex to override the arterial baroreceptor response. During severe hemorrhage, the ventricular receptors can be excited by abnormal squeezing of the myocardium owing to vigorous contraction around a nearly empty chamber.
In 1915 Bainbridge demonstrated that intravenous infusion of saline solution or blood in the anesthetized dog produced tachycardia. The elimination of the response following transection of the cardiac autonomic nerve supply and injection of the anticholinergic drug atropine demonstrated that the tachycardia was reflexive in origin, with the vagus nerves constituting the afferent limb and withdrawal of vagal tone the primary efferent limb. The increase in venous return is detected by stretch receptors in the right and left atria. The Bainbridge reflex is present in primates, including man, but is much less prominent than in the dog, attributed to a more dominant arterial baroreceptor reflex in humans. The Bainbridge reflex is obtunded or absent when the heart rate is high. A “reverse” Bainbridge reflex has been proposed to explain decreases in heart rate observed under conditions in which venous return is reduced, such as during spinal and epidural anesthesia and controlled hypotension.
Oxygen serves as an electron acceptor in oxidative phosphorylation, enabling production of adenosine triphosphate (ATP) along efficient aerobic pathways. The high-energy phosphate bonds of ATP provide energy for functional and biochemical cellular processes within the cell, such as contraction of muscle proteins and ion transport.
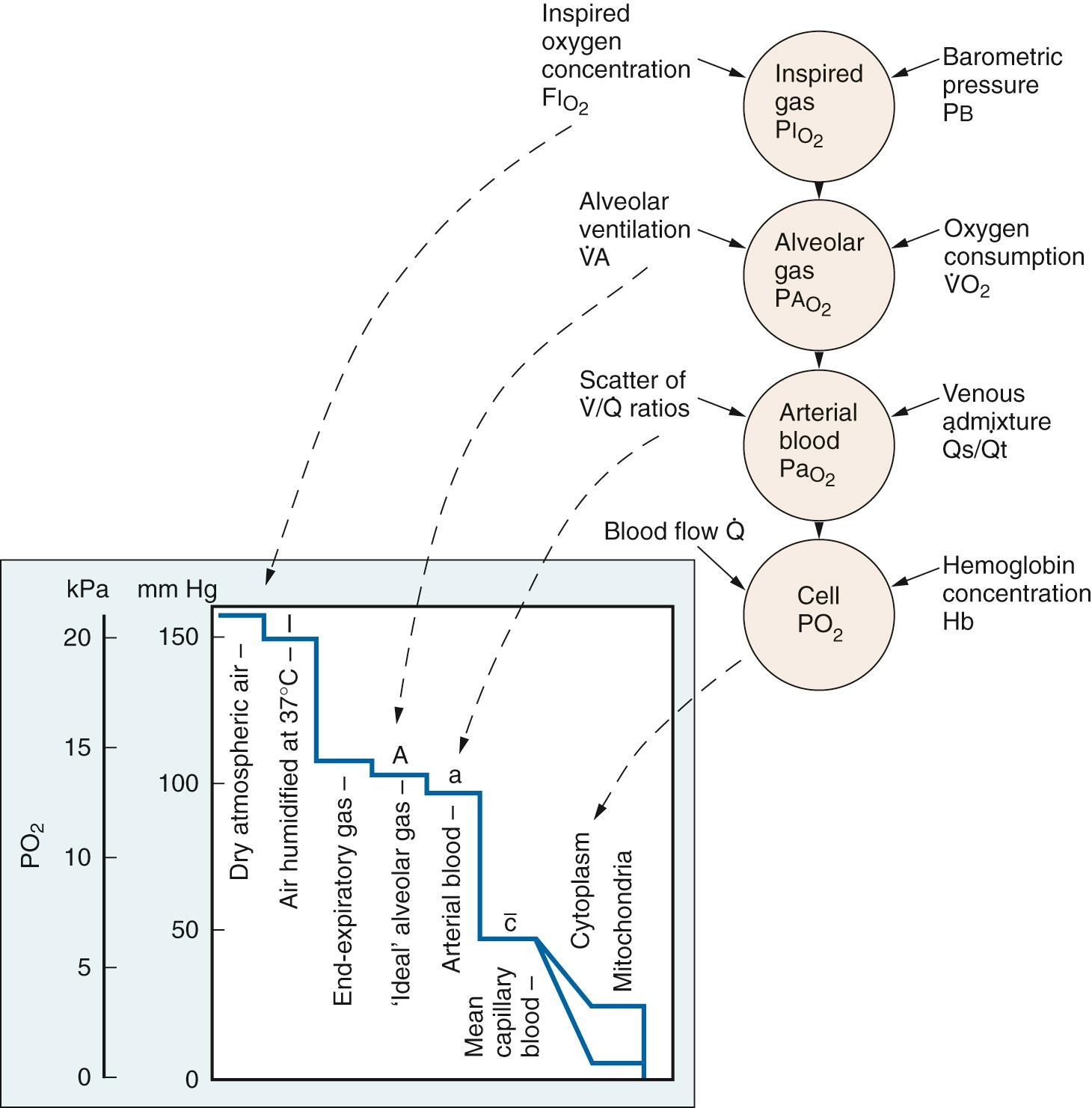
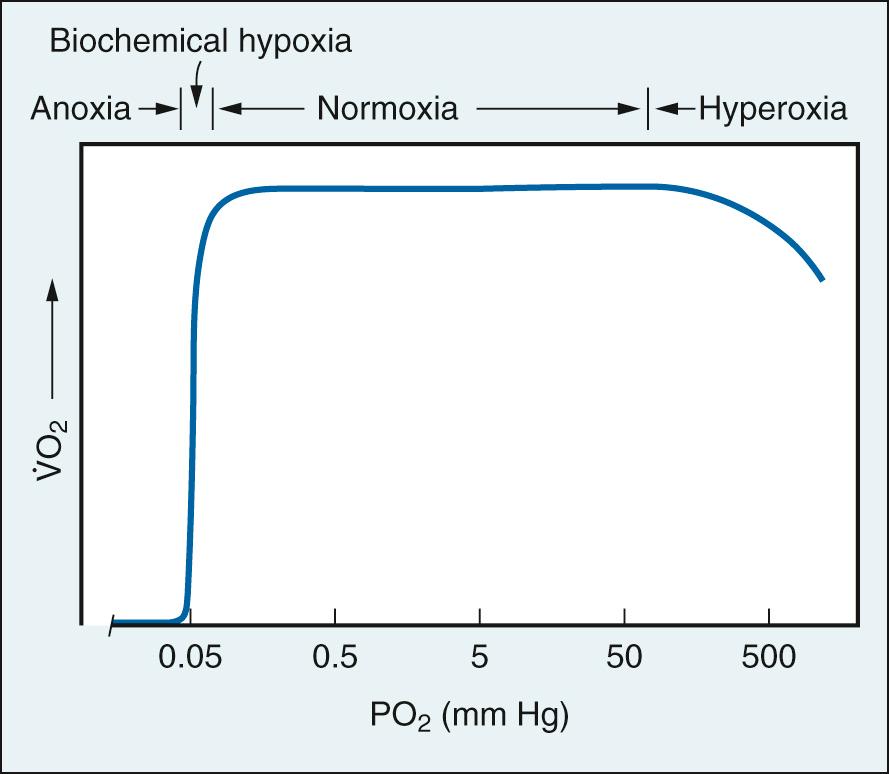
The cardiovascular system acts in concert with the respiratory system in transporting oxygen to tissue mitochondria from its source in inspired air. Oxygen transport is comprised of a series of steps down a gradient in partial pressure, each associated with a P o 2 cost ( Fig. 24.19 ). Normal oxygen delivery maintains P o 2 in the vicinity of mitochondria at 0.1 mm Hg, the level required for unimpaired O 2 consumption (V̇ o 2 ) ( Fig. 24.20 ). The only effect of greater P o 2 values is to provide a gradient for diffusion of oxygen to mitochondria remote from capillaries.
The amount of oxygen carried from the lungs to tissues by circulating blood (i.e., convective systemic oxygen delivery [DO 2 ]), is given by the equation:
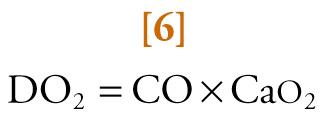
where CO is cardiac output in L/min and Ca o 2 is the arterial oxygen content in volume percent.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here