Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital anomalies of the gastrointestinal tract, (including those of the anterior abdominal wall) account for approximately 15% of congenital abnormalities detected by routine prenatal ultrasound.
The prenatal detection rates from routine ‘screening ultrasound’ either in the first trimester (usually between 11 to 13 +6 weeks) or in the second trimester (18 to 22 weeks), are dependent upon the type of congenital anomaly as well as factors such as fetal position and maternal body mass index. Congenital anterior abdominal wall anomalies are more likely to be detected early in the first trimester of pregnancy or in association with an elevated maternal serum α-fetoprotein (second-trimester quadruple screening for aneuploidy). However, fetal gastrointestinal malformations are diverse and conditions such as bowel atresia may not be apparent on ultrasound until the late second or third trimesters as part of the investigation of polyhydramnios.
Embryologically, the anterior abdominal wall forms from the fusion of four ecto-mesodermic folds (cephalic, caudal and two lateral). During the 6th to 7th week of embryonic development, the flat embryonic disk folds in four directions, each converging at the site of the umbilicus, obliterating the extra-embryonic coelom. The lateral folds form the lateral portions of the abdominal wall and the cephalic and caudal folds make up the epigastrium and hypogastrium. During the 8th week of development the abdominal cavity is temporarily too small to accommodate all of its contents, resulting in protrusion of the small bowel into the base of the umbilical cord as the ‘physiologic midgut herniation’.
After this gestational age, it is possible to demonstrate the integrity of the fetal abdominal wall outlined by amniotic fluid on 2D prenatal ultrasound. Transverse sections (TS) should demonstrate not only the insertion of the umbilical cord but also the urinary bladder within the fetal pelvis ( Figure 17-1 ). This lower abdominal portion can be obscured if the fetal legs are flexed and a midline sagittal section may allow improved imaging.
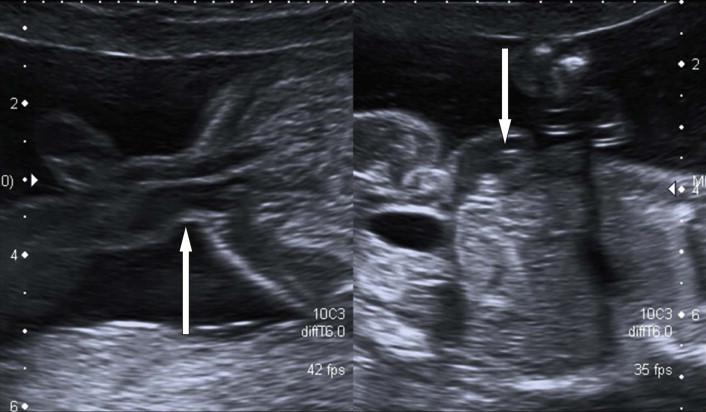
The features of the physiological midgut herniation ( Figure 17-2 ) are as follows:
Present in embryos between 8 weeks 3 days and 10 weeks 4 days.
The midgut herniation is small (6–8 mm) and echogenic, never more than 8 mm in transverse measurement.
The bowel returns to the abdominal cavity by the time of a fetal crown rump length (CRL) measurement of 45 mm.
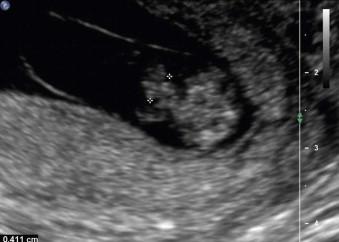
The ultrasound appearances of herniated bowel through the umbilical cord ring is abnormal after 12 weeks gestational age. Difficulties in assessing the abdominal wall can occur if the fetus is lying adjacent to the maternal uterine wall or placenta. Major difficulties arise when there is significant oligohydramnios/anhydramnios in which distortion of the anterior abdominal wall mimics a defect, the so-called ‘pseudo-exomphalos’ of oligohydramnios.
The incidence of exomphalos (omphalocoele) is 1 in 4000 to 1 in 7000 live births, but 1 in 300 to 1 in 4000 pregnancies. Exomphalos is more commonly associated with increasing maternal age.
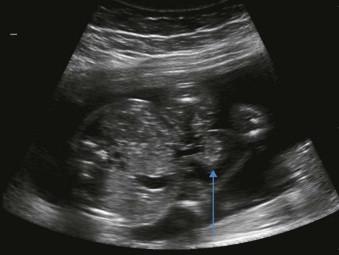
An exomphalos is a herniation of intra-abdominal viscera through an open umbilical ring into the base of the umbilical cord. This pathological herniation is covered by a clear, three-layered membrane of peritoneum, Wharton's jelly and amnion. This covering distinguishes it from an umbilical hernia which is skin covered (and rarely noted in utero) and gastroschisis. The herniation may be small (containing only Meckel's diverticulum) or large and although it most commonly contains bowel, it may contain any of the abdominal fetal organs. Small bowel-only omphalocoeles account for 10% to 20% and liver will be present in 80%.
A mass of varying size and echogenicity depending on the organs affected and the volume of tissue herniated (bowel is usually hyper-echoic, liver hypo-echoic) ( Figures 17-3 and 17-4 ).
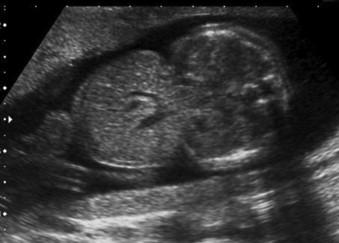
In the majority of cases the umbilical cord inserts directly into the membrane covering the defect, but variants with the cord insertion above and below have been reported and omphalocoeles may be subdivided into different types based on the location of the defect in relation to the insertion of the umbilical cord, i.e. epigastric or hypogastric.
Eighty percent of abdominal wall defects will be detected using prenatal ultrasound with only occasional misdiagnosis as gastroschisis (5%). Classically, prenatal detection was prompted by the presence of elevated maternal serum α-fetoprotein on second-trimester serum aneuploidy screening. Improvements in first-trimester combined screening with an associated anomaly ultrasound examination, facilitate detection of this anomaly in the first trimester.
An exomphalos may be differentiated from other fetal anterior abdominal wall defects by noting the presence of the peritoneal membrane/covering sac and the insertion of the umbilical cord into the membrane.
Diagnostic difficulties may arise when thickened loops of bowel in gastroschisis mimic a membrane covering, or in the rare scenario when the sac of the exomphalos has ruptured.
In approximately 40% of cases there will be an underlying chromosomal abnormality, usually autosomal trisomies (trisomy 13 or 18). The association is more prevalent with a small bowel-only exomphalos (67%) than with large liver-containing defects (16%) ( Figure 17-5 A and B ).
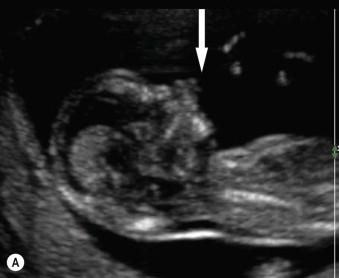
Additional congenital structural abnormalities are visualized in up to 80% of cases once associated aneuploidy has been excluded. Cardiac defects are the most frequently reported anomaly (30% of cases) in association with omphalocoeles with normal karyotype. The ultrasound visualization of associated cord cysts or other fetal abnormalities will of course increase the chances of an underlying chromosomal abnormality.
An exomphalos is a significant feature in many non-chromosomal syndromes and associations such as:
Beckwith–Wiedemann syndrome, which may occur in up to 10% of prenatal exomphalos cases. The additional features supporting this diagnosis are:
macrosomia;
big bright kidneys;
hepatomegaly;
macroglossia.
Cloacal exstrophy (see below).
Pentalogy of Cantrell (see below).
Meckel–Gruber syndrome. The additional features supporting this diagnosis are:
encephalocoele and/or Dandy–Walker malformation;
enlarged echogenic kidneys;
polydactyly;
cleft lip.
The prenatal management of fetal exomphalos requires:
Accurate diagnosis.
Exclusion of other structural abnormalities.
Offering prenatal karyotype.
Offering detection of Beckwith-Weidemann. This requires an amniotic fluid sample for DNA analysis.
A giant omphalocoele is a rare abdominal defect that usually wider than 5 cm in diameter, which is due to failure of abdominal wall closure in the region of the umbilical ring, and the entire liver usually protrudes through the defect. It is believed to be due to an early teratogenic insult affecting the embryonic lateral folding at 6 weeks' gestation. Because the defect has developed so early and is so big there is extra-abdominal development of the liver and small intestine as well as the stomach and spleen. As a result of herniation and extra-abdominal development of the liver, the liver vascular pedicle may be elongated and abnormal hepatic portal vessels may develop as a consequence. There is a significant risk of hepatic thrombosis with any degree of liver rotation pre- or perinatally, in particular after visceral reduction.
Ongoing pregnancies should be regularly monitored by ultrasound to assess fetal biometry and to exclude the development of polyhydramnios and bowel dilatation.
Multidisciplinary consultation and counselling is mandatory. There is no advantage to pre-term delivery in these fetuses. In modern obstetric care, all but very small omphalocoeles are delivered by Caesarean section, usually at 39 weeks’ gestation. In an exomphalos containing liver, delivery by elective Caesarean section prevents the small risk of liver rupture ( Figure 17-6 ).
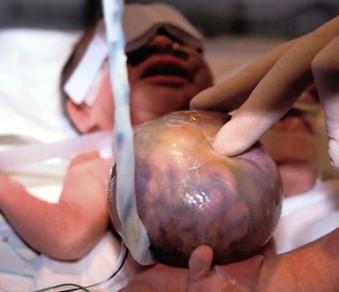
Postnatal/neonatal care for these neonates is dependent on the:
size of the anterior abdominal wall defect;
gestational age of delivery;
presence of other associated anomalies.
At birth parenteral fluid replacement is commenced and a large nasogastric tube passed. Early surgical review is mandatory and timing and type of surgery is individualized. Where the defect is small, a primary closure can take place with excision or inversion of the sac and closure of the fascia and skin.
In a large exomphalos with liver herniation, there is considerable loss of space in the developing peritoneal cavity; if primary replacement of organs is undertaken the increased intra-abdominal pressure will lead to significant respiratory compromise. In addition, giant exomphalos can be associated with a functional kyphosis which may further complicate neonatal resuscitation and be associated with cardiopulmonary difficulties. Primary closure in such cases may be difficult or even undesirable. There are now several techniques to aid primary closure using either abdominal flaps, tissue expanders or patches. Long-term follow-up has shown that isolated cases with a small exomphalos do well with no long-term sequelae; however this may represent as few as 10% of cases initially diagnosed.
The incidence of complications in those with larger defects is high, with morbidity due to gastro-oesophageal reflux, pulmonary insufficiency, recurrent lung infections, obstructive airways disease and feeding difficulties. There is ‘failure to thrive’ in up to 60% of cases.
Exomphalos is normally a sporadic condition, although there are some reported familial cases consistent with inherited genetic disease (X-linked, recessive and autosomal dominant pedigrees).
Gastroschisis occurs in 1 : 2500 to 1 : 3000 live births. The condition is more common in women under the age of 20 years. Gastroschisis is thought to be due to an ischaemic event leading to interruption or abnormal blood flow in the right umbilical vein or omphalomesenteric artery after the physiological closure of the anterior abdominal wall. This results in gastroschisis, herniation of the small and occasionally large bowel (any structure may extrude including bladder, uterus and ovaries) through an opening on the right side of a normally inserted umbilical cord ( Figure 17-7 ).
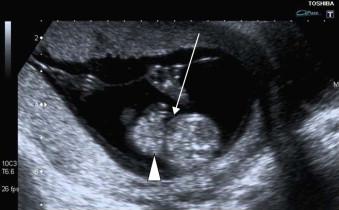
Epidemiological data have documented increasing prevalence of gastroschisis over recent years, the ‘gastroschisis epidemic’, with an observed association with use of tobacco and cocaine. In the UK the increase in incidence through all age groups (by 10 to 20-fold in the past two decades) has been a cause for concern.
Prenatal diagnosis using ultrasound has a high sensitivity and specificity for detection, with direct visualization of fetal bowel lying free in the amniotic cavity.
Gastroschisis is an isolated defect in 95% of cases with a very low association with aneuploidy. The main problem is not the abdominal wall defect itself, but the associated bowel complications that occur because of the defect; complication rates of 26–30% have been reported. These bowel complications evolve over time and so the appearance of the bowel changes throughout gestation. In the late second and third trimesters, ultrasound imaging commonly demonstrates an increase in bowel wall thickness, bowel shortening and luminal distension. The thickened bowel wall is thought to be due to exposure to fetal urine causing a ‘mild peritonitis’ as amniotic fluid contains increased concentrations of inflammatory cytokines.
Intestinal atresia or obstruction secondary to volvulus and/or ischaemia at the hernial orifice occurs in up to 30% of cases and the visualization of intra-abdominal and/or exteriorized bowel with a luminal diameter of greater than 17 mm on ultrasound scan is suspicious of this ( Figure 17-8 ).
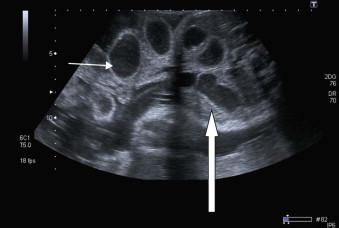
The survival rate of affected infants depends on the presence or absence of associated bowel abnormalities and if intestinal complications could be predicted antenatally, a better risk assessment and prognostic evaluation could be made.
Features which may have predictive significance:
The degree of bowel dilatation and the degree of thickening of the wall of extruded bowel. Various attempts have been made to correlate these findings on prenatal ultrasound with outcome, but neither bowel wall thickening to >3 mm or bowel dilatation >20 mm are predictive of bowel atresia obstruction or even of severe bowel dysmotility.
Polyhydramnios. The presence of polyhydramnios on the other hand, seems to predict an adverse neonatal outcome as polyhydramnios may indeed be a sign of severe bowel damage, due either to intestinal atresia and/or decreased or absent gut motility.
Improving gastroschisis. Total atresia of the exteriorized loops can occur and one should always ‘beware the gastroschisis that appears to get better’. This appearance is associated with a very poor long-term prognosis, as it implies that ischaemia and torsion have produced avulsion of much of the exteriorized bowel loops with the result that short bowel syndrome postnatally is inevitable.
A fetus with gastroschisis requires serial ultrasound surveillance for:
Fetal biometry.
Liquor volume assessment.
Doppler insonation of the umbilical artery waveform.
Twenty-five percent of cases will be associated with intrauterine growth restriction, which is inherently difficult to diagnose, as measurements of the fetal abdominal circumference are more technically difficult to obtain and may be smaller than expected. Many centres combine ultrasound assessment with non-stressed cardiotocography (biophysical assessment) due to an increased association with unexplained intrauterine death and fetal distress in the third trimester.
There is no advantage to delivering these babies by elective Caesarean section. However, because of the association with late unexplained fetal demise, elective induction of labour is often advised between 38–39 weeks. In labour, there is also an increase in suspicious or pathological cardiotocographic changes in fetal heart rate that lead to expedited delivery and high rates of emergency Caesarean section. However, the association with intrapartum acidosis and hypoxemia is relatively low ( Figure 17-9 ).
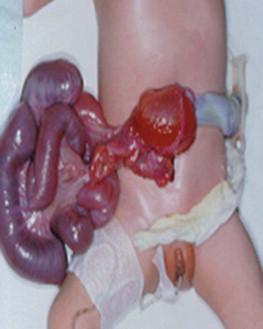
At birth the initial management is designed:
to prevent intestinal distension with gastric decompression (by insertion of a large-bore nasogastric tube);
to initiate parenteral fluid resuscitation;
to protect the exteriorized small bowel by correct fetal positioning, the use of a plastic wrap to reduce evaporation and to aid temperature stability.
Surgical options include primary reduction with operative closure of the fascia, silo placement, serial reduction and delayed closure or primary or delayed reduction without fascial closure.
The choice of treatment is not only dependent on the size of the lesion, but also the experience of the paediatric surgeons and the centre managing the neonate. The outcome of these neonates is largely dependent on the amount of intestinal damage that occurred in fetal life:
Up to 10% will have jejuna or ileal atresia which is also commonly associated with poor bowel perfusion and ischemia and the risk of bowel resection is increased. In the short term there is associated abnormal gut motility, which may be managed pharmacologically but in some individuals parenteral feeding via a long line is required. There is recent evidence that primary closure of Gastroschisis is associated with better long term outcome than those where a ‘silo’ method is utilized. Parentral feeding is associated with an increased risk of long line sepsis and necrotising enterocolitis. In the long term these babies generally have an excellent prognosis with the majority achieving normal growth and development. In a small number (<5% of cases) because of spontaneous or iatrogenic short bowel syndrome with hepatic, cholestatic complications of parenteral nutrition childhood combined small bowel hepatic transplant is necessary.
This is one of the rarest and most severe abdominal wall defects occurring in 1 : 7500 fetuses. It is usually a sporadic condition thought to be due to early amnion rupture, vascular disruption early in embryonic life, or a germinal disc abnormality. The condition is seen more frequently in a younger age group and in cocaine abuse.
Prenatal diagnosis may be made early in the first trimester as the fetus often has multiple structural anomalies which may include ( Figure 17-10 ):
A large anterior abdominal wall defect (abdominoschisis) often affecting the left side.
Shortened umbilical cord.
Extensive lower spine neural tube defect.
Exencephaly/encephalocoele.
Cleft lip.
Numerous subtypes of limb anomalies.
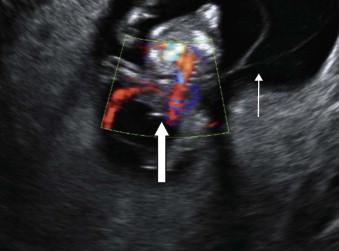
Characteristically on ultrasound there is the appearance of failure of fusion of the amnion and chorion with the amnion forming a continuous sheet between the anterior abdominal wall and placenta. The fetuses are cytogenically normal.
This condition is uniformly fatal either in utero or in the early neonatal period. There are anecdotal case reports of recurrence but in general such cases are thought to be sporadic.
This is a rare condition characterized by herniation of the heart though the chest wall defect (true ectopic cordis) or through a thoraco-abdominal wall defect ( Figure 17-11 ).
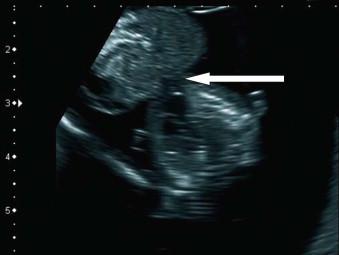
Ectopia cordis is diagnosed usually at first ultrasound examination when a pulsating heart is seen outside the chest cavity. This condition is likely to be fatal although few survivors have been reported and all ‘component defects’ are potentially amenable to repair.
Pentalogy of Cantrell is a variant of this syndrome defined by the association of five features:
Epigastric abdominal wall defect.
Defect in the lower sternum.
Diaphragmatic hernia.
Defect in the epicardium with displacement of the heart.
Intracardiac anomalies (most commonly ventral septal defect, atrial septal defect, or right-sided complex cardiac anomalies).
The syndrome is rare, complicating 1 in 200,000 births. It is usually first suspected by the detection of an epigastric omphalocoele. Although the heart in this condition is positioned within the fetal chest, the apex is oriented vertically at the superior edge of the abdominal wall defect. In association with the five classic features of this syndrome there are also commonly abnormalities of the spine, hands, head and face.
Knowledge of the outcomes of these pregnancies is limited due to the rare nature of the condition, but is thought to be determined mostly by the cardiac abnormalities. The newborn will need to be managed by a multidisciplinary surgical team. The condition is usually a sporadic one, but it has been suggested that there may be an X-linked inheritance.
Cloacal exstrophy has an incidence of 1 in 300,000 live births which is likely to be an underestimation as many affected fetuses die in utero. It is also known as the OEIS complex (omphalocoele, exstrophy, imperforate anus, spinal abnormalities).
This is a sporadic condition thought to be a result of maldevelopment of the cloacal membrane that prevents the normal migration of mesenchymal tissue. The cloacal membrane separates the coelomic cavity from the amniotic space in early development and depending on the timing of disruption of the membrane, varying complex fetal abnormalities result.
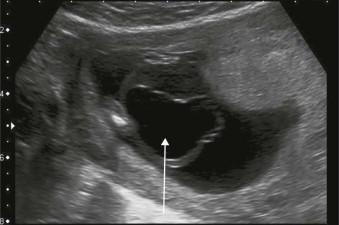
Cloacal exstrophy has different sonographic appearances in the first and second trimesters:
First trimester
Cystic mass arising from the lower anterior abdomen ( Figure 17-12 ).
Second trimester
Major criteria:
low anterior abdominal wall defect with exomphalos;
absence of the fetal bladder in the pelvis;
lumbosacral meningocoele (70%);
± a persistent cloacal membrane (cystic structure) ( Figure 17-13 );
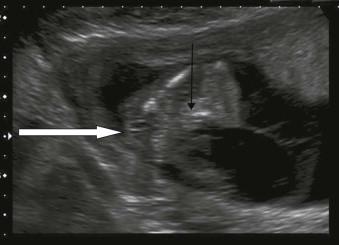
low insertion of the cord;
normal liquor volume;
talipes, renal anomalies, ascites, single umbilical artery.
The main differential diagnosis includes:
Isolated bladder exstrophy, omphalocoele and gastroschisis.
Amniotic band syndrome (scoliosis, facial clefting, limb defects).
Limb–body wall complex.
Due to the complex nature of the condition, mothers should be managed at a tertiary centre. Post delivery these neonates require assessment by a multidisciplinary surgical team. With advances in paediatric surgery, overall survival rates are now quoted been 80% and 100%; however the surgery required is extensive and long-term complication of faecal and urinary incontinence are common.
This is a rare sporadic condition seen in 1 in 10,000 live births, more commonly in the male fetus, M : F ratio 2 : 1. As with cloacal exstrophy this condition results from an abnormality of the cloacal membrane, but after the descent of the urorectal septum, producing bladder exstrophy rather than cloacal exstrophy.
This condition is diagnosed sonographically based on the following features:
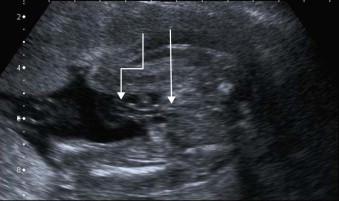
Non-visualization of the fetal bladder in a fetus with normal liquor volume and normal kidneys ( Figure 17-14 ). It is very important to realize that not all fluid-filled lesions in the pelvis represent the fetal bladder; a urachal cyst may be very misleading.
A mass on the anterior surface of the suprapubic region abdominal wall with umbilical arteries running along either side ( Figure 17-15 ).
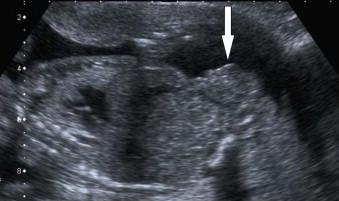
It may be possible to appreciate an abnormal widening of the iliac crest.
Genital abnormalities:
In the male fetus the penis is small and the scrotum is anteriorly displaced.
In the female fetus the there is a hemi-clitoris on either side of the bladder and there may be a vaginal orifice and uterine duplication, although ovaries and the fallopian tubes are usually normal.
This condition is not usually seen in association with other abnormalities or complications in the antenatal period. These fetuses do not benefit from delivery by Caesarean section.
The treatment for this condition is staged functional reconstruction, with initial surgical intervention in the first few days of life to close the bladder and protect the upper urinary tract. Long-term achievement of continence appears to be around 70%.
There is an increased risk of adenosarcoma of the bladder in these patients that is 400 times higher than the general population. There is also an increased risk of recurrence of 1 in 275 in siblings. In offspring of affected parents the risk may be as high as 1 in 70.
On prenatal ultrasound scan the upper abdominal cavity is filled predominantly by the liver on the right side ( Figure 17-16 ).
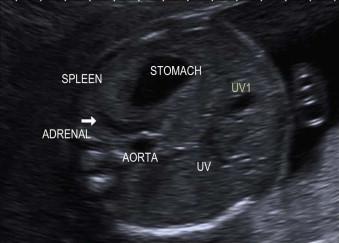
The liver is a uniform echodense structure containing a number of transonic structures:
Hepatic veins: predominantly the intrahepatic portion of the umbilical vein as it joins the portal vein.
Gall bladder.
The spleen is of similar density to the liver and visualized in the fetal left hypochondrium.
The fetal stomach is often visualized on ultrasound in the first trimester and should be easily visualized at 20 weeks in the left of the abdomen below the diaphragm ipsilateral to the abdominal aorta. Due to gastric filling and emptying, it will be of variable size and overall size will vary with gestation. It may take repeated scanning over a couple of hours to visualize the fetal stomach. The importance attached to finding a small fetal stomach will depend on the liquor volume.
The fetal kidneys are easily distinguished on prenatal ultrasound by their position within the posterior aspect of the upper abdomen and slightly echogenic cortex in comparison to the renal medulla and adjacent liver. The normal ureters are not routinely seen on ultrasound. The fetal adrenal glands can be seen in the second and third trimesters. The fetal bladder is of variable size and can be identified from the first trimester.
The small bowel is visible in most fetuses by 20 weeks and in all cases thereafter. It has a slightly echodense appearance in comparison to the liver. Normal small bowel has a luminal diameter of less than 6 mm. The large bowel is seen in all cases after 25 weeks and can become echogenic. The luminal diameter increases with gestation measuring 5 mm at 26 weeks’ gestation and increasing to 20 mm by term ( Figure 17-17 ).
There is a nomogram for normal large bowel calibre with age. The large bowel may be distinguished from the small bowel by its position and the presence of haustrations.
There are several anomalies in the liver that may be visualized using prenatal ultrasound:
Hepatic hyperechogenicities.
Simple cysts.
Hepatic tumours.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here