Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
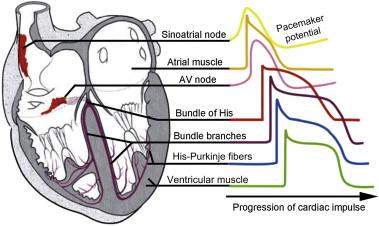
The cardiac conduction system is a network of specialized cells responsible for the initiation and co-ordination of the heartbeat. Relative to the myocytes responsible for regulating cardiac contraction in a normal heart (~1 × 10 9 ), the cells that make up the cardiac conduction system are relatively few in number, but are essential for cardiac electrical signaling and normal physiology. The three main components of the system are the sinoatrial node (SAN), the atrioventricular node (AVN) and the His–Purkinje system (HPS). The SAN, located in the right atrium, is the primary pacemaker of the heart and thus is responsible for the normal initiation of the cardiac action potential (sinus rhythm; Figure 7.1 ). Cells in the conduction system (SAN, AVN, and Purkinje cells) are unique in their ability to generate an electrical impulse or action potential without an external stimulus. This property of automaticity requires a distinct ion channel profile and a finely controlled intracellular electrical coupling. With respect to SAN function, pacemaking requires a synchronized effort because of the principle of entrainment (whereby faster discharging cells are slowed by cells firing more slowly) in a highly heterogeneous complex. The innervation of the sinus node consists of post-ganglionic adrenergic and cholinergic terminals. Most of the efferent vagal fibers converge at the superior vena cava–aortic root fat pad in the right atria, which is also the site of the highest concentrations of norepinephrine. Subsequently the SAN is triggered to discharge after catecholamines bind to sympathetic nerve terminals, causing a positive heart-rate-dependent response via β-adrenergic receptors and the cyclic adenosine monophosphate signaling pathway. A negative chronotropic response is caused by vagal stimulation via acetylcholine binding to muscarinic receptors. Internodal tracts then lead from the SAN to the AVN to continue conduction.
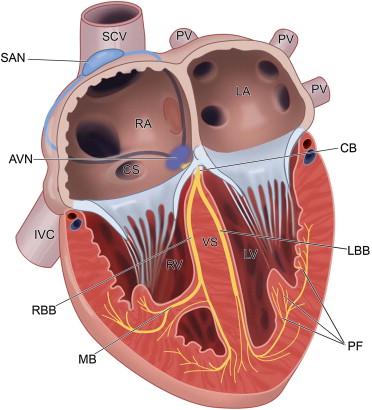
Following initiation in the sinoatrial node, the cardiac action potential propagates to the atrioventricular node (AVN; Figure 7.1 ), located at the apex of a triangle formed by the tricuspid annulus and the tendon of Todaro. The atria and the ventricles are separated by a ring of fibrous tissue, and the only conduction pathway between the two sets of chambers is the AVN, which is located at the base of the right atrium. Conduction of the action potential through the atrioventricular node is relatively slow, consistent with its role as a functional delay between atrial and ventricular systole to allow the atria to pump blood into the ventricles before they in turn contract. Due to automaticity of AVN cells, the AVN may serve as a secondary pacemaker in case of SAN failure (e.g. due to aging or disease). In the event of atrial tachyarrhythmia (e.g. atrial fibrillation or atrial flutter), the AVN plays an important role in limiting the number of action potentials conducted to the ventricles. The main function of the AVN is transmission of the atrial impulse to the His–Purkinje system (HPS) and the ventricles to stimulate chamber contractions.
The HPS in the ventricles consists of a common bundle (the bundle of His), the left and right bundle branches (which arise from the bundle of His), and a network of terminal Purkinje fibers (which arise from the bundle branches; Figure 7.1 ). The function of the HPS is to conduct the action potential rapidly (at velocities up to four meters/second; compared to 0.3–1 meter/second in ventricle ) to the ventricles to ensure that the ventricular muscle contracts simultaneously. The bundle of His or the penetrating portion of the AVN sends out extensions to the actual bundle branches. These fibers connect with the terminal Purkinje fibers on the endocardial surface of the ventricles. Here they form multicellular bundles in longitudinal strands that transmit the atrial action potential to the ventricle to stimulate myocyte contraction.
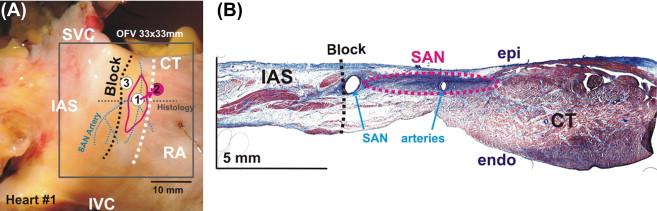
Originally described by the pioneering work of Keith, Mackenzie, Leipzig, Aschoff, Tawara, and Flack over a century ago, the human sinoatrial node ( Figure 7.2 ) is called a ‘spindle’- or ‘cresent’-shaped collection of excitable cells located in a subepicardial location juxtaposed with the crista terminalis (terminal crest). The cells are surrounded by a dense and fibrous tissue environment usually 10–20 mm in length and 2–3 mm in width. The position of the node within the right atria is most often located 1 mm from the surface of the epicardium, laterally in the sulcus terminalis of the right atria (near the junction of the right atrium and the superior vena cava; Figure 7.2 ). The precise position of the SAN may vary by individual, but is usually localized in an environment that includes a nodal artery.
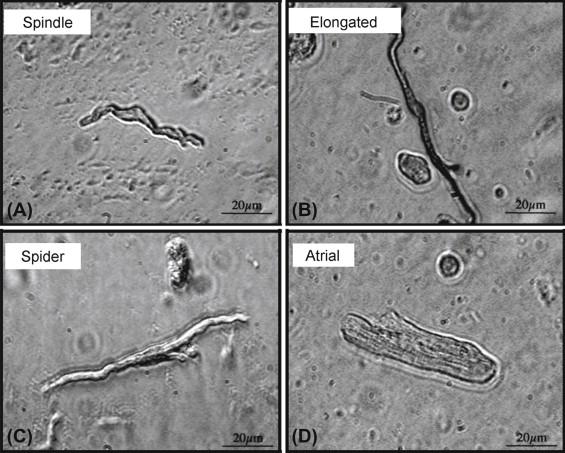
The pacemaker is a collection of weakly coupled, heterogeneous cells, including pacemaker cells as well as non-pacemaker cells such as atrial myocytes, adipocytes and fibroblasts. Within the node, pacemaker cells vary by size and electrophysiological properties with smaller cells showing slower intrinsic firing concentrated in the SAN center and larger, faster-firing cells in the periphery. Pacemaker cells have been successfully isolated from a wide range of species and can be separated into three main categories based on gross morphology. Spindle-shaped cells are relatively small with total membrane capacitance less than 30 pF and sparse apparent myofilaments ( Figure 7.3 ). Elongated cells are larger (membrane capacitance between 35 pF and 50 pF) especially in the longitudinal axis and have a higher myofilament density. Finally, spider cells are distinguished by their branched appearance, although the functional consequences are unclear. It is commonly believed that spindle-shaped cells are concentrated in the central SAN and serve as the leading pacemakers, while elongated cells are more likely found in the periphery. As discussed in the next section, these morphologically distinct cells possess unique electrophysiological properties that are critical for synchronized pacemaking.
The SAN action potential is distinct from that measured in atrial or ventricular cells ( Figure 7.4 ). Most importantly, unlike ventricular or atrial cells, the SAN action potential shows a phase 4 spontaneous depolarization phase with a maximum diastolic value around –60 mV (compared to rest potential close to –90 mV for ventricular myocytes) that eventually reaches the threshold of action potential generation without an external depolarizing stimulus. Control of this spontaneous depolarization phase is critical for pacemaking and involves the coordinated effort of multiple ion channels/transporters/exchangers.
Pacemaking in automatic cells from the SAN depends on a unique electrophysiological profile that allows for generation of spontaneous action potentials. One key channel highly expressed in pacemaker cells is the f- or ‘funny’ channel (primarily HCN4 in the SAN), a hyperpolarization-activated channel that is permeable to both Na + and K + . F-channel current ( I f ) is unique in that it was the first voltage- and time-dependent current that was discovered to be activated upon hyperpolarization of the membrane (rather than depolarization like most other channels). The mixed conductance of Na + and K + provides a reversal potential for the channel around –10 mV so that during diastole, the current is depolarizing. I f is believed to be a key determinant of intrinsic excitability of SAN pacemaker cells. Importantly, these channels are heavily regulated by β-adrenergic and muscarinic receptor activation mediated in large part by direct activation by cAMP.
Expression of Na + channels is highly heterogeneous in the pacemaker with variability across species. In general, I Na is higher in mouse than in larger animals (e.g. rabbit). Both tetrodotoxin (TTX)-sensitive neuronal-type channels and TTX-resistant cardiac isoforms have been found in adult mouse SAN cells. In rabbit, I Na is found in larger cells from the SAN periphery but not in smaller, central SAN. Furthermore, I Na measured in larger rabbit cells shows increased TTX sensitivity compared to that measured in ventricular myocytes, indicating a greater contribution from neuronal isoforms (e.g. Na v 1.1). These findings are significant for the design of Na+ channel-based therapies for cardiac conduction disease.
L-type voltage-gated Ca 2+ channels are essential for generating the SAN action potential upstroke, which as a consequence is much slower than the upstroke recorded in ventricular or atrial myocytes. In SAN cells, Ca 2+ current is contributed to by a variety of channels including multiple isoforms of L- and T-type channels. L-type Ca 2+ current in SAN cells is likely the result of both Ca v 1.2 and Ca v 1.3 channels, unlike ventricular I Ca , which is predominantly Ca v 1.2. Ca v 1.3 channels activate earlier (at more negative potentials) and inactivate more slowly compared to Ca v 1.2.
An important electrophysiological feature of SAN cells is their low expression of inward rectifier K + channels that regulate I K1 , a current responsible for maintaining the stable resting potential in atrial and ventricular myocytes ( Figure 7.4 ). The lack of this major repolarizing current facilitates the spontaneous diastolic depolarization phase in these cells and increases the importance of other K + channel currents (e.g. I Kr , I Ks ) in controlling the maximum diastolic potential. In mouse and rabbit SAN, I Kr block using E-4031 depolarizes the maximum diastolic potential, decreases the action potential amplitude, and delays repolarization leading to a decrease in SAN cell spontaneous firing rate, although to varying degrees depending on species. In contrast, larger animals such as the pig seem to depend more heavily on I Ks for control of maximum diastolic potential. The transient outward K + current ( I to ) is also expressed in SAN and displays a heterogeneous distribution with increasing density as cells become larger (i.e. from SAN center to periphery). I to likely regulates SAN repolarization but not maximum diastolic potential or firing rate. In addition to I Kr , I Ks , and I to, multiple additional K + channel currents play important regulatory roles in the SAN. Notably, SAN cells express acetylcholine-activated K + channels (Kir3.1/Kir3.4) that give rise to an inward rectifying current ( I KACh ), that is activated by muscarinic and adenosine receptor agonists. Also found in SAN cells are K ATP channels ( I KATP ) that activate under conditions of low ATP (e.g. myocardial ischemia) to help decrease pacemaker activity and slow heart rate.
Normal homeostasis of intracellular ions (Na + , Ca 2+ , K + , Cl – ) is tightly controlled in SAN cells through similar mechanisms as found in atrial and ventricular cells. In particular, just as in other cell types, the sarcolemmal Na + /Ca 2+ exchanger (NCX) exploits the Na + gradient to extrude Ca 2+ from inside the cell (forward mode) and help maintain normal homeostasis of intracellular Ca 2+ . Importantly, in the forward mode, NCX generates a depolarizing current, providing a potential link between intracellular Ca 2+ /Na + levels and membrane excitability. In particular, I NCX has been proposed to be an important determinant of the diastolic depolarization rate. In contrast, the Na + /K + ATPase extrudes three Na + from the cell for two K + generating a net repolarizing current that is thought to help set the maximum diastolic potential. Finally, while diastolic depolarization rate (DDR) is heavily influenced by activity of I f , recent studies demonstrate that intracellular Ca 2+ cycling is yet an additional important pathway for controlling DDR and thus heart rate. Specifically, local Ca 2+ release from sarcoplasmic reticulum (SR) ryanodine receptor Ca 2+ channels has been measured during diastole and is believed to facilitate spontaneous depolarization through forward-mode Na/Ca 2+ exchange, which generates a depolarizing current that can affect the rate of spontaneous diastolic depolarization.
Catecholamine-dependent increases in heart rate (positive chronotropy) depend on activation of beta-adrenergic receptors and subsequent activation of a myriad of sarcolemmal and SR ion channels, exchangers and transporters. Increases in cAMP associated with beta-adrenergic receptor activation directly modulate the activities of several channels, including I f , while downstream activation of protein kinase A leads to enhanced phosphorylation and activity of L-type Ca 2+ channels, SR uptake, and release.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here