Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Endovascular aneurysm repair (EVAR) of infrarenal abdominal aortic aneurysms (AAA) produces clinical results similar to those of open surgery after observation periods of 6 and 10 years, respectively, with EVAR having an advantage in postoperative mortality and morbidity.
Unlike open repair where the branch vessels to the aneurysm are ligated, perigraft blood flow outside the endoprosthesis but inside the aneurysm sac may occur after EVAR as the branch vessels are not occluded. This phenomenon was coined endoleak (EL) by White et al.
Endoleak, defined by the persistence of blood flow outside the lumen of the endoluminal graft but within an aneurysm sac or adjacent vascular segments being treated by the graft, is due to incomplete sealing or exclusion of the aneurysm sac or vessel segment, as evidenced by imaging studies (e.g., contrast-enhanced computed tomography [CT] scanning, ultrasonography [US], angiography).
White et al. proposed the first systematic classification of ELs. This original classification was modified in a consensus conference of vascular surgeons and interventional radiologists, and the widely accepted classification was established.
Another classification of EL is based on their source and differentiates five types ( Table 23.1 ).
| Type I | Attachment Site Leak | |
| A | Proximal | |
| B | Distal | |
| C | Iliac plug in aortouniiliac stent-grafts | |
| Type II | Reperfusion Leak | |
| A | Simple single inflow/outflow leak | |
| B | Complex side branch perfusion | |
| Hyperdynamic on US | Quick wash-in | |
| Hypodynamic on US | Slow wash-in | |
| Type III | Device-related Leak | |
| A | Midgraft hole | |
| B | Junctional separation | |
| C | Any other leak caused by device failure | |
| Type IV | Porosity Leak | |
| Type V | Endotension | |
| A | Sac enlargement without demonstration of EL with any imaging modality | |
| B | Sac enlargement, currently no EL discernible, but type I or II EL in previous examinations | |
| C | Type I or III EL proven at surgery, not shown during follow-up imaging | |
| D | Type II EL proved at surgery, not shown during follow-up imaging |
Details for the classification depicted in Table 23.1 follow:
Type I ELs: In this type of EL, inflow occurs from the attachment sites of the endograft, so these ELs are also called attachment site endoleaks. If the leak occurs at the proximal attachment site, it is called a type IA EL ( Fig. 23.1 ). If the leak is around the distal attachment site, it is a type IB leak. If aorto-uni-iliac stent-grafts have been inserted and an EL occurs at the iliac occluder, it is a type IC EL.
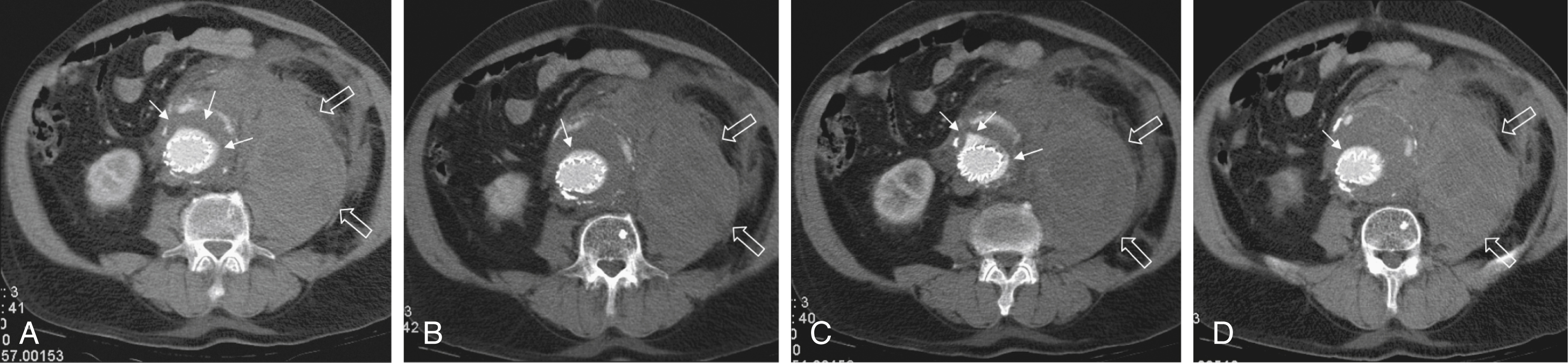
Type II ELs: These ELs are also called reperfusion leaks. The aneurysm sac is partially perfused via arterial side branches originating from the aortic lumen. These side branches can be the inferior mesenteric artery (IMA), lumbar arteries, or in rare cases, accessory renal arteries ( Fig. 23.2 ). Direct arterial inflow into the sac occurs via this side branch perfusion despite tight sealing at the proximal and distal attachment sites. Typically, an inflow and outflow situation is present in type II ELs. Type II ELs can be further divided into simple single-vessel inflow and single-vessel outflow leaks ( type IIA ) and complex inflow and outflow vessel leaks, typically via multiple lumbar arteries ( type IIB ).
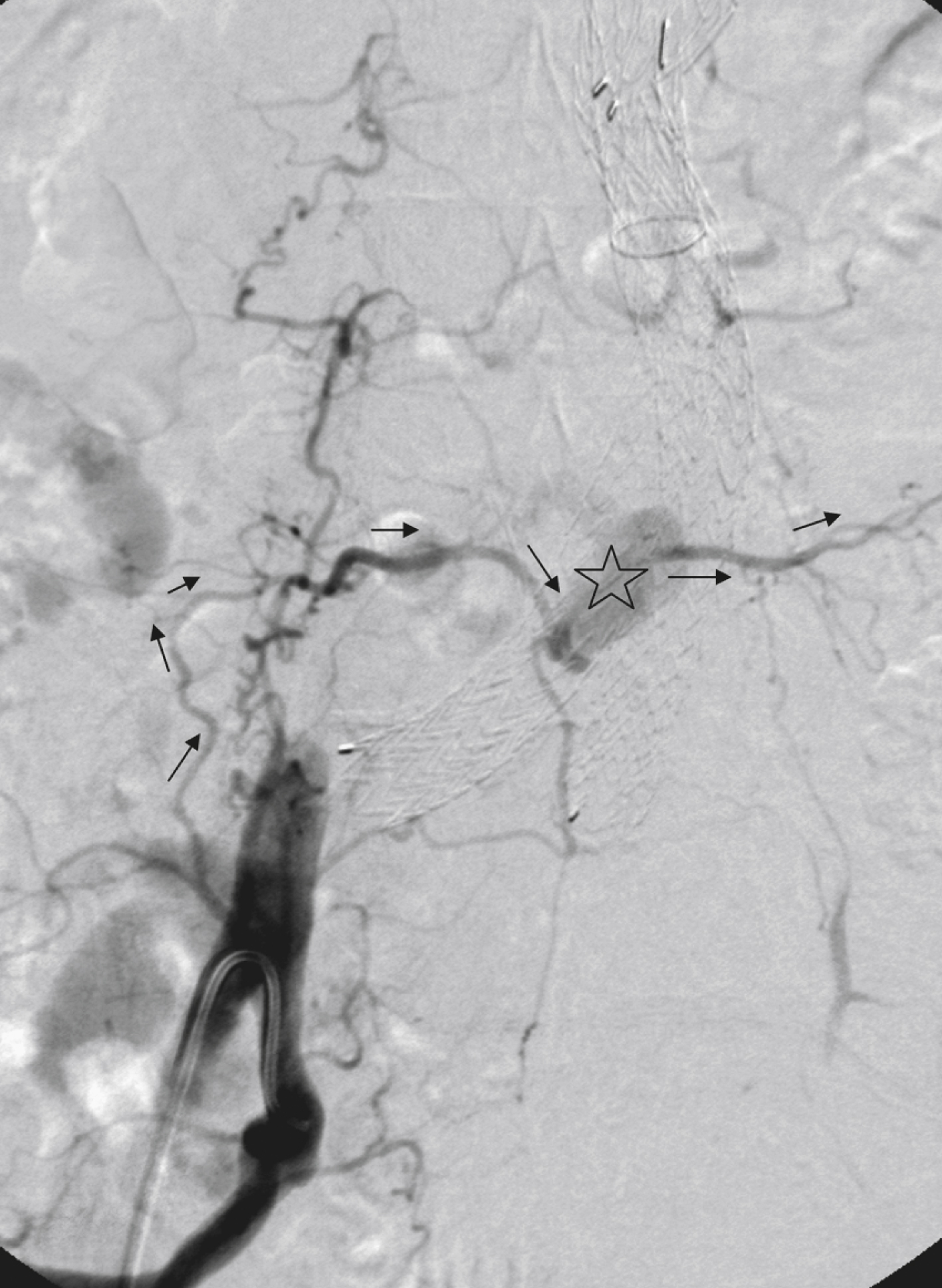
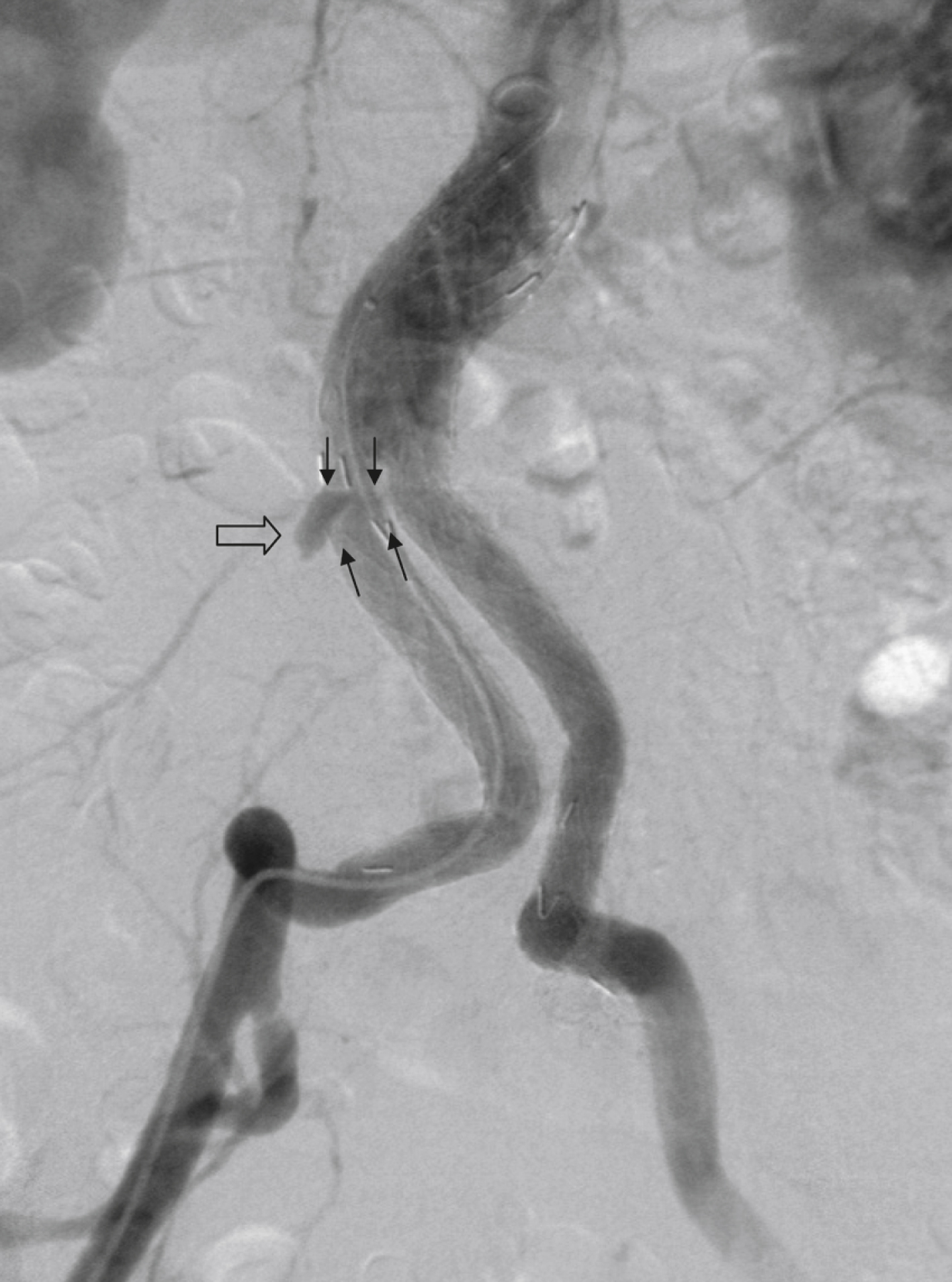
Type III ELs: Type III ELs are caused by graft failure. Typically these types of ELs occur during long-term follow-up and are due to some type of fatigue of the fabric material or fracture of the stent component or both. The stent-graft is under constant mechanical microstress caused by the steady impact of arterial pulsation. Over time, stent wires may break, and holes can develop in the fabric material. Stent-graft distortion may also lead to a type III EL. Eventually, remodeling of the aneurysm may occur and lead to increased bending of the graft. Again, this can be a reason for wire fracture and perforation of the fabric material. With early modular stent-graft designs, junctional separations were observed and resulted in type III ELs ( Figs. 23.3 and 23.4 ). In these stent-graft designs, the overlapping zones at the junctions were too short. This problem has been addressed in newer stent-graft designs simply by extending the junctional overlapping zones. Type III ELs can also be subcategorized. A type IIIA EL is present if the EL is caused by a midgraft hole. A type IIIB EL is a junctional leak, and a type IIIC EL is caused by any other type of graft failure. Generally speaking, with newer stent-graft designs, the incidence of type III ELs has decreased. However, long-term follow-up beyond a period of 10 years is needed to demonstrate whether modern stent-graft designs are durable enough.
![Fig. 23.3, (A)–(C) Computed tomographic angiography follow-up 12 months after endovascular repair. Slippage of the right iliac limb out of the short right common iliac artery has resulted in a distal type III endoleak ( white arrows ). Multiplanar reconstruction ([C]) nicely shows relationship of dislocated iliac limb ( solid arrow ) to parent vessel ( open arrow ). Fig. 23.3, (A)–(C) Computed tomographic angiography follow-up 12 months after endovascular repair. Slippage of the right iliac limb out of the short right common iliac artery has resulted in a distal type III endoleak ( white arrows ). Multiplanar reconstruction ([C]) nicely shows relationship of dislocated iliac limb ( solid arrow ) to parent vessel ( open arrow ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/EndoleaksClassificationDiagnosisandTreatment/2_3s20B9780323612043000233.jpg)
Type IV ELs: These types of ELs are caused by graft porosity. The phenomenon of angiographic blush can be observed during the immediate postimplantation angiogram. Stent-graft designs differ in graft material composition, and immediately after implantation some graft materials are temporarily slightly permeable. The degree of graft permeability is influenced by the degree of anticoagulation. This porosity is self-limiting and resolves within hours after EVAR. In most currently available stent-graft designs, graft material porosity has been reduced to a minimum, so type IV ELs are rarely a problem and have no real clinical relevance. The only important issue is that it can sometimes be difficult to differentiate a type IV arterial blush on the postimplantation angiogram from other clinically relevant forms of ELs. Some authors do not even consider a type IV EL to be a true EL.
Type V ELs: Type V ELs are summarized under the term endotension. These types of ELs were not described in White’s original 1997 classification but were added by the same authors 2 years later. A type V EL, or endotension, is present if the diameter of the aneurysmal sac increases during follow-up without a discernible EL. A subcategorization differentiates type VA, VB, VC, and VD ELs. Type VA EL is the actual endotension. Neither dual-phase contrast-enhanced CT nor any other imaging modality is capable of detecting an EL, and no EL has been shown in previous control studies, but the diameter of the sac increases. Type VB EL is present if the diameter of the sac increases and no EL is shown at a given follow-up CT, but a type I or II EL was shown on previous studies. Therefore an EL might be still present but is not apparent on the given CT scan owing to flow dynamics. Type VC and VD ELs are surgically proved type I and III or type II ELs that have not been shown on CT or other imaging modalities.
Type I and III ELs are also called device-related ELs because a persistent channel of blood flow into the sac is present as a consequence of insufficient sealing of the graft at the proximal or distal attachment site, or at the junction zone of modular stent-grafts.
Primary ELs can be distinguished from secondary ELs according to the timing of development:
Primary ELs occur during the first 30 days after EVAR. These ELs usually develop immediately after EVAR, and the aneurysm was never actually completely excluded from the arterial circulation.
Secondary ELs occur later. Typically, the aneurysm has been excluded from the arterial circulation primarily. However, during follow-up, an EL not seen before is diagnosed. This type of EL is often caused by stent-graft distortion or migration.
Development of an EL is the most common undesired event during or after EVAR. The reason a primary type I EL develops is improper patient selection in most cases. It has been shown that a short aneurysmal neck, severe angulation and calcification, and extensive thrombus lining of the neck are risk factors for a type I EL. If patients with these conditions are not considered for EVAR, primary type I ELs are rare events. Type III ELs are related to graft failure and are not under direct influence of the operator. These types of ELs were frequent with first- and second-generation stent-grafts. The incidence decreased significantly with third-generation devices. Type II ELs are not typical complications because their development can hardly be influenced by the operation; they are inherent phenomena of EVAR. Type IV ELs are not true ELs because they represent an angiographic feature without any clinical consequence. Type V ELs seem to be related to type II ELs and can just barely be called complications.
In an analysis of 6787 patients from the registry database of the European Collaborating Groups on Stent-Graft Techniques for Abdominal Aortic Aneurysm Repair (EUROSTAR), the annual incidence of device-related ELs (i.e., type I and III ELs) was 6% and that of type II ELs was 5%. In this analysis, the authors showed a clear difference in performance of different stent-graft designs with regard to EL occurrence. Device-related ELs occur less frequently with second- and third-generation stent-grafts because they provide a better proximal and distal seal and better modular stability.
There is clear evidence that ELs directly influence the clinical outcome of EVAR. It has been shown that type I and III ELs are significant risk factors for rupture after EVAR. The incidence of these types of ELs varies between 0% and 10% and strongly depends on patient selection.
The situation is less clear for type II ELs. Gelfand et al. summarized the results of 10 EVAR trials involving a total of 2617 patients. The incidence of type II ELs was 6% to 17% at 30 days, 4.5% to 8% at 6 months, and 1% to 5% at 1 year. A EUROSTAR database analysis of 3595 patients showed significantly higher rates of secondary interventions in patients with type II ELs than in patients without ELs. However, conversion to open repair or post-EVAR rupture was not significantly associated with type II ELs. In contrast, rupture of aneurysms with a type II EL has been described, although this occurs rarely.
The frequency of type V ELs is less well defined. Mennander et al. reported an incidence of 3.1% in a series of 160 patients; aneurysm rupture occurred in 3 of the 5 patients with endotension they followed. However, no significant intraperitoneal or retroperitoneal bleeding was observed.
Because of their clinical relevance, most authors assume that diagnosis of ELs is important. Adequate follow-up care of patients after EVAR can be provided only if the responsible physician knows whether the patient has an EL and, if such is the case, which type of EL it is. Sufficient information about the morphologic evolution of the aneurysmal sac is vital, whether it be maximum sac diameter or, even better, sac volume.
Imaging modalities used for patient follow-up after EVAR and for diagnosis of ELs are CT, US, magnetic resonance imaging (MRI), intraarterial digital subtraction angiography, and plain abdominal radiology. As an alternative method of monitoring patients after EVAR, intraluminal sac pressure monitoring has been proposed. Each imaging modality is discussed separately in the following sections.
The diagnostic CT criterion for an EL is a contrast-enhancing area outside the stent-graft lumen but inside the aneurysmal sac. For correct classification of an EL, the location of the contrast-enhancing area, its relationship to the proximal and distal sealing zones, and the presence of possibly perfused side branches have to be evaluated. Additional desirable information includes stent-graft integrity, deformity, and migration.
Typically, multiphase examinations are performed with multislice CT scanners, including an unenhanced, arterial, and delayed-phase scan. Unenhanced images may be helpful in differentiating small perigraft leaks from areas of calcified thrombus or metallic structures of the stent-graft. Typically the arterial-phase acquisition clearly shows perigraft flow if present. However, Rozenblit et al. showed that a delayed CT acquisition in addition to the arterial-phase scan can help detect additional ELs ( Fig. 23.5 ). These authors also showed that the number of indeterminate findings can be reduced when an additional delayed-phase CT is acquired, so a triphasic CT acquisition protocol, including a delayed-phase scan acquired 50 seconds after injection of contrast material, has now been adopted in many institutions.
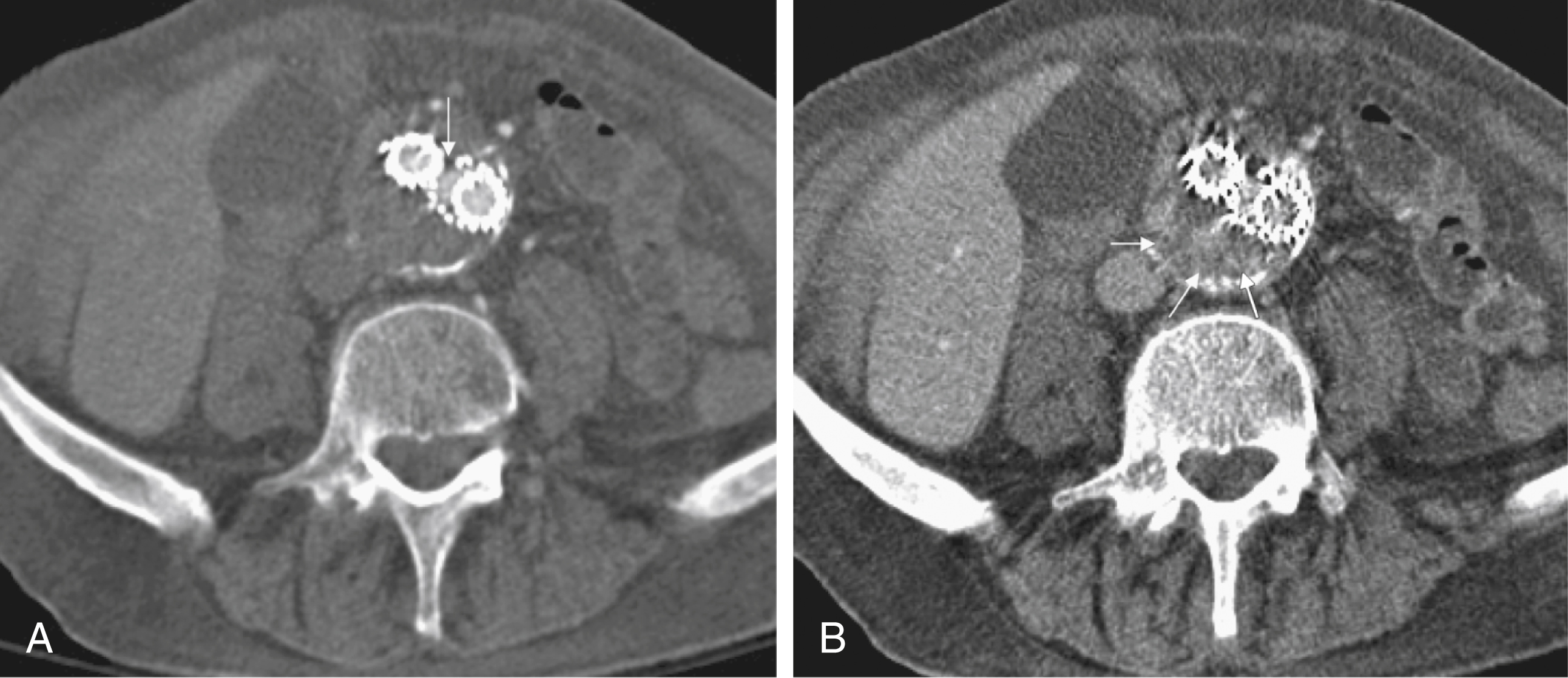
In the case of a type I EL, direct communication of the parent vessel with the sac lumen may be depicted. Sometimes this communication can be displayed best in a multiformatted reconstruction (see Fig. 23.1 ).
The diagnostic clue to a type II EL is seeing contrast-enhancing side branches with direct communication to the area of contrast enhancement in the sac ( Fig. 23.6 ).
![Fig. 23.6, Axial computed tomography (CT) slices ( [A] and [B]) show a type II endoleak ( small white arrows ). Note that lumbar arteries of segment L5 are well perfused and therefore permit an inflow/outflow situation ( open arrows ). However, CT is unable to show the direction of flow. Fig. 23.6, Axial computed tomography (CT) slices ( [A] and [B]) show a type II endoleak ( small white arrows ). Note that lumbar arteries of segment L5 are well perfused and therefore permit an inflow/outflow situation ( open arrows ). However, CT is unable to show the direction of flow.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/EndoleaksClassificationDiagnosisandTreatment/5_3s20B9780323612043000233.jpg)
In type III ELs, the EL is usually in direct communication with the stent-graft, and direct communication between the lumen of the stent-graft and sac can often be seen. Disintegration of the stent-graft and metallic strut fractures is a strong indicator of a type III EL. Although the metallic skeleton of the stent-graft is typically evaluated by plain abdominal films, the same information can be provided by modern multislice CT scanners.
Type IV ELs do not play a clinically significant role, as already mentioned.
Type V ELs, by definition, are characterized by increasing diameter of the sac without a detectable EL.
Although CT has been advocated as the gold standard for EL detection, it has several drawbacks. One is that a considerable amount of potentially nephrotoxic contrast medium is required. Depending on the multislice CT scan protocol, 80 to 120 mL of contrast medium per examination is used. Over a 5-year period, Azizzadeh et al. found impaired renal function in 83% of their 398 patients who underwent EVAR. The authors conclude that periprocedural renal protection and the use of alternative contrast agents during follow-up might be beneficial in selected patients.
The second drawback of multislice CT is radiation exposure. Although several technical features are implemented in modern CT scanners to reduce radiation exposure, multislice CT remains a radiologic examination responsible for more than two-thirds of the total patient radiation dose associated with medical imaging. Based on an equation published by the International Commission for Radiation Protection, the lifetime risk for induction of cancer is 280 per million patients after a single abdominal CT scan. White and Macdonald estimate a total effective radiation exposure of 145 to 205 mSv for 70-year-old patients, including planning CT, EVAR, and surveillance CT at 1, 3, 6, and 12 months and yearly thereafter. In their calculation, this leads to a lifetime cancer risk of 0.42%, or 1 in 240. These numbers become more worrisome with decreasing patient age. For a 50-year-old patient, the cancer risk would be 0.73%, or 1 in 140. However, a mitigating factor is that we are dealing with an elderly patient population usually well above the age of 65, and it has been well documented that radiation risk is significantly lower in the elderly.
Nevertheless, contrast medium load and radiation exposure are important concerns when serial CTs are performed for long-term follow-up of patients after EVAR. For this reason, two studies evaluated whether the commonly used multiphase CT protocol can be reduced by eliminating the early arterial phase or the unenhanced or delayed phase or both. The authors of the first study concluded that the arterial phase may not be necessary for routine EL detection, whereas the authors of the latter study found that the combination of unenhanced and arterial-phase CT affords the highest positive predictive value for detection of ELs after 1 month, and that the delayed phase does not significantly increase the sensitivity for detection of ELs, although ELs not seen on early-phase CT may be seen on delayed-phase CT. In another study, Bley et al. showed that serial volumetric analysis of nonenhanced CT may serve as an adequate screening test to identify ELs that cause a volumetric increase of more than 2%. Recently, dual-energy CT has been evaluated for EL detection after EVAR. With dual-energy CT, only one single delayed scan is acquired with a dual-source CT scanner. From this dataset, a virtual nonenhanced scan is calculated. Compared with a triphasic CT protocol, there was no significant difference in the numbers of ELs detected, but the radiation dose was up to 61% less.
The third drawback of CT is that it cannot display flow dynamics. In some cases, correct EL classification depends on evaluating the direction of perigraft flow and the direction of flow in aneurysmal side branches. This problem is discussed in the sections describing the value of intraarterial digital subtraction angiography and US in patient follow-up after EVAR.
The ideal CT protocol has not yet been defined. Although it is of utmost importance to apply the CT protocol with the best trade-off between radiation and contrast medium exposure and diagnostic yield, proper timing and selection of the imaging modality used during follow-up have the largest influence.
Ultrasonography is being used more frequently as an alternative to multiphase CT in patient follow-up after EVAR. It is less expensive, and no radiation or nephrotoxic contrast material is required ( Fig. 23.7 ). The reliability of US in detecting ELs has been evaluated in numerous studies. In an early study, Sato et al. reported a 74% specificity and 97% sensitivity of color-coded US (ccUS) for detection of ELs. Better results were reported by Zannetti et al., who found an encouragingly high 91.7% sensitivity and 98.4% specificity of ccUS for EL detection, with CT serving as the standard of reference. In contrast, Raman et al. found only 42.9% sensitivity of ccUS in detecting ELs. Its specificity was 96%, and the positive predictive value was 53.9%, with only modest correlation of the results of ccUS and CT. Based on their experience with 281 patients, the authors concluded that ccUS cannot effectively replace CT after EVAR.
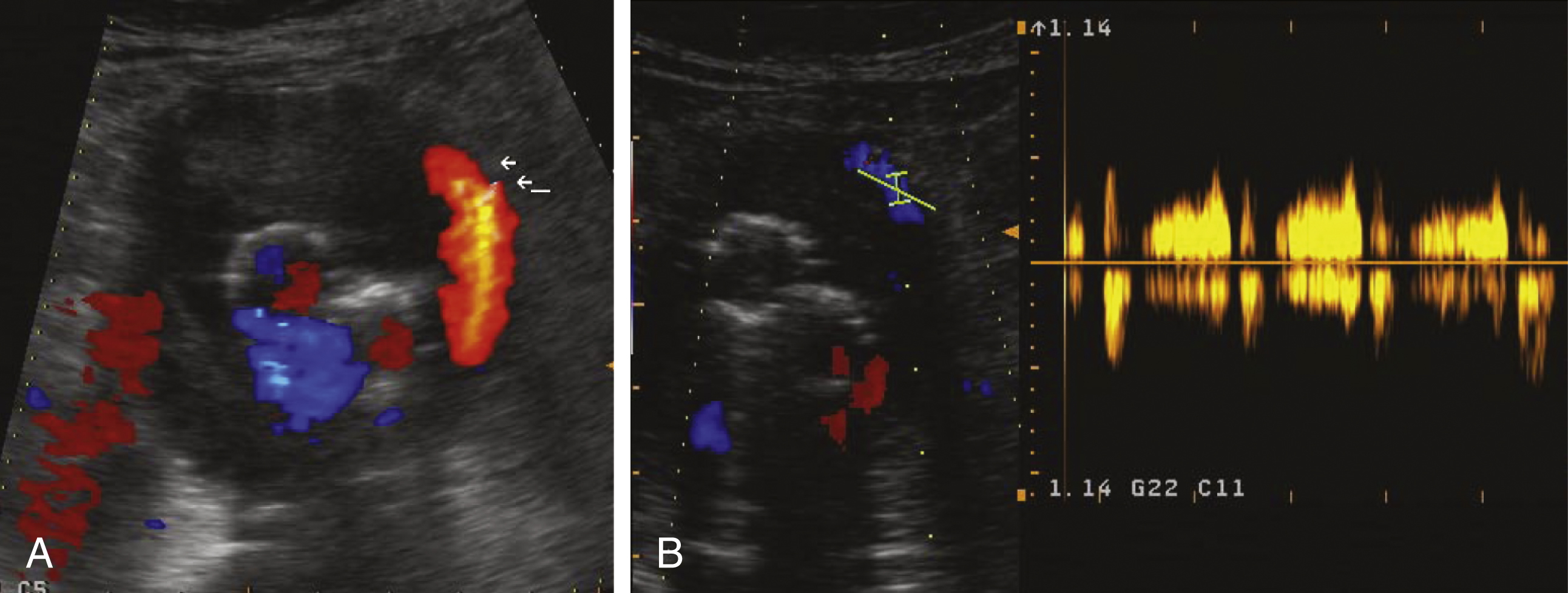
In more recent studies, contrast-enhanced US (CEUS) has been compared with CT. Encouraging results have been reported by Giannoni et al. and Napoli et al. In both studies, CEUS showed ELs not detected by other imaging modalities. One potential advantage of US over other imaging modalities is its ability to characterize blood flow dynamics. For instance, a persistent EL is typically associated with an inflow and outflow situation. In contrast to CT, these flow phenomena can be evaluated with US. In a small but interesting series of 18 patients, Bargellini et al. used CEUS to analyze the hemodynamic flow pattern of type II ELs and found a correlation of type II EL flow dynamics and AAA size. Type II ELs with wash-in and washout times less than 100 and 520 seconds, respectively, were called hyperdynamic ELs , in contrast to hypodynamic ELs , which had longer wash-in and washout times. The authors showed that a volume increase of 1 mL per month or greater might be associated with hypodynamic type II ELs. They hypothesized that insufficient outflow from the sac in a hypodynamic EL could lead to pressurization of the aneurysm and possible enlargement. On the other hand, in hyperdynamic type II ELs, a widely patent inflow and outflow tract might allow free flow through the aneurysm without significant effects on pressure. In a later study, Beeman et al. also tried to determine Doppler sonographic flow parameters, which may be associated with the persistence of type II ELs and an increase in AAA sac diameter. In contrast to the Bargellini study, they found no correlation between the intrasac flow velocity and increased AAA size after EVAR. However, the presence of multiple type II ELs and a bidirectional intrasac flow were identified as possible predictors of an increase in sac diameter. In another study, Parent et al. showed that a to-and-fro flow pattern in a type II EL is more often associated with spontaneous sealing of the EL than is the case with a monophasic or biphasic flow pattern.
Despite these interesting observations, the roles of ccUS and CEUS are still under evaluation. In a systematic review performed by Ashoke et al. and based on eight published and two unpublished studies, the sensitivity and specificity of US were 69% and 91%, respectively. The authors concluded that US does not have sufficient diagnostic accuracy for detection of all ELs in routine clinical practice. However, this conclusion was only partially confirmed by Mirza et al., who performed a more recent and more extensive systematic review. Twenty-one studies comparing ccUS with CT and seven studies comparing CEUS with CT were included. Similar to Ashoke’s analysis, sensitivity for ccUS (77%) was rather low, but specificity was 94%. However, CEUS had a sensitivity and specificity of 98% and 88%, respectively.
Another important parameter during patient follow-up after EVAR is sac diameter. US was thought to be less reliable than CT for sac measurement, but several recent studies have shown them to be equally reliable.
The fact that CEUS may detect ELs not shown by CT angiography (CTA) explains why CTA can no longer be considered the standard of reference. Most endoleaks are shown by both imaging modalities, but some are only seen on CTA and some only by CEUS.
In general, ccUS and especially CEUS are valid methods for patient follow-up after EVAR, and in many centers, US has replaced CTA as the standard method.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here