Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the genitourinary system as in other organ systems, radiologically guided interventional procedures have grown in usage. Interventional genitourinary radiology procedures are used widely, so familiarity and experience with these procedures are necessary in most clinical settings. Growth in the use of these procedures is due to their minimally invasive nature. Advantages of these procedures include shortened hospital stay, diminished need for anesthesia, lower cost, and rapid recuperation after the procedures. Also driving the increase in popularity for interventional procedures have been improvements in equipment, including imaging equipment improvements and catheter and guidewire technology advances.
Before percutaneous puncture of the urinary system is undertaken, a basic coagulation profile [international normalized ratio (INR) and platelet count] should be obtained and abnormalities reversed whenever possible. In patients with thrombocytopenia, platelet transfusion can be performed before and during percutaneous urinary tract procedures. Similarly, a coagulopathy may be corrected if necessary. A platelet count higher than 50,000 and INR less than 1.5 are desirable before percutaneous renal puncture, although slight deviations may be acceptable depending on the urgency of the procedure.
Antibiotics should be administered before urinary tract puncture ( Box 10-1 ). If no urinary infection is suspected, a broad-spectrum antibiotic (usually a cephalosporin) can be administered immediately before the procedure. Antibiotics can be discontinued after the procedure if the patient has no evidence of infection when discharged. If urinary tract infection is suspected, but no specific organism has been cultured, the combination of an aminoglycoside and ampicillin or a similar penicillin derivative should be administered immediately before the procedure. Patients in this category include those with evidence of ongoing urinary tract infection, and those with a high risk of asymptomatic urinary tract infection (e.g., patients with urinary conduit diversions or with infection-based stones). Ideally, culture-specific antibiotics should be administered before percutaneous urinary intervention in patients with known urinary tract infection. In addition, a bladder catheter should be placed in most patients because procedures may be protracted and urination with the patient in the prone position will be difficult. Finally, signed informed consent should be obtained before the procedure, with its indications and risks explained to the patient or surrogate ( Box 10-2 ).
Ideal: culture-specific antibiotics
Suspected infection: penicillin derivative and aminoglycoside
No suspected infection: broad-spectrum cephalosporin
Serum coagulation profile
Serum platelet count
Prophylactic antibiotics (see Box 10-1 )
Informed consent
Most percutaneous urinary tract procedures involve transrenal puncture. Although access to the collecting system is feasible by fluoroscopy alone, ultrasound (US) guidance is now commonly used to direct a needle into a calyx, followed by passage of a wire and further manipulations observed with fluoroscopy. Computed tomography (CT) may be used in rare cases. CT is especially useful to ensure a safe transrenal puncture in patients whose congenital urinary tract anomalies or abnormalities of surrounding organs can be visualized only with CT guidance.
Before planning transrenal access, any available cross-sectional imaging should be reviewed to assess the position of the colon. A far lateral needle trajectory on either side, but particularly on the left, may risk traversing the colon.
Antegrade pyelography, which entails simple percutaneous puncture followed by contrast injection, is performed for radiographic evaluation of the ureter and collecting system. Antegrade pyelography may be useful for patients with suspected urinary tract abnormalities who have contraindications to intravascular administration of contrast material, or those who have poorly functioning or obstructed kidneys. Note that retrograde pyelography is usually the initial test of choice in this setting because it is less invasive. Antegrade pyelography procedure may also be performed as a prelude to percutaneous nephrostomy (PN) drainage or Whitaker testing.
The Whitaker test is used to evaluate and quantify suspected ureteral obstruction and is an extension of antegrade pyelography ( Box 10-3 ). Both require fine-needle puncture and opacification of the intrarenal collecting system. With Whitaker testing, active infusion of dilute contrast material is used to evaluate the capacitance of the urinary system to transmit varying fluid volumes. Following or during dynamic infusion, pressure gradients are measured between the renal pelvis and the bladder, and obstructions, when present, can be quantified. The Whitaker test is the most objective measure of the urodynamic significance of areas of ureteral narrowing or of collecting system dilatation. The test yields objective, reproducible data that can be essential for treatment planning. Specifically, Whitaker testing can be used to evaluate ureteropelvic junction (UPJ) strictures or other ureteral strictures to determine the need for percutaneous or open surgical repair. In addition, percutaneous pyelography and Whitaker testing are often useful in the evaluation of a hydronephrotic transplant kidney. Because of the potential nephrotoxicity of intravascular contrast material and the minimal risk associated with antegrade pyelography, this procedure is often selected to evaluate transplant kidneys in patients with suspected obstructive uropathy.
Distinguish obstruction from unobstructed ectasia
Evaluate ureteral narrowing for possible intervention
Evaluate the ureter of transplant kidney
Both antegrade pyelography and the Whitaker test are performed after fine-needle puncture of the intrarenal collecting system. A 21- or 22-gauge thin-wall needle is adequate for these procedures. Fine-needle puncture of the kidney is associated with low risk of vascular injury and sepsis. Numerous transrenal punctures with a 21- or 22-gauge needle may be performed with relative impunity because of the low risk of significant complications.
When the bladder has been catheterized and the patient is in the prone position on the fluoroscopy table, the flank is cleansed and draped in accordance with standard sterile techniques. Similarly, access to a transplant kidney is achieved with patient in the supine position, usually from an inferolateral approach. The kidney is localized with either fluoroscopy or US. Sometimes a previously obtained abdominal radiograph can be used as a reference for identifying the renal position. If the kidney is not readily visible, a puncture site can be empirically selected 2 to 3 cm lateral to the top of the L2 vertebral body. Before puncture, local anesthetic should be administered. In the case of fluoroscopic guidance, the skinny needle is passed in a vertical direction ( Fig. 10-1 ) while the patient suspends respiration. In patients with normal body habitus, the needle should be advanced 10 to 12 cm with a single pass. The needle stylet should then be removed and a syringe attached to the needle. The needle can then be withdrawn while continuous aspiration is applied. Alternatively using US, the needle is directed into a posterolateral calyx (usually inferior pole). An echo-tip needle may be helpful. When aspiration returns urine, withdrawal is halted. The urine aspirate should be saved for culturing. Iodinated contrast material can then be injected into the urinary system through the skinny needle. Once satisfactory needle position has been confirmed fluoroscopically with opacification of the intrarenal collecting system, more contrast material may be injected under intermittent fluoroscopic monitoring. Overdistension of the collecting system should be avoided to minimize the risk of sepsis. Overdistension can lead to pyelovenous backflow of urine, carrying along any pathogens contained in the urine. To avoid overdistension, a transfusion technique should be employed. With this technique, an equal volume of pure contrast material is injected after the withdrawal of a volume of urine and diluted contrast material through the needle. With this procedure, opacification of the urinary system is slower, but overdistension is avoided because the volume of contrast material injected matches the volume of urine withdrawn.

For antegrade pyelography, spot films are obtained as needed to demonstrate the entire pelvicalyceal system and ureter, with particular attention to the sites of suspected abnormality. If significant obstruction is identified, a PN drain can be placed. Alternatively, if no significant obstruction is seen, or a drain is not required, antegrade pyelography can be terminated and the needle is withdrawn. If no obstruction is encountered and a drain is not placed during this procedure, antibiotics should be continued for at least 24 hours after the procedure is terminated.
The Whitaker test is performed after opacification of the collecting system for antegrade pyelography. Opacification is performed to ensure that the needle is in the correct position, without extravasation outside the urinary tract. The equipment needed for the Whitaker test is somewhat complex ( Fig. 10-2 ). With the needle in place, the bladder is emptied, and manometers, infusion lines, a power infusion pump, and a bladder drainage conduit are connected. The two manometers should be placed at an equal height above the floor. Bladder and renal pelvic pressures are measured just before and immediately after each timed infusion. For the Whitaker test, dilute contrast material is infused into the renal pelvis at a rate of 5 mL/minute for 10 minutes. Pressures are measured and recorded. The second infusion is performed at 10 mL/minute for 10 minutes; and finally a third infusion is performed at 15 mL/minute for 10 minutes. Infusions should be discontinued immediately if adjusted renal pelvis pressure exceeds 40 cm H 2 O, of if severe flank pain develops, or significant contrast extravasation occurs. If results are equivocal from this initial series of infusions, the test is repeated, but with the bladder filled. Occasionally, mild obstruction may become evident with increased pressure only with a full bladder. The patient should be instructed to notify personnel during the procedure if symptoms are reproduced during infusion of contrast material. The reproduction of symptoms, such as flank pain, supports the presence of urodynamically significant obstruction.

Net renal pelvic pressures are calculated by subtracting the bladder pressure from the renal pelvic pressure, thereby removing the component of general intra-abdominal pressure from the measurements. Table 10-1 outlines a system for classifying the numerical results of the Whitaker tests and converting them to degree of ureteral obstruction.
| Differential Pressure (cm H 2 O) | Degree of Obstruction |
|---|---|
| 0-12 | None |
| 13-20 | Mild |
| 21-34 | Moderate |
| >34 | Severe |
Indications for PN drainage are numerous. The most common indications are decompression of an obstructed kidney, urinary diversion for treatment of urinary tract fistula or perforation, decompression of an infected urinary tract, or as a prelude to additional urinary tract interventions, such as stent placement or endoscopy. The risks of PN ( Table 10-2 ) include septicemia (2%), life-threatening hemorrhage (1% to 2%), and adjacent organ injury (1%). The only absolute contraindication is the presence of an uncorrected bleeding disorder.
| Complication | Rate |
|---|---|
| Sepsis | 2% |
| Hemorrhage requiring transfusion | 2% |
| Adjacent organ injury | 1% |
With this procedure as with other percutaneous transrenal procedures, antibiotics should be preadministered. The choice of antibiotics has been outlined earlier (see Box 10-1 ).
Selection of the transrenal puncture site is important to avoid unnecessary vascular injury. Ideally, the nephrostomy tract should traverse the kidney near the junction of the ventral two thirds and the dorsal one third of the kidney. This area is known as Brodel line or the avascular plane ( Fig. 10-3 ) of the kidney. It represents a watershed zone that is supplied by only small-vessel ramifications from the anterior and posterior renal artery branches ( Fig. 10-4 ). This plane has been used for renal surgical procedures to minimize postoperative renal atrophy.


The location of the avascular plane can be estimated using real-time sonography, CT, or fluoroscopy. In most instances the simplest technique for guiding transrenal puncture is fluoroscopy with opacification of the collecting system. This can be performed via US or with antegrade pyelography through a skinny needle, with intravenous urography, or with retrograde injection of contrast material through a stent or through a urinary conduit diversion. Posterior calyces should be identifiable with opacification. These calyces are usually positioned more medially than are anterior calyces. In addition, they are generally seen en face when imaged on the anteroposterior plane ( Fig. 10-5 ). Identification of posterior calyces can be enhanced in the prone patient by injecting small volumes (5 to 10 mL) of room air into the calyces; the air will flow to fill these dorsally oriented structures.

When the posterior calyces have been identified, selection of the exact calyx to be punctured will depend on the position of the calyx and the intention of the procedure. If the procedure is being performed to divert urine or decompress an obstructed system, a posterior calyx located below the 12th rib should be selected for transrenal puncture. If further interventions such as stent placement are likely, puncture of a calyx in the upper pole will make access to the ureter or to specific areas of the kidney easier. In any case the selected posterior calyx should be punctured from a posterior obliquity of 25 to 30 degrees from the vertical position ( Figs. 10-6 and 10-7 ). This can be achieved by leaving the patient in the prone position and angling the fluoroscopy tube 25 to 30 degrees. The kidney can then be punctured along the axis of the fluoroscopic beam, assuring an appropriate approach. Alternatively, the patient's ipsilateral flank can be elevated 25 to 30 degrees when vertical fluoroscopy is employed.

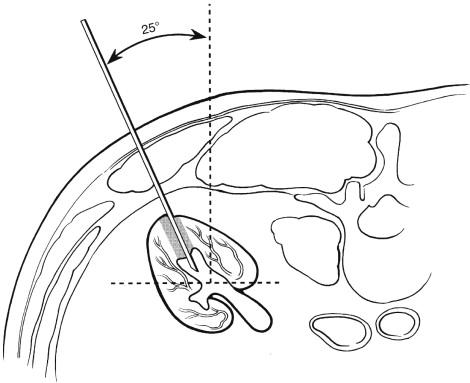
After the puncture site is identified with fluoroscopic localization, the area of skin in line with the planned site of calyceal entry should be anesthetized with 1% lidocaine without epinephrine. Both superficial and deep skin anesthetization should be performed along the anticipated puncture tract before transrenal puncture. A small skin incision is made with a scalpel at the site of intended puncture. The calyx is then punctured with an 18- to 22-gauge needle under continuous fluoroscopic monitoring while the patient suspends respiration ( Fig. 10-8 ). Use of a radiolucent needle holder ( Fig. 10-9 ) allows for fluoroscopic needle guidance while avoiding radiation exposure to, and obscuration by, the operator's hand. Actual puncture of the calyx can be seen fluoroscopically ( Fig. 10-10 ) or sonographically. Urine can be aspirated to confirm needle position.



US guidance may alternatively be employed with patient in the same positioning. Using a curved or vector transducer, the kidney is visualized in its longitudinal axis. The transducer may be angled up from a subcostal approach to target a posterolateral inferior pole calyx. The planned trajectory should be anesthetized with 1% lidocaine to a depth just shy of the renal cortex. Then under direct visualization, an 18- to 22-gauge needle is directed into the targeted calyx. An echo-tip needle may be helpful for visualization. Urine can be aspirated to confirm needle position.
An angiographic guidewire (0.035 or 0.038 in.) should then be advanced into the collecting system. Once an adequate length of guidewire has been placed within the intrarenal collecting system, the needle is removed and an angiographic catheter can be advanced along the guidewire ( Fig. 10-11 ). The catheter selected will depend on the operator's preference, but a standard catheter shaped like a hockey stick works well in most situations. The catheter is used to steer the guidewire into a stable position for further tract dilatation. Ideally, the guidewire can be advanced well into the ureter to decrease the risk of dislodging the guidewire during further manipulations. Once the guidewire is positioned, the catheter is removed and the tract is dilated over the guidewire to an appropriate size for nephrostomy tube placement ( Fig. 10-12 ). For simple urine drainage, an 8- to 10-F tube is adequate. For a solitary kidney, or for drainage of viscous urine (i.e., infected or hemorrhagic urine), tubes 12 to 14 F in diameter are recommended. To avoid unnecessary accidental dislodgment of the PN tubes, only self-retaining nephrostomy tubes should be used ( Fig. 10-13 ). Before terminating the procedure, contrast material should be injected through the PN tube to confirm satisfactory position and function of the drainage catheter.



Urine output should be monitored carefully while the patient is hospitalized. Some hematuria is expected for up to 72 hours after PN tube placement. In addition, bacteriuria occurs in nearly all patients with prolonged PN drainage. This bacteriuria is of no clinical significance provided urinary drainage is adequate, that is, the nephrostomy tube is patent. Nephrostomy tubes should be changed prophylactically every 4 to 8 weeks to avoid tube obstruction. Shorter tube-change intervals may be required in some patients, but intervals greater than 8 weeks should be avoided because tube occlusion can lead to dramatic and rapid onset of significant clinical problems. Obstructed urinary outflow coupled with bacteriuria can lead to rapid development of pyonephrosis and septicemia. Further, exchange of an obstructed PN tube is technically more demanding than exchange of a patent tube.
Ureteral stents can be placed for the maintenance of ureteral patency in patients with urolithiasis and in those with benign or malignant strictures. In addition, ureteral stents can enhance ureteral healing while reducing the risk of stricturing in patients with ureteral fistulas or those who have undergone endoluminal ureteral manipulations, including endopyelotomy, which entails transmural incision of the ureteral wall, and ureteral balloon dilatation. Patients are usually referred for antegrade placement after failure of retrograde cystoscopic placement.
The risks associated with placement of percutaneous antegrade ureteral stents are similar to those associated with placement of PN tubes. Ureteral perforation occasionally occurs during attempts at ureteral stent placement. Although ureteral perforation may interfere with completion of stent placement, the perforations are self-limited and of no clinical significance if adequate renal drainage is maintained after the procedure.
Usually, percutaneous ureteral stent placement is requested after PN drainage. In any case, transrenal percutaneous puncture and tract dilatation should be performed as outlined earlier for PN drainage procedures. For stent placement, transrenal access through a mid or upper calyx is preferred to provide the most favorable approach down the ureter. Before further manipulations, two guidewires should be placed. This task can be accomplished using a sheathed dilator ( Fig. 10-14 ). One of these wires will be used as a working wire, and the other should remain in place as a safety guidewire for use in case the working wire becomes dislodged or kinked. With standard angiographic techniques, the working wire can then be steered through the UPJ. Often, use of a hydrophilic guidewire facilitates these manipulations. The angiographic guidewire should then be advanced down the length of the ureter and into the bladder. An angiographic catheter could be advanced over the guidewire so that its tip reaches the bladder lumen.

The next step, determining the correct stent length, can be achieved with various methods, but the most direct uses the bent-guidewire technique ( Fig. 10-15 ). With the catheter tip within the bladder, a guidewire is advanced so that its tip is within the catheter just below the ureterovesical junction. This guidewire is then kinked at its exit from the catheter hub outside the patient's flank. The guidewire is then further retracted until the tip, which is still within the angiographic catheter, is in the renal pelvis. Its location is readily visible with fluoroscopy. A second kink is made where the guidewire exits the catheter hub outside the patient's flank. The guidewire is then completely removed and the catheter is left in place. The distance between the two guidewire kinks provides an exact measurement between the ureterovesical junction and the renal pelvis, and therefore determines the length of the straight segment of the ureteral stent to be placed. A new working guidewire should then be advanced into the catheter. A stiff angiographic guidewire is preferred for stent placement. The catheter is then removed, and the stiff working guidewire is left in place.

A tapered 8-F angiographic catheter is then advanced over the guidewire into the bladder. This catheter is used to test for stenotic areas that might impede placement of the ureteral stent. If no resistance is encountered, an 8-F or smaller stent can be placed without further manipulations. If resistance is encountered, the 8-F catheter is removed and replaced over the guidewires with a 9-F sheathed dilator. This sheath should be advanced as close to the bladder as possible to facilitate stent placement. The dilator segment is then removed, and the ureteral sheath is left in place over the working guidewire. With or without the sheath, the stent is then advanced over the guidewire until the tip of the stent is well within the bladder. If an adequate length of stent has been advanced into the bladder, then, upon retracting the working guidewire, the lower loop of the ureteral stent will form within the bladder lumen. If the length is inadequate, the guidewire should be readvanced through the stent, followed by further advancement of the stent itself. If ureteral stenosis prevents adequate advancement of the stent, balloon dilatation of the stenotic ureter should be performed. Balloons 6 to 10 mm in diameter are routinely used for ureteroplasty. The entire stenotic segment should be dilated with a balloon before an attempt is made to replace the ureteral stent. High-pressure balloons are often required to dilate malignant or fibrotic benign ureteral strictures.
After advancing an adequate length of the stent into the bladder, the guidewire is retracted and the lower coil of the stent reconstituted within the bladder. Fluoroscopy of the renal end of the stent is then performed to estimate whether an adequate length of stent remains to allow for reconstitution of the upper loop within the renal pelvis. If the stent has been advanced too far distally, applying traction to the suture threaded through its proximal end can retract it. Once positioned for final stent placement, the introducer sheath, if used, is removed. The retracting suture is then removed while the stent is maintained in a stable position with gentle pressure on the pusher catheter. After removal of the suture, the guidewire is further retracted so that only the flexible portion of the guidewire remains within the proximal end of the ureteral stent. This allows for the upper loop to begin to reconstitute and avoids inadvertent advancement of the stent into an unsatisfactorily low position. With only the floppy portion of the guidewire in the stent, the pusher is used to advance the stent through the remaining segment of the nephrostomy tract and into the renal pelvis while the guidewire is simultaneously withdrawn. This technique causes the upper loop of the stent to form within the renal pelvis.
Once the stent has been placed, the safety guidewire can be used to position a small-bore nephrostomy catheter for temporary maintenance of the nephrostomy tract. The nephrostomy catheter should be left in place for 12 to 24 hours to confirm that the ureteral stent is functional. If no symptoms of ureteral occlusion develop, the nephrostomy tube can be removed after a nephrostogram demonstrates satisfactory position and function of the ureteral stent. The nephrostomy tube should be removed under fluoroscopic guidance to ensure that the ureteral stent is not inadvertently dislodged during tube removal. The nephrostomy tract will close spontaneously during the next 4 days.
In some patients a ureteral stent may be desirable, but the nephrostomy tract should be maintained for further percutaneous procedures or stent changes. In these patients an alternative method of stenting is placement of an internal/external ureteral stent (also called percutaneous nephroureterostomy ). This technique employs a single catheter that extends from the patient's flank through the kidney, down the ureter, and into the bladder ( Fig. 10-16 ). These catheters are commercially available, or they may be tailored from an extralength nephrostomy tube. Varying numbers of side holes may be placed along the length of this catheter, extending from the renal pelvis to the bladder. For treatment of ureteral fistulas or perforations, side holes should be placed above and below the site of ureteral injury, and placement of side holes in the area of ureteral leakage should be avoided. This facilitates healing of the ureteral wall while supplying adequate urinary drainage. These internal/external ureteral stents should be used when ureteral stenting is indicated, but prolonged percutaneous renal access is desired. Once placed, the external limb of these catheters may be capped, and the catheter then functions as an internal ureteral stent, but with preservation of the transrenal tract. In the author's institution this type of catheter is routinely used for treatment of ureteral fistulas and perforations, as well as for following ureteral dilatation because follow-up imaging studies such as pyelography and repeated dilatation procedures are usually required.

The procedure for placement of these stents closely parallels placement of internal ureteral stents. The bent-wire technique is used to determine the location of the side holes to be placed in the catheter for drainage. Modification of these standard nephrostomy catheters entails fashioning side holes along the desired length of the catheter shaft. The catheter must be positioned so that side holes in the catheter are advanced beyond the renal parenchyma. If not, parenchymal bleeding will persist and lead to premature tube occlusion.
Urologists assume long-term management of internal ureteral stents. Once these stents are placed, they can be removed or replaced in a retrograde fashion using a cystoscope. Internal/external ureteral stents should be changed prophylactically every 4 to 6 weeks to avoid stent occlusion. Pyelography should be performed whenever a stent is replaced to assess the status of the ureter and to confirm that the new ureteral stent is appropriately positioned.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here