Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Odontogenic tumors are classified as follows: (1) epithelial tumors with mature fibrous stroma without ectomesenchyme, (2) mixed epithelial and mesenchymal tumors, and (3) mesenchymal tumors with or without scarce epithelium (usually in the form of rests) ( Fig. 15.1 ). Epithelial tumors putatively result from activation of odontogenic rests and mesenchymal tumors from precursor multipotent mesenchymal cells that recapitulate formation of the dental papilla, dental follicle, periodontal tissues, and osteocementum. Those that produce significant amounts of calcified tissues will exhibit radiopacities on imaging studies. Some odontogenic neoplasms exhibit histopathologic patterns of several tumors, and these are called combined or hybrid odontogenic tumors . Attention has focused on molecular findings in odontogenic tumors, particularly on changes in the Wnt signaling pathway that is involved in odontogenesis, cell proliferation and migration, and cell fate, as well as mutations in BRAF (specifically BRAF V600E) and dysregulation of the mitogen-activated protein kinase (MAPK) signaling pathway ( Table 15.1 ). Theoretically, any of the tumors may have malignant counterparts and only the better-characterized ones are discussed in this chapter.
| Tumor | More Common | Less Common |
|---|---|---|
| Keratocystic odontogenic tumor/odontogenic keratocyst | PTCH1 | P53 , MCC SUFU in syndromic cases |
| Ameloblastoma | BRAF, SMO | RAS , FGFR2 , CTNNB1 , PIK3CA , SMARCB1, EGFR, PTEN, CDKN2A, APC |
| Adenomatoid odontogenic tumor | KRAS | |
| Calcifying odontogenic tumor | PTEN, PIK3CA, PTCH1, JAK2, MEC | |
| Squamous odontogenic tumor | ? Notch | |
| Calcifying cystic odontogenic tumor | CTNNB1 | |
| Dentinogenic ghost cell tumor | CTNNB1 | |
| Ghost cell odontogenic carcinoma | APC | |
| Ameloblastic carcinoma | BRAF | |
| Ameloblastic fibrosarcoma | BRAF, NRAS | |
| Ghost cell odontogenic carcinoma | CTNNB1 | TCF4::PTPRG fusion |
| Odontogenic carcinoma | APC | CTNNB1 |
| Clear cell odontogenic carcinoma | EWSR rearrangement , with ATF partner, less often CREB | |
| Central cementoossifying fibroma | CDC73 | CTNNB1 |
| Familial florid cementoosseous dysplasia | ANO5 | |
| Gnathodiaphyseal dysplasia | ANO5 | |
| Hyperparathyroidism-jaw tumor syndrome | CDC73 | |
| Odontogenic myxoma | PRKAR1A, HMGA2 rearrangement | |
| Cementoblastoma | FOS rearrangement |
Clinically, lesions may cause jaw expansion when large or be entirely asymptomatic and discovered on routine radiography. Malignant lesions tend to perforate the cortical plate and present as an ulcerated soft tissue mass although ameloblastoma may also present in this manner. Depending on the rate of growth and size of the lesion, teeth may be displaced or rendered mobile, and roots resorbed. Impingement of the inferior alveolar nerve leads to paresthesia.
Any odontogenic tumor may occur within the soft tissue of the gingiva (peripheral or extraosseous), and produce a painless, localized, nondescript gingival swelling. The most common odontogenic tumors are the peripheral odontogenic fibroma that comprises approximately 50% of all cases of peripheral odontogenic tumors, peripheral ameloblastoma, and peripheral calcifying cystic odontogenic tumor/calcifying odontogenic cyst. Even odontomas may occur in an extraosseous site, and all behave in a benign fashion.
Keratocystic odontogenic tumor/odontogenic keratocyst (KCOT/OKC) is one of the defining lesions in the nevoid basal cell carcinoma syndrome (Gorlin-Goltz or Gorlin syndrome). In 2005 this lesion was reclassified by the World Health Organization as a cystic neoplasm because of its locally aggressive behavior, high proliferation indices, high recurrence rate, and importantly, identification of mutation of PTCH1 in >80% of syndromic cases and some nonsyndromic cases. This was reversed in 2017, when studies showed that sporadic cases exhibited PTCH1 mutation in only 30% of cases, among other arguments. However, data show that the mutation is present in at least 90% of sporadic cases.
Forty percent of sporadic cases occur in the third and fourth decades of life, with a 2:1 male predilection. Lesions in syndromic cases are generally diagnosed in the first and second decades of life, and patients tend to develop multiple lesions. Painless swelling tends to occur in larger, long-standing lesions.
Approximately 80% of cases present as unilocular and the rest as multilocular radiolucencies, usually in the posterior mandible and/or ramus area (70% of cases). The radiolucency expands along the long axis of the jaw bones anteroposteriorly rather than buccal-lingually or superoinferiorly. Twenty-five to 33% of cases are associated with an impacted tooth, or occur in locations favored by lateral periodontal cyst ( Fig. 15.2 A–C).
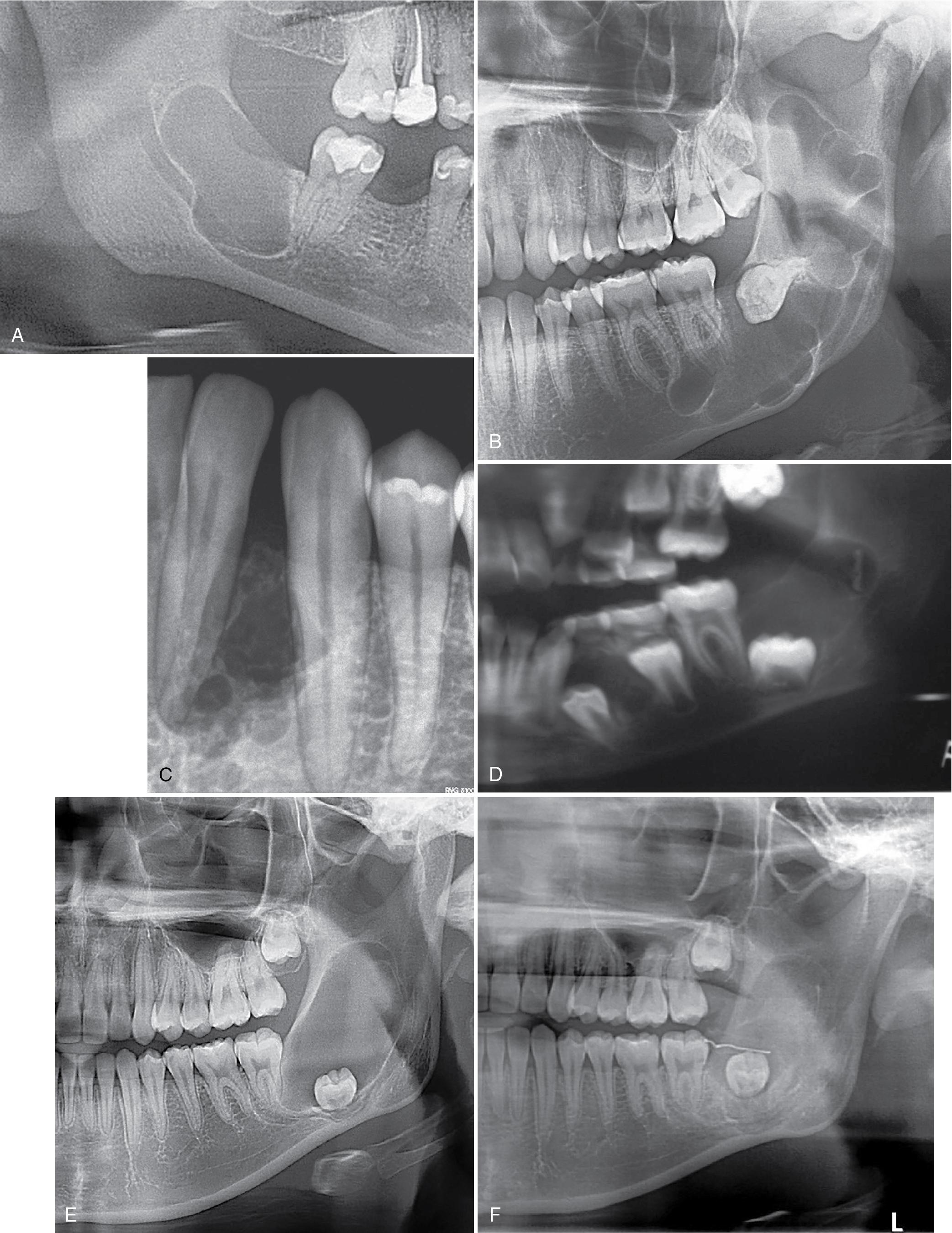
Those arising in the midline of the maxilla in the vicinity of the incisive (nasopalatine) canal occur in patients in the seventh decade of life or older, with a 3:1 male predilection. Whether this represents a primordial cyst of a mesiodens is uncertain.
Patients with the nevoid basal cell carcinoma syndrome, an autosomal dominant condition associated with mutation of PTCH1, have not only multiple cystic lesions in the jaw in the first and second decades ( Fig. 15.2 D) but also many basal cell carcinomas starting within the first three decades of life, as well as hypertelorism, rib abnormalities (such as bifid, missing, or fused ribs), calcification of the falx cerebri, palmar-plantar pits, cutaneous epidermal cysts, ovarian fibromas, medulloblastomas, and other malformations ( Table 15.2 ). In one study, 44% of children newly diagnosed with the syndrome presented with a single KCOT/OKC and were younger. As such, a KCOT/OKC in a young child should raise suspicions for the syndrome.
| Diagnosis of NBCC made in the presence of two major or one major and two minor criteria |
| Major criteria |
|
|
|
|
|
|
| Minor criteria |
| Any one of the following features: |
|
|
|
|
|
|
This cystic tumor has been reported in peripheral or extraosseous sites, such as the gingiva, especially the maxillary anterior gingiva and vestibule, as well as in the buccal mucosa.
Mutation in PTCH1, a tumor suppressor gene is seen in KCOT/OKC in the nevoid basal cell carcinoma syndrome, an autosomal dominant condition, in over 80% of syndromic and in approximately 90% of sporadic cases of this cystic tumor. Allelic losses have also been identified for other tumor suppressor genes (namely, TP53 and MCC ). Infrequently, patients with Gorlin syndrome exhibit germline mutations in SUFU .
The epithelium is uniformly 5 to 10 cells thick, with parakeratosis, often with a corrugated surface, palisading of the basal cell nuclei, and a flat interface, the last leading to epithelial detachment; there may be very little keratin present but palisading of basal cell nuclei is always present. Basal cell hyperplasia and dysmaturation with occasional mitotic figures are not uncommon, as are epithelial buds although these findings do not appear to be associated with recurrence but may be seen more frequently in syndromic cases ( Fig. 15.3 ). However, if inflammation is present, these typical features are lost or may be subtle or focal and may lead to a misdiagnosis if the specimen is small and from an inflamed portion of the lesion ( Fig. 15.4 ). The lining may show mucous and ciliated cell metaplasia and epithelial plaques, and Rushton bodies are frequent within inflamed epithelium (see Fig. 15.4 B–D). Focal areas may be covered by orthokeratin but the presence of any parakeratin renders the cyst a KCOT/OKC.
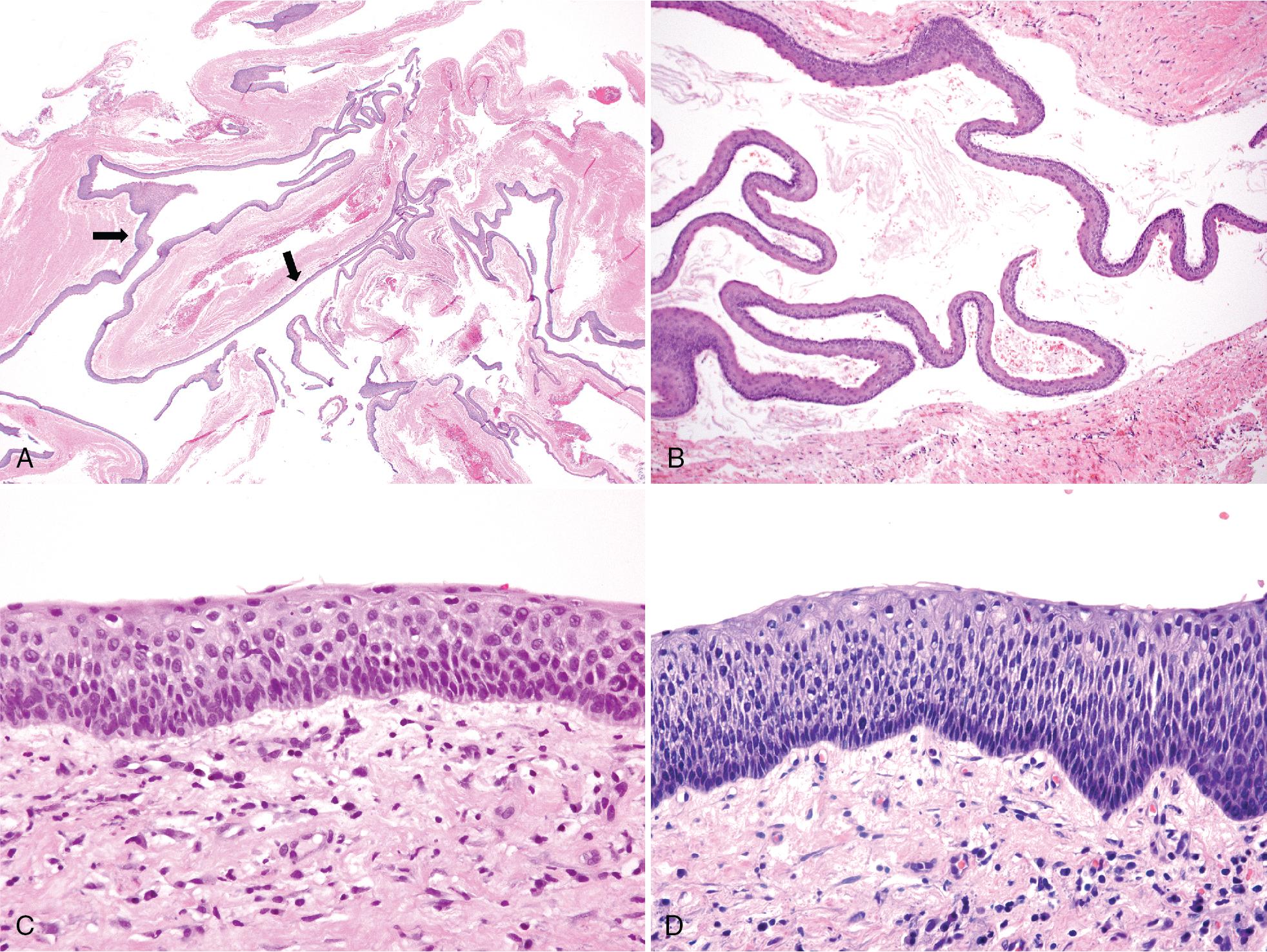
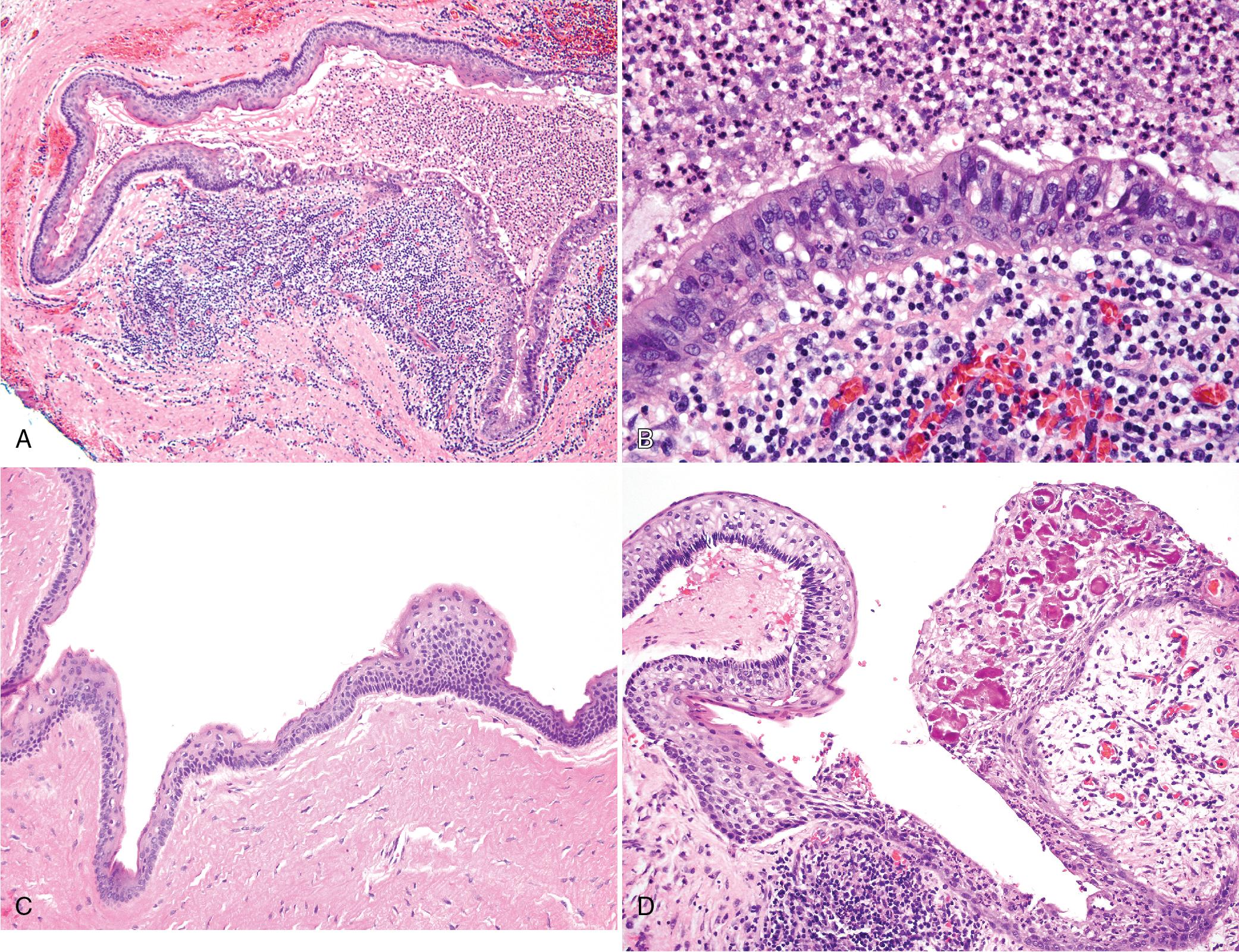
Marsupialization or exteriorization of the cyst and flushing with a caustic agent results in inflammation and loss of the parakeratin layer and the typical features of KCOT/OKC. However, residual lesional tissue may still be present and levels should be performed if the cyst is not present in the initial sections (see Fig. 15.2 E–F).
Satellite cysts are often present within the wall and sometimes within the deep lamina propria of the oral mucosa if the cortical plate is perforated; not all satellite cysts contain keratin and some display macrophages within the lumen ( Fig. 15.5 ). Cortical perforation may be associated with a higher recurrence rate and should be reported. Syndromic cases have a higher frequency of satellite cysts. Perforation of maxillary lesions into the sinus is not uncommon (see Fig. 15.5 E).
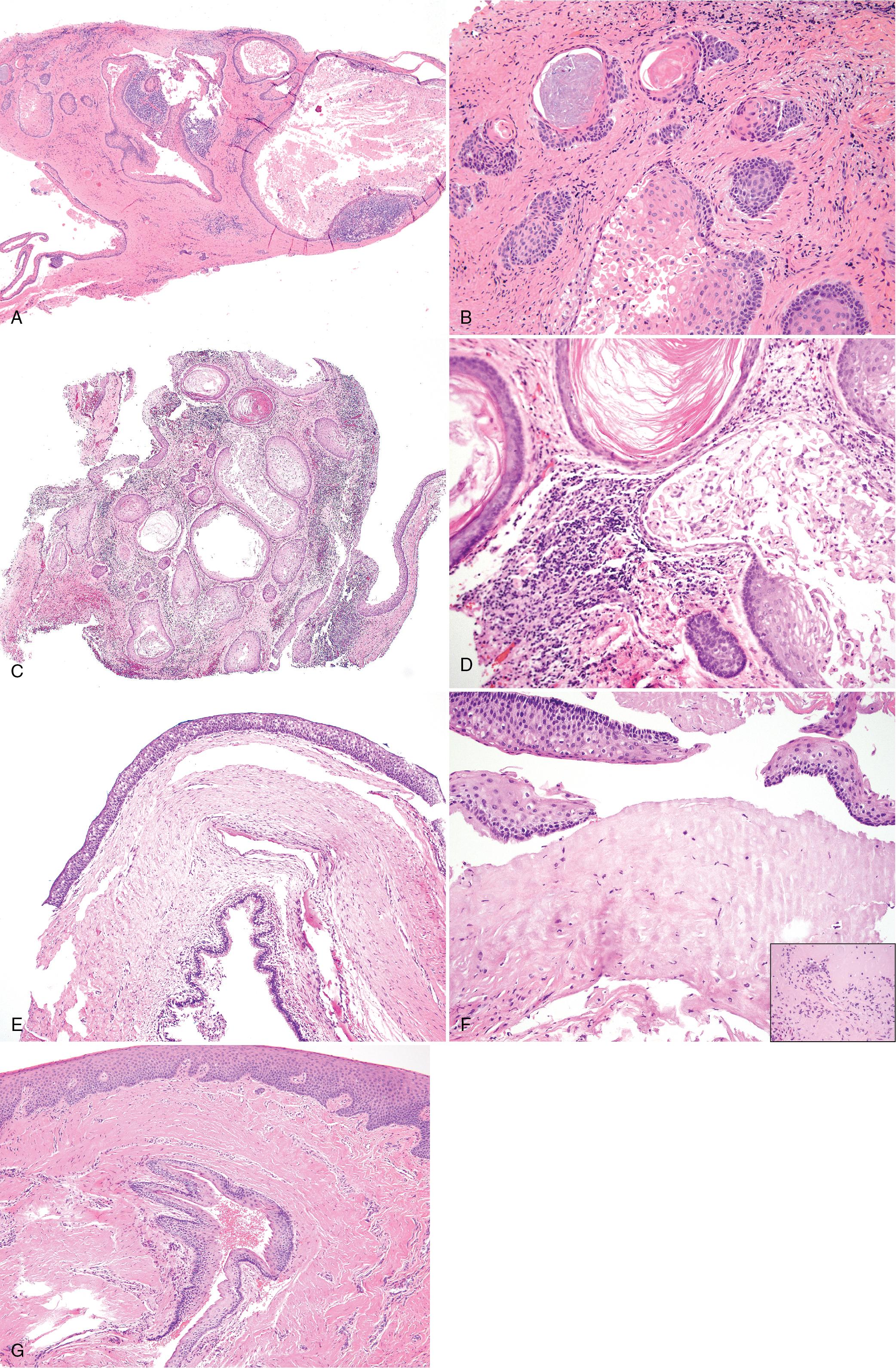
Cartilage and melanin pigment may be present (see Fig. 15.5 F).
A solid variant has been reported and is composed of back-to-back cysts filled with keratin. The relationship of this cyst with keratoameloblastoma is unclear (see later).
K4, K13, K17, and K19 have been identified in the cyst epithelium. Compared with other odontogenic cysts, there are high proliferating cell nuclear antigen and Ki-67 indices, and positivity for BCL2 in basal cells, and SOX2 throughout the epithelium (see Fig. 15.6 C–D).
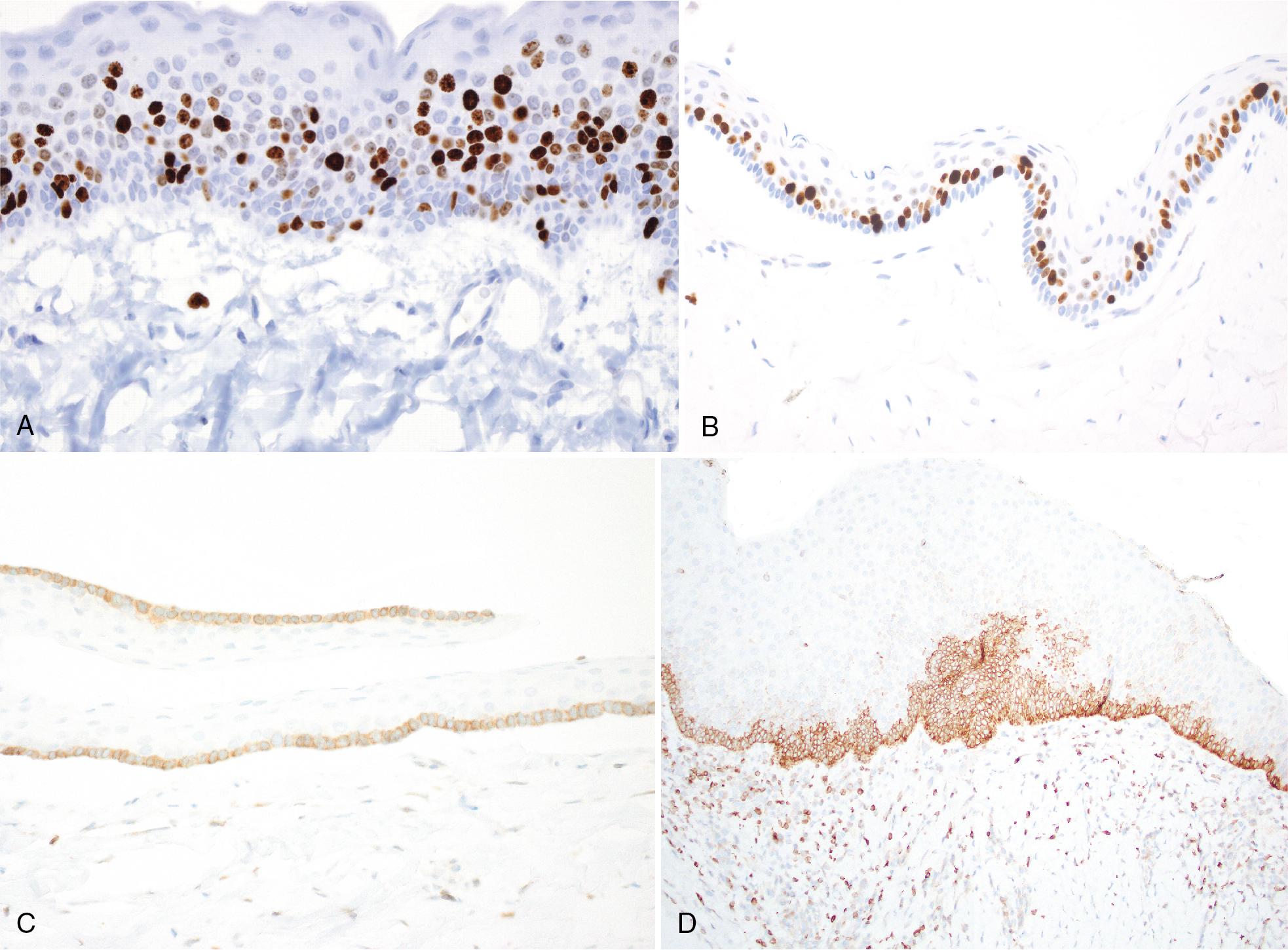
Recurrence may be associated with cases exhibiting Ki-67 labeling index >10%.
Peripheral lesions are uncommon but extraosseous extension from an intraosseous lesion must always be ruled out ( Fig. 15.3 G).
Orthokeratinized odontogenic cyst is lined entirely by orthokeratin with a granular layer, does not exhibit palisaded basal cells or satellite cysts, is negative for BCL2, and has a low Ki-67 index and a low recurrence rate of <10%.
Some basal cell hyperplasia and dysmaturation is acceptable but if this is significant, epithelial dysplasia occurring within the lining should be considered ( Fig. 15.3 F).
Cystic ameloblastoma has no parakeratosis, although the luminal cells are large, with abundant eosinophilic cytoplasm, and exhibit stellate reticulum-like areas and palisaded basal cell nuclei with reverse nuclear polarization.
Recurrence rate varies from 10% to 40% with an overall rate of 23% in all cases and 34% in syndromic cases. Unilocular versus multilocular lesions have recurrence rates of 14% versus 26%, respectively.
Enucleation/curettage versus segmental resection is associated with a recurrence rate of 23% versus 3%.
Enucleation with adjunctive measures such as application of 5-fluorouracil, Carnoy solution, peripheral ostectomy, cryosurgery, and electrocautery is associated with a low recurrence rate and is tissue-sparing.
Marsupialization and daily irrigation followed by cystectomy is also associated with a low recurrence rate and is organ-sparing.
En bloc excision may be necessary for recurrent lesions that have failed other therapies but is not usually first-line therapy.
Vismodegib, a hedgehog pathway inhibitor, has been shown to shrink these cystic tumors in syndromic patients.
Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol . 2014;150:542-545.
Al-Moraissi EA, Dahan AA, Alwadeai MS, et al. What surgical treatment has the lowest recurrence rate following the management of keratocystic odontogenic tumor? A large systematic review and meta-analysis. J Craniomaxillofac Surg . 2017;45:131-144.
Al-Ramadhan S, Fitzpatrick S, Bhattacharyya I, et al. Histopathologic features as strong predictor of syndromic odontogenic keratocysts. Oral Surg Oral Med Oral Pathol Oral Radiol . 2022;133:e124.
Aragaki T, Michi Y, Katsube K, et al. Comprehensive keratin profiling reveals different histopathogenesis of keratocystic odontogenic tumor and orthokeratinized odontogenic cyst. Hum Pathol . 2010;41:1718-1725.
August M, Faquin WC, Troulis MJ, Kaban LB. Dedifferentiation of odontogenic keratocyst epithelium after cyst decompression. J Oral Maxillofac Surg . 2003;61:678-683, discussion 683-684.
Buchner A, Merrell PW, Carpenter WM. Relative frequency of central odontogenic tumors: a study of 1,088 cases from Northern California and comparison to studies from other parts of the world. J Oral Maxillofac Surg . 2006;64:1343-1352.
Chi AC, Owings JR Jr, Muller S. Peripheral odontogenic keratocyst: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2005;99:71-78.
Chrcanovic BR, Gomez RS. Recurrence probability for keratocystic odontogenic tumors: an analysis of 6427 cases. J Craniomaxillofac Surg . 2017;45:244-251.
Fornatora ML, Reich RF, Chotkowski G, Freedman PD. Odontogenic keratocyst with mural cartilaginous metaplasia: a case report and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2001;92:430-434.
Geng N, Lv D, Chen QM, et al. Solid variant of keratocystic odontogenic tumor with ameloblastomatous transformation: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol . 2012;114:223-229.
Gurgel CA, Ramos EA, Azevedo RA, et al. Expression of Ki-67, p53 and p63 proteins in keratocyst odontogenic tumours: an immunohistochemical study. J Mol Histol . 2008;39:311-316.
John AM, Schwartz RA. Basal cell nevus syndrome: an update on genetics and treatment. Br J Dermatol . 2016;174:68-76.
Kaminagakura E, Almeida JD, Carvalho YR, et al. Keratocyst of the buccal mucosa: case report and immunohistochemical comparative study with sporadic intraosseous keratocystic odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol . 2013;116:e387-e392.
Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet . 1997;69:299-308.
Kuroyanagi N, Sakuma H, Miyabe S, et al. Prognostic factors for keratocystic odontogenic tumor (odontogenic keratocyst): analysis of clinico-pathologic and immunohistochemical findings in cysts treated by enucleation. J Oral Pathol Med . 2009;38:386-392.
Karhade DS, Afshar S, Padwa BL. What is the prevalence of undiagnosed nevoid basal cell carcinoma syndrome in children with an odontogenic keratocyst? J Oral Maxillofac Surg . 2019;77:1389-1391.
Lafuente-Ibanez de Mendoza I, Aguirre-Urizar JM, Villatoro-Ugalde V, Magana-Quinones JJ, Lana-Ojeda J, Mosqueda-Taylor A. Peripheral odontogenic keratocyst: clinicopathological and immunohistochemical characterization. Oral Dis . 2022;28:1198-1206.
Ledderhof NJ, Caminiti MF, Bradley G, Lam DK. Topical 5-fluorouracil is a novel targeted therapy for the keratocystic odontogenic tumor. J Oral Maxillofac Surg . 2017;75:514-524.
Pan S, Dong Q, Sun LS, Li TJ. Mechanisms of inactivation of PTCH1 gene in nevoid basal cell carcinoma syndrome: modification of the two-hit hypothesis. Clin Cancer Res . 2010;16:442-450.
Silva BS, Silva LR, Lima KL, et al. SOX2 and BCL-2 expressions in odontogenic keratocyst and ameloblastoma. Med Oral Patol Oral Cir Bucal . 2020;25:e283-e290.
Stojanov IJ, Schaefer IM, Menon RS, et al. Biallelic PTCH1 inactivation is a dominant genomic change in sporadic keratocystic odontogenic tumors. Am J Surg Pathol . 2020;44:553-560.
Central (intraosseous) ameloblastoma, the third most common odontogenic tumor after KCOT/OKC and odontoma, is a locally aggressive neoplasm, whereas peripheral (extraosseous) ameloblastoma behaves in an indolent fashion.
Solid/conventional ameloblastoma:
This constitutes two-thirds of lesions and is generally seen in the fourth to fifth decades of life with equal sex distribution, with patients in South America and Africa being at least a decade younger. If large, it may cause pain, paresthesia, teeth mobility, and swelling of the face. Approximately 80% of cases occur in the mandible and it is usually described as a solid tumor at surgery, although microcystic spaces may be present. Perforation of the cortical plate, especially on the lingual aspect, is often encountered.
It presents as a unilocular or multilocular radiolucency with almost equal frequency, most often in the posterior mandible and/or ramus area, although in Black patients it may have a predilection for lesions in the anterior mandible. Some ameloblastomas are associated with impacted teeth ( Fig. 15.7 ).
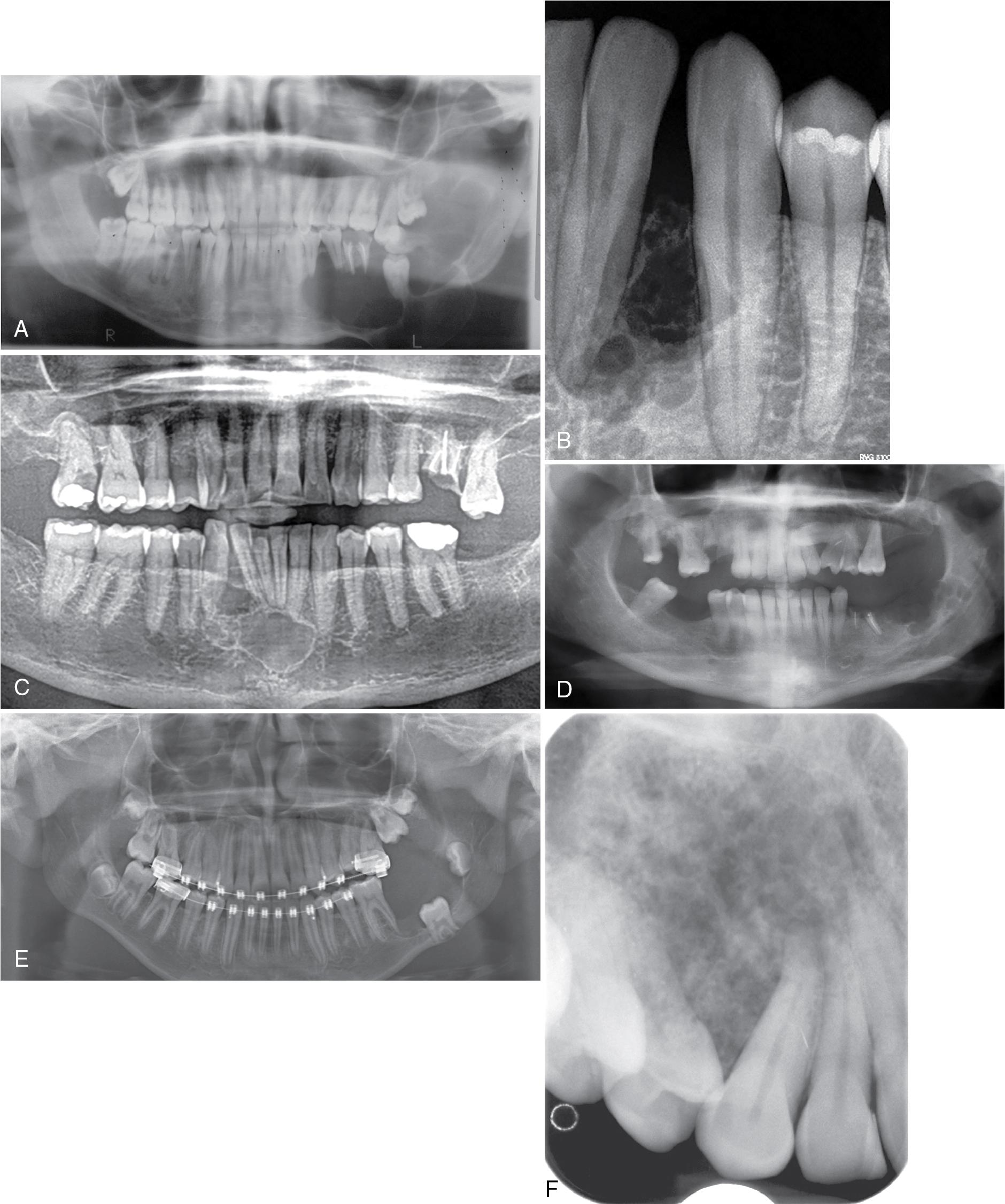
Desmoplastic (scirrhous) ameloblastoma:
This variant constitutes 4% of cases, has equal mandibular and maxillary frequency with equal sex distribution, and unlike conventional solid ameloblastoma, 40% to 50% of cases occur in the anterior jaws.
Two-thirds present as a mixed radiolucent-radiopaque mass suggesting a fibroosseous lesion (see Fig. 15.7 E).
Keratoameloblastoma, a histologic variant which produces abundant keratin, may also have a mixed radiolucent-radiopaque appearance.
Unicystic or unicystic plexiform ameloblastoma:
This represents 20% to 25% of ameloblastomas and presents in the second and third decades of life and is often associated with an impacted tooth, often with jaw and facial swelling. The most common sites are mid- and posterior mandible especially associated with the impacted third molar. Lesions unassociated with an impacted tooth tend to occur at least one decade later.
It occurs as a unilocular radiolucency in the posterior mandible and is associated with a displaced impacted mandibular molar in 50% to 80% of cases (see Fig. 15.7 F). It is described as a cystic lesion at surgery.
Peripheral (extraosseous) ameloblastoma:
This represents approximately 1% of ameloblastomas and occurs in the fifth to sixth decades of life, with a male predilection of 2:1, with the mandible twice as likely to be affected. It occurs as a sessile nodule on the gingiva in the mandibular premolar-molar area (50% of cases). Radiographs must be taken to rule out an intraosseous ameloblastoma with perforation of the cortical bone. Rare cases occur at extragingival sites such as the buccal mucosa, although some authorities believe that these represent an unusual form of salivary gland tumor.
Ameloblastoma differentiates toward and recapitulates ameloblasts and stellate reticulum of the developing tooth germ, without deposition of dental hard tissues or their matrix. Mutations in BRAF, RAS, and FGFR2 are present in 88% of cases with activating mutation in BRAF V600E present in 60% to 70% of mandibular tumors with the exception of adenoid ameloblastoma. Mutation in SMO is present in 56% of maxillary tumors especially those with plexiform histopathology, and CTNNB1, PIK3CA, SMARCB1, EGFR, PTEN, CDKN2A, and JAK3 have also been identified in ameloblastoma but much less frequently. Mutation in BRAF V600E usually occurs in younger patients and is an independent predictor of recurrence-free survival. Peripheral ameloblastoma may arise from rests of Serres in the gingiva or from the basal cell layer of the surface epithelium. The rare extragingival ameloblastoma of which less than 10 cases exist in the English literature, may arise from multipotent stem cells in the basal cells of the mucosal epithelium that proliferate and undergo odontogenic differentiation, or even represent an adamantinomatous pattern of a salivary gland tumor.
Ameloblastoma is classified histopathologically by gross pattern and constituent cells.
Solid/conventional ameloblastoma patterns:
Follicular ameloblastoma exhibits islands or follicles of epithelial cells, typically with peripheral basal cells showing nuclear palisading and reverse polarization of the nuclei. Areas of microcystic and macrocystic change within the epithelial islands are common ( Fig. 15.8 ).

Plexiform ameloblastoma shows interconnected broad and thin trabeculae and cords of epithelial cells with basal cell nuclear palisading and reverse nuclear polarization, although these changes may be subtle in this pattern ( Fig. 15.9 ).

Desmoplastic or scirrhous ameloblastoma is a variant of the follicular ameloblastoma in which islands or follicles of epithelium appear compressed by markedly desmoplastic stroma; the epithelium is present as slender cords and irregularly shaped islands of hyperchromatic cells. Classic and acanthomatous cytology are seen in some of the larger tumor islands ( Fig. 15.10 ).

Constituent cells:
Many tumors contain several cell types, the most common being classic and acanthomatous.
Classic lesions exhibit loose, stellate reticulum-like areas (see Figs. 15.8 and 15.9 ); classic and acanthomatous variants often coexist in follicular tumors while most plexiform tumors exhibit stellate reticulum ( Figs. 15.8 and 15.9 ).
Acanthomatous lesions exhibit large, polygonal squamous cells with abundant eosinophilic cytoplasm or keratinization (see Fig. 15.8 B and F).
Granular cell lesions contain large, pale cells with granular cytoplasm rich in lysosomes ( Fig. 15.11 A–D).

Basal cell lesions contain mostly basaloid cells; this is a rare variant (see Fig. 15.11 E–F).
Keratoameloblastoma is a rare form of ameloblastoma producing cystic spaces filled with compact parakeratin and orthokeratin, some with intraluminal papilliferous projections. Its relationship with solid KCOT/OKC is unclear ( Fig. 15.12 ).

Adenoid ameloblastoma is a new entity with a pattern of plexiform ameloblastoma but forming ductlike structures (resulting from degenerated stroma) with a pseudocribriform architecture, basophilic stroma, epithelium spherules, and presence of dentinoid, ghost cells, and hyaline droplets reminiscent of ghost cell lesions. It tends to occur in the maxilla (80% of cases) and has a high recurrence rate ( Fig. 15.13 ).

A rare osteoplastic type that induces bone formation within fibrous stroma has been reported.
Intraosseous ameloblastoma that perforates the cortical plate and fuses with the surface epithelium, often causes papillary epithelial hyperplasia (see Fig. 15.9 C).
Tumors with areas of hypercellularity, pleomorphism, and mitotic activity should be diagnosed as ameloblastoma with atypia and must be carefully evaluated for malignant transformation.
Some lesions are highly vascular but these probably do not require a separate designation.
Unicystic ameloblastoma:
Unicystic ameloblastoma has three patterns ( Fig. 15.14 ).

Luminal pattern: The cyst lining is generally 5 to 15 cells thick and exhibits eosinophilic luminal cells, underlying stellate reticulum-like cells, and palisaded, hyperchromatic basal cell nuclei with reverse polarization, giving the lining a “red-white-blue” appearance ( Fig. 15.15 ). These changes are referred to as the Vickers-Gorlin criteria and are identical to those seen in cystic changes within epithelial islands in solid ameloblastoma (see Fig. 15.8 C and G).

Intraluminal pattern (plexiform unicystic ameloblastoma): In addition to changes within the cyst lining, there is a plexiform proliferation of ameloblastomatous epithelium that protrudes into the lumen ( Fig. 15.16 ). Inflammation results in loss of the diagnostic features of this lesion and may lead to misdiagnosis of this cystic tumor as a radicular cyst or an inflamed dentigerous cyst (see Fig. 15.16 D).

Mural pattern: If there is mural proliferation within the wall of a unicystic ameloblastoma which usually is in the form of a follicular ameloblastoma, this must be reported because it will behave in a fashion similar to a solid ameloblastoma ( Fig. 15.16 G).
Tumor cells exhibit cytoplasmic positivity for K14 and K19 and are negative for K7 and K18. Calretinin is positive within the stellate reticulum cells of solid ameloblastoma and the luminal cells of unicystic ameloblastoma, and CD56 is also often positive ( Fig. 15.17 A). Two-thirds of tumors are positive for BRAF ( Figs. 15.16 F and 15.17 B). High Ki-67 labeling and large numbers of CD10+ stromal cells may correlate with recurrence.

Peripheral ameloblastoma: This usually represents a follicular ameloblastoma, classic and/or acanthomatous type. Fifty percent of cases are in continuity with the overlying epithelium ( Figs. 15.18 and 15.19 ).


Extragingival ameloblastoma: Typical appearing, usually follicular ameloblastoma is present within the soft tissues of the buccal mucosa.
Dentigerous cyst or other odontogenic cyst does not exhibit the “red-white-blue” Vickers-Gorlin criteria, as noted in unicystic ameloblastoma; however, if the unicystic ameloblastoma is inflamed, these criteria may be lost.
Squamous odontogenic tumor consists of islands of squamous cells that do not show palisading of the peripheral basal cells.
Ameloblastic carcinoma shows obvious features of malignancy with hypercellularity, pleomorphic cells, many mitotic figures, and sometimes necrosis.
Dentinogenic ghost cell tumor closely resembles adenoid ameloblastoma and contains ghost cells and dentinoid. At this time, it is unclear how the two are related: both are positive for β-catenin, and adenoid ameloblastoma is negative for BRAF.
Adenomatoid odontogenic tumor contains enameloid while adenoid ameloblastoma exhibits dentinoid and ghost cells.
Basaloid squamous cell carcinoma of the mucosa shows significant epithelial atypia and synchronous conventional squamous cell carcinoma and is usually readily distinguished from peripheral ameloblastoma.
Oral basal cell carcinoma has been reported to occur on gingiva that is BerEP4+, similar to cutaneous basal cell carcinoma.
Recurrence varies greatly depending on the type of ameloblastoma and modality of treatment.
Solid ameloblastoma: The recurrence rate is 5% to 17% if treated with marginal or segmental resection, and 30% to 35% if the tumor is curetted and/or enucleated.
Reduction in tumor volume, especially for recurrent and metastatic tumors, was noted in patients using drugs that target the MAPK signaling pathway such as dabrafenib, trametinib, and vemurafenib.
Unicystic ameloblastoma: The recurrence rates for those without and with mural proliferation are 6% and 37%, respectively, and most authorities recommend treating a unicystic ameloblastoma with mural proliferation as a solid ameloblastoma.
Peripheral (extraosseous) ameloblastoma: This is treated with simple excision and does not recur.
Ahlem B, Wided A, Amani L, et al. Study of Ki67 and CD10 expression as predictive factors of recurrence of ameloblastoma. Eur Ann Otorhinolaryngol Head Neck Dis . 2015;132:275-279.
Anpalagan A, Tzortzis A, Twigg J, Wotherspoon R, Chengot P, Kanatas A. Current practice in the management of peripheral ameloblastoma: a structured review. Br J Oral Maxillofac Surg . 2021;59:e1-e8.
Brown NA, Betz BL. Ameloblastoma: a review of recent molecular pathogenetic discoveries. Biomark Cancer . 2015;7(suppl 2):19-24.
Chrcanovic BR, Gomes CC, Gomez RS. Desmoplastic ameloblastoma: a systematic review of the cases reported in the literature. Int J Oral Maxillofac Surg . 2020;49:709-716.
Childers ELB, Taddasse-Heath L, Bonnick A, Naab T. Vascularized ameloblastoma: a case report and clinicopathologic review of 18 cases from the literature. Oral Surg Oral Med Oral Pathol Oral Radiol . 2020;129:e264-e268.
DeVilliers P, Liu H, Suggs C, et al. Calretinin expression in the differential diagnosis of human ameloblastoma and keratocystic odontogenic tumor. Am J Surg Pathol . 2008;32:256-260.
Gultekin SE, Aziz R, Heydt C, et al. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch . 2018;472: 807-814.
Heikinheimo K, Huhtala JM, Thiel A, et al. The mutational profile of unicystic ameloblastoma. J Dent Res . 2019;98:54-60.
Gonzalez-Gonzalez R, Lopez-Verdin S, Lavalle-Carrasco J, et al. Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review. World J Clin Oncol . 2020;11:31-42.
Hendra FN, Natsir Kalla DS, Van Cann EM, de Vet HCW, Helder MN, Forouzanfar T. Radical vs conservative treatment of intraosseous ameloblastoma: systematic review and meta-analysis. Oral Dis . 2019;25: 1683-1696.
Hendra FN, Van Cann EM, Helder MN, et al. Global incidence and profile of ameloblastoma: a systematic review and meta-analysis. Oral Dis . 2020;26:12-21.
Ide F, Mishima K, Miyazaki Y, et al. Peripheral ameloblastoma in-situ: an evidential fact of surface epithelium origin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2009;108:763-767.
Ide F, Mishima K, Yamada H, Kikuchi K, Saito I, Kusama K. Intraosseous ameloblastoma with a prominent extraosseous component: pitfalls in diagnosis. Head Neck Pathol . 2010;4:192-197.
Isomura ET, Okura M, Ishimoto S, et al. Case report of extragingival peripheral ameloblastoma in buccal mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2009;108:577-579.
Itoh Y, Nakahara H, Itoh R, et al. Osteoplastic ameloblastoma: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol . 2012;113:e23-e28.
Lee C, Park BJ, Yi WJ, et al. Keratoameloblastoma: a case report and a review of the literature on its radiologic features. Oral Surg Oral Med Oral Pathol Oral Radiol . 2015;120:e219-e225.
Li TJ, Wu YT, Yu SF, Yu GY. Unicystic ameloblastoma: a clinicopathologic study of 33 Chinese patients. Am J Surg Pathol . 2000;24:1385-1392.
Loyola AM, Cardoso SV, de Faria PR, et al. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol . 2015;120:368-377.
Philipsen HP, Reichart PA, Nikai H, et al. Peripheral ameloblastoma: biological profile based on 160 cases from the literature. Oral Oncol . 2001;37:17-27.
Philipsen HP, Reichart PA. Unicystic ameloblastoma: a review of 193 cases from the literature. Oral Oncol . 1998;34:317-325.
Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol . 1995;31B:86-99.
Sun ZJ, Wu YR, Cheng N, Zwahlen RA, Zhao YF. Desmoplastic ameloblastoma a review. Oral Oncol . 2009;45:752-759.
Woods TR, Cohen DM, Islam MN, et al. Intraoral basal cell carcinoma, a rare neoplasm: report of three new cases with literature review. Head Neck Pathol . 2014;8:339-348.
You Z, Liu SP, Du J, Wu YH, Zhang SZ. Advancements in MAPK signaling pathways and MAPK-targeted therapies for ameloblastoma: a review. J Oral Pathol Med . 2019;48:201-205.
Yuwanati MB, Singh A, Gadbail AR, Mhaske S. Hybrid peripheral ameloblastoma of cheek mucosa. BMJ Case Rep . 2013;2013:bcr2013009510.
Zhang J, Gu Z, Jiang L, et al. Ameloblastoma in children and adolescents. Br J Oral Maxillofac Surg . 2010;48:549-554.
Zhang X, Tian X, Hu Y, Zhang C, Wei C, Yang X. Oral peripheral ameloblastoma: a retrospective series study of 25 cases. Med Oral Patol Oral Cir Bucal . 2018;23:e277-e281.
Zheng CY, Cao R, Hong WS, Sheng MC, Hu YJ. Marsupialisation for the treatment of unicystic ameloblastoma of the mandible: a long-term follow up of 116 cases. Br J Oral Maxillofac Surg . 2019;57:655-662.
This occurs most frequently in the second and third decades of life, with a 2:1 female predilection. At least two-thirds of cases occur in the maxilla, and two-thirds are associated with the canine, usually maxillary. There may be slight painless swelling, but the most common complaint is failure of eruption of a tooth.
It presents as a unilocular radiolucency and variable radiopaque flecks (“snowflake” opacities) around the crown of an impacted tooth ( follicular type) ( Fig. 15.20 ). Those unassociated with a tooth are referred to as extrafollicular tumors .

Peripheral/extraosseous lesions are rare.
Approximately 70% of cases exhibit KRAS mutation.
The tumor has a thick capsule and consists of sheets, nodules, and whorls of spindled, cuboidal, and polygonal epithelial cells forming ductlike and rosette-like structures, sometimes even with a cribriform architecture. The cells have pale or clear cytoplasm and pale nuclei with dispersed chromatin and small nucleoli. Interlacing, delicate strands of epithelium surrounding the nodules contain darker nuclei with more eosinophilic cytoplasm. Macro- or microcystic areas may be present ( Figs. 15.21 and 15.22 ).


Eosinophilic, deep pink, or basophilic droplets of amorphous material termed enameloid are characteristic, and this likely represents a matrix protein, amelotin (see Figs. 15.21 D and 15.22 D).
Infrequently, dentinoid is encountered focally, calcifications may be present, and the stroma may be delicate, myxoid/mucinous, or densely collagenous.
Areas resembling calcifying epithelial odontogenic tumor are present in 25% of cases and are considered by some to be within the normal histopathologic spectrum of this tumor while others believe this represents a hybrid odontogenic tumor.
Tumor cells are positive for K5, K14, K17, and K19; Ki-67 index is 1% or less and BCL2 is present in approximately 25% of cases. Some cases exhibit nuclear and cytoplasmic β-catenin expression.
Adenoid ameloblastoma does not have a thick capsule or formation of enameloid but rather exhibits dentinoid deposition and sometimes, ghost cells.
Adenomatoid dentinoma and adenomatoid odontogenic hamartomas may represent developing odontomas with an unusual adenomatoid epithelial proliferation. These two entities are not yet well-characterized.
Enucleation is curative, and the recurrence rate is insignificant.
Allen CM, Neville BW, Hammond HL. Adenomatoid dentinoma: report of four cases of an unusual odontogenic lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 1998;86:313-317.
Carlos-Bregni R, Vargas PA, Santos Silva AR, et al. Adenomatoid odontogenic hamartoma: concerns about correct nomenclature and 2 additional case reports. J Oral Maxillofac Surg . 2009;67:1779-1780, author reply 1780.
Chrcanovic BR, Gomez RS. Adenomatoid odontogenic tumor: an updated analysis of the cases reported in the literature. J Oral Pathol Med . 2019;48:10-16.
Crivelini MM, Felipini RC, Miyahara GI, de Sousa SC. Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med . 2012;41:272-280.
de Matos FR, Nonaka CF, Pinto LP, de Souza LB, de Almeida Freitas R. Adenomatoid odontogenic tumor: retrospective study of 15 cases with emphasis on histopathologic features. Head Neck Pathol . 2012; 6:430-437.
Ide F, Mishima K, Saito I, Kusama K. Rare peripheral odontogenic tumors: report of 5 cases and comprehensive review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2008;106:e22-e28.
Leon JE, Mata GM, Fregnani ER, et al. Clinicopathological and immunohistochemical study of 39 cases of adenomatoid odontogenic tumour: a multicentric study. Oral Oncol . 2005;41:835-842.
Mosqueda-Taylor A, Carlos-Bregni R, Ledesma-Montes C, et al. Calcifying epithelial odontogenic tumor-like areas are common findings in adenomatoid odontogenic tumors and not a specific entity. Oral Oncol . 2005;41:214-215.
Philipsen HP, Reichart PA, Siar CH, et al. An updated clinical and epidemiological profile of the adenomatoid odontogenic tumour: a collaborative retrospective study. J Oral Pathol Med . 2007;36:383-393.
Razavi SM, Tabatabaie SH, Hoseini AT, Hoseini ET, Khabazian A. A comparative immunohistochemical study of Ki-67 and Bcl-2 expression in solid ameloblastoma and adenomatoid odontogenic tumor. Dent Res J (Isfahan) . 2012;9:192-197.
Philipsen HP, Khongkhunthiang P, Reichart PA. The adenomatoid odontogenic tumour: an update of selected issues. J Oral Pathol Med . 2016;45:394-398.
Reichart PA, Philipsen HP, Khongkhunthian P, Sciubba JJ. Immunoprofile of the adenomatoid odontogenic tumor. Oral Dis . 2017;23:731-736.
Santos HBP, Medeiros HCM, Mafra RP, Miguel MCC, Galvao HC, de Souza LB. Regulation of Wnt/beta-catenin pathway may be related to Reggamma in benign epithelial odontogenic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol . 2019;128:43-51.
This is most frequent in the fourth and fifth decades of life with equal sex distribution, and the mandible is involved in 60% to 70% of cases, mostly in the posterior aspect; a swelling may be present and almost half of cases exhibit cortical perforation.
It presents usually as a unilocular (and much less often multilocular) radiolucency with variable calcifications (70% of cases), and 50% are associated with an impacted tooth ( Fig. 15.23 ).

Five to 6% are extraosseous.
This tumor recapitulates cells of the stratum intermedium of the enamel organ. Mutations in PTEN, PIK3CA, PTCH1. JAK3, and MET have been reported infrequently.
The tumor consists of cords, islands, and sometimes sheets of large, often polygonal cells with abundant eosinophilic cytoplasm and sometimes intercellular bridges. Variation in nuclear shape and size that may be marked, and pseudoinclusions are common, but mitotic activity is not seen unless the tumor is malignant, an extremely rare occurrence. Cystic and microcystic change may be present. Congophilic amyloid (identified as odontogenic ameloblast-associated protein) is always present and generally in abundance ( Fig. 15.24 ).

Approximately one-third of cases do not exhibit calcifications which may take the form of concentric lamellar rings (Liesegang rings) or dystrophic calcifications and the stroma is densely collagenous.
Tumor cells are positive for K7, K8, K14, K13, and K19.
Clear cell variant: Although clear cells may be present very focally in up to 46% of tumors, this variant is composed primarily of clear cells. (see Fig. 15.24 F).
Noncalcifying Langerhans cell–rich variant: This is a rare variant, and occurs most often in the anterior maxilla, although peripheral cases exist. Calcifications are not present and Langerhans cells that are usually apposed to the epithelium, are readily identified with CD1a and CD207 (langerin) ( Fig. 15.25 ).

Foci of calcifying epithelial odontogenic tumor are often seen within an adenomatoid odontogenic tumor and very small such foci within dentigerous cysts (see Fig. 14.29 ).
Peripheral lesions are less likely to exhibit calcifications.
Malignant forms are extremely rare.
Metastatic carcinomas have significantly more atypia, mitotic figures and desmoplasia, and lack amyloid.
Central odontogenic fibroma may exhibit amyloid and CD1a+ cells but even the epithelium-rich variant does not contain as much epithelium as the calcifying epithelial odontogenic tumor. However, some regard the Langerhans cell–rich calcifying epithelial odontogenic tumor to be an amyloid-rich central odontogenic fibroma because it behaves less aggressively and tends to occur more frequently in the anterior maxilla, a more common site for central odontogenic fibroma.
Malignant calcifying epithelial odontogenic tumor exhibits hypercellularity, hyperchromatic nuclei with coarse chromatin, mitotic figures, and other features of malignancy.
Primary clear cell odontogenic carcinoma does not produce amyloid.
Complete excision or enucleation is the treatment of choice, and the recurrence rate is up to 10% to 15%, especially if the tumor is curetted.
Abrahao AC, Camisasca DR, Bonelli BR, et al. Recurrent bilateral gingival peripheral calcifying epithelial odontogenic tumor (Pindborg tumor): a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2009;108:e66-e71.
Afrogheh A, Schneider J, Mohamed N, Hille J. Calcifying epithelial odontogenic tumour with clear Langerhans cells: a novel variant, report of a case and review of the literature. Head Neck Pathol . 2014;8:214-219.
Chrcanovic BR, Gomez RS. Calcifying epithelial odontogenic tumor: an updated analysis of 339 cases reported in the literature. J Craniomaxillofac Surg . 2017;45:1117-1123.
de Oliveira MG, Chaves AC, Visioli F, et al. Peripheral clear cell variant of calcifying epithelial odontogenic tumor affecting 2 sites: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2009;107:407-411.
Demian N, Harris RJ, Abramovitch K, et al. Malignant transformation of calcifying epithelial odontogenic tumor is associated with the loss of p53 transcriptional activity: report of a case with review of the literature. J Oral Maxillofac Surg . 2010;68:1964-1973.
de Sousa SF, Diniz MG, Franca JA, et al. Cancer genes mutation profiling in calcifying epithelial odontogenic tumour. J Clin Pathol . 2018; 71:279-283.
Gopalakrishnan R, Simonton S, Rohrer MD, Koutlas IG. Cystic variant of calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2006;102:773-777.
Mosqueda-Taylor A, Carlos-Bregni R, Ledesma-Montes C, et al. Calcifying epithelial odontogenic tumor-like areas are common findings in adenomatoid odontogenic tumors and not a specific entity. Oral Oncol . 2005;41:214-215.
Murphy CL, Kestler DP, Foster JS, et al. Odontogenic ameloblast-associated protein nature of the amyloid found in calcifying epithelial odontogenic tumors and unerupted tooth follicles. Amyloid . 2008;15:89-95.
Peacock ZS, Cox D, Schmidt BL. Involvement of PTCH1 mutations in the calcifying epithelial odontogenic tumor. Oral Oncol . 2010;46:387-392.
Philipsen HP, Reichart PA. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol . 2000;36:17-26.
Rydin K, Sjostrom M, Warfvinge G. Clear cell variant of intraosseous calcifying epithelial odontogenic tumor: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol . 2016;122:e125-130.
Sanchez-Romero C, Carlos R, de Almeida OP, Romanach MJ. Microcystic calcifying epithelial odontogenic tumor. Head Neck Pathol . 2018;12:598-603.
Santosh N, McNamara KK, Kalmar JR, Iwenofu OH. Non-calcifying Langerhans cell-rich variant of calcifying epithelial odontogenic tumor: a distinct entity with predilection for anterior maxilla. Head Neck Pathol . 2019;13:718-721.
Sedghizadeh PP, Wong D, Shuler CF, et al. Multifocal calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2007;104:e30-e34.
Siriwardena B, Speight PM, Franklin CD, Abdelkarim R, Khurram SA, Hunter KD. CEOT variants or entities: time for a rethink? A case series with review of the literature. Head Neck Pathol . 2021.15(1):186-201.
This rare odontogenic tumor occurs most frequently in the third and fourth decades of life without sex predilection, equally affecting the body and posterior mandible, and anterior maxilla. Patients report painless swelling and teeth mobility. Multifocal lesions occur in 18% of cases and tends to occur in Blacks at a younger age.
It presents as a well-demarcated semicircular or triangular usually unilocular radiolucency around the roots of teeth. ( Fig. 15.26 ).

There has been a suggestion that NOTCH may play a role in the pathogenesis of this tumor.
The tumor consists of irregularly shaped islands of mature squamous cells that are evenly spaced, with some islands containing scattered apoptotic cells. Importantly the cells at the periphery of the islands are squamous and not basaloid. Some islands may exhibit cystic change or may contain globular, eosinophilic, amorphous deposits reminiscent of enameloid or Rushton bodies, calcifications, or even mucous cells ( Figs. 15.27 and 15.28 ). The stroma is densely collagenous although there may mucinous/myxoid areas.


Some tumors exhibit positivity for Notch1, 3, and 4, and their ligands Jagged1 and Delta1.
Follicular ameloblastoma, acanthomatous type, shows palisading of basal cell nuclei with stellate reticulum, and mandibular lesions are often positive for BRAF on immunohistochemistry.
Primary intraosseous and metastatic carcinomas show significant cytologic atypia and are invasive.
Squamous odontogenic-like proliferations composed of similar irregular islands of benign epithelium are sometimes seen in the walls of radicular cysts (see Chapter 14 ).
The recurrence rate is 20% although enucleation, curettage, or excision is generally curative. Lesions that are large, multiloculated, multifocal, and/or recurrent may need en bloc resection.
Chrcanovic BR, Gomez RS. Squamous odontogenic tumor and squamous odontogenic tumor-like proliferations in odontogenic cysts: an updated analysis of 170 cases reported in the literature. J Craniomaxillofac Surg . 2018;46:504-510.
Jones BE, Sarathy AP, Ramos MB, Foss RD. Squamous odontogenic tumor. Head Neck Pathol . 2011;5:17-19.
Parmar RM, Brannon RB, Fowler CB. Squamous odontogenic tumor-like proliferations in radicular cysts: a clinicopathologic study of forty-two cases. J Endod . 2011;37:623-626.
Philipsen HP, Reichart PA. Squamous odontogenic tumor (SOT): a benign neoplasm of the periodontium. A review of 36 reported cases. J Clin Periodontol . 1996;23:922-926.
Siar CH, Nakano K, Ng KH, Tomida M, Nagatsuka H, Kawakami T. Squamous odontogenic tumor of the mandible: a case report demonstrating immunoexpression of Notch1, 3, 4, Jagged1 and Delta1. Eur J Med Res . 2010;15:180-184.
Upadhyaya JD, Banasser A, Cohen DM, Kashtwari D, Bhattacharyya I, Islam MN. Squamous odontogenic tumor: review of the literature and report of a new case. J Oral Maxillofac Surg . 2021;79:164-176.
Primary carcinomas within the bone can be divided into those with overt odontogenic differentiation such as ameloblastic carcinoma, those without overt odontogenic differentiation and appear nonspecific and are labeled primary intraosseous carcinoma, those that differentiate towards salivary gland tumors, such as clear cell odontogenic carcinoma, and others such as sclerosing odontogenic carcinoma ( Table 15.3 ). Odontogenic carcinosarcoma is exceedingly rare. Odontogenic carcinomas are rare and putatively arise from proliferation of odontogenic rests, within the bone.
| Odontogenic Carcinomas |
|
|
|
|
|
The term malignant ameloblastoma is sometimes used to include two entities, the ameloblastic carcinoma and the metastatic ameloblastoma. Ameloblastic carcinoma, the more common of the two, displays many features of ameloblastoma, except that it has marked cytologic atypia, is more infiltrative, may metastasize, and has a poor prognosis. Metastatic ameloblastoma, a very rare condition, shows minimal cytologic atypia, although it has metastasized usually to lymph nodes or more often to the lungs. It is unclear whether all such lesions have benign histopathology and the original biopsies were not always available for review in reports on this entity and as such, some question the existence of this entity.
Ameloblastic carcinoma:
Most cases occur in the sixth decade of life and beyond, with a 2:1 to 3:1 male predilection, although the age range is wide with 18% occurring in those under 25. More than two-thirds occur in the mandible, mostly in the posterior, and pain, mucosal ulceration, and paresthesia are often noted.
Lesions appear as poorly demarcated, destructive radiolucencies.
Spindle cell variant: Only a handful of cases have been reported. The mean age is in the fifth decade, slightly younger than patients with conventional ameloblastic carcinoma with a slight male predilection and most lesions are mandibular.
Metastatic ameloblastoma:
The mean age is in the fifth decade with almost equal sex distribution with 80% occurring in the mandible. They occur at a median of 10 years after the initial diagnosis of ameloblastoma in the jaws. Most patients had been treated with enucleation and curettage initially and there is an average of four local recurrences. Seventy percent of lesions present with lung lesions often bilaterally, and 20% to 30% with nodal metastases, with 20% to 25% of patients exhibiting multisite metastases. Sixty percent of the original tumors were treated by enucleation or curettage. Hypercalcemia has been reported in some cases with pulmonary metastases.
Radiographically, the primary lesion may appear circumscribed and discrete, rather than poorly demarcated as one expects for a primary carcinoma.
The majority of ameloblastic carcinomas arise de novo, with approximately 25% arising from preexisting ameloblastomas, and this has no bearing on its behavior or prognosis. One-third of these tumors are positive for BRAF V600E mutation.
Metastatic ameloblastoma may occur as a result of multiple surgical manipulations of multiple recurrences of the primary tumor resulting in hematogenous and lymphatic spread and as such, are often discovered many years after removal of the original jaw tumor. Most are positive for mutations in BRAF .
Ameloblastic carcinoma:
These tumors are plexiform or follicular in pattern with palisading of the basal cell nuclei and reverse nuclear polarity which may be subtle. Stellate reticulum-like areas are usually difficult to discern and there is hypercellularity with tumor cells exhibiting spindling or pseudosarcomatoid appearance, pleomorphism, necrosis, hyperchromasia, and many mitotic figures (average of 4 per high-power field), as well as atypical mitotic figures ( Fig. 15.29 ). Necrosis and perineural invasion may be present.

Keratinization, ghost cells, clear cells, and focal dentinoid production may be present.
Tumor cells express K14, K18, and K19 and there is a high Ki-67 proliferation index as expected which helps to differentiate this from ameloblastoma. SOX2 and OCT4 are expressed in >85% of tumors, as is PITX2.
Spindle cell variant (sarcomatoid ameloblastic carcinoma): While ameloblastic carcinoma may show foci of spindle cells within tumor islands, this lesion consists of a proliferation of atypical spindle/sarcomatoid cells representing epithelial-mesenchymal transition from ameloblastic carcinoma. Both spindle cells and carcinomatous cells are positive for vimentin and p40.
Metastastatic ameloblastoma:
The cytology is that of a conventional ameloblastoma without or with minimal evidence of cytologic atypia.
Primary intraosseous carcinoma and ameloblastic carcinoma are likely related lesions, with the former showing less ameloblastic differentiation, in that basal cell nuclear palisading or formation of stellate reticulum-like areas is minimal or insignificant.
Whether the spindle cell variant of (sarcomatoid) ameloblastic carcinoma is distinct from an ameloblastic carcinosarcoma or odontogenic carcinosarcoma is unclear.
Metastatic carcinoma must be ruled out.
Calcifying epithelial odontogenic tumor often exhibits nuclear pleomorphism but tumor cells are large and eosinophilic and there is insignificant mitotic activity, hypercellularity, or necrosis.
Ameloblastic carcinoma:
Treatment is with en bloc resection with radiation and/or chemotherapy as appropriate. The recurrence rate is close to 30%. Twenty to twenty-five percent develop metastases, and distant metastases are to lungs, bone, and brain. Five-year overall survival overall without and with metastasis are approximately 70% and 20%, respectively.
Metastatic ameloblastoma:
Patients are treated with surgery, chemotherapy, and/or radiation. Five-year survival is 37% and 62% with pulmonary and nodal metastases respectively, although some patients survive for decades.
Chou YH, Jhuang JY, Chang MH, et al. Metastasizing ameloblastoma with localized interstitial spread in the lung: report of two cases. Int J Surg Pathol . 2014;22:343-346.
Diniz MG, Gomes CC, Guimaraes BV, et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol . 2015;36:5649-5653.
Dissanayake RK, Jayasooriya PR, Siriwardena DJ, Tilakaratne WM. Review of metastasizing (malignant) ameloblastoma (METAM): pattern of metastasis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2011;111:734-741.
García-Muñoz A, Rodríguez MA, Licéaga-Escalera C, et al. Expression of the transcription factor PITX2 in ameloblastic carcinoma. Arch Oral Biol . 2015;60:799-803.
Ghiam A, Al Zahrani A, Feld R. A case of recurrent metastatic ameloblastoma and hypercalcaemia successfully treated with carboplatin and paclitaxel: long survival and prolonged stable disease. Ecancermedicalscience . 2013;7:323.
Giridhar P, Mallick S, Upadhyay AD, Rath GK. Pattern of care and impact of prognostic factors in the outcome of ameloblastic carcinoma: a systematic review and individual patient data analysis of 199 cases. Eur Arch Otorhinolaryngol . 2017;274:3803-3810.
Hall JM, Weathers DR, Unni KK. Ameloblastic carcinoma: an analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2007;103: 799-807.
Khan W, Augustine D, Rao RS, Sowmya SV, Haragannavar VC, Nambiar S. Stem cell markers SOX-2 and OCT-4 enable to resolve the diagnostic dilemma between ameloblastic carcinoma and aggressive solid multicystic ameloblastoma. Adv Biomed Res . 2018;7:149.
Loyola AM, Cardoso SV, de Faria PR, et al. Ameloblastic carcinoma: a Brazilian collaborative study of 17 cases. Histopathology . 2016;69: 687-701.
Matsushita Y, Fujita S, Yanamoto S, et al. Spindle cell variant of ameloblastic carcinoma: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol . 2016;121:e54-e61.
McLean-Holden AC, Bishop JA, Kessler HP, et al. Spindle-cell variant of ameloblastic carcinoma: a report of 3 cases and demonstration of epithelial-mesenchymal transition in tumor progression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2019;128:e113-e121.
Niu Z, Li Y, Chen W, et al. Study on clinical and biological characteristics of ameloblastic carcinoma. Orphanet J Rare Dis . 2020;15:316.
Pandiar D, Anand R, Kamboj M, Narwal A, Shameena PM, Devi A. Metastasizing ameloblastoma: a 10 year clinicopathological review with an insight into pathogenesis. Head Neck Pathol . 2021;15(3):967-974.
Richardson MS, Muller S. Malignant odontogenic tumors: an update on selected tumors. Head Neck Pathol . 2014;8:411-420.
Van Dam SD, Unni KK, Keller EE. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg . 2010;68:2962-2974.
Yoon HJ, Jo BC, Shin WJ, et al. Comparative immunohistochemical study of ameloblastoma and ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2011;112:767-776.
The median age is in the sixth decade of life, with a 2:1 male predilection, and pain, mobility of teeth, and paresthesia are common. Eighty to ninety percent occur in the mandible. Lymph node metastasis is present in 20% to 50% of cases.
It presents as a poorly demarcated radiolucency of the mandible, often with cortical perforation ( Fig. 15.30 ).

Tumors arise either from preexisting odontogenic cysts (30% of cases) or de novo. Approximately 60% are associated with a residual or radicular cyst, 16% with a dentigerous cyst, and 14% with a KCOT/OKC. There is likely overlap between this entity and the ameloblastic carcinoma because both putatively arise from odontogenic rests within the jaw bones, except that one has a more squamous cell carcinoma phenotype and the other a clearly ameloblastomatous phenotype.
Primary intraosseous carcinomas are generally squamous cell carcinomas with approximately half being well-differentiated and keratinizing. Most arise from odontogenic cysts, the lining of which exhibits varying degrees of dysplasia or carcinoma in situ ( Fig. 15.31 ).

Features suggesting odontogenic differentiation may be seen in approximately half the cases and include a follicular pattern, palisading of the peripheral basal cells, and the presence of clear cells ( Fig. 15.32 ). Tumors may manifest as verrucous or papillary squamous carcinoma or sarcomatoid carcinoma.

Ameloblastic carcinoma shows obvious palisading of the peripheral cells. Differentiation between this and primary intraosseous carcinoma may be moot because both are primary carcinomas in bone.
Primary squamous cell carcinoma of the overlying mucosa or the maxillary sinus invading bone, as well as metastatic squamous cell carcinoma, must be ruled out.
Calcifying epithelial odontogenic tumor contains cells with pleomorphic nuclei but do not exhibit increased mitotic activity or other features of malignancy.
Mucoepidermoid carcinoma contains mucous cells although this may be scant, and ductal structures may be present. Most will exhibit CRCT-MAML2 rearrangement.
Desmoplastic (scirrhous) ameloblastoma contains irregularly shaped islands of basaloid cells without cytologic atypia, some islands show typical ameloblastoma, and the stroma is desmoplastic.
Odontogenic carcinosarcomas have been reported, but they are extremely rare and would be difficult to distinguish from a primary intraosseous sarcomatoid squamous cell carcinoma.
Resection is the treatment of choice with or without lymph node dissection, with adjuvant radiation therapy and/or chemotherapy as appropriate. The recurrence rate is 60%. Ten percent develop distant metastases to lungs, bones, and brain, and 2-year and 5-year survival are 60% and 40%, respectively.
Bodner L, Manor E, Shear M, van der Waal I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst: a clinicopathologic analysis of 116 reported cases. J Oral Pathol Med . 2011;40:733-738.
Chaisuparat R, Coletti D, Kolokythas A, et al. Primary intraosseous odontogenic carcinoma arising in an odontogenic cyst or de novo: a clinicopathologic study of six new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2006;101:194-200.
Chikosi R, Segall N, Augusto P, Freedman P. Odontogenic carcinosarcoma: case report and literature review. J Oral Maxillofac Surg . 2011;69:1501-1507.
de Morais EF, Carlan LM, de Farias Morais HG. Primary intraooseous squamous cell carcinoma involving the jaw bones: a systematic review and update. Head Neck Pathol . 2021;15:608-616.
Huang JW, Luo HY, Li Q, Li TJ. Primary intraosseous squamous cell carcinoma of the jaws: clinicopathologic presentation and prognostic factors. Arch Pathol Lab Med . 2009;133:1834-1840.
Li K, Yang L, Qiao YJ, Liang YJ, Wang X, Liao GQ. Risk factors and prognosis for the primary intraosseous carcinoma of the jaw. Int J Oral Maxillofac Surg . 2019;48:157-162.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here