Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We thank Drs. Kerem Ozcan and Aslihan Yavas for their contributions in the preparation of this chapter.
As discussed in other chapters, gallstones and inflammatory disorders constitute the vast majority of biliary tract pathology. Neoplasms and tumor-like lesions (pseudotumors) are relatively rare but form an important category with challenging diagnostic issues. As a result of advances in radiographic modalities and widespread use of laparoscopic cholecystectomy, the frequency of gallbladder and biliary tumors that have come to clinical attention has increased in recent years. These techniques have also led to an increase in the diagnosis of mass-forming lesions that would have otherwise gone undetected, including incidentalomas such as cholesterol polyps. Nevertheless, a significant proportion of gallbladder neoplasms, including adenocarcinomas, are detected as “clinically unapparent” in cholecystectomies performed for cholecystitis.
Recent work has focused on reevaluation of the terminology used for gallbladder and bile duct tumors and, in particular, for preinvasive neoplasms. This was done to standardize the approach to tumors across the entire pancreatobiliary system.
A significant proportion of gallbladder carcinomas are unapparent both clinically and grossly, discovered only after microscopic examination of cholecystectomy specimens performed for cholecystitis. Therefore a sampling protocol addressing this possibility should be utilized routinely. , Our approach is the following , : The cystic duct margin is sampled en-face and unopened. The serosal and hepatic surfaces of the gallbladder are inked in different colors, and the gallbladder is opened from the serosal aspect. If no overt lesions are noted, a through-and-through section, proceeding from the fundus to the cystic duct, is taken, rolled up, and submitted in the same cassette along with the cystic duct margin. If a lymph node is present, this is also included in the same cassette, if possible.
If epithelial atypia or low-grade dysplasia is identified in this random sample, four additional tissue blocks with multiple strips are obtained. If high-grade dysplasia is identified, then the entire gallbladder is examined because of its association with invasive carcinoma. The prognosis changes dramatically if a patient has invasive carcinoma rather than high-grade dysplasia. Similarly, if a pTis, pT1a, or pT1b carcinoma is identified, then the entire gallbladder should be examined to rule out a pT2 carcinoma (invasion through the tunica muscularis) because the clinical outcome of the latter-stage tumor is far worse. In fact, a diagnosis of early gallbladder cancer (pTis or pT1) must not be rendered unless the entire gallbladder is examined. , It has been recently shown in an analysis from a high cancer-risk population that although high-grade dysplasia and invasive carcinoma are likely to be identified in initial sections, accurate staging requires total sampling.
If a polyp or papillary lesion is present, it is submitted entirely for microscopic examination. If it is neoplastic, a minimum of four tissue blocks with multiple strips are obtained from the uninvolved gallbladder to evaluate any other dysplastic lesions. If the polyp shows high-grade dysplasia, then the entire gallbladder should be examined because of its association with invasive carcinoma as mentioned earlier.
For a pT2 or greater carcinoma, documentation should include, in addition to the cystic duct margin, the presence or absence of spread to the serosal versus hepatic surfaces, respectively. Typically, proper documentation of the deepest region requires at least 12 tissue blocks with multiple strips because foci of carcinoma are often grossly unapparent.
For high-risk lesions, such as hyalinizing cholecystitis or xanthogranulomatous cholecystitis, which are notorious for being associated with grossly unapparent carcinomas, extensive sampling (minimum 20 sections) should be performed. If there is pancreatobiliary maljunction (anomalous union of pancreatobiliary ducts where the common bile duct and main pancreatic duct adjoin above the sphincter of Oddi and thus lead to reflux and cancers), the entire gallbladder should be examined, irrespective of gross findings.
Patients with a clinical history of high-risk disease for carcinoma, such as primary sclerosing cholangitis, should also be sampled more extensively, preferably with at least four tissue blocks with multiple strips during the initial random sampling procedure. ,
Similar to the remainder of the pancreatobiliary tract, mass-forming (>1 cm), preinvasive neoplastic lesions also occur in the gallbladder. , , As such, these lesions represent gallbladder counterparts of intraductal neoplasms of the pancreas and bile ducts , or intra-ampullary papillary tubular neoplasms , and duodenal surface adenomas of the ampulla, and much of the information elucidated about these tumors is also applicable to the gallbladder. Of course, there are also organ-specific characteristics. , , , In essence, these are gallbladder examples of what is known in other organs as adenoma-carcinoma sequence . Because they are relatively rare and the experience with them is relatively limited, the views about these tumors have been in flux. However, at the same time, there are numerous characteristics that have been delineated and agreed on in the past decade. They are all considered premalignant, although the risk of invasive carcinoma may vary greatly by subtype (discussed later).
In the past, various terms have been employed for these lesions ( Table 39.1A ). In the 2019 World Health Organization (WHO) classification of tumors of the digestive system, these lesions are regarded in two broad categories of intracholecystic papillary neoplasm and pyloric gland adenoma ( Box 39.1 ) . However, intracholecystic papillary neoplasm –type lesions often show areas of pyloric gland differentiation, and pyloric gland adenoma –type lesions often have papillary architecture or even mixed components. In addition, in the pancreatobiliary tract, pyloric gland adenoma –type lesions are now classified as intraductal papillary mucinous neoplasms (IPMNs) and intraductal papillary neoplasms of the bile ducts [IPNBs]). , Therefore we have proposed a unifying term of intracholecystic papillary-tubular neoplasms (ICPNs) for these lesions. Recently, new entities, namely intracholecystic tubular nonmucinous neoplasm (ICTN) and mural intracholecystic neoplasm arising in adenomyomatous nodules, have been described. In this text, all of these entities are collectively referred to as intracholecystic neoplasms, and distinct subtypes are discussed in the following sections.
| Mass-Forming Lesion | Authors’ Approach | Old Literature | 2019 WHO Classification |
|---|---|---|---|
| Polypoid pyloric gland collections in the mucosa | <1 cm, not well demarcated, and without atypia: polypoid pyloric gland metaplasia ≥1 cm and distinct from the neighboring mucosa: Intracholecystic papillary-tubular neoplasm (ICPN), exclusively pyloric phenotype |
Pyloric gland adenoma | <0.5 cm and not well demarcated: polypoid pyloric gland metaplasia Distinct from the neighboring mucosa: pyloric gland adenoma |
| Papillary, tubulopapillary, or tubular and mucinous preinvasive neoplasm in the mucosa (including gastric pyloric, gastric foveolar, biliary, intestinal, and oncocytic cell types, which often occur in a mixture) | Intracholecystic papillary-tubular neoplasm (ICPN) Document:
|
Biliary adenoma Intestinal type adenoma Tubular adenoma Tubulopapillary adenoma Papillary adenoma Papillary neoplasm Papillary carcinoma |
Pyloric gland adenoma or Intracholecystic papillary neoplasm |
| Complex tubular and nonmucinous preinvasive neoplasm | Intracholecystic tubular nonmucinous neoplasm (ICTN) | Pyloric gland adenoma Tubulopapillary adenocarcinoma |
No specific information |
| Cystic papillary/tubular and mucinous lesion on the wall (arising in adenomyoma) | Mural intracholecystic neoplasm arising in adenomyomatous nodule | Mural IPMN-like tumor | No specific information |
Adenoma
Biliary intraepithelial neoplasia (dysplasia) (BilIN)
Intracholecystic papillary neoplasm (gallbladder)
Intraductal papillary neoplasm (bile ducts)
Mucinous cystic neoplasm
Carcinomas
Adenocarcinoma, pancreatobiliary type
Adenocarcinoma, intestinal type
Adenocarcinoma, clear cell type
Poorly cohesive carcinoma
Mucinous carcinoma
Adenosquamous carcinoma
Squamous cell carcinoma
Intracholecystic papillary neoplasm with associated invasive carcinoma
Intraductal papillary neoplasm with associated invasive carcinoma
Mucinous cystic neoplasm with associated invasive carcinoma
Well differentiated neuroendocrine tumor (NET G1, G2, and G3)
Poorly differentiated neuroendocrine carcinoma
Large cell neuroendocrine carcinoma
Small cell neuroendocrine carcinoma
Mixed neuroendocrine–nonneuroendocrine neoplasm (MINEN)
Clinically significant (>1 cm) ICPNs have certain recognized characteristics. They occur in older adults who are typically in their early 60s at the time of diagnosis. Patients may present with abdominal pain, or the tumor may be detected incidentally. Gallstones in these patients are less common compared with patients with other types of gallbladder tumors. Rarely, patients have Peutz-Jeghers syndrome, , Gardner syndrome, , or an anomalous union of pancreatobiliary ducts. Some cases are associated with a Brunner gland hamartoma of the duodenum.
Radiologically, nearly 50% of cases are classified as cancer because of the mucosal mass that defines these tumors, whereas others are classified as polypoid tumors . About 10% are not detected clinically, presumably because they are mistaken for stones or sludge.
There are geographic differences in the incidence and association with invasive carcinoma. For instance, both the overall incidence of ICPNs and its relative proportion in intraepithelial neoplasia is higher in the East than in the West (ICPNs constitute 52.5% of gallbladder intraepithelial neoplasia in the East and only 26% in the West). Moreover, ICPNs, although lower in relative frequency, may be more prone to advance to invasive carcinoma in the West (ICPN-associated invasive carcinomas constitute 42% of the gallbladder carcinomas in the West and 29% in the East).
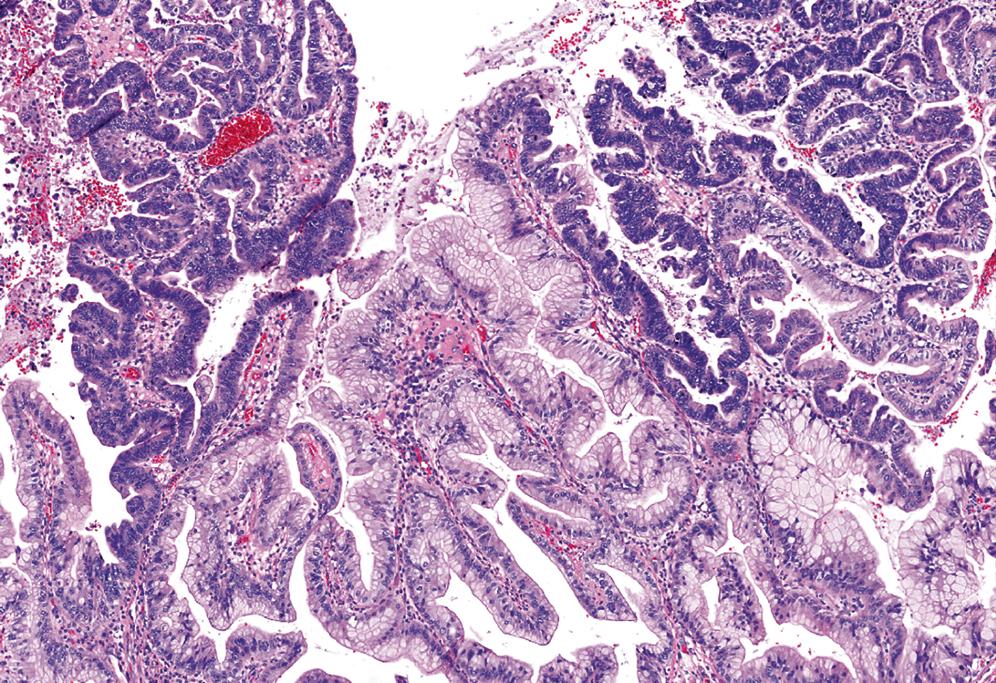
ICPNs are characterized by the presence of large, papillary (or villous) growths with a feathery pattern or by smooth-surfaced polypoid projections that may be pedunculated or sessile ( Fig. 39.1 ). , They occur more commonly in the body or fundus of the gallbladder. They may grow up to 8 cm in the maximum dimension and even fill the entire gallbladder in some cases. A substantial proportion, especially predominantly papillary examples, present as multifocal sessile lesions and have a field effect risk to the remainder of the biliary tract, with some noninvasive cases developing biliary tract cancers several years after the cholecystectomy. Of note, ICTNs (discussed later) invariably form unifocal pedunculated lobulated polyps that easily detach from the surface and present as debris in the lumen. Similarly, intracholecystic neoplasms arising in adenomyomatous nodules (discussed later) are localized mural nodular tumors that typically do not even show significant involvement of the gallbladder mucosa and remain mostly confined to the adenomyomatous nodule on the wall.
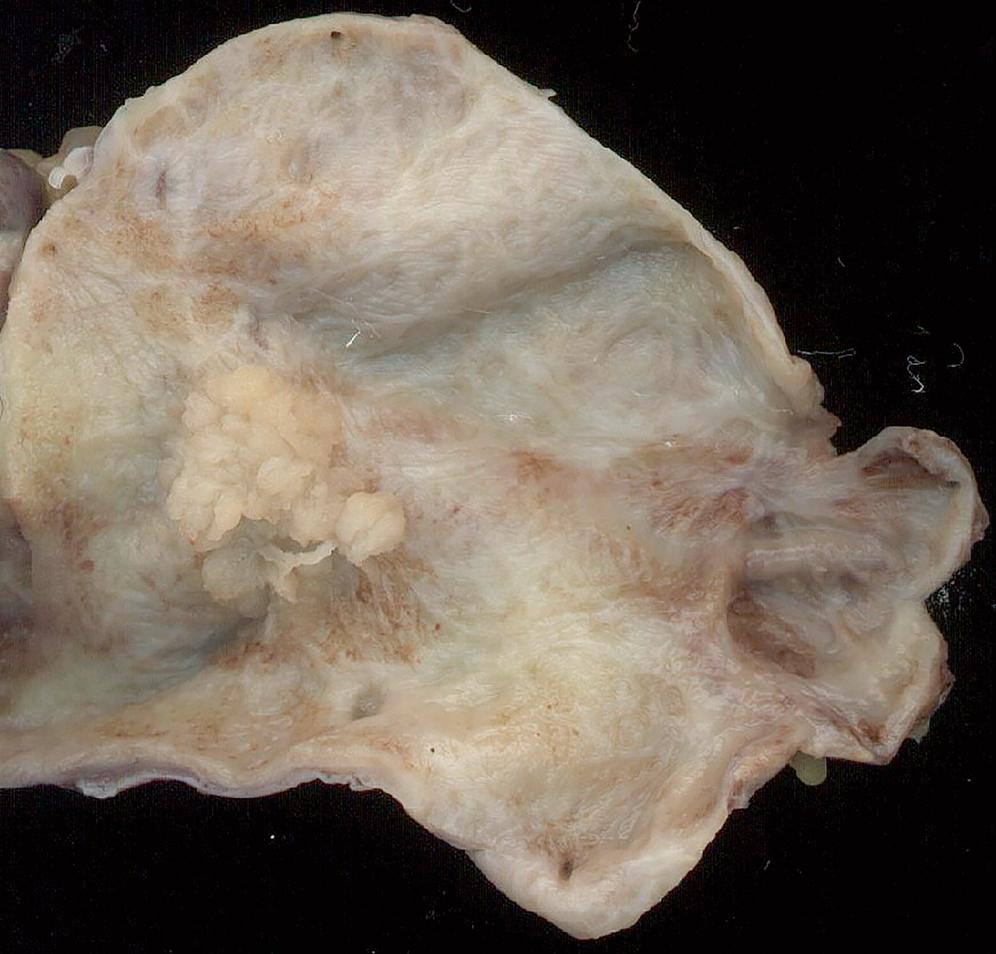
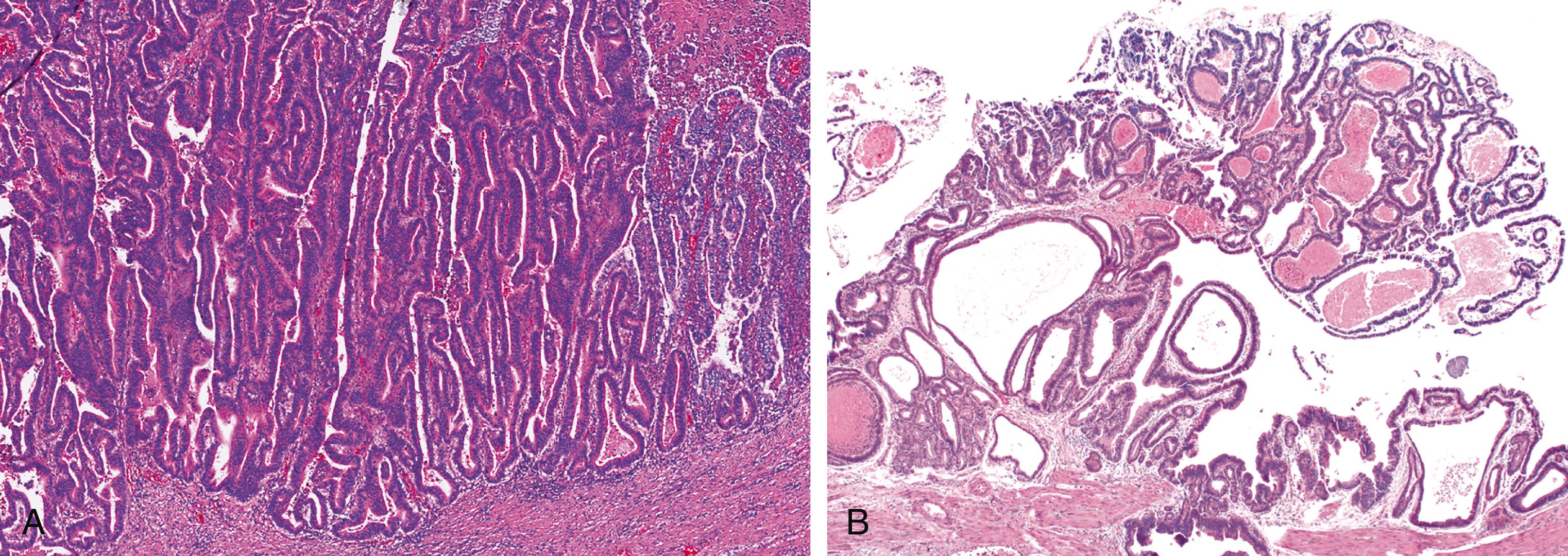
The lesions are overtly exophytic and distinct from neighboring tissues. They may be predominantly papillary (or villous) or predominantly tubular, but a significant proportion show a mixed pattern of growth ( Fig. 39.2 ).
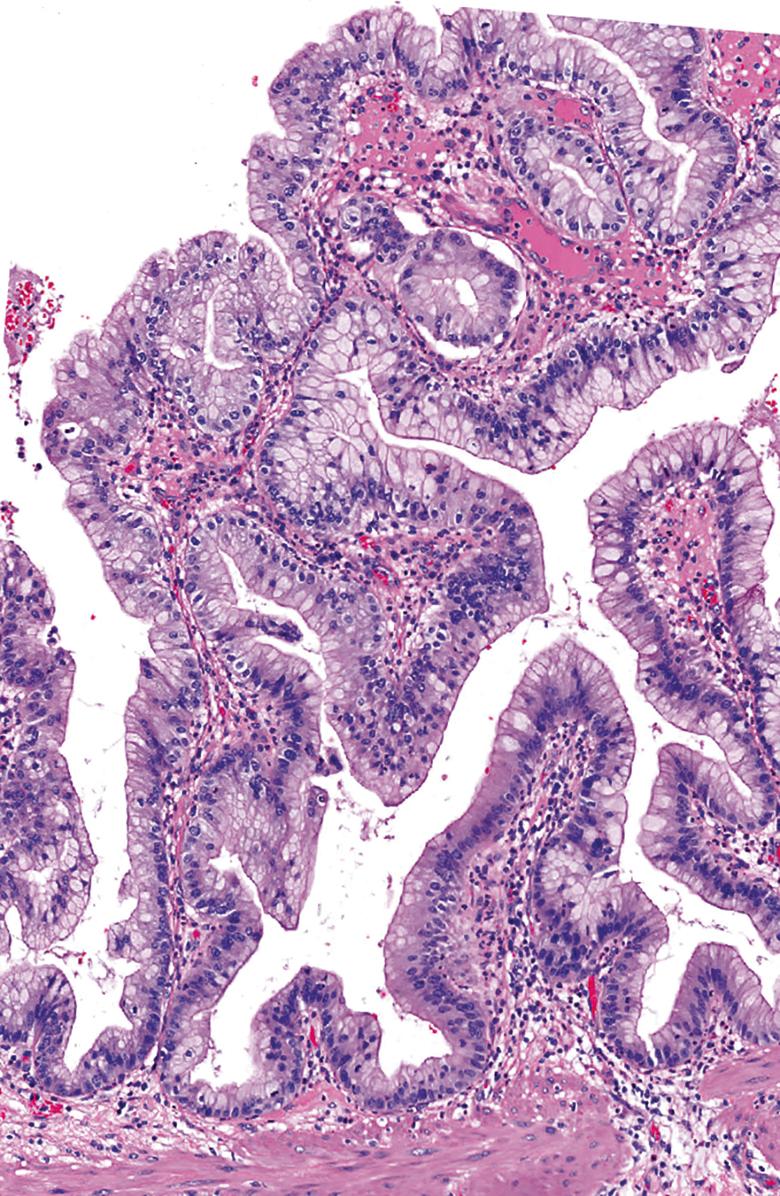
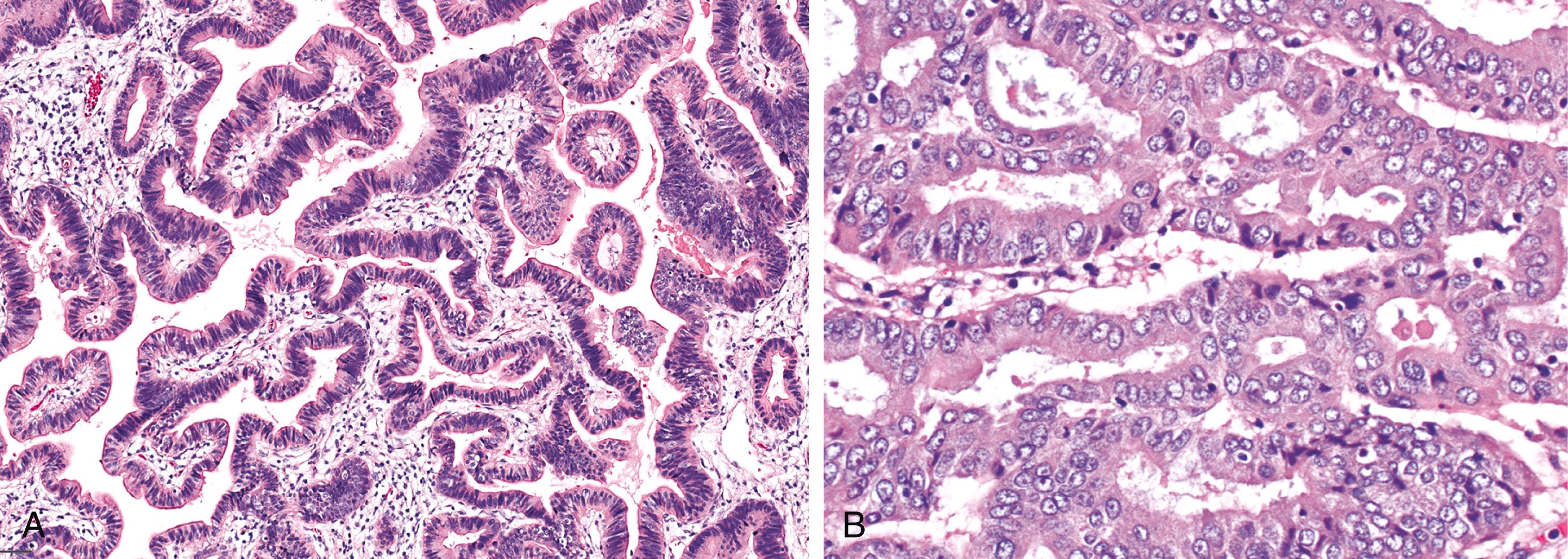
On the basis of the highest degree of cytoarchitectural atypia in the epithelium, dysplasia in ICPNs is classified as low-grade or high-grade. ICPNs with low-grade dysplasia reveal only mild architectural and cytological atypia. Relatively uniform but enlarged and hyperchromatic atypical nuclei are still mostly confined to the lower aspect of the epithelium ( Fig. 39.3 ). High-grade dysplasia, which is present in a significant proportion of ICPNs that are >1 cm in size, are characterized by severe architectural and cytological atypia, and they may present in different patterns. In most cases, the main histological finding is pseudostratification of cells with loss of polarity and nuclear pleomorphism. Others exhibit a cribriform arrangement of cells, either with or without clear cell features, or a single layer of epithelium with centrally located and markedly abnormal nuclei ( Fig. 39.4 ). A transition from low-grade dysplasia to high-grade dysplasia is common in these tumors ( Fig. 39.5 ). ,
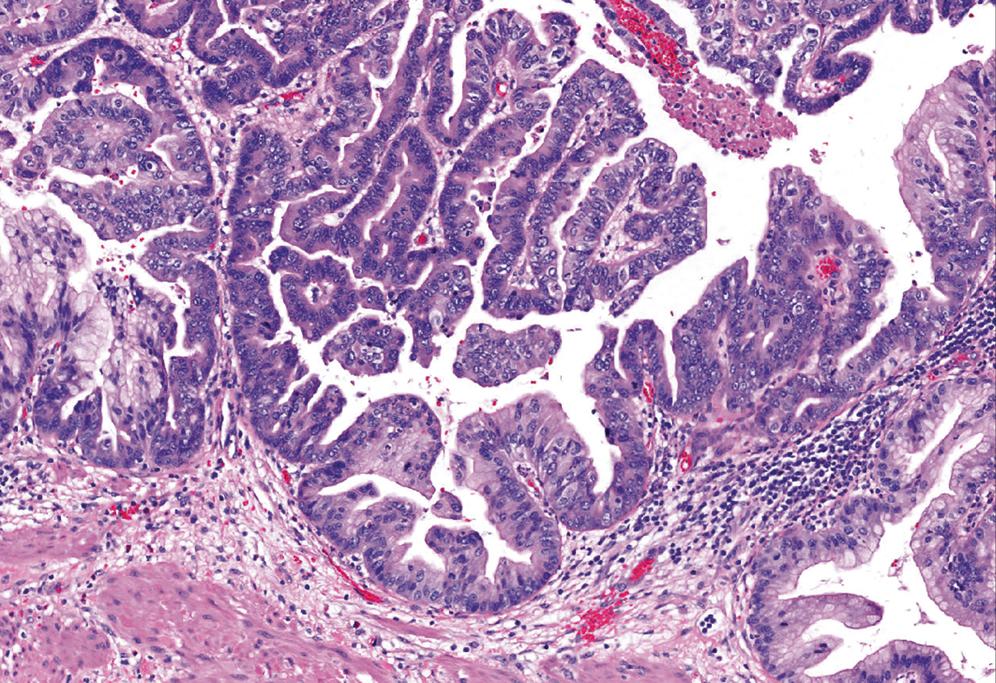
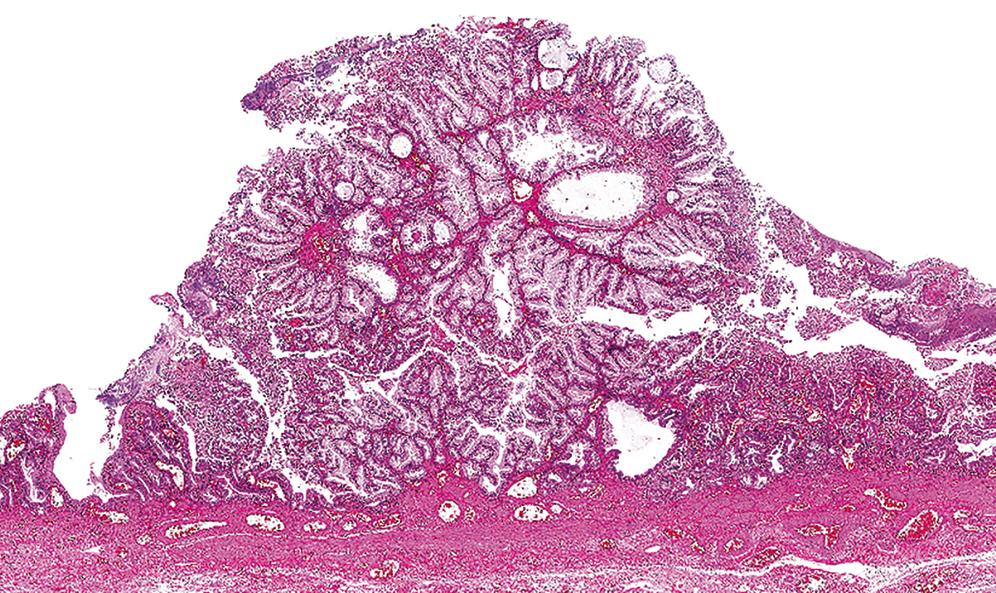
Once intracholecystic neoplasms , , as well as polypoid pyloric metaplasias and fibromyoglandular polyps , , were better characterized, ICPNs with a predominantly papillary growth pattern became the most common among mass-forming (>1 cm) preinvasive neoplasias in the gallbladder. As mentioned earlier, these are recognized in the 2019 WHO classification as intracholecystic papillary neoplasms and are distinguished from pyloric gland adenomas ; however, they often exhibit gastric pyloric–type (or Brunner gland–like) mucinous glands as well.
There are also different cell lineages observed in this group of ICPNs ( Table 39.1B ). The majority exhibit biliary phenotype ( Fig. 39.6 ), characterized by round cuboidal cells with prominent nucleoli and thus by nature, dysplasia is classified as high-grade. These lesions commonly express MUC1 and are often associated with invasive carcinoma. That is why they were classified as papillary adenocarcinomas in the past. Prominent lymphoplasmacytic and neutrophilic inflammation may involve the papillary cores and the epithelium. Follicular cholecystitis may also occur in patients with this type of tumor. ,
| Authors’ Classification | Key Features |
|---|---|
| Intracholecystic papillary-tubular neoplasm (ICPN), exclusively pyloric phenotype |
|
| Intracholecystic papillary-tubular neoplasm (ICPN), predominantly foveolar phenotype |
|
| Intracholecystic papillary-tubular neoplasm (ICPN), predominantly biliary phenotype |
|
| Intracholecystic papillary-tubular neoplasm (ICPN), predominantly intestinal phenotype |
|
| Intracholecystic papillary-tubular neoplasm (ICPN), predominantly oncocytic phenotype |
|
| Intracholecystic tubular nonmucinous neoplasm (ICTN) |
|
| Mural intracholecystic neoplasm arising in adenomyomatous nodules |
|
Some ICPNs have a gastric foveolar phenotype ( Fig. 39.7 ), which is characterized by the presence of tall columnar cells that have abundant, pale, mucinous cytoplasm and MUC5AC expression. These lesions may be analogous to the foveolar dysplasia that is being increasingly recognized. In the gallbladder, they appear very innocuous and do not seem overtly atypical; however, their association with invasive carcinoma is high.
A small percentage of ICPNs resemble intestinal- type adenomas ( Fig. 39.8 ). They are characterized by pseudostratified columnar epithelium with cells that contain cigar-shaped nuclei with conspicuous nucleoli, arranged in a tubular and/or villous architecture. In low-grade foci, the neoplastic cells are well oriented, have small and uniform nuclei, lack nucleoli, and have atypical mitoses ( Fig. 39.9 ). High-grade foci are characterized by severe atypia with irregular branching papillae and budding of cell clusters into the lumen, cuboidal-shaped cells with stratification, loss of polarity, pleomorphism, and prominent nucleoli; mitoses are frequent ( Fig. 39.10 ). As expected, these tumors are commonly positive for CK20, CDX2, and MUC2. Of note, goblet cells may be found in any type of ICPN. ,


Rare cases may be oncocytic ( Fig. 39.11 ), revealing features similar to those of intraductal oncocytic papillary neoplasms of the pancreas and bile ducts, , although these are more prone to have intracytoplasmic hyaline globules. Interestingly, oncocytic morphology in ICPNs does not correlate with the characteristic immunoprofile of intraductal oncocytic papillary neoplasms in the pancreas. , , , Instead, there is diffuse MUC1 expression and an absence of MUC6.

As mentioned earlier, ICPNs that are composed almost entirely of gastric pyloric–type (or Brunner gland–like) mucinous glands are classified as a separate category of pyloric gland adenomas ( Fig. 39.12 ). However, these often have papillary architecture or even mixed components.

In the older literature, pyloric gland adenoma -type lesions were regarded as the most common type of “adenomatous” process in the gallbladder, but this was because noninvasive papillary neoplasms were regarded as part of adenocarcinomas. , , Moreover, the older literature is mostly based on series that included cases as small as 2 mm in largest dimension (mean size ranged from 0.6 to 0.9 cm). It is now well known that pyloric gland metaplasia is seen in 60% of cholecystitis patients, and these lesions often form polypoid collections. It appears that a significant proportion of the lesions reported as pyloric gland adenomas actually represent exuberant polypoid examples of pyloric gland metaplasia, rather than true adenomas. , Perhaps as importantly, fibromyoglandular polyps of the gallbladder often have prominent collections of pyloric glands, and we have seen these polyps also designated erroneously as pyloric gland adenomas . To address these issues, the 2019 WHO classification recommends that small (<0.5 cm) pyloric gland nodules arising in a background of pyloric gland metaplasia in the adjacent mucosa should not be designated as pyloric gland adenomas .
A true adenoma typically forms a true polyp and is sharply distinct from the neighboring mucosa. By definition, the majority of the lesions reveal uniform mucinous glands with abundant apical mucinous cytoplasm and well-polarized nuclei located at the periphery of the cell cytoplasm, and they are thus classified as low grade ( Fig. 39.13 ). However, in large (>1 cm) pyloric gland adenomas, foci of high-grade dysplasia, characterized by cytoarchitectural complexity (disorganized appearance and highly atypical nuclei), may be present ( Fig. 39.14 ). These cases are occasionally associated with invasive carcinoma. ,


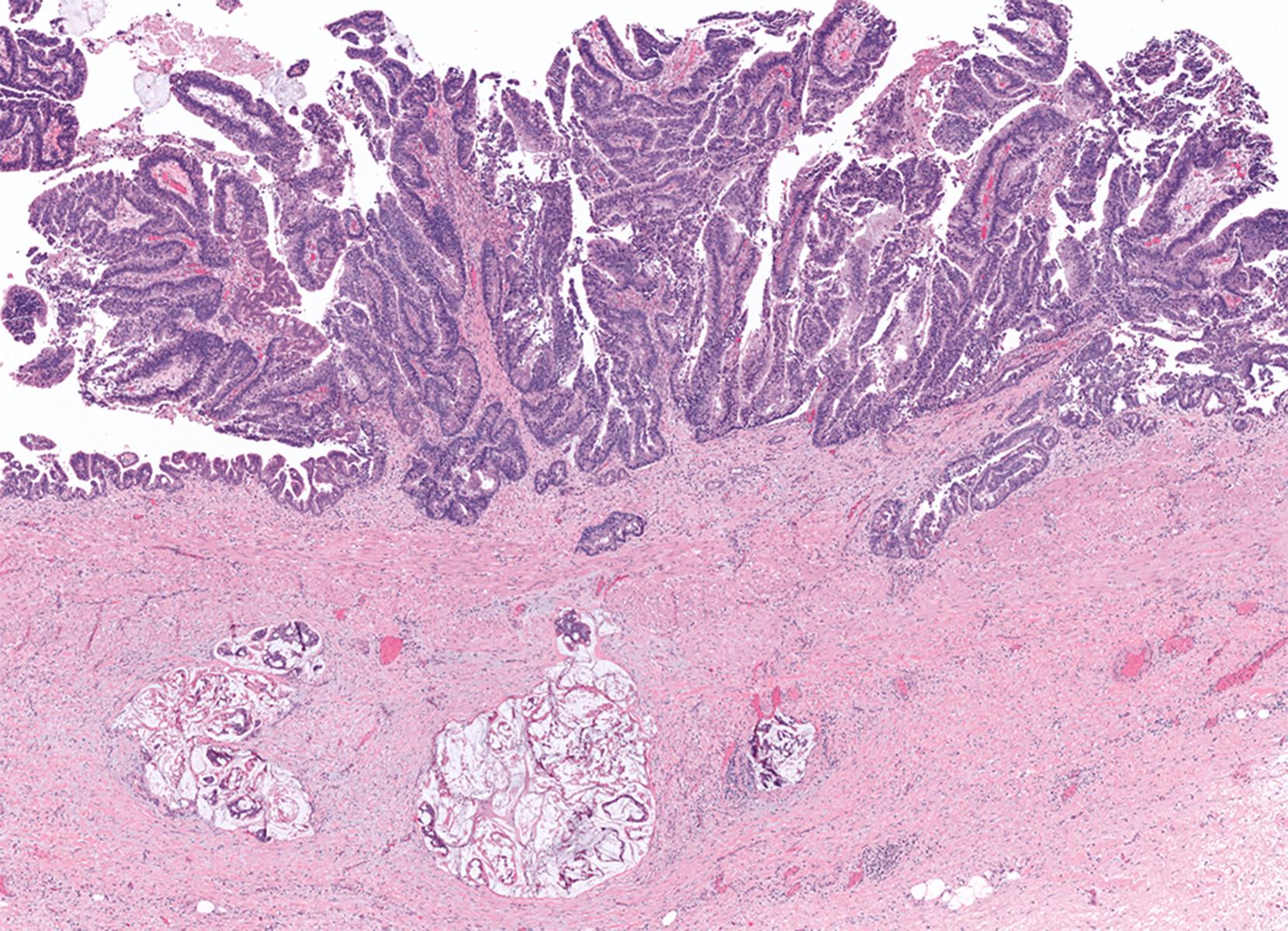
Regardless of the subtype, the most important task in a patient with ICPN is to rule out invasive carcinoma. In fact, in the presence of invasive carcinoma, typing of the preinvasive lesion becomes much less significant. Invasive carcinoma is difficult to detect in the gallbladder in general. This becomes even a bigger issue in patients with ICPNs, where invasive elements can be masked, in addition to inflammatory reactive changes, by the preinvasive dysplastic process. Moreover, especially in patients with predominantly papillary ICPNs, invasive carcinoma can be hidden away from the main lesion. For this reason, it is imperative that extensive, if not entire, sampling is employed before a case can be classified as noninvasive. If an invasive carcinoma is identified, its size, extent, and type should be documented separately. Most invasive carcinomas that arise in patients with these tumors are ordinary tubule-forming adenocarcinomas (pancreatobiliary type), although unusual types such as mucinous (colloid), squamous, and neuroendocrine carcinomas may also occur and may in fact be proportionally more common. If invasive carcinoma is not found, it is helpful to document the extent of high-grade dysplasia, the amount of papillary growth, and the cell lineages (especially biliary and gastric foveolar phenotypes) because these parameters have been found to be associated with a higher incidence of invasive carcinoma elsewhere in the biliary tract and with disease progression. ,
The immunophenotype of ordinary ICPNs corresponds to their line of differentiation. Most are cytokeratin 7 (CK7) positive, and many express mucin-related glycoproteins and oncoproteins, including carcinoembryonic antigen (CEA). The MUC1 response is typically confined to the apical membrane of cells in areas of biliary differentiation or high-grade dysplasia. MUC2 is expressed in intestinal areas, MUC5AC in gastric foveolar areas, and MUC6 in gastric pyloric or Brunner gland–like areas. ,
Current evidence indicates that molecular alterations of ICPNs are different from those observed in the conventional dysplasia-carcinoma sequence in the gallbladder. They are more similar to those described in intraductal neoplasms of the intrahepatic and extrahepatic bile ducts. Although KRAS mutations are common, , the GNAS codon 201 mutation, which is seen in about two-thirds of pancreatic IPMNs, is rarely seen in ICPNs. This disparity is presumably related to the rarity of the intestinal variant, which appears to be linked with this mutation.
Typically, pyloric gland adenoma –type lesions form polyps that are clearly distinct from the background mucosa, both in architecture and cytology. They display large (usually >1 cm) collections of uniform, back-to-back glands without intervening stroma. In contrast, polypoid metaplasia and fibromyoglandular polyps are typically composed of smaller focus of variably sized glands with intervening stroma. The 2019 WHO classification recommends that small (<0.5 cm) pyloric gland nodules arising in a background of pyloric gland metaplasia in the adjacent mucosa should not be designated as pyloric gland adenomas .
Occasionally, an ICPN that is smaller than 1 cm but that displays all characteristics of the larger examples (including clear-cut evidence of dysplastic changes) are encountered in a gallbladder. These should prompt total sampling of the gallbladder if this has not been performed. If no other lesions are discovered, then the case can be classified as an incipient ICPN with proper descriptions, as has been employed in the pancreatobiliary tract. These do not appear to imply the field effect risk that larger and more extensive ICPNs signify.
ICPNs also should be distinguished from ordinary nontumoral (flat) dysplasia, which often reveals papillary structures or tubular units forming small collections. In some cases, these papillae may be tall and exuberant, but these cases should not be regarded as ICPNs unless they form a distinct clinically evident mass ( Fig. 39.15 ).

Recognition of the presence or absence of invasive carcinoma within or under polypoid tumors may be problematic because of architectural complexity and tangential sectioning. Features that favor carcinoma include wide separation of glandular units, their irregular contours ( Figs. 39.16 and 39.17 ), cytological differences from the surface lesion (including paradoxical differentiation with more abundant cytoplasm and tubule formation), and foamy gland features and cell clusters that reside in clefts. , Extension of dysplastic epithelium into Rokitansky-Aschoff sinuses creates a pseudo-invasive appearance ( Fig. 39.18 , Table 39.2 ). The distribution of the units, their spatial connection to the overlying mucosa, and the finding of remnant normal epithelium are helpful clues in determining that the lesion is not invasive. Unlike invasive adenocarcinomas, Rokitansky-Aschoff sinuses are often flask-shaped or elongated, with the longitudinal axis oriented perpendicular to the surface ( Fig. 39.19 ). Paradoxically, these sinuses often have concentric bands of loose stroma that resembles desmoplasia ( Fig. 39.20 ). Most gallbladder adenocarcinomas do not exhibit significantly desmoplastic stroma.



| Invasive Carcinoma | Involvement of Rokitansky-Aschoff Sinuses by Neoplasia |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|


Small (<1 cm) noninvasive ICPNs are typically clinically inconsequential. However, when larger (≥ 1 cm) ICPNs are analyzed separately, even the pyloric gland adenoma– type lesions (previously thought to be innocuous) may be associated with or progress to invasive carcinoma. In our experience, invasive carcinomas occur in about 65% of ≥1-cm ICPNs with prominent papillary architecture. Biliary and foveolar cell phenotypes seem to be more frequently (60% to 70%) associated with invasive carcinoma, but the incidence of invasion is low (approximately 15%) in pyloric gland adenoma– type lesions. From the opposite perspective, 6% of invasive gallbladder carcinomas arise in association with tumoral intraepithelial neoplasms. , This association seems to be even stronger in patients with chemical carcinogenesis, as exemplified in pancreatobiliary maljunction cases. In pancreatobiliary maljunction cases, the reflux of the pancreatic acinar enzymes leads to reflux cholecystopathy and ultimately to invasive carcinoma. One fourth of the pancreatobiliary maljunction–associated gallbladder carcinomas proved to be through the ICPN pathway.
The 3-year survival rate for noninvasive ICPNs is 90%, compared with 60% for invasive tumors. Some noninvasive cases are found to have recurrences and metastases on long-term follow-up that cannot be explained by undersampling or missed invasion. This suggests that there may be a field-effect phenomenon responsible for the development of new biliary tract carcinomas years after the original gallbladder tumor was diagnosed. Thus these cases, especially the papillary and extensive/multifocal examples, should not only be carefully evaluated for the presence of invasion, but they also should be placed on a surveillance protocol, although currently there are no specific guidelines for this. Of note, ICTNs (discussed later) and intracholecystic neoplasms arising in adenomyomatous nodules (discussed later) seem to lack the field-effect phenomenon.
There is some evidence that, similar to the intraductal neoplasms of the pancreas/biliary tract, invasive carcinomas that arise from ICPNs have a more favorable survival rate than those that arise from flat dysplasia (3-year survival, 60% vs. 30%). This survival advantage is maintained even in stage-matched cases.
These cases are viewed differently by different experts. Some authors (especially in Asia and South America) typically classify these as tubular adenocarcinomas. Although they are not mucinous, because of some morphological similarities to pyloric gland adenomas and MUC6 positivity, we and some others have regarded these as a peculiar form of pyloric gland lesion in the past. , However, more analyses disclosed that they have several features clearly distinguishing them from other intracholecystic neoplasms and warranting their separate classification as ICTNs, akin to intraductal tubulopapillary neoplasms (ITPNs) of the pancreas and bile ducts.
Unlike ICPNs, these form pedunculated polyps with very thin stalks, and they often become detached from the mucosa and can be easily dismissed as “debris” during gross examination. We have seen several examples in which a revisit to the specimen container brought in the main diagnostic material.
Microscopically they are characterized by the presence of a distinctive lobulated, complex tubular pattern of small, back-to-back, round, or compressed glandular units with minimal lumen formation ( Fig. 39.21 ). The lobules are often covered by unremarkable gallbladder epithelium, and scattered cystically dilated glands with acidophilic granular secretions are often evident within the lobules. The glands are lined by cuboidal cells without overt intracytoplasmic mucin formation ( Fig. 39.22 ). Paneth cells and neuroendocrine cells may also be identified.


In some examples, the nuclei are more elongated and reveal overlapping features and chromatin clearing, which creates a pattern reminiscent of papillary thyroid carcinomas, which is not seen in other intracholecystic neoplasms ( Fig. 39.23 ). In addition, morules composed of squamoid to spindle cells in a vague whorled pattern, similar to the squamoid morules found in pancreatoblastoma, pulmonary blastoma, cribriform-morular thyroid carcinoma, or endometrioid adenocarcinoma, are seen in more than 66% of ICTNs. The nuclei in these morules often have biotin-rich, optically clear pseudoinclusions ( Fig. 39.24 ). These morules are not seen in other intracholecystic neoplasms other than rare pyloric gland adenoma–type lesions, which often show transition to ICTNs. This kinship to other morule-forming tumors, also known as biotin-rich, optically clear nuclei [BROCN]-forming tumors, , also involves expression of estrogen receptors and mutations in β-catenin. , Recently it has been reported that ICTNs appear to be driven by the Notch and Wnt/CTNNB1 signaling pathways, bearing mutations in APC2 and MLL2 (two known regulators of β-catenin signaling) and demonstrate aberrant nuclear CTNNB1 protein expression. ,


Another important distinguishing feature of ICTNs is the association with cholesterol polyps. The overall cauliflower-like architecture, which is consistent in every case, is something that is highly characteristic of a cholesterol polyp ( Fig. 39.25 ). , , , The fact that the lobules are typically outlined by normal epithelium, combined with the common presence of cholesterolosis in the stroma and the lack of significant injury in the remainder of the gallbladder , , (a common feature in patients with cholesterol polyps), makes this association even more impressive.

Although the level of cytoarchitectural atypia warrants a high-grade dysplasia classification, surprisingly ICTNs do not appear to be associated with invasive carcinoma (undoubtedly, there must be cases, but we have not yet seen an example). From the opposite perspective, we also have not identified this growth pattern in the background of invasive adenocarcinomas. It is also important to note that the dysplastic process is largely confined to the polypoid lesion and does not show multifocality. More importantly, unlike ICPNs, ICTNs do not seem to pose any field-effect risk for the remainder of the biliary tract.
Intracholecystic neoplasms arising in adenomyomatous nodules also appear to form a distinct category (see Table 39.1B ). They are typically discovered in elderly patients. Unlike other intracholecystic neoplasms, they form distinct mural nodules on the gallbladder wall in the fundic region, mostly preserving the surface mucosa. Microscopically, they are characterized by papillary proliferations lined by gastric/endocervical-like mucinous epithelium ( Figs. 39.26 and 39.27 ). Focal intestinal-type epithelium may also be seen (16%; Fig. 39.28 ). They predominantly reveal low-grade dysplasia (see Fig. 39.27 ), have a low incidence of associated invasive carcinoma (16%), and do not appear to bear risk for the remainder of the biliary tract. As such, they have some analogy to pancreatic branch duct–type IPMNs, which are more innocuous, typically of gastric phenotype, and progress to invasive carcinoma in only 15% of cases. In contrast, surface intracholecystic neoplasms (ICPNs and ITPNs) are like pancreatic main duct–type IPMNs, which tend to be much more complex, extensive, and multifocal, and show high (>50%) propensity for invasion and dissemination. ,



Hepatobiliary mucinous cystic neoplasms (with ovarian-type stroma) is another type of tumoral intraepithelial neoplasm that is worth mentioning. On careful scrutiny, the majority of mucinous cystic neoplasms identified in the gallbladder originate in the bile ducts and liver (discussed later). They secondarily involve the gallbladder. Convincing cases that originate in the gallbladder mucosa/wall independent of the large bile ducts of the liver are extremely rare. In a few cases, adenomyomatous nodules with hypercellular stroma have also been misconstrued as mucinous cystic neoplasms.
As in the pancreas and the bile ducts, there are microscopic forms of dysplasia that occur in the gallbladder, discovered either incidentally or adjacent to invasive carcinomas. Essentially these are the gallbladder counterparts of pancreatic intraepithelial neoplasia (PanIN) and biliary intraepithelial neoplasia (BilIN). In fact, BilIN, a term originally proposed for the bile ducts, is now used synonymously for the gallbladder as well, as depicted in the 2019 WHO classification. However, many authorities prefer the term dysplasia for the gallbladder, and this is considered acceptable as well.
These nontumoral (“flat”) forms of intraepithelial neoplasms (dysplasia) have many characteristics that distinguish them from the tumoral (mass-forming) intraepithelial neoplasms discussed previously. These will be presented in detail in the following sections. However, it should be noted that dysplasia often has a microscopic papillary configuration as well as zones of tubule formation, and it may not be distinguishable from the tumoral intraepithelial neoplasm if taken in isolation (on high-power examination). However, by definition, dysplasia does not form a grossly or radiographically recognizable mass lesion. ,
Patients with dysplasia are identified mainly in their 50s to 60s. Dysplasia cases without invasive carcinoma are diagnosed at a mean age of 57 years compared with 66 years for dysplasia cases with invasive carcinoma.
All risk factors that have been established for gallbladder adenocarcinoma (see later), including geography, gallstones, sclerosing cholangitis, and anomalous union of pancreatobiliary ducts, are considered risk factors for dysplasia as well.
Dysplastic lesions are normally detected incidentally in gallbladder specimens removed for other disorders because dysplasia is clinically unapparent. Low-grade dysplasia, which is subjective and difficult to define, is more likely to be detected when multiple sections of the gallbladder are examined, whereas high-grade dysplasia is often extensive at the time of diagnosis. The overall incidence of dysplasia varies greatly among different populations, but it parallels the incidence of adenocarcinoma. In one North American population, routine cholecystectomies for gallstones and morbid obesity contained low-grade dysplasia in less than 5% of cases, high-grade dysplasia in less than 1%, and frank carcinoma in less than 0.2%. The rate approaches 15% in high-risk regions such as Mexico and Chile. Dysplasia is associated with 40% to 60% of invasive gallbladder carcinomas. However, it is sometimes difficult to distinguish true dysplasia from colonization of the surface epithelium by invasive carcinoma cells. This makes analysis of dysplasia in invasive carcinoma cases more difficult.
By definition, gallbladder dysplasia is difficult to appreciate grossly. Some cases, especially those with a prominent papillary configuration, may reveal granularity or a feathery change to the mucosa, but most appear hyperemic or are entirely normal by macroscopic examination.
Microscopically, gallbladder dysplasia is graded based on the highest degree of cytoarchitectural atypia in the epithelium as either low-grade or high-grade . The terms high-grade dysplasia and carcinoma in situ (CIS) are interchangeable, but the former is recommended. The 2019 WHO classification also advocates use of the term high-grade dysplasia . Of note, cases are categorized as indefinite if the cytological features approach but do not quite reach those of definite dysplasia or if the epithelium shows atypical features suggesting dysplasia, but a definitive diagnosis cannot be established because of ulceration, inflammation, or technical reasons.
Low-grade dysplasia is characterized by columnar cells that show mild pseudostratification, nuclear enlargement, an increased nucleus-to-cytoplasm (N:C) ratio, and nuclear irregularity. In low-grade dysplasia, the nuclei are usually limited to the basal portion of the cell cytoplasm, and there is only a minimal amount of architectural distortion and loss of nuclear polarity. Mitoses are increased in number, but atypical mitoses are rare ( Fig. 39.29 ). Similar to the esophagus, if the surface epithelium lining the tips of the mucosal folds are not involved, then the diagnosis of dysplasia should be regarded dubious. High-grade dysplasia is characterized by cells with loss of polarity, marked nuclear enlargement, a markedly increased N:C ratio, significant nuclear irregularity, and hyperchromasia ( Fig. 39.30 ). Mitotic activity is brisk, but it should be kept in mind that mitotic activity also can be brisk in some cases of regeneration. High-grade dysplasia is most commonly characterized by nuclei that appear round, but some cases have a more elongated and pencillate appearance.


Because of a lack of prospective (outcome) studies, it is difficult to determine the biological and clinical significance of low-grade dysplasia in the gallbladder. However, it is clear that high-grade dysplasia is often associated with invasive carcinoma. Therefore thorough sampling is crucial in any case of high-grade dysplasia. Recurrences and metastasis seen in some patients with high-grade dysplasia are attributable to missed areas of invasion and/or a field-effect phenomenon. In fact, a case should not be classified as mere high-grade dysplasia only (noninvasive) unless total sampling is performed.
Gallbladder dysplasia has various growth patterns, and any one case may have multiple growth patterns ( Fig. 39.31 ). However, the diagnosis is established solely on the basis of cytological findings rather than architecture. The tubular pattern can be difficult to distinguish from invasive well-differentiated adenocarcinomas (see Fig. 39.31 ). The tall papillary pattern is seen in about 25% of cases (see Fig. 39.31 ). If the papillae form is a grossly visible, compact polypoid mass, the lesion is best classified as an intracholecystic neoplasm (discussed earlier).

In some cases, the glands reveal partially preserved nonneoplastic epithelium, indicating that the dysplasia represents pagetoid involvement of nonneoplastic glands by dysplastic cells ( Fig. 39.32 ). Unusual types of carcinoma, such as adenosquamous, squamous cell, and neuroendocrine, also can have intramucosal components.

Adsay and his colleagues reported that gallbladder dysplasia reveals different cell lineages, either alone or in combination ( Fig. 39.33 ). , Most cases are the biliary type, characterized by relatively monotonous-appearing, large, cuboidal-shaped cells with abundant cytoplasm; centrally or suprabasally located nuclei; cherry-red macronucleoli; and relatively fine chromatin. The cytoplasm may reveal clear cell (centrally located nuclei and nuclear contour irregularities), chromophobe-like (perinuclear cytoplasmic clearing), or oncocytoid (abundant acidophilic granular cytoplasm) features ( Fig. 39.33A ). The latter two often contain smoother nuclear contours. Some cases are the intestinal type, showing features similar to those of colonic adenomas, such as basophilia and pseudostratified, cigar-shaped nuclei ( Fig. 39.33B ). Gastric foveolar– type dysplasia reveals abundant, apical, pale cytoplasm and basally located, mildly enlarged nuclei with marked nuclear irregularity and hyperchromasia or prominent nucleoli ( Fig. 39.33C ). This is not to be confused with pyloric gland metaplasia that forms small antral-type (or Brunner-like) glandular units in the subsurface. Gastric foveolar–type dysplasia is characterized by an alteration in the surface epithelium. , The clinical significance of these cell types has not yet been determined, therefore it is not imperative to determine the type for diagnostic or clinical purposes. ,

The differential diagnosis of dysplasia and reactive changes is a well-known problem in gallbladder pathology ( Figs. 39.34 and 39.35 ; Table 39.3 ). Molecular studies to document abnormalities in cancer-associated genes (findings that could help establish the neoplastic nature of morphologically defined lesions) have been limited. Therefore dysplasia is defined and distinguished from other epithelial lesions based primarily on morphological principles, drawing in part from experience with early neoplastic changes in the pancreatobiliary tract.


| Feature | Reactive Changes | Low-Grade Dysplasia | High-Grade Dysplasia |
|---|---|---|---|
| Architectural growth pattern, such as tall papillary, micropapillary/tufting, cribriform arrangements | – | – | + |
| Nuclear chromasia | Hyperchromatic/hypochromatic | Hyperchromatic | Hyperchromatic |
| Nuclear enlargement | – | + | ++ |
| Very large, prominent “cherry-red” nucleoli | – | – | +/– |
| Surface maturation | + | – | – |
| Dislodged stone– related changes | + | +/– | +/– |
| Inflammatory cells | ++ | +/– | +/– |
| Stromal hemorrhage and edema | ++ | –/+ | –/+ |
| Goblet cells | –/+ | ++ | ++ |
| Mitotic figures | ++ | +/– | ++ |
| Loss of polarity | – | +/– | ++ |
The architectural growth pattern, such as a tall papillary configuration with cytological atypia, micropapillary or tufting pattern, cribriform arrangements, fused units, piled cells, and severe dispolarity of cells, is very helpful in diagnosing dysplasia (see Fig. 39.31 ). These patterns of growth are unusual in reactive lesions, although there are always exceptions. In the biliary type, cells typically lose their columnar shape and often acquire a monotonous appearance. Nuclear enlargement (two or three times normal) and prominent, cherry-red nucleoli are characteristic features of dysplasia, not seen in reactive lesions (see Fig. 39.33A ). Some cases are associated with abundant neutrophils, so inflammatory cells should not always be regarded as definite evidence of reactive changes ( Fig. 39.36 ). The gastric foveolar type of dysplasia is typically composed of a single layer of innocuous-appearing cells on the tips of the folds, with pale cytoplasm and eccentric nuclei. Some cases have foamy or microvesicular cytoplasm. Nuclei are hyperchromatic when compressed at the periphery, but they typically have a raisinoid appearance or prominent nucleoli when they are suprabasal. Mitotic figures, including atypical forms, can be prominent in areas of regeneration and are therefore not helpful in this differential diagnosis. Detached reactive cells can also exhibit signet ring cell–like features ( Fig. 39.37 ).


High-grade dysplasia shows a “wildfire” phenomenon in the gallbladder, which means it is typically extensive at the time it is detected and involving a significant proportion of epithelium. , , , However, focal epithelial atypia in a background of well-preserved nondysplastic epithelium is more likely to represent reactive changes. Moreover, reactive changes usually show surface maturation ( Fig. 39.38 ). Deeper aspects of the epithelium show atypical basophilic cells with an attenuated appearance, molding of slightly disorganized nuclei, and cells with a high N:C ratio, but the surface is usually mature.

Reactive changes in areas of ulceration can be even more challenging. In the setting of hemorrhage in adjacent stroma, epithelial atypia should be evaluated with caution. Atypical cells in these areas often have washed-out chromatin and nucleoli, but they are not as large as in high-grade dysplasia. Nuclear enlargement is also not as striking (see Fig. 39.35 ). Moreover, reactive changes tend to wax and wane throughout the epithelium, gradually changing from areas with marked atypia to areas without atypia.
In cases of hyalinizing cholecystitis with minimal or no calcifications (i.e., incomplete porcelain gallbladder in which the entire gallbladder wall transforms into a thin, uniform, paucicellular, fibrotic band with distinctive clefting), any type of “clinging” epithelium on the surface should be regarded as highly suspicious for dysplasia. However, in well-preserved (nonhyalinized) gallbladders, the pathologist should refrain from rendering a diagnosis of dysplasia in detached epithelial cells because they often acquire degenerative changes that resemble dysplasia.
In gallbladders with invasive carcinoma, secondary involvement of the epithelium, referred to as cancerization or colonization, may be difficult or even impossible to distinguish from true dysplasia. If the process has direct continuity with and morphological similarity to an underlying invasive carcinoma, it is best regarded as cancerization rather than dysplasia. Abrupt transition from normal epithelium to atypical cells also favors cancerization ( Fig. 39.39 ).

For early gallbladder carcinomas (i.e., pTis, pT1a with lamina propria invasion, and pT1b with tunica muscularis invasion), it may be difficult to distinguish “in situ” changes from true early invasive carcinomas, if the dysplastic changes occur in areas of the gallbladder with peribiliary mucous glands (i.e., neck and cystic duct region), in areas with pyloric gland metaplasia (i.e., common in injured gallbladders and seen in up to 60% of cholecystectomy specimens), or in areas with Rokitansky-Aschoff sinuses. The gallbladder epithelium normally shows undulations, and there is no muscularis mucosa to separate the mucosa from the submucosa. More importantly, the tunica muscularis (there is no muscularis propria in the gallbladder) is highly irregular and porous in normal gallbladders. Dysplastic glands can often be seen lying within or deep to the tunica muscularis ( Fig. 39.40 ). There are no basal or myoepithelial cells that can help distinguish native epithelium from invasive carcinoma. Nevertheless, features that favor true invasive carcinoma include invasion of nerves or blood vessels, a monotonous population of atypical glands (rather than a mixture of neoplastic and benign or metaplastic glands), a haphazard infiltrative pattern, variable size and shape of the glands, angulated contours, lack of luminal bile, and lack of a connection to benign epithelium at the surface or adjacent submucosal glands (see Figs. 39.42 to 39.48 ). Fortunately, this distinction may not be clinically significant because data from high-risk regions have concluded that cases classified as early gallbladder cancer (i.e., pTis, pT1a, and pT1b) have a very good long-term survival rate, much more favorable than those with advanced carcinomas.








It is far more important to exclude an advanced carcinoma than to distinguish high-grade dysplasia from early gallbladder carcinoma. In fact, a case should not be classified as pTis, pT1a, or pT1b unless complete sampling of the gallbladder is performed. The reported differences in survival rates of pT1 cases in Western populations compared with high-risk regions, such as Chile and Southeast Asia, may be caused by undersampling in the West, resulting in downstaging of pT2 carcinomas. For cases of early gallbladder carcinoma identified by a thorough sampling of the gallbladder with the protocol established by Roa and colleagues, in which advanced carcinomas are definitely ruled out, the 10-year survival rate approaches 90%. ,
Immunohistochemically, mCEA and MUC1 typically show a linear pattern on staining at the apical border of dysplastic cells. In high-grade dysplasia, intercellular membranes may also be accentuated by CEA; however, intracytoplasmic labeling with mCEA and MUC1 is uncommon. Therefore dense intracytoplasmic staining favors cancerization over dysplasia. TP53 nuclear staining occurs in more than 30% of cases of dysplasia and tends to be more common (and stronger) in high-grade lesions. However, it should be kept in mind that it can also be seen in areas of regenerative changes. Similarly, although the Ki67 labeling index is high in cases of dysplasia and increases by grade, it can also be high in areas of regenerative changes.
Analysis of normal and dysplastic gallbladder epithelium has shown that allelic losses of chromosome 8p occur in normal mucosa. In low-grade dysplasia, allelic loss of chromosome 3p is common and regarded as an intermediate change, which also corresponds to progressive loss of fragile histidine triad (FHIT) protein in dysplastic lesions of increasing grade. Loss of heterozygosity of 5q has been found in various grades of dysplasia. Oncogenic mutations of the KRAS gene is uncommon in upper biliary (gallbladder and proximal bile duct) dysplasia. , Telomere shortening is very common in dysplasia, similar to invasive carcinomas, and it is typically absent in normal epithelium. However, metaplastic changes also commonly show telomere shortening.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here