Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors thank Dr. Christiane Gruner for her contribution to the previous edition of this chapter.
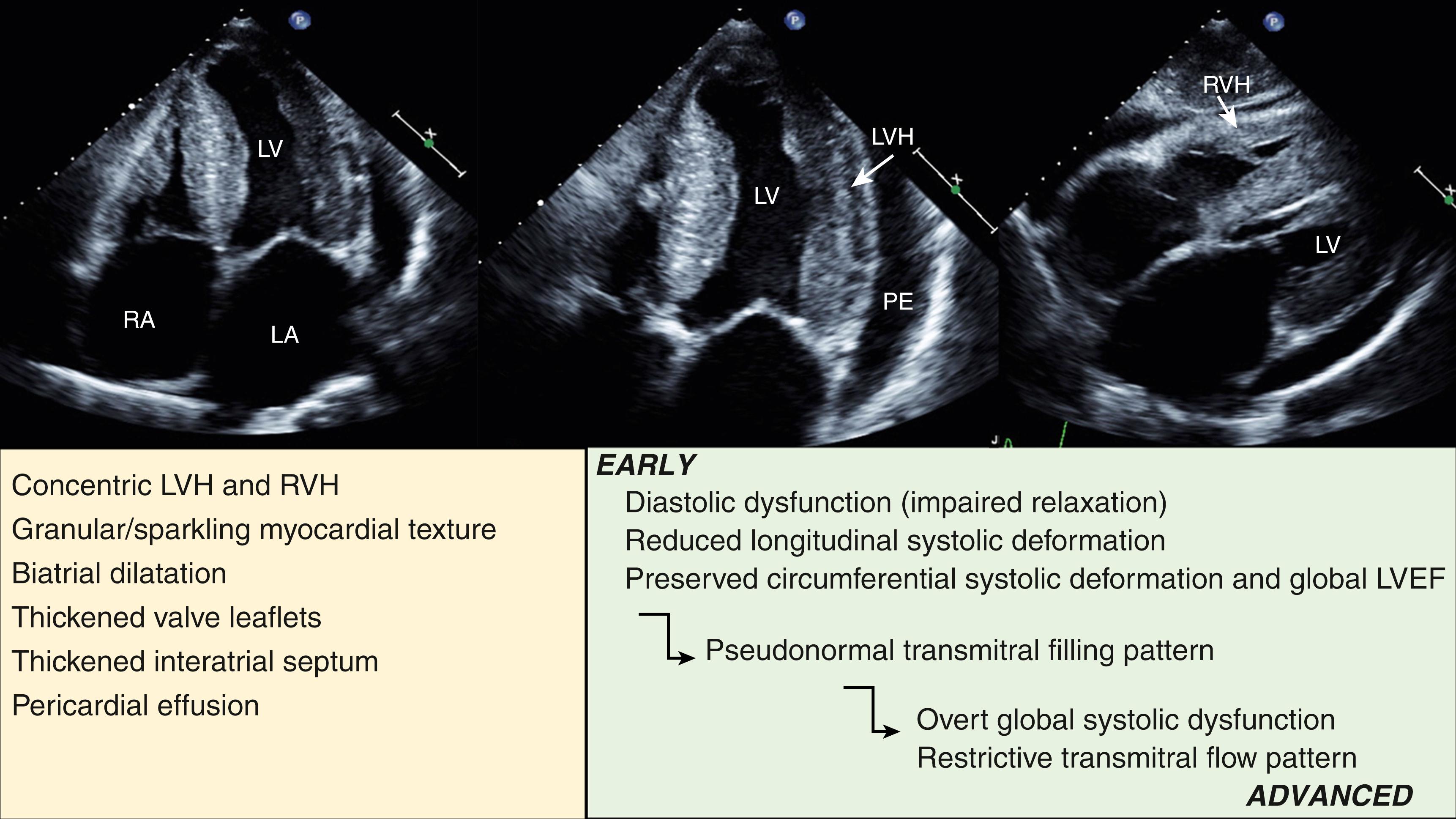
Left ventricular (LV) hypertrophy (LVH) can represent either a physiologic or pathologic cardiac process that can be secondary to abnormal loading conditions or a variety of cardiac and systemic diseases ( Fig. 60.1 ). Echocardiography is not only the most commonly used method but also the most feasible method to visualize and quantify LVH in clinical practice. Although hypertrophic cardiomyopathy (HCM) is the most prevalent genetic primary cardiac disease that occurs in 0.2% of the general population, several other conditions can mimic the phenotypic appearance of HCM and present with increased myocardial thickness in echocardiography. Correct identification of the underlying cause of increased myocardial thickness is of critical importance in order to distinguish between HCM, benign physiological conditions, and cardiac or systemic disorders. HCM is a genetic disease that can cause premature sudden cardiac death (SCD) and can lead to considerable morbidity. It requires proper treatment and SCD risk stratification, as well as family screening. Moreover, some mimics of HCM have specific treatments with prognostic implication. The purpose of this chapter is to highlight echocardiographic features that may help to distinguish HCM from other causes of LVH. Emphasis is on the multidisciplinary and multimodality approach required for the accurate diagnosis of the underlying condition causing the LVH (see Fig. 60.1 ).
Arterial hypertension represents a common and important health problem, with an estimated prevalence of 28% in North America and 44% in Europe. With the prevalence of HCM being 1 in 500, co-existence of both disorders is common, making the diagnosis in this setting more challenging. Long-standing hypertension causes LV pressure overload and an increase in wall stress, which often results in compensatory hypertrophy. Early in the disease process, a basal septal bulge is often prominent because of the inhomogeneity of the regional LV wall stress, which is greatest in the basal septum. With time, the typical concentric pattern of hypertrophy becomes more evident. An absolute wall thickness of 15 mm or greater in any myocardial segment, which is diagnostic for HCM, can also be found in hypertensive heart disease (HHD). Therefore, it is important to also consider using septal/posterior wall thickness ratio for an accurate diagnosis. Although a septal/posterior wall thickness ratio greater than 1.3:1 is supportive of HCM diagnosis in nonhypertensive patients, a ratio greater than 1.5:1 is generally applied for patients with a history of systemic hypertension. However, these data were generated from a predominantly white population and should be used with caution in other populations, especially Afro-Caribbean patients who usually display a greater propensity for LVH in response to hypertension. Systolic anterior motion (SAM) of the mitral valve, which is associated with left ventricular outflow tract (LVOT) obstruction, is not pathognomonic for HCM and can also be found in patients with HHD and other conditions that cause LVH.
Advanced echocardiographic techniques, such as tissue Doppler imaging and speckle-tracking echocardiography (STE), are potentially helpful in distinguishing HHD from HCM. Global LV systolic function remains preserved in most cases of HCM and HHD, but using STE reveals changes between the two. A recent study demonstrated that although both HCM and HHD patients show decreased LV longitudinal strain compared with normal control participants, patients with HCM show lower values than those with HHD. Additionally, LV radial strain was increased in patients with HCM compared with patients with HHS and control participants. Another study demonstrated changes in strain patterns of the right ventricle (RV); although HHS patients had normal RV longitudinal strain, in patients with HCM, RV longitudinal strain was significantly impaired.
The accurate diagnosis of HCM in individuals participating in competitive sports is challenging yet crucial because of the potential risk of patients with HCM for SCD. HCM has been identified as the predominant cause of SCD in young athletes. To date, the largest study showed that fewer than 2% of elite athletes had a maximal wall thickness greater than 12 mm in the interventricular septum. Racial differences exist in the propensity to develop hypertrophy in response to exercise, and as many as 12% of Afro-Caribbean athletes demonstrate LV wall thickness greater than 12 mm. However, the upper limit of physiological hypertrophy in athletes of all ethnicities appears to be an absolute wall thickness of 16 mm. , Asymmetric hypertrophy is encountered rarely in athletes, with the typical distribution of LVH being concentric. Chamber dilatation found in athletes, in contrast to the normal or reduced cavity size seen in HCM, is another clue to the underlying cause. In athletes, LV end-diastolic cavity dimensions frequently exceed 55 mm, and in up to 5% of cases, these dimensions may exceed 60 mm. A practical algorithm to allow better differentiation between physiologic and pathologic hypertrophy is provided in Fig. 60.2 .
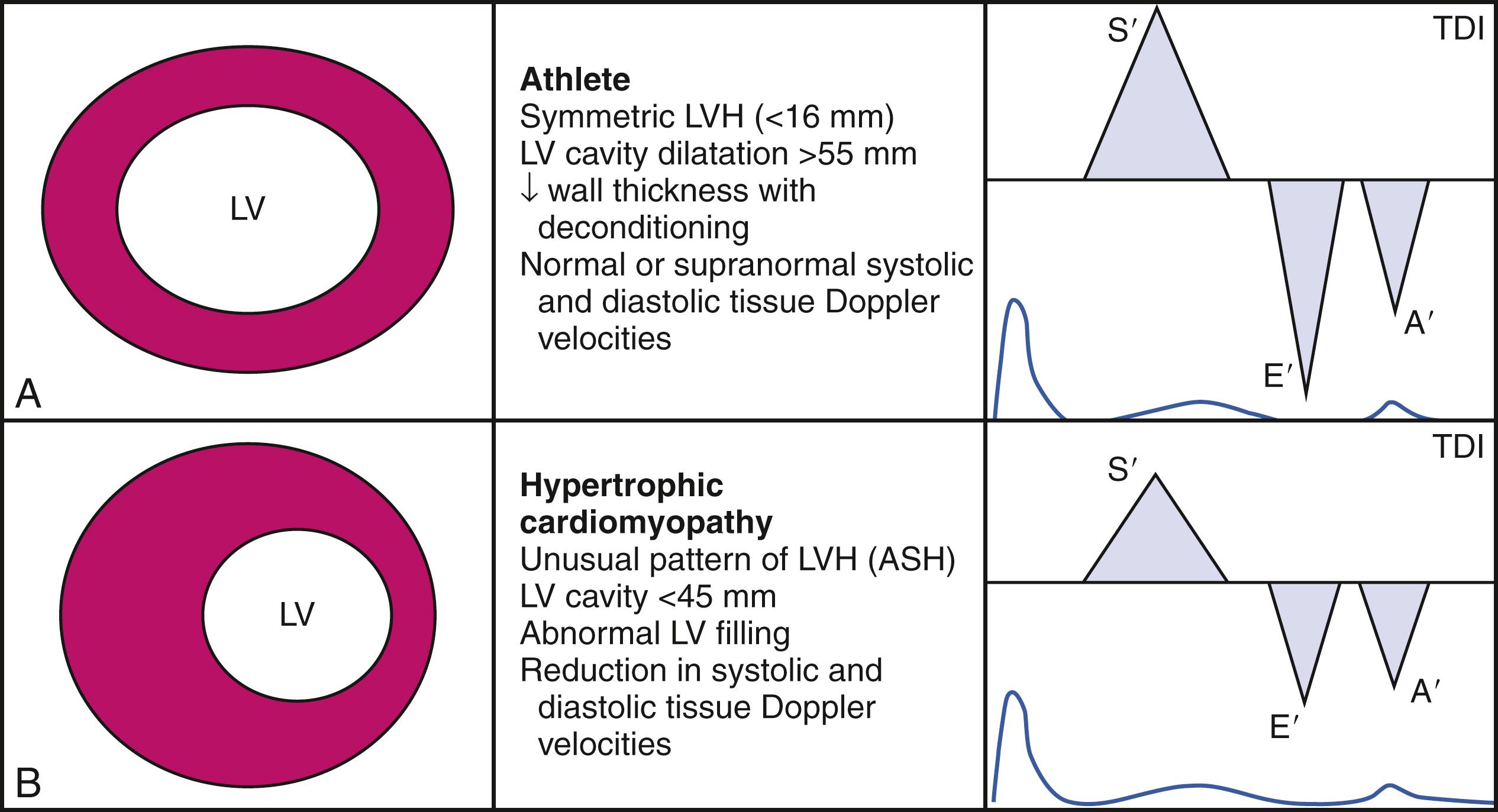
Accumulating evidence suggests that advanced echocardiographic techniques may play a contributory role in the differentiation between athlete’s heart and HCM. Peak LV longitudinal strain and strain rate are typically normal or high-normal in athletes with physiologic hypertrophy in contrast to HCM ( Fig. 60.3 ), in which longitudinal systolic strain (both regional and global) are impaired. Moreover, diastolic function is preserved and often enhanced in athletes’ hearts, demonstrated by normal diastolic tissue Doppler velocities and an increase in twist and untwist rates. , In contrast, the pathologic hypertrophy of HCM is associated with marked attenuation in diastolic tissue Doppler velocities and delayed untwist. Another difference lies in the left atrium; although mild left atrial (LA) dilatation can be seen in athletes’ hearts, LA function is typically preserved when measured by STE. In comparison, patients with HCM not only display an increase in LA volumes but also have a significant impairment in LA myocardial deformation, with reductions in both strain and strain rates.
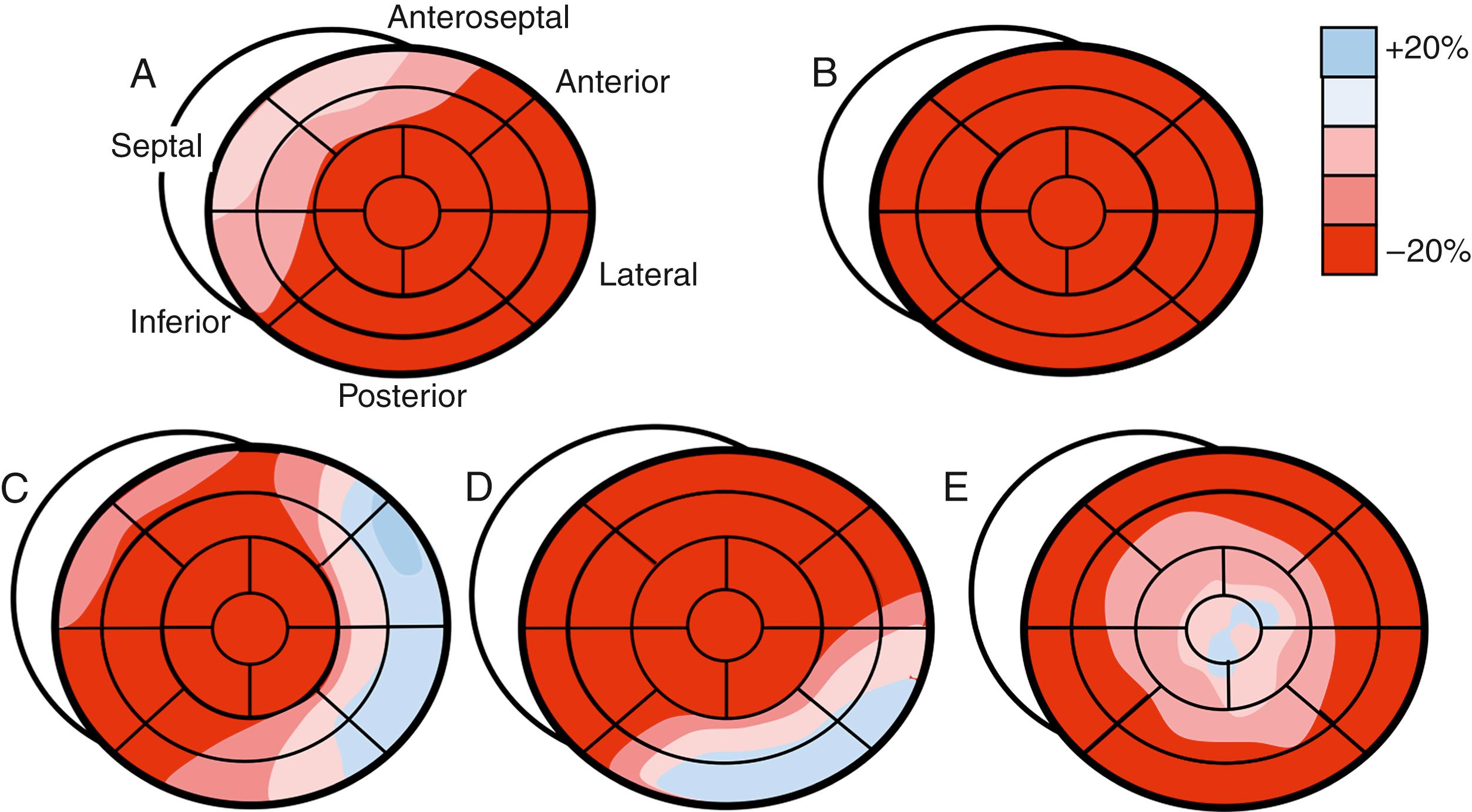
Amyloidosis is a systemic disease characterized by the deposition of extracellular interstitial misfolded proteins in various organs, including the heart. Cardiac involvement is common in hereditary transthyretin amyloidosis (ATTRm), wild-type transthyretin amyloidosis (ATTRwt), and AL (light-chain) amyloidosis. Amyloid can infiltrate the ventricles, atria, and interventricular septum and damage the ventricular function, atrial function, and valvular function as well as the conduction system. Additionally, in AL amyloidosis, light chain toxicity can contribute to cardiac dysfunction. The typical echocardiographic features of cardiac amyloid are concentric hypertrophy, diastolic dysfunction, and LA enlargement ( Fig. 60.4 ). Thickening of the RV wall and interatrial septum are also common, as well as LV systolic dysfunction, which is more prevalent in advanced cardiac amyloidosis (CA) than in HCM. Electrocardiography plays a complementary role and shows the characteristic feature of a low QRS voltage in up to 50% of patients. Although LV global systolic function is typically preserved early in the disease process, systolic longitudinal strain and strain rates are typically severely reduced in a homogenous pattern, in contrast with HCM, in which the reduction in strain may be inhomogeneous and most marked in the hypertrophied segments. A distinctive pattern of regional variations in myocardial dysfunction has been described in CA, with relative sparing of the apical segments represented by better strain values. This finding could aid in the differentiation of CA from other causes of LVH (see Fig. 60.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here