Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The use of probiotics to promote a healthy state has been widely studied.
Oral probiotics have been shown to aid in the prevention of atopic dermatitis in select populations.
Oral probiotics may also help in the treatment of atopic dermatitis, though data to support this are less clear.
Details on specific probiotic strain, dose, and duration of treatment for efficacy are lacking.
Multiple topical modalities to alter the skin microbiome are being explored, many with promising results.
As disruption of the healthy microbiome, referred to as dysbiosis, has been recognized as a risk factor for the development and exacerbation of atopic dermatitis (AD), treatments aimed at restoring balance in the microbiome have been explored. Over the past few decades, numerous studies and subsequent reviews and meta-analyses have been conducted, investigating the use of oral probiotics for both treatment and prevention of AD. Though results are mixed, and study methodologies varied, certain populations appear to benefit from their use. Probiotics are considered generally safe, though they should be used with caution in select populations. More recently, topical preparations have been explored in small studies with varying results.
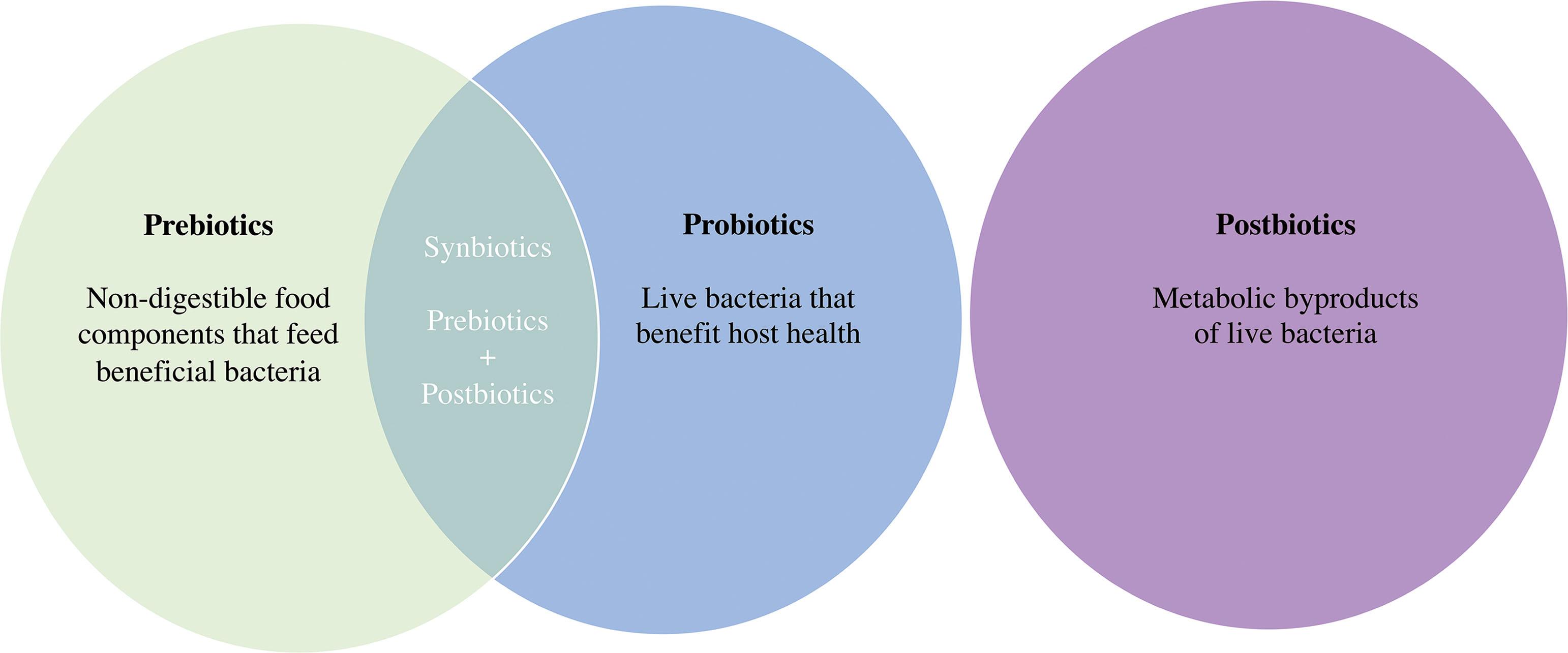
As defined by the International Scientific Association for Probiotics and Prebiotics, probiotics refers to “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” ( ), while a prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” ( ). In short, probiotics are active bacterial cultures, and prebiotics are what feed them. Synbiotics are a combination of probiotics and prebiotics. The term postbiotics refers to metabolic byproducts of live bacteria (either secreted or released through lysis) that, like probiotics, provide a health benefit to the host ( ) ( Fig. 24.1 ).
Gut commensals in the microbiome aid human health through a variety of mechanisms, including competitive inhibition of pathogenic organisms, protection of the gut epithelial barrier, assisting in nutrient metabolism, and immunomodulation ( ). For example, intake of macronutrients such as complex carbohydrates are metabolized by gut bacteria through fermentation into end products used by humans for energy, such as short-chain fatty acids (SCFAs) ( ). To be effective, bacterial strains used in oral probiotics should be able to survive passage through the acidic environment and digestive enzymes of the upper gastrointestinal (GI) tract, adhere to intestinal epithelial cells, colonize the lower GI tract, and be safe for use (i.e., nonvirulent, nontoxin producing) ( ).
Dosage of probiotics is measured in colony-forming units (CFU), which indicate the number of live or viable organisms per serving (i.e., capsule or gram). Probiotic dosage typically ranges from 1 to 10 billion CFU, and in guidelines from the World Gastroenterology Organisation (WGO), due to variation in dosage and strains used in studies, there is no one recommended dose; instead, dosage should be based on the studies of the disorder treated ( ). Though probiotics and prebiotics are readily available as supplements in drugstores and supermarkets, several common foods contain live microbes and prebiotics as well ( Table 24.1 ).
| Foods Contaning Live Microbes ( ) |
| Dairy Products |
| Yogurt |
| Frozen yogurt |
| Kefir |
| Cultured buttermilk |
| Select cheeses |
| Fermented Vegetables |
| Sauerkraut |
| Olives |
| Pickles |
| Kimchi |
| Other Fermented Products |
| Miso |
| Tempeh (fermented soy beans) |
| Fish sauce |
| Fermented meats (i.e., sausage, salami) |
| Fermented tea (kombucha) |
| Select beer (i.e., sour beer) |
| Select cereals (i.e., fermented porridge) |
| Foods Containing Prebiotics ( ) |
| Asparagus |
| Garlic |
| Leeks |
| Onions |
| Bananas |
| Jerusalem artichoke |
| Chicory root |
| Wheat (bran, flour) |
| Barley |
Dysbiosis has been purported to play a role in the development and exacerbation of AD, and as such, probiotic use has been explored in both the prevention and treatment of the disease. The exact mechanism of action of probiotics is unclear, though likely through influencing both the microbiome and the immune system ( Fig. 24.2 ). Like commensals, probiotics aid in barrier function, compete for nutrients or displace pathogenic organisms, produce AMPs, and stimulate the immune system to target pathogenic organisms ( ). Lactobacillus , a genus of commensal bacteria in the phylum Firmicutes (a predominant gut phylum), is commonly used in probiotics. Studies have shown a range of benefits by different species within Lactobacillus . For example, Lactobacillus rhamnosus GG can protect the gut barrier by minimizing mucosal lining permeability and protecting against oxidative stress-induced damage to tight junctions, the intercellular connections that prevent the passage of molecules ( ). Another Lactobacillus species, Lactobacillus reuteri , produces reuterin, an antimicrobial compound with broad coverage against gram-positive bacteria, gram-negative bacteria, fungi, yeasts, and protozoa ( ).

Both the innate and adaptive immune systems are influenced by probiotic administration. Depending on the strain, this is accomplished through different mechanisms. L. reuteri and Lactobacillus casei , for example, induce increased inhibitory regulatory T-cell (Treg) differentiation after detection by tolerogenic dendritic cells, while Lactobacillus acidophilus , Lactobacillus bulgaricus , and Bifidobacterium bifidum increase protective immunoglobulin G (IgG) and IgA antibodies ( ). Regardless of species and strain differences, probiotics can inhibit the differentiation of naïve T cells to T helper type 2 (Th2) cells and stimulate the production of antiinflammatory cytokines such as interferon-gamma and interleukin-10 (IL10) ( ). Prebiotics also play a role by increasing production of SCFAs, byproducts of fermentation by gut commensals, which have antiinflammatory effects and decrease pH, creating a more favorable environment for commensal over pathogenic bacteria and promoting gut homeostasis ( ).
Numerous studies, systematic reviews, and meta-analyses on the use of probiotics for the treatment of AD have been published, with conflicting results and conclusions. The most recent Cochrane review published by analyzed 39 randomized clinical trials (RCT) on the use of probiotics (specifically Lactobacillus and Bifidobacteria ) in 2599 participants with at least mild AD of all ages. Key findings from the review were that the use of probiotics as treatment did not help with AD symptoms or quality of life, concluding that the “use of probiotics for the treatment of eczema is currently not evidence-based” ( ). This review was an update to the 2008 Cochrane review of 12 RCTs with 781 participants (pediatric patients only), which also found lack of benefit with probiotic use as treatment for AD ( ).
A meta-analysis specifically investigating improvements in the scoring of AD (SCORAD) measure following probiotic therapy was conducted by and identified 25 RCTs with 1599 participants. Results from this analysis conflict with the Cochrane reviews and showed a benefit in the use of probiotics in children at least 1 year of age (–5.74-point difference in SCORAD compared to controls) and adults (–9.69-point difference in SCORAD compared to controls), though this benefit was not seen in infants less than 1 year old. Differences in benefit were influenced by type of probiotic and severity of AD, with greater benefits seen in those supplemented with Lactobacillus or a mixture of bacteria rather than Bifidobacterium alone ( ). In subgroup analysis, this benefit from probiotics was limited to those with moderate to severe AD rather than those with mild disease ( ). However, some studies included in the meta-analysis allowed patients to continue using topical emollients and medications in addition to probiotics, making these results challenging to interpret. Similar results were seen in a more recent meta-analysis focused on infantile AD, which identified a significant benefit of probiotic supplementation (specifically with Lactobacillus ) in children ages 1 to 18 years with moderate to severe AD ( ).
The benefits of probiotics also vary dependent upon the population studied. A meta-analysis conducted by found that only certain strains of Lactobacillus improved SCORAD in children, showing that Lactobacillus fermentum and Lactobacillus salivarius (as well as probiotics of varying combinations of Lactobacillus and Bifidobacterium strains) led to significant improvement, while single strain treatment with L. rhamnosus GG or L. plantarum did not. Differences in benefit were also age dependent, and even geography dependent, with significant improvements in SCORAD only seen in children age greater than 1 year and in those in Asia, though not in Europe ( ). This suggests that there may be different phenotypes of AD that respond more favorably to probiotics than others.
Given the potential role the gut microbiome plays in the development of AD, multiple studies have investigated the use of probiotics in the prevention of disease. Conclusions of meta-analyses have largely agreed that, within certain parameters, there is evidence to support the use of probiotics in a preventative role. A large systematic review and meta-analysis by analyzed 28 studies that included 6907 infants and children exposed to probiotics prenatally and/or after birth prior to diagnosis of AD. In this study, significant reduction in the incidence of AD was found when probiotics were given both to the mother in utero and to the infant postnatally, but not if only one or the other. This benefit was only seen when treatment occurred through the age of 6 months, with no decreased risk in the development of AD in those treated for more than 1 year. Contrary to studies on treatment of AD, this benefit of disease prevention was seen across strains, including Bifidobacterium, Propionibacterium , and Lactobacillus strains, with certain Lactobacillus strains ( L. rhamnosus and Lactobacillus paracasei ) showing greater efficacy ( ). This benefit continued to be sustained on long-term, at least 5-year, follow-up ( ). Multiple other systematic reviews and meta-analyses have also concluded that early probiotic use decreases risk of AD development ( ).
The use of prebiotics, given as oligosaccharides (i.e., fructo- and galactooligosaccharide) for prevention of AD has also been evaluated. A Cochrane review published in 2013 by Osborn and Sinn investigated the role of prebiotics in infants for the prevention of allergic disease. A significant risk reduction was observed in the development of AD (four studies, 1218 infants), though not in the development of allergy or asthma ( ). A strength of this review is that there was no significant heterogeneity among AD studies. Contradictory to these findings, a meta-analysis published that same year included subgroup analysis of three RCTs investigating prebiotic use and found no benefit in the prevention of AD, though heterogeneity among the included studies was moderate (I 2 of 48%) ( ).
The use of synbiotics for both the prevention and treatment of AD has also been investigated. In a meta-analysis published in 2016, Chang et al. included six studies on the treatment and two studies on the prevention of AD in children and found that use of synbiotics were shown to help in the treatment but not the prevention of AD. In this meta-analysis, significant improvements in SCORAD were seen in those ages 1 to 18 years treated for 8 weeks, and when treated with mixed bacterial strains rather than a single strain. Synbiotics were associated with lower incidence of AD in the two individual studies used in the meta-analysis; however, when combined, the pooled relative risk was not significant ( ). Theoretically, synbiotics should perform as well as or better than either separate component; however, to date, there are no studies specifically comparing probiotics to synbiotics in the prevention and treatment of AD, and comparisons of separate meta-analyses is challenging in the setting of study heterogeneity ( ).
Translating current meta-analyses into evidence-based recommendations is challenging given the heterogeneity among studies, particularly in strains used, doses used, timing and duration of supplementation, concomitant treatments, and outcome assessment. Though recognizing the low quality of evidence, the World Allergy Organization (WAO) currently recommends considering supplementation in the following scenarios:
a.“using probiotics in pregnant women at high risk for having an allergic child;
b.using probiotics in women who breastfeed infants at high risk of developing allergy; and
c.using probiotics in infants at high risk of developing allergy” ( ).
The WAO also recommends use of prebiotics specifically in “not-exclusively breastfed infants” ( ), though no specific strains or regimens are provided. A follow-up meta-analysis to these recommendations, specifically evaluating use of L. rhamnosus GG (LGG) in the abovementioned three groups, found that LGG supplementation did not reduce the risk of AD ( ). In their guidelines on the management of AD, the American Academy of Dermatology (AAD) acknowledges the current interest in the study of probiotics but does not currently recommend the use of probiotics or prebiotics for treatment of established AD based on the available evidence ( ). Other expert groups have not endorsed the use of probiotics, prebiotics, or synbiotics for the prevention of allergic disease, including the American Academy of Pediatrics, National Institute of Allergy and Infectious Diseases, European Academy of Allergy and Clinical Immunology, European Society of Paediatric Gastroenterology, Hepatology, and Nutrition, and the Food and Agriculture Organization of the United Nations/World Health Organization ( ).
Though oral probiotics have been studied in other cutaneous diseases as well, such as psoriasis ( ) and acne ( ), many questions remain regarding their mechanism of action. In addition to unanswered questions already discussed, there are questions regarding how long probiotics need to stay in the gut to work, how many CFU are needed to make a compositional change, and whether a transient encounter in the gut is enough to change the immune milieu or behavior of the microbial community.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here