Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The formation of the spine occurs from the second to sixth weeks of gestation through:
Gastrulation (weeks 2–3): conversion of a bilaminar to trilaminar layer with the middle layer of the mesoderm.
Primary neurulation (weeks 3–4): the notochord interacts with the overlying ectoderm to form the neural plate ( Fig. 16.1A ). The neural plate then bends to begin formation of the neural tube ( Fig. 16.1B ). Continued infolding of neural plate ( Fig. 16.1C ). Disjunction is the separation of the neural tube from the ectoderm ( Fig. 16.1D ).
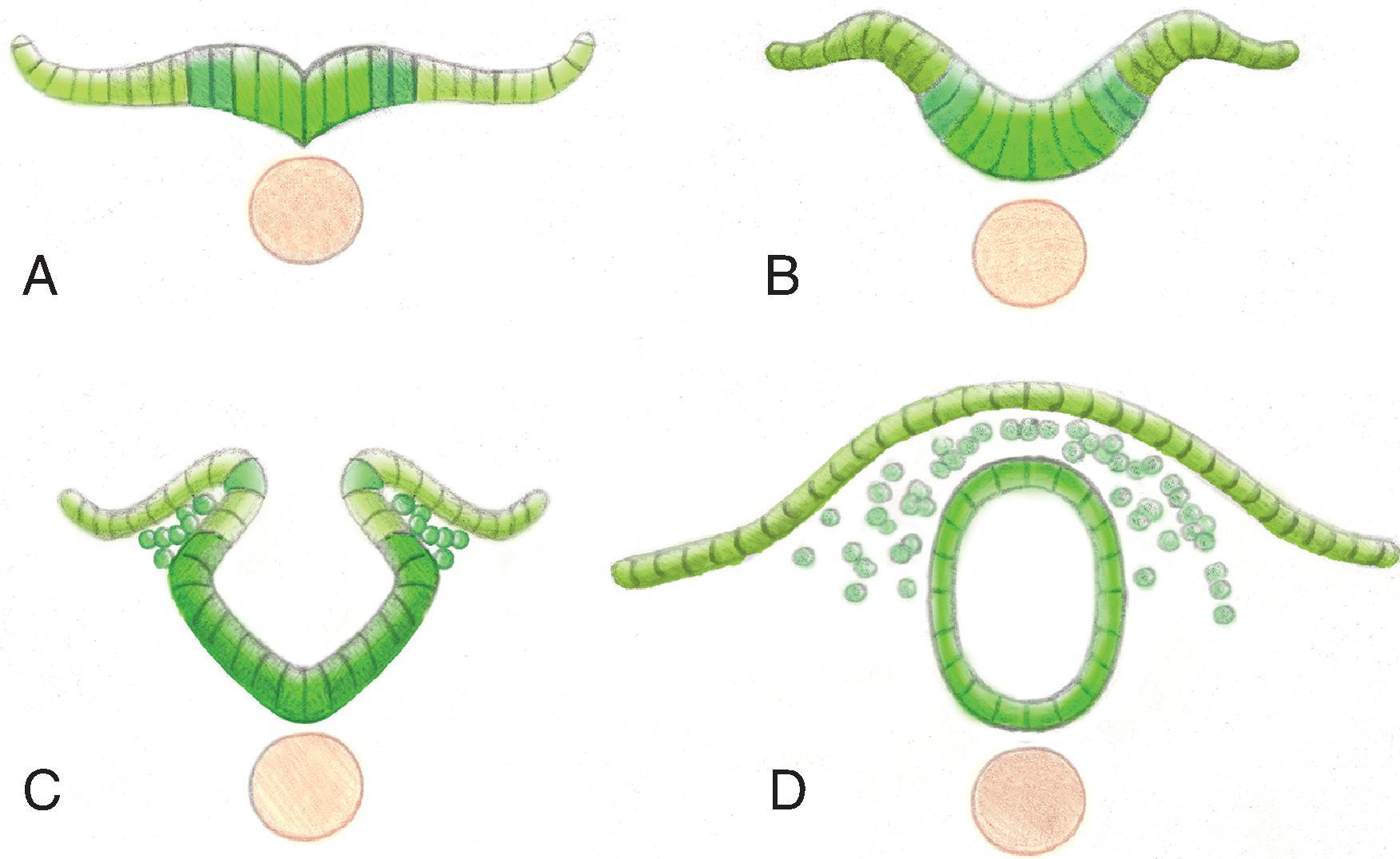
Secondary neurulation and retrogressive differentiation.
Spinal dysraphisms occur when these processes are disrupted.
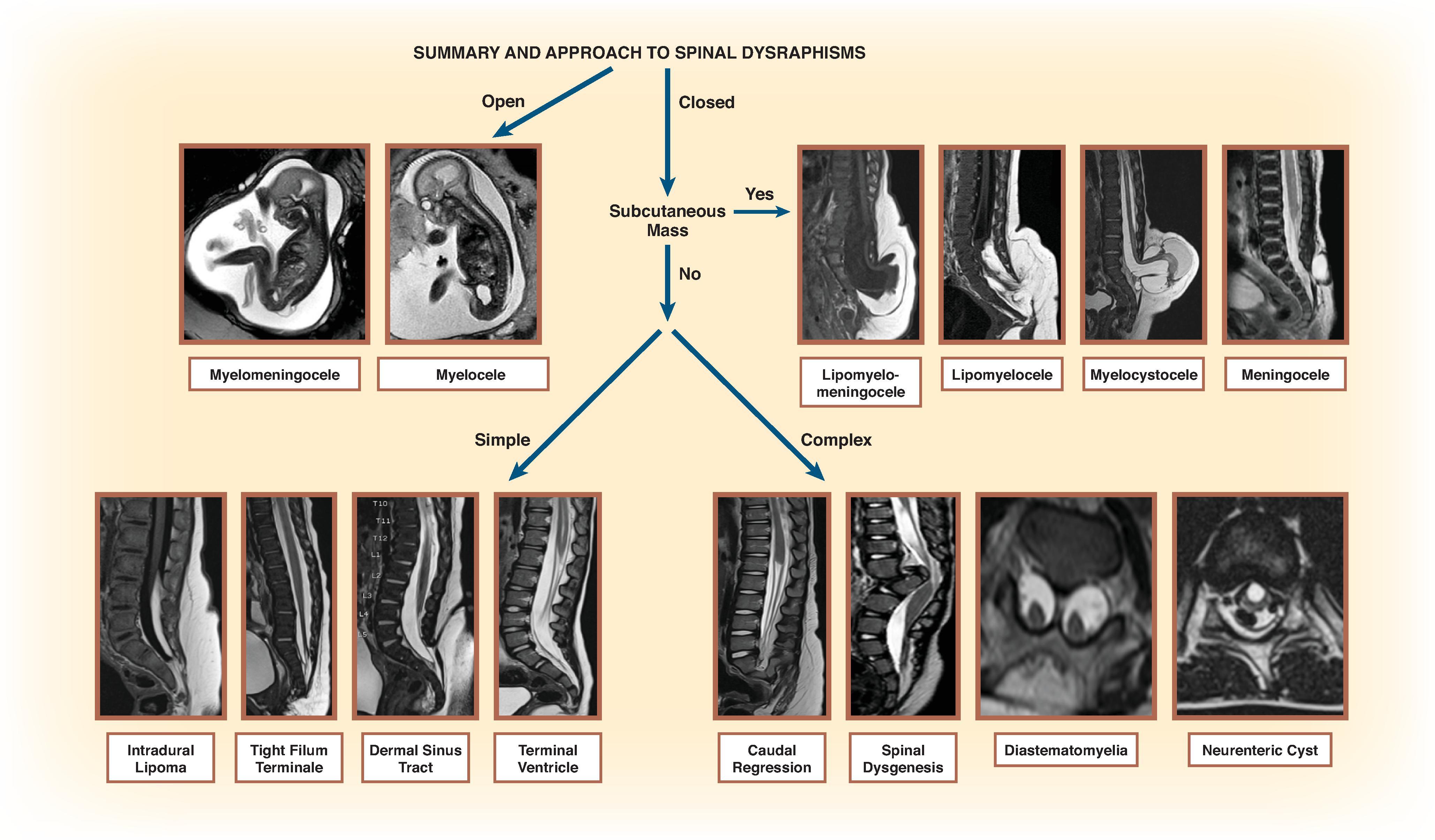
Spinal dysraphisms can be classified by embryologic anomaly ( Box 16.1 ). This classification is important; however, an imaging algorithm as follows is more clinically useful ( Fig. 16.2 ):
Determine whether the malformation is exposed to the skin to determine whether the malformation is a closed or open defect.
Determine whether there is a subcutaneous mass or not.
Determine the necessary imaging features for final diagnosis.
Midline notochord integration disorder:
Dorsal enteric fistula
Neurenteric cyst
Diastematomyelia
Dermal sinus tract
Notochord formation disorder:
Caudal regression
Segmental spinal dysgenesis
Myelomeningocele
Myelocele
Lipomyelomeningocele
Lipomyelocele
Intradural lipoma
Nonterminal myelocystocele
Filar lipoma
Tight filum terminale
Terminal ventricle
Terminal myelocystocele
Meningocele
This section will demonstrate the many types of spinal malformations, associated findings, and other genetic disorders that are associated with spinal malformations.
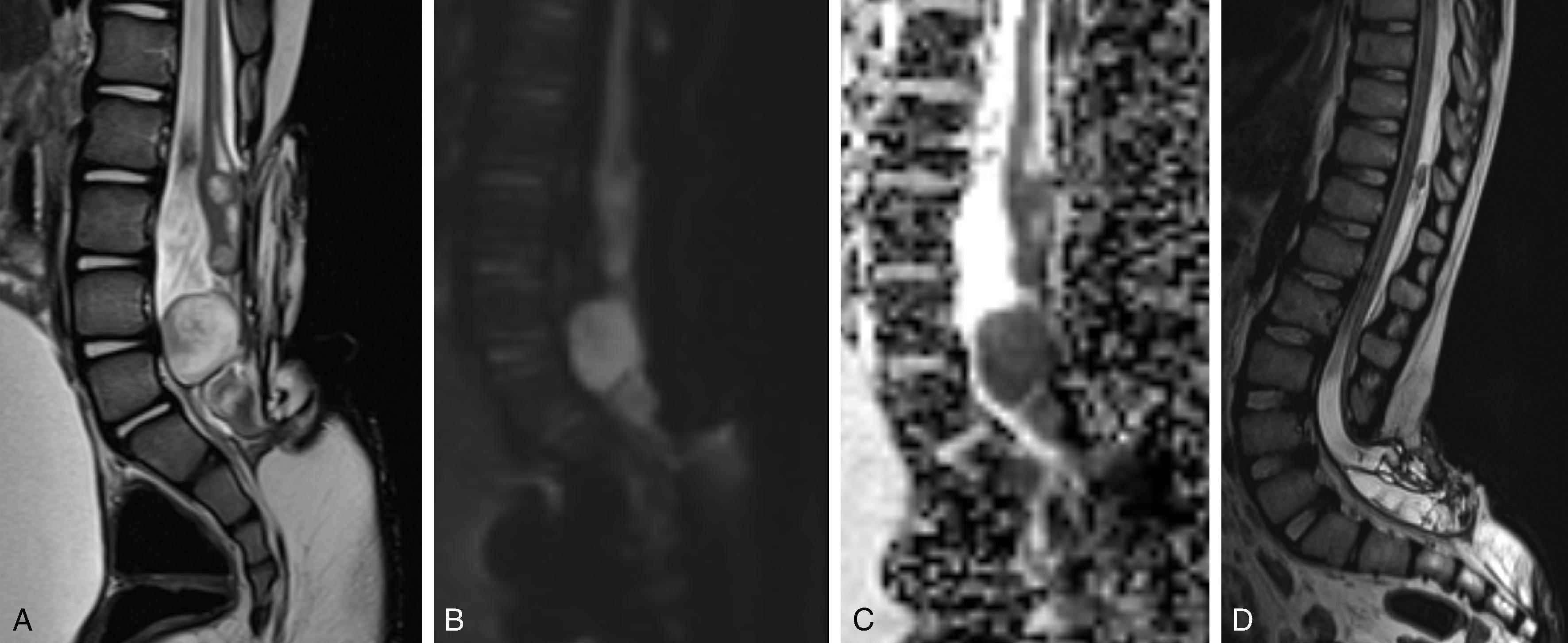
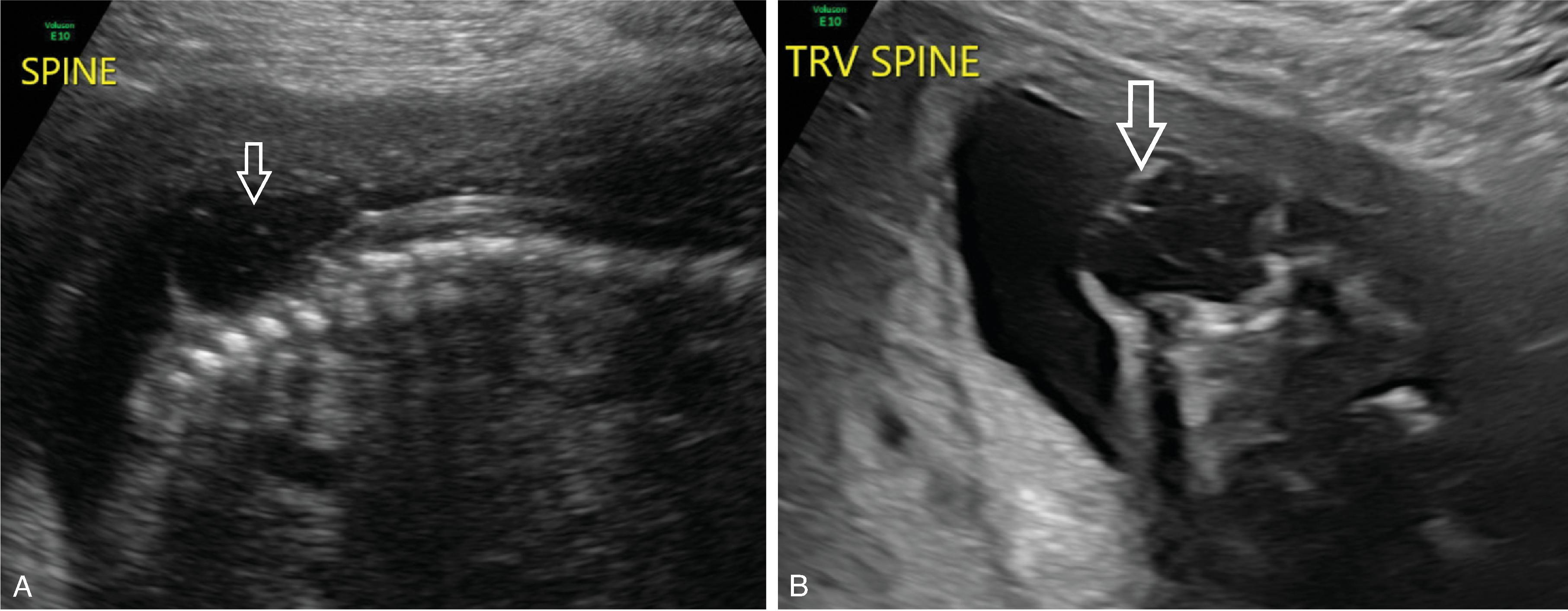
Open spinal dysraphism and primary neurulation disorder due to failure of closure of the neural tube and nondisjunction.
Neurological disabilities: paraplegia, incontinence, sexual dysfunction, skeletal deformities, pulmonary hypoplasia, breech position.
The presumed etiology is multifactorial. Folate administration during pregnancy reduces incidence.
Elevated amniotic fluid alpha-fetoprotein and acetylcholinesterase.
Fetal/intrauterine surgery may limit neural placode injury, preventing/limiting the intrauterine “dual hit” injury of the exposed neural placode. Compared to postnatal repair, in utero repair is associated with decreased CSF shunting, less hindbrain herniation, and improved lower extremity motor function.
Accounts for ~70% of open spinal dysraphisms.
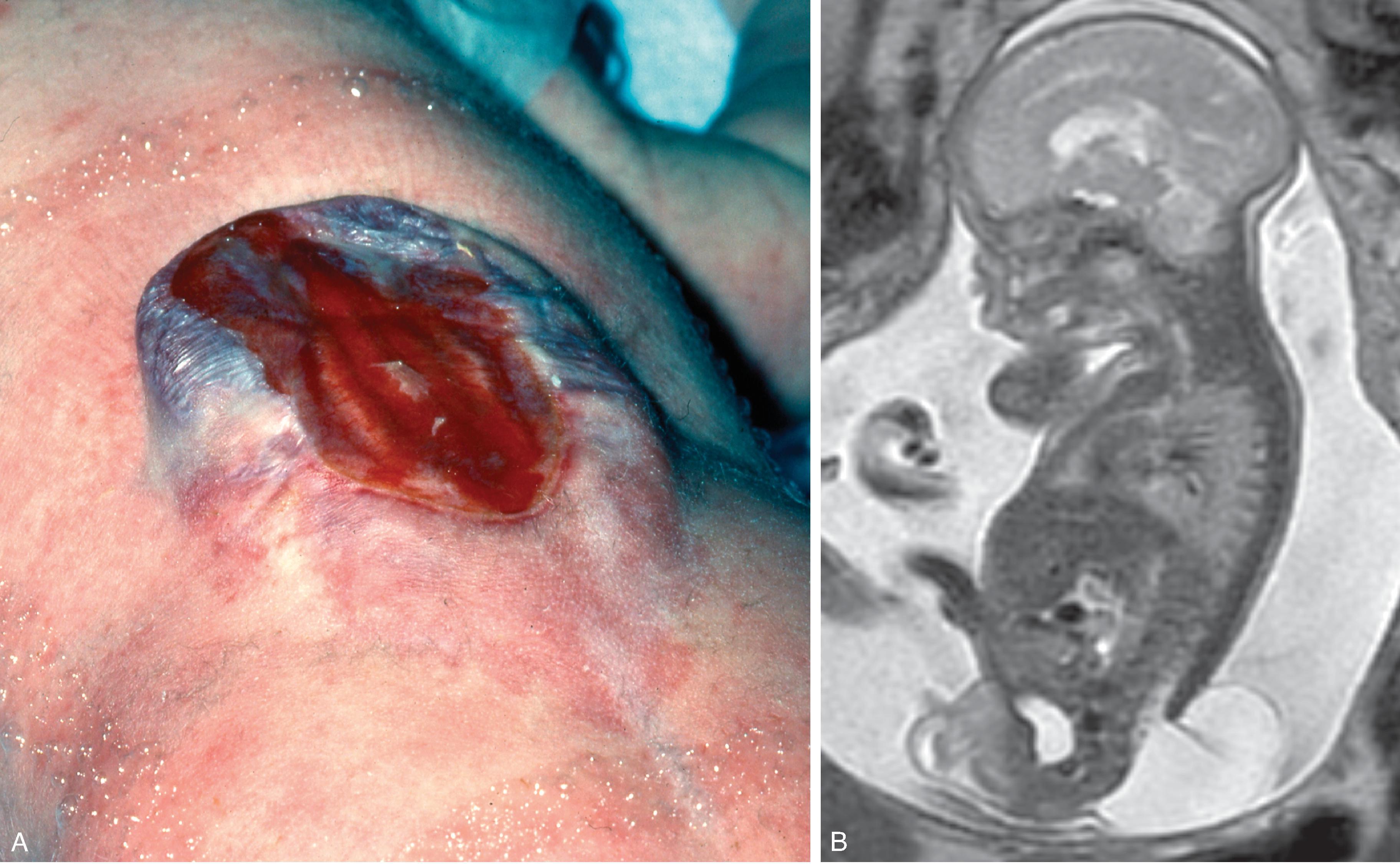
An open/non-skin covered spinal dysraphism in which the neural placode and meninges herniate outside the spinal canal.
Post-operative myelomeningoceles are associated with intrathecal adhesions, epidermoid cyst, and syrinx.
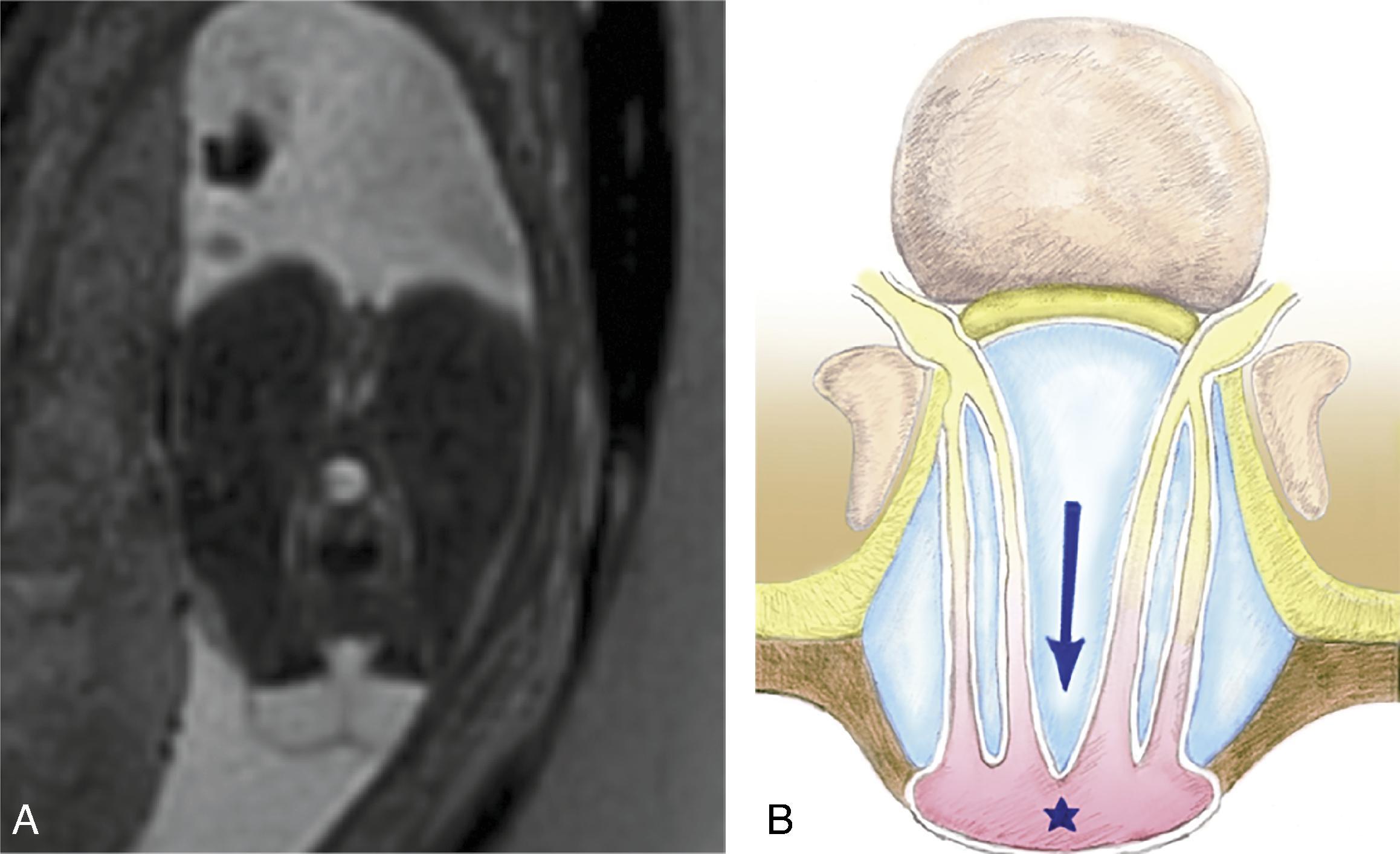
Associated with Chiari 2 malformation .
Differentiation of open spinal dysraphisms from closed spinal dysraphisms on fetal ultrasound and MRI can be challenging. The subcutaneous fat cannot always be differentiated from the neural placode on ultrasound and the expected T1W hyperintensity from fat in the defect is not reliably seen on MRI. Several reported imaging findings have been reported to help differentiate open vs closed spinal dysraphism as follows:
The wall thickness of the myelomeningocele sac was found to be thinner (0.7+/−0.6 mm) than with closed spinal dysraphisms (2.9+/−1.3 mm).
Continuity of the skin and subcutanoues tissues with the wall of the herniating sac or defect was seen in 94% of closed dysraphisms compared to 5% of open dysraphisms.
The lack of Chiari 2 malformation may also indicate a closed spinal dysraphism. Severe hindbrain herniation was seen in 6% of closed spinal dysraphisms compared to 82% with open spinal dysraphisms.
Ventriculomegaly was only present in 12.5% of closed spinal dysraphisms compared to 86% with open spinal dysraphisms.
Lastly, the clivus-supraoccipit angle was found to be larger (75 degrees +/−11 degrees) compared to open dysraphisms (53 degrees +/−10 degrees).
Larger volumes of the myelomeningocele sac are associated with lower Chiari 2 grades (i.e. less effacement of the fourth ventricle and cisterna magna) but did not correlate with ventricular size or clubfoot. Larger areas of skin defect are associated with larger volumes of the lateral ventricles but did not correlate with the Chairi 2 grade or clubfoot.
Myelomeningoceles repaired in utero demonstrate less hindbrain herniation on follow-up imaging. Intraventricular hemorrhage can be seen on both preoperative and post-operative fetal MRI.
Hydrocephalus occurs in ~80%. Following in utero repair of a myelomeningocele the ventricles can increase in size on a follow up fetal MRI, however ultimately there is a postnatal decrease the incidence of hydrocephalus. Larger ventricular size on fetal MRI in open spinal dysraphisms has been shown to correlate with increased reuqirement of postnatal shunt placement.
All myelomeningoceles are tethered by imaging, however the tethered cord syndrome occurs in 3–32% indicating the requirement of associated symptoms and urodynamic studies for evaluation. Tethering occurs as the spinal canal lengthens and due to adhesions. In utero repair is associated with equal or greater risk of tethered cord syndrome and greater risk of epidermoid cyst formation compared to postnatal repair. Retethering occurs at ~ 5–9 years of age which is the time of rapid growth of the spine. Patients with a conus position at or below S1 have poorer outcomes after detethering.
Also known as myeloschisis
A myelocystocle is an open spinal dysraphism similar to myelomeningocele but differs from a myelomeningocele in that the exposed neural placode is flush with the skin and there is no herniating sac
Myeloceles account for ~30% of open spinal dysraphisms
Clinical implications of a myelocele as compared to a myelomeningocele have not been fully evaluated. One study has shown that myeloceles were more positively correlated with independent ambulation after prenatal repair compared to myelomeningocele.
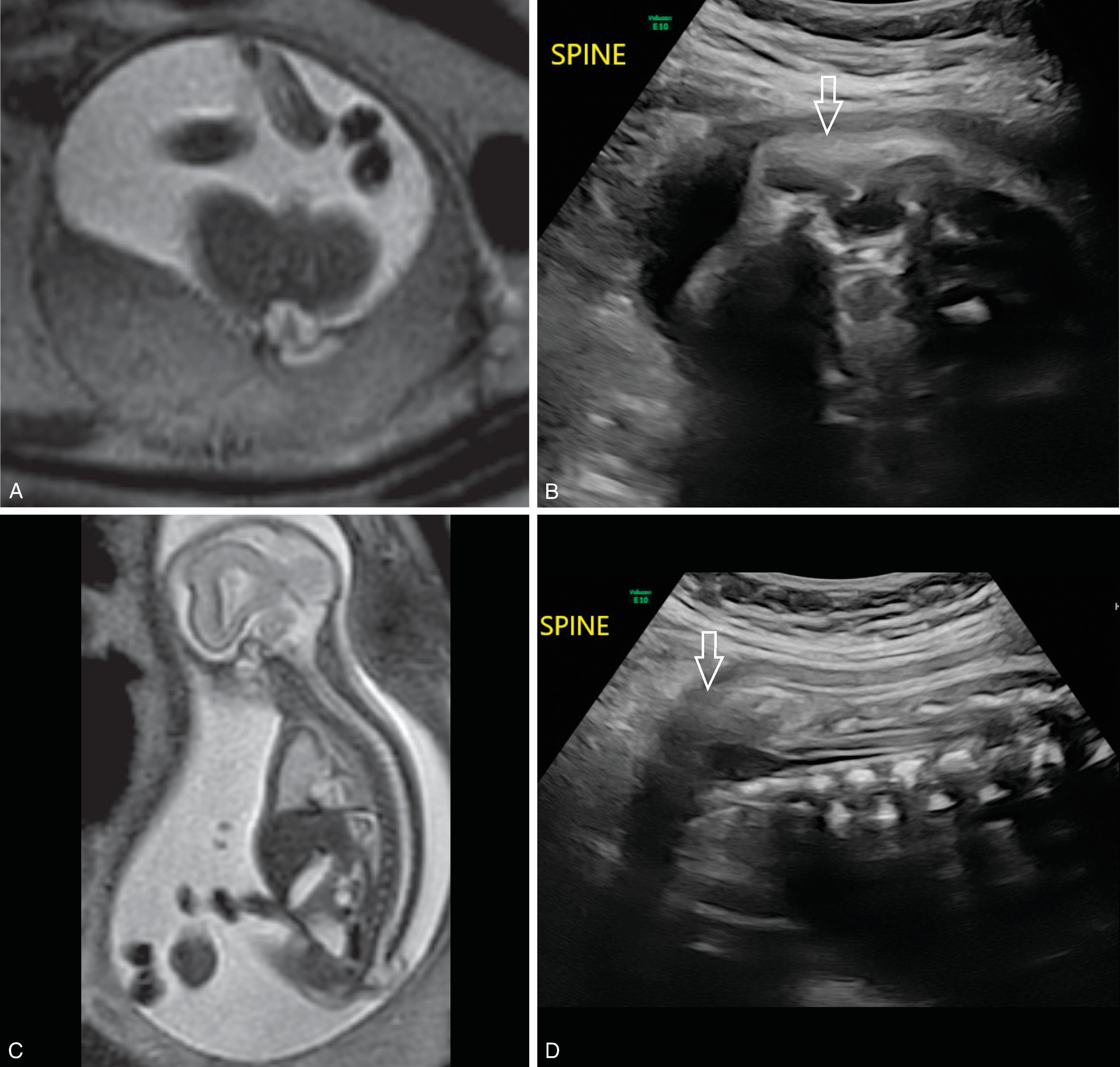
Skin covered closed spinal dysraphism and primary neurulation disorder caused by premature disjunction of neural ectoderm from the cutaneous ectoderm while the overlying ectoderm closes, allowing mesodermal precursors to gain access to the central core of the open/nonclosed neural tube, enhancing the development of a coexisting lipoma

The neural placode attaches outside the confines of the spinal canal onto fat which is in continuity with the enlarged subcutaneous fat.
Clinical: Patients present with tethered cord signs and symptoms. Bladder dysfunction usually occurs first around 2 years of age followed by motor and sensory dysfunction by the teenage years. Progressive neurologic dysfunction occurs without detethering and there is limited recovery if detethering is performed after symptom progression.
Herniation of spinal cord and meninges outside the spinal canal and attached to the lipoma that is in continuity with the prominent or mass-like subcutaneous fat
Associated with syrinx, diastematomyelia, spinal segmentation anomaly, caudal regression, and anorectal and genitourinary malformations
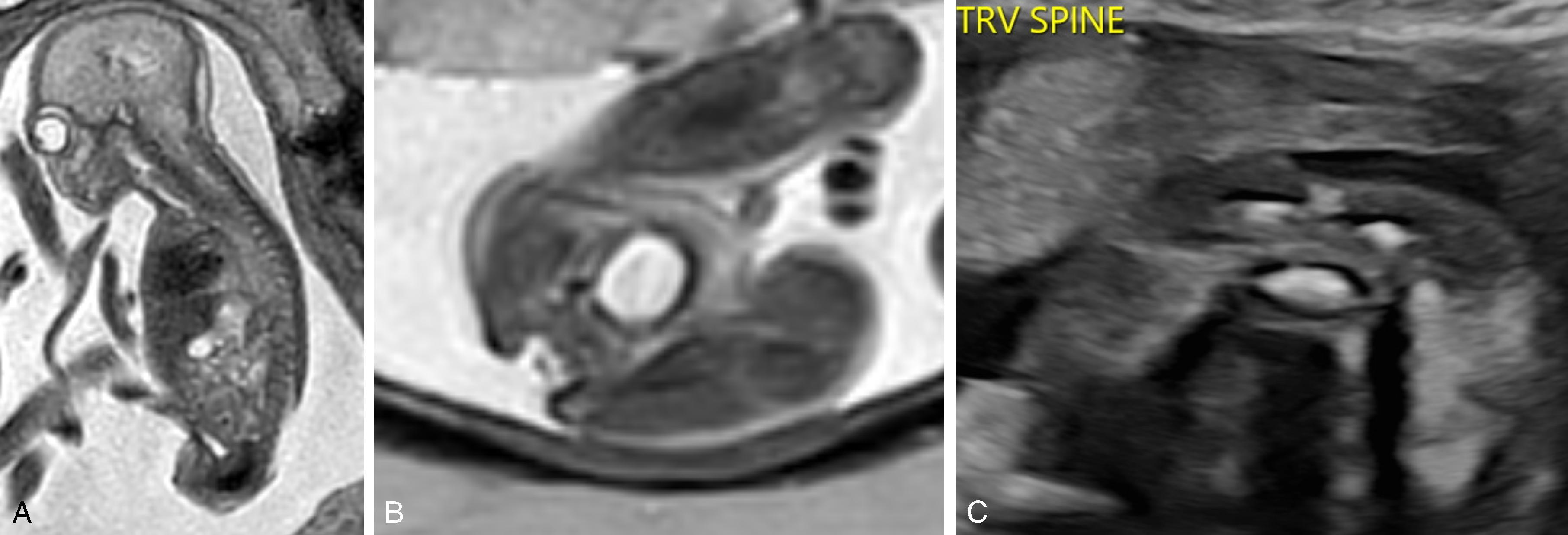
Closed spinal dysraphism with subcutaneous mass and primary neurulation disorder caused by premature disjunction of neural ectoderm from the cutaneous ectoderm
May present with lower extremity symptoms, back pain, and bowel/bladder dysfunction
Early surgical intervention recommended to reduce neurological impairment
The spinal cord-lipoma attachment is inside the spinal canal and in continuity with prominence/mass-like subcutaneous fat. The lack of herniation of the cord and meninges differentiates a lipomyelocele from a lipomyelomeningocele.
Associated with vertebral anomalies, terminal diastematomyelia, anorectal and genitourinary anomalies, epidermoid/dermoid, and dermal sinus tract
Closed spinal dysraphism with unknown embryological etiology
Herniation of fluid-filled meningocele sac through a defect in sacral or coccygeal vertebrae
Symptoms secondary to result of pressure on the bowel, bladder, or nerve roots
Fluid signal sac with communication to the spinal canal.
Associated with NF-1 and Marfan syndrome.
Closed spinal dysraphism .
A terminal myelocystocele consists of a skin-covered lumbosacral spinal dysraphism, an arachnoid-lined meningocele that is directly continuous with the spinal subarachnoid space, and a low-lying hydromyelic spinal cord that traverses the meningocele and then expands into a large terminal cyst which does not communicate with the subarachnoid space and is lined by ependyma and dysplastic glia. Terminal myelocystoceles are presumed to be a disorder of secondary neurulation caused by inability of CSF to exit the early neural tube causing the expansion and disrupting the overlying mesenchyme. Terminal myelocystoceles are associated with cloacal exstrophy, omphalocele, imperforate anus, ambiguous genitalia, caudal regression, and renal anomalies. Terminal myelocystoceles typically present with no bowel or bladder control and poor lower extremity function.
A nonterminal myelocystocele consists of a skin-covered spinal dysraphism, a CSF filled cyst, a meningocele and variable amount of dorsal fat continuous with the subcutaneous fat. Type 1 consists of the meningocele sac traversed by a fibroneurovascular stalk attached to the dome of the sac. Type 2 consists of focal hydromyelia that has displaced the posterior wall of the spinal cord into the meningocele sac. Nonterminal myelocystoceles are suspected to be disorders of primary neurulation with incomplete fusion of the dorsal neural folds/failure of the neural ectoderm to separate from the cutaneous ectoderm. Nonterminal myelocystoceles are associated with Chiari 2 malformations, hydrocephalus, ectopic cerebellar tissue, heterotopic renal tissue, and diastematomyelia. Nonterminal myelocystoceles often demonstrate no neurologic dysfunction at presentation.
Etiology unknown. Possibly secondary to teratogens based on animal studies with retinoic acid.
Early surgical repair maximizes neurological function
Divided into terminal and nonterminal myelocystoceles based on location.
Hindbrain herniation can be seen in ~40% of patients with myelocystoceles.
Skin covered spinal dysraphism/malformation with herniated spinal cord and meninges . The central canal is expanded and herniates into the meningocele.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here