Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
At the end of the third week of gestation, the primitive gastrointestinal (GI) tract of an embryo consists of a straight tube suspended from the esophagus to the rectum by the dorsal mesentery, which contains the vascular supply. In the dorsal mesentery, the portion connected to the stomach is known as the dorsal mesogastrium. As the embryo lengthens, the caudal portion of the septum transversum thins and becomes the ventral mesentery. It attaches the stomach and duodenum to the ventral wall of the abdominal cavity. Because there is no ventral mesentery below the foregut, the ventral mesentery is the same as the ventral mesogastrium. The sagittally oriented ventral and dorsal mesogastrium, along with the upper GI tract, divide the upper abdominal cavity into equally sized right and left peritoneal cavities.
During the fourth gestational week, cords of tissue within the ventral mesogastrium begin to grow rapidly, forming the liver, bile ducts, and ventral pancreas within the ventral mesogastrium, while the spleen and dorsal pancreas arise within the dorsal mesogastrium. The remainder of the mesogastrium become the ligaments and omenta. The anterior part of the ventral mesogastrium between the anterior abdominal wall and the liver becomes the falciform ligament, and the posterior part between the liver and the stomach becomes the lesser omentum. The posterior portion of the dorsal mesogastrium between the spleen and posterior abdominal wall becomes the splenorenal ligament, while the anterior portion between the spleen and the stomach becomes the gastrosplenic ligament, which is a part of the greater omentum. The stomach initially develops as a fusiform bulge in the foregut. This bulge rapidly becomes asymmetric owing to the more rapid growth of its dorsal margin (greater curvature) than of its ventral margin (lesser curvature). The stomach then undergoes a 90-degree counterclockwise rotation along with the ventral and dorsal mesogastrium ( Fig. 47-1 ).
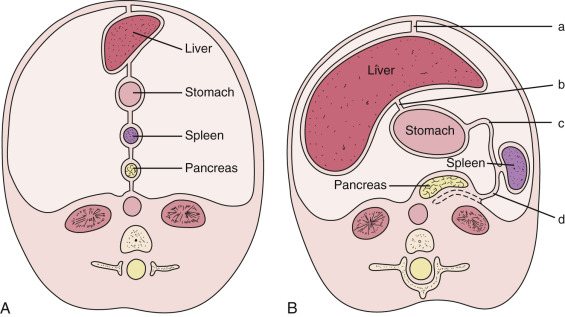
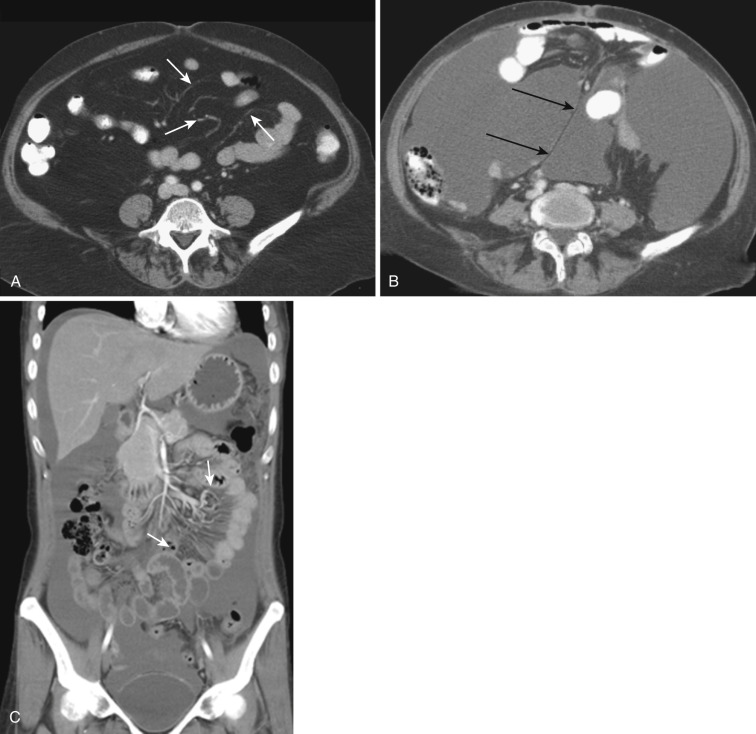
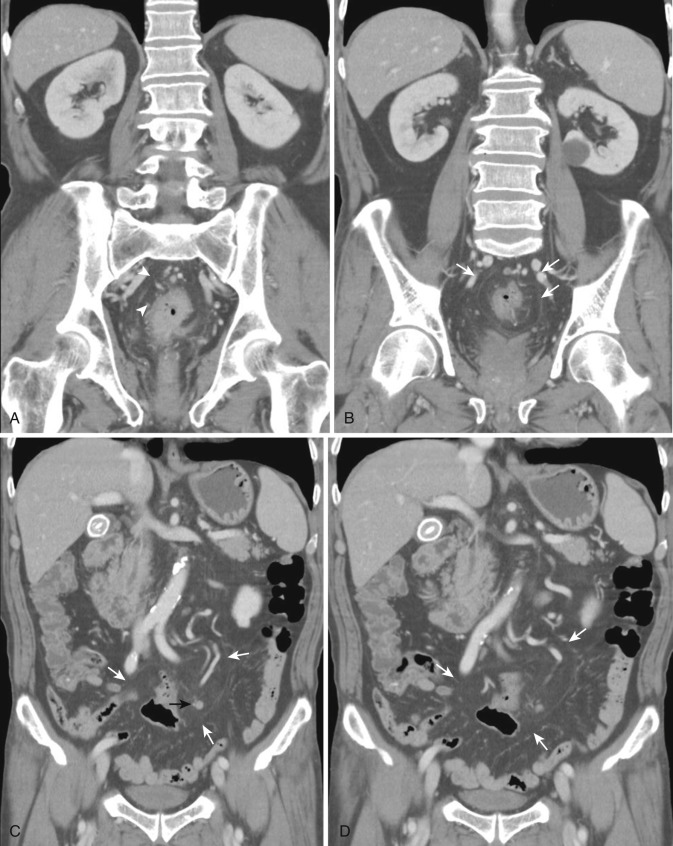
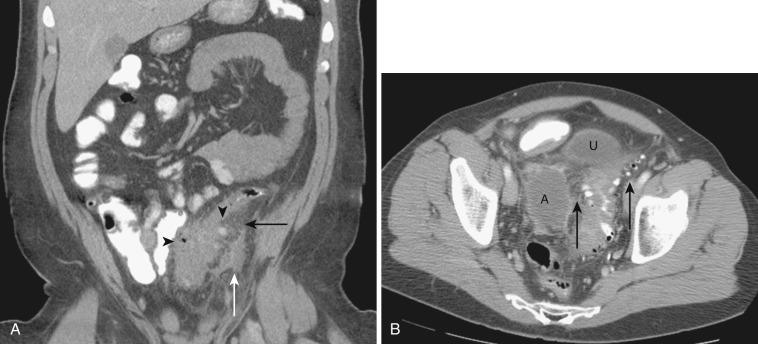
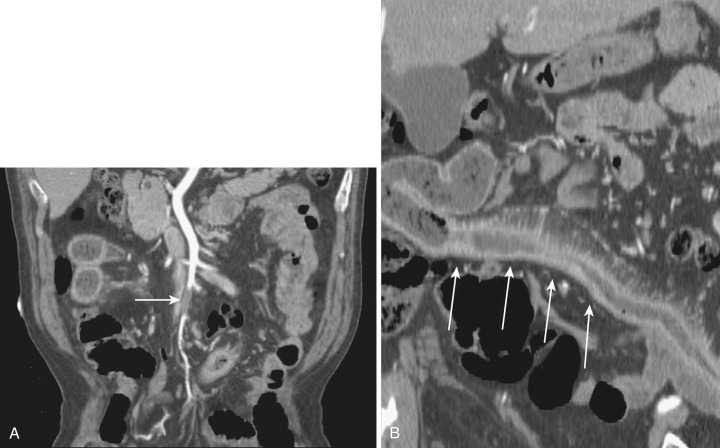
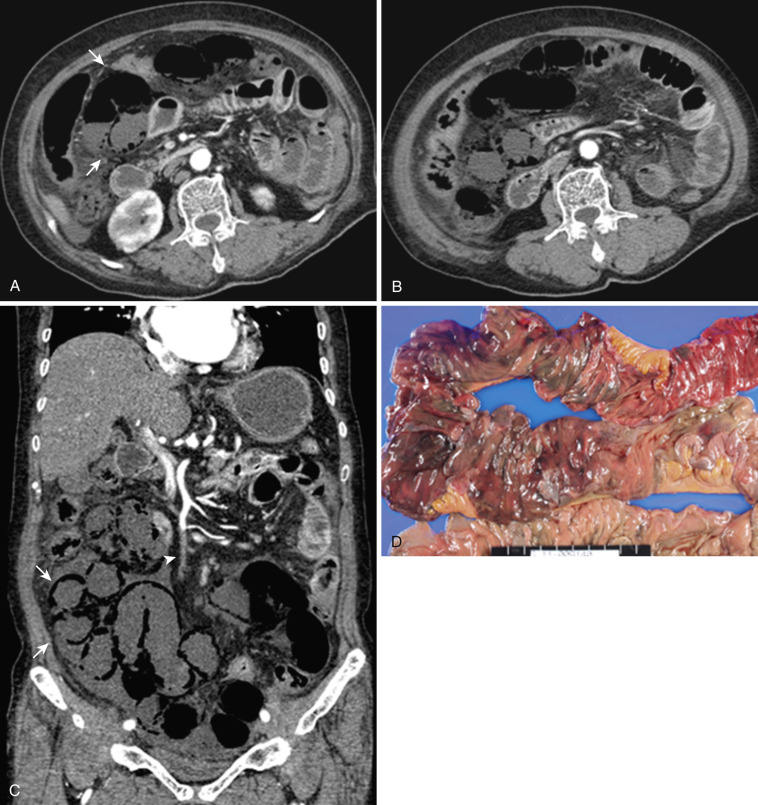
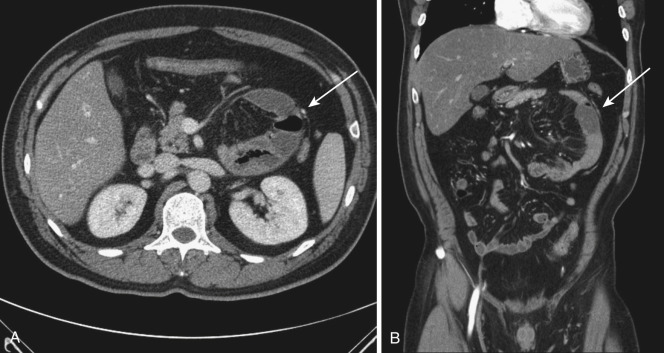
The dorsal mesentery inferior to the stomach evolves into small bowel mesentery that attaches the jejunum and ileum to the posterior abdominal wall and mesocolon in the transverse and sigmoid colon. The dorsal mesentery of the duodenum, along with the pancreas, is fused with the posterior abdominal wall and loses its mesentery. Rarely when they are not completely fused with the posterior abdominal wall, the duodenum and pancreas can become intraperitonealized ( Fig. 47-2 ). The dorsal mesentery attached to ascending and descending colon and rectum is lost once these segments of colon are fused with the posterior abdominal and pelvic walls. Not infrequently the ascending and descending colon are incompletely fused with the posterior abdominal wall and become intraperitonealized ( Fig. 47-3 ). Incomplete fusion with the posterior pelvic wall can cause the rectum to be intraperitonealized ( Fig. 47-4 ).
In its broad meaning, mesentery may include any membranous derivatives of the embryonic dorsal and ventral mesentery (e.g., falciform ligament, lesser omentum, gastrosplenic and splenorenal ligaments). When it is defined as a membranous fold attached to various organs, its definition can be even broader and may include even the broad ligament of the uterus, mesosalpinx, gastropancreatic fold, and other structures. The usual definition of the mesentery is, however, limited to the derivatives of the embryonic dorsal mesentery below the foregut—the membranous fold connecting the small and large bowel to the posterior body wall.
Mesenteries are double-layered folds of peritoneum within which lie continuations of the subperitoneal space, which contains various amounts of adipose tissue within which the arteries, veins, lymphatics, and nerves of the bowel course. On normal cross-sectional images, although the supplying and draining vessels can be traced to and from the major mesenteric vessels, the actual leaves of the mesentery are not discernible unless they are separated by intervening ascites or peritoneal thickening ( Fig. 47-5 ). Mesenteries can act as barriers as well as conduits for the spread of intraperitoneal and extraperitoneal diseases.
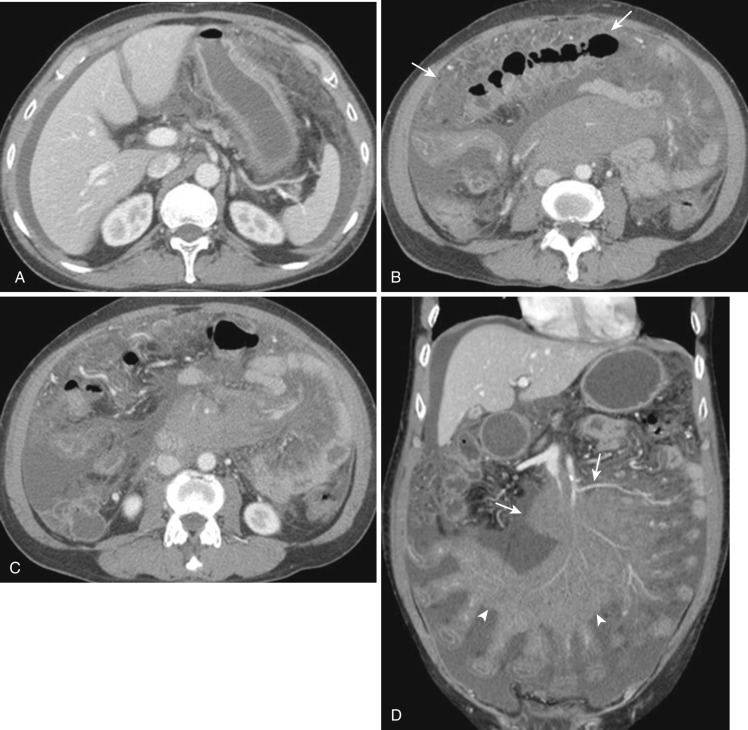
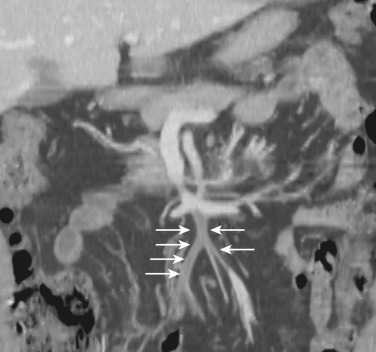
The narrowest definition of the mesentery is the small bowel mesentery, also known as the mesentery proper or mesenterium. The small bowel mesentery is a broad fan-shaped fold of peritoneum connecting the jejunum and ileum to the root of the mesentery. The mesenteric root is approximately 15 cm long and is directed obliquely downward to the right from the duodenojejunal flexure to the ileocecal junction. The intestinal border of the mesentery is about 6 m long and is formed into numerous pleats. The mesentery consists of two layers of the peritoneum between which lie the jejunal and ileal branches of the superior mesenteric artery (SMA), with accompanying veins, nerve plexuses, lymphatics, lymph nodes, connective tissue, and fat ( Fig. 47-6 ). The gastrocolic trunk is an anatomic landmark between the transverse mesocolon and root of the small bowel mesentery.
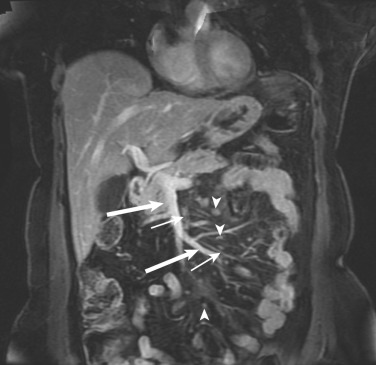
On computed tomography (CT) and magnetic resonance imaging (MRI), the mesentery appears as a fat-containing structure inseparable from the other fat-containing peritoneal folds (e.g., greater omentum) or even from retroperitoneal fat (see Fig. 47-5 ). Normal mesenteric fat is similar in density to subcutaneous fat (−100 to −160 Hounsfield units [HU]) on CT and has the same signal intensity on MRI. The jejunal and ileal vessels can be identified as distinct round or linear densities within the mesenteric fat. Normal-sized lymph nodes within the mesentery are routinely identified, particularly following contrast enhancement and in coronal images ( Fig. 47-7 ).
The mesocolon is composed of two layers of peritoneum that connect the colon to the posterior abdominal wall; it contains its related blood vessels, lymphatics, nerves, and a variable amount of adipose tissue. The transverse colon and sigmoid colon have a well-formed mesocolon. The cecum is attached to the ileum by the ileocecal fold and has a complete peritoneal covering with no mesocolon in most cases. On CT and MRI, the mesocolon is not usually easily discernable, but it can be traced by identifying its blood vessels from the marginal vessels to the superior or inferior mesenteric vessel or vice versa ( Fig. 47-8 ).
The transverse mesocolon is a broad fold connecting the transverse colon to the posterior abdominal wall. The transverse mesocolon is typically absent close to the hepatic flexure, and therefore the colon is usually in direct posterior contact with the second portion of the duodenum as it crosses. The mesocolon is short over the first part of the head of the pancreas but is much longer where it is attached to the anterior border of the body of the pancreas. It ends at the splenic flexure but usually extends farther laterally as a peritoneal fold called the phrenicocolic ligament. Because of its intimate relationship with almost the entire length of the pancreas, pancreatitis easily extends to the transverse mesocolon ( Fig. 47-9 ).
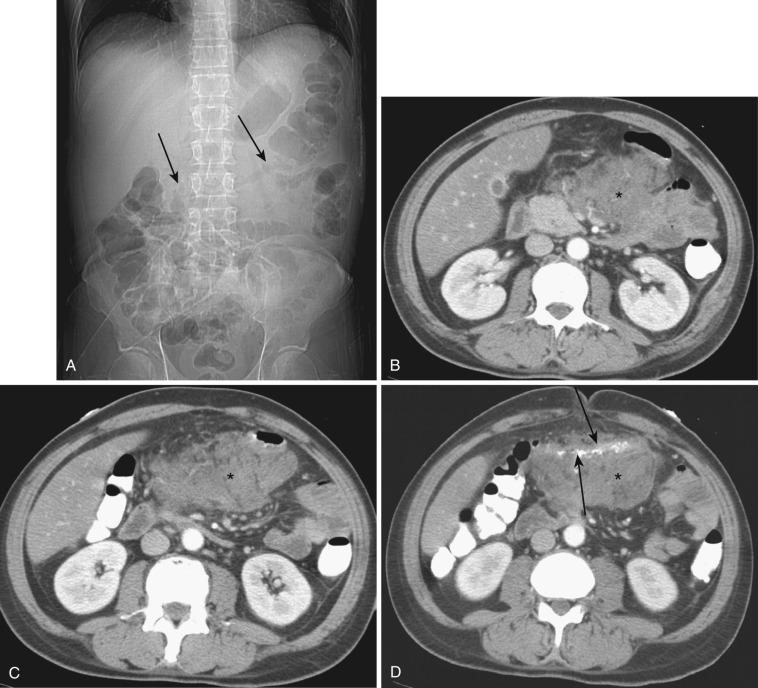
The transverse mesocolon is formed by two layers of peritoneum; the upper layer is adherent to but separable from the greater omentum ( Fig. 47-10 ). Between the two layers of the transverse mesocolon are the middle colic arteries and veins, lymphatics, and nerves of the transverse mesocolon. Because of the transverse mesocolon, the transverse colon has a great deal of mobility, and the position and orientation of vessels in the transverse mesocolon vary depending upon the position of the transverse colon.
The sigmoid mesocolon is a fold of peritoneum that attaches the sigmoid colon to the posterior pelvic wall. Its line of attachment is shaped like an inverted V, the apex of which is near the division of the left common iliac artery; the left line descends medially to the left psoas major muscle, and the right line descends into the pelvis and ends in the median plane at the level of the third sacral vertebra. Because of this attachment, a triangular-shaped fluid collection with a floating mesocolon is seen frequently in the presence of ascites. For the same reason, when a volvulus of the sigmoid colon occurs, the twisted colonic loop orients toward the right upper quadrant of the abdomen ( Fig. 47-11 ). Between the layers of the sigmoid mesocolon are the sigmoidal and superior hemorrhoidal vessels.
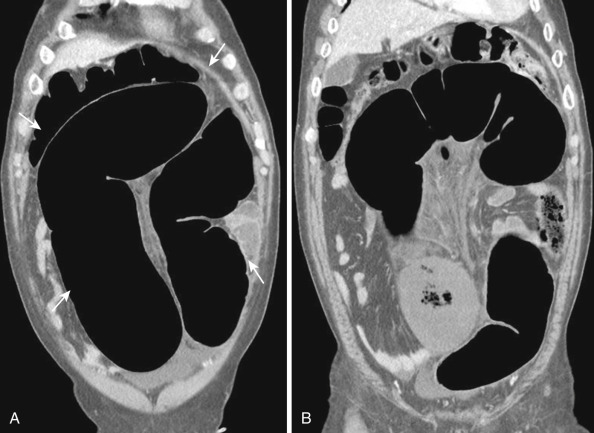
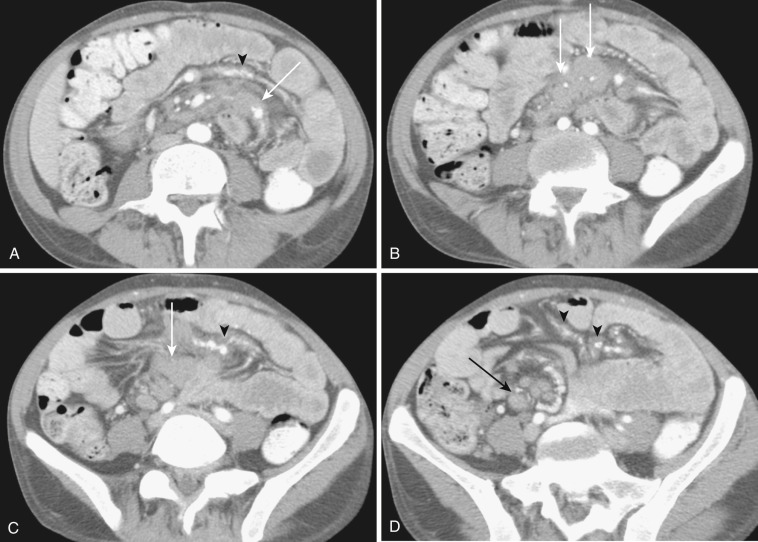
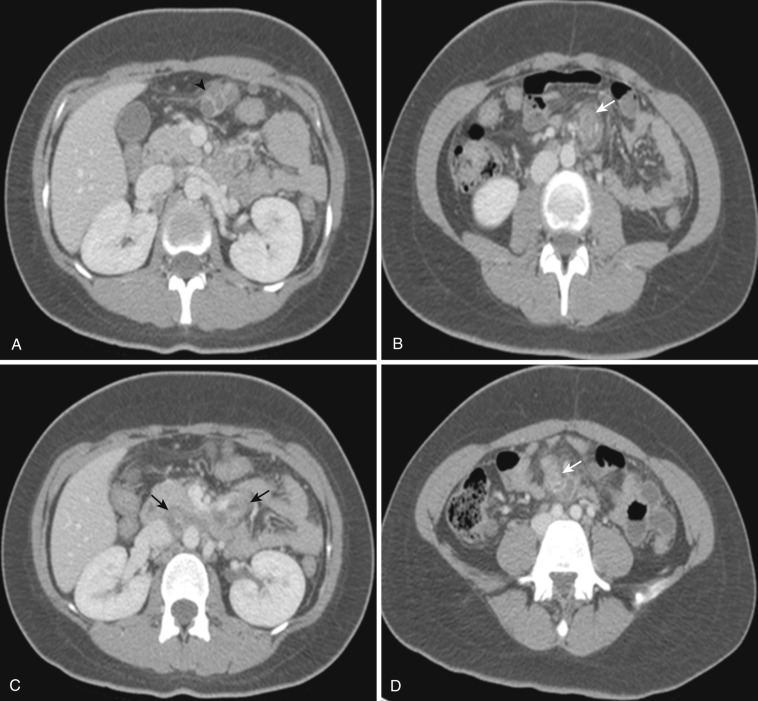
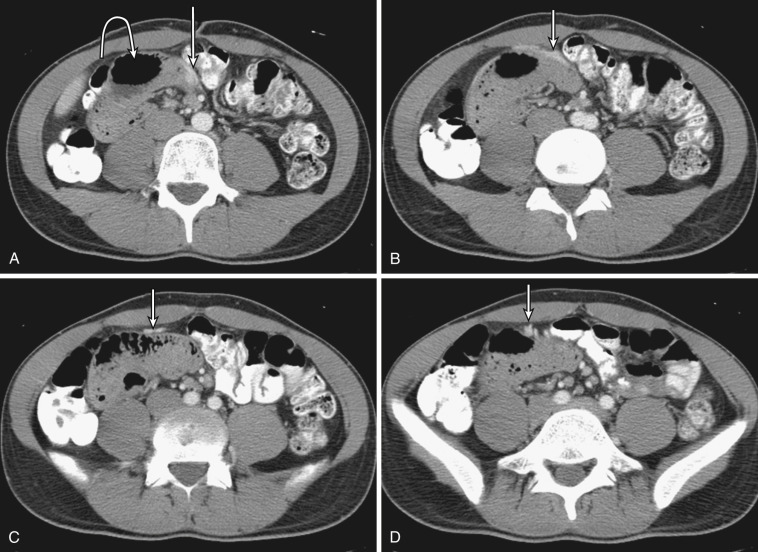
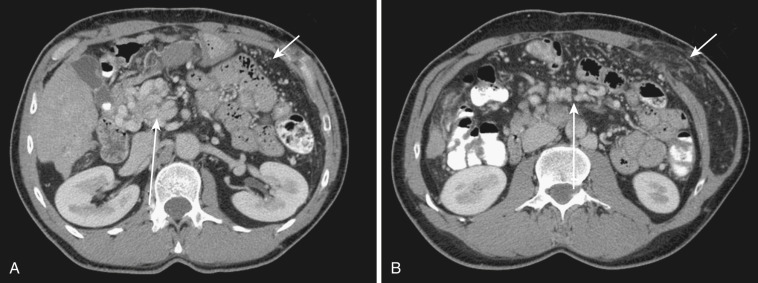
Both the ascending and descending colons are normally retroperitoneal organs but can be partially or totally intraperitoneal. The ascending colon possesses a mesocolon in up to 26% of cases, and the descending colon in up to 36% of cases (see Fig. 47-3 ). In such instances the ascending and descending colons can be more mobile. Ascites and seeded metastases can be found posterior to the ascending and descending colons, thereby mimicking a retroperitoneal collection or mass. Even the rectum is rarely peritonealized by having its own mesorectum (see Fig. 47-4 ). In this situation an intraperitoneal organ or mass can be mistaken for retroperitoneal structures.
The omentum is a fold of peritoneum extending from the stomach to adjacent organs. Different from the small bowel mesentery and mesocolon, which connect the bowels to the posterior abdominal wall, the omentum connects two interperitoneal organs: the lesser omentum (between the stomach and liver) and the greater omentum (between the stomach and spleen or transverse colon). Like the small bowel mesentery and mesocolon, the greater omentum is formed by two layers of peritoneum within which lie vessels, lymphatics, lymph nodes, nerves, and varying amounts of adipose tissue ( Fig. 47-12 ; see Fig. 47-10 ).
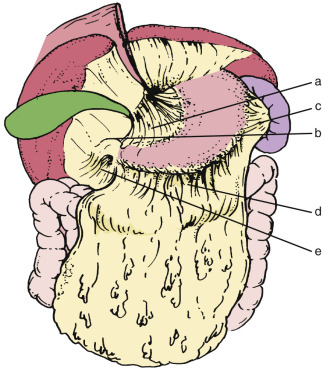
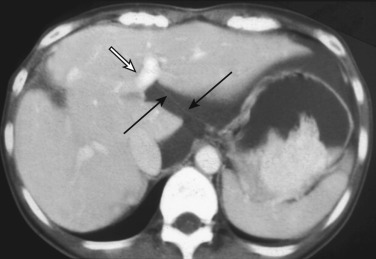
The lesser omentum extends from the lesser curvature of the stomach and first portion of the duodenum to the liver at the porta hepatis into the fissure for the ligamentum venosum. The portion of the lesser omentum extending between the liver and stomach is called the gastrohepatic ligament, and that between the liver and duodenum, the hepatoduodenal ligament. The left gastric artery acts a marker for the gastrohepatic ligament, and the hepatic artery proper acts as an anatomic marker for the hepatoduodenal ligament. At its right free margin, the lesser omentum encloses the portal triad with its lymphatics and lymph nodes; this free margin is the anterior edge of the epiploic foramen. The lesser omentum contains the left and right gastric arteries and corresponding veins as well as lymphatics and lymph nodes. When there is sufficient ascites, it may appear as an undulating fat-containing plate in the fissure for the ligamentum venosum and serve as an anatomic landmark separating the lesser sac from the greater sac ( Fig. 47-13 ). The left gastric vessels are easily identified at the lesser curvature aspect of the lesser omentum. Normal lymph nodes up to 8 mm in diameter are also frequently seen in the lesser omentum. The dilated coronary vein can easily be visualized in patients with portal hypertension.
The greater omentum is the largest peritoneal fold, consisting of a double sheet folded on itself so that it is made of four layers in early development. The inner two layers are fused below the transverse colon and lose their mesothelial lining during fetal life (see Fig. 47-10 ). This fusion limits the inferior extent of the lesser sac. It stretches from the greater curvature of the stomach and first portion of the duodenum downward in front of the small intestine for a variable distance. It adheres to but is separable from the upper layer of the transverse mesocolon. The gastrocolic ligament is the part of the greater omentum stretching from the stomach to the transverse colon, where as the duodenocolic ligament it extends from the first portion of the duodenum to the transverse colon (see Fig. 47-12 ). The gastrosplenic ligament connects the stomach to the spleen and is the part of the greater omentum.
Contained within the greater omentum are the gastroepiploic arteries and veins. On cross-sectional images the greater omentum appears as a thin, broad, fat-containing area just beneath the anterior abdominal wall and ventral to the stomach, transverse colon, and small bowel loops. The greater omentum is frequently found wrapped about the organs in the upper part of the abdomen—only occasionally is it evenly dependent anterior to the intestines. Especially in the presence of a large amount of ascites, it is wrapped on itself and often misplaced at a particular area. It may limit the spread of infection by forming adhesions with areas of inflammation in the peritoneal cavity. It is frequently involved by peritoneal diseases, either infective or malignant.
Mesenteric disease is difficult to separate from peritoneal or bowel disease, because the mesentery is intimately related to the peritoneum and bowel anatomically and physiologically. Therefore primary peritoneal and bowel diseases often accompany changes of the mesentery and vice versa. The peritoneum is indeed a mesenteric component because it is the lining membrane of the mesentery. Peritonitis, particularly tuberculous peritonitis, and peritoneal carcinomatosis cause or are associated with mesenteric abnormality. By the same token, primary mesenteric disease processes cause and are associated with abnormal peritoneal findings like thickening and enhancement of peritoneal membrane or ascites or changes of bowel ( Figs. 47-14 and 47-15 ). Inflammatory, infectious, and neoplastic diseases of bowel bring mesenteric fat changes and regional lymphadenopathy. Sclerosing mesenteritis induces, on the other hand, thickening and edema of bowel wall and ascites secondary to vascular and/or lymphatic involvement (see Fig. 47-14 ). Because mesenteric space is continuous with extraperitoneal space as subperitoneal space, it becomes a conduit transmitting disease processes arising in the retroperitoneal space to GI organs and vice versa.
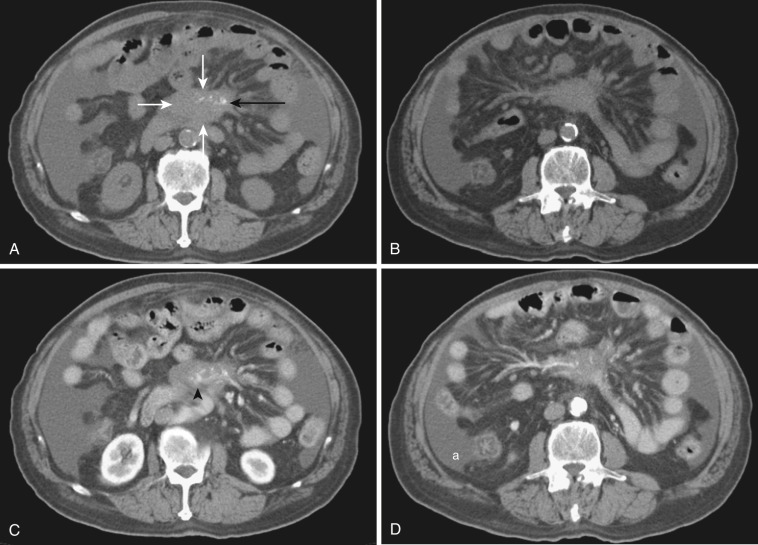
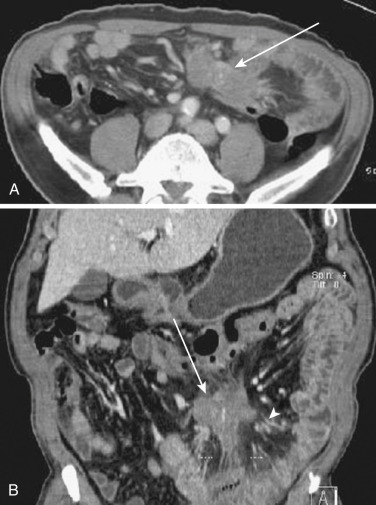
Whether abnormal mesenteric changes are due to primary mesenteric causes or are secondary effects due to disease processes of neighboring organs or structures, they manifest distinct and limited radiologic findings. These findings come together in different combinations or independently.
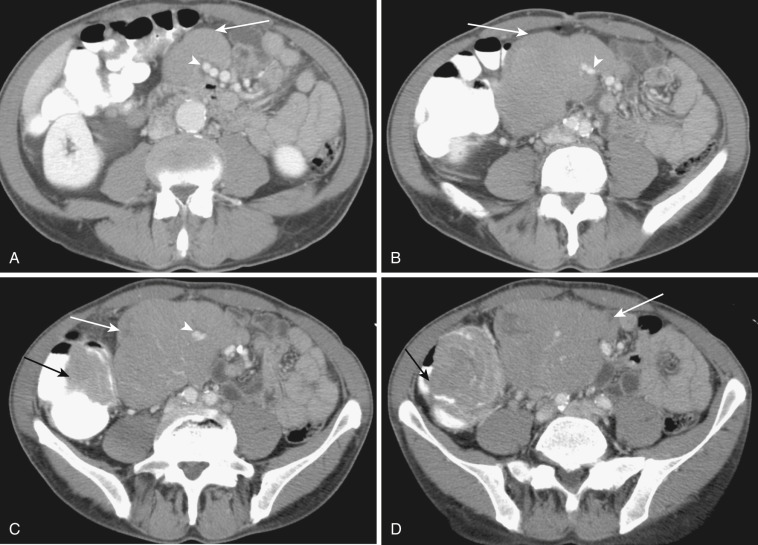
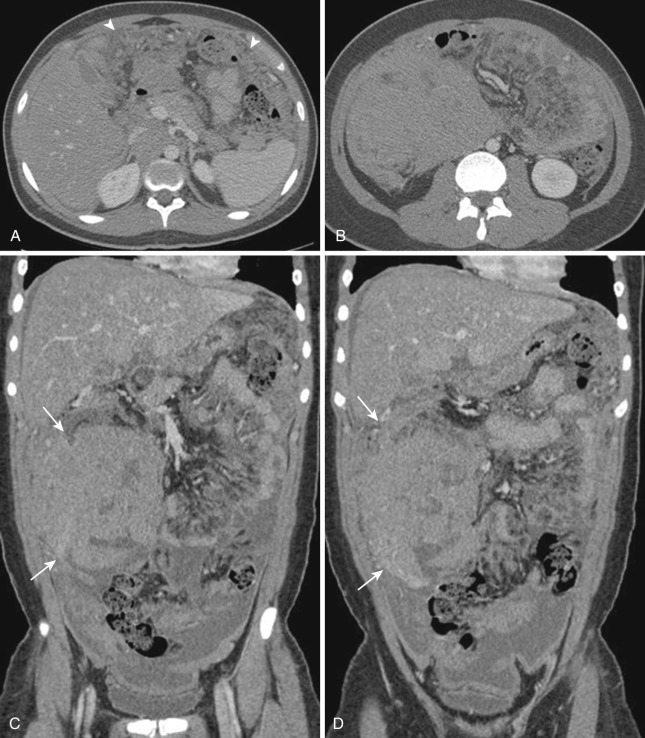
The most frequent radiologic manifestation of mesenteric abnormalities is alteration of fat density or intensity. The mesenteric fat becomes hazy due to lymphedema (see Fig. 47-24 ), edema ( Fig. 47-16 ), hemorrhage ( Fig. 47-17 ), cellular infiltration by inflammation or neoplasm ( Figs. 47-18 to 47-20 ; see Fig. 47-15 ), or fibrosis (see Fig. 47-14 ). It is described as “misty mesentery.” Focal misty mesentery can be due to local etiologies such as trauma, strangulated bowel obstruction, infectious and inflammatory bowel disease (IBD), pancreatitis (see Fig. 47-16 ), and ischemic mesenteric disease ( Fig. 47-21 ). It is diffuse when it is due to systemic causes like portal hypertension ( Fig. 47-22 ), hypoalbuminemia, or heart failure. Whether localized or diffuse, it is usually ill-defined but is sometimes well demarcated, as in segmental misty mesentery ( Figs. 47-23 to 47-25 ; see Fig. 47-16 ) and mesenteric panniculitis. It is sometimes demarcated by a so-called tumoral pseudocapsule (see Figs. 47-16 and 47-23 ), which is a peripheral band of soft tissue attenuation.
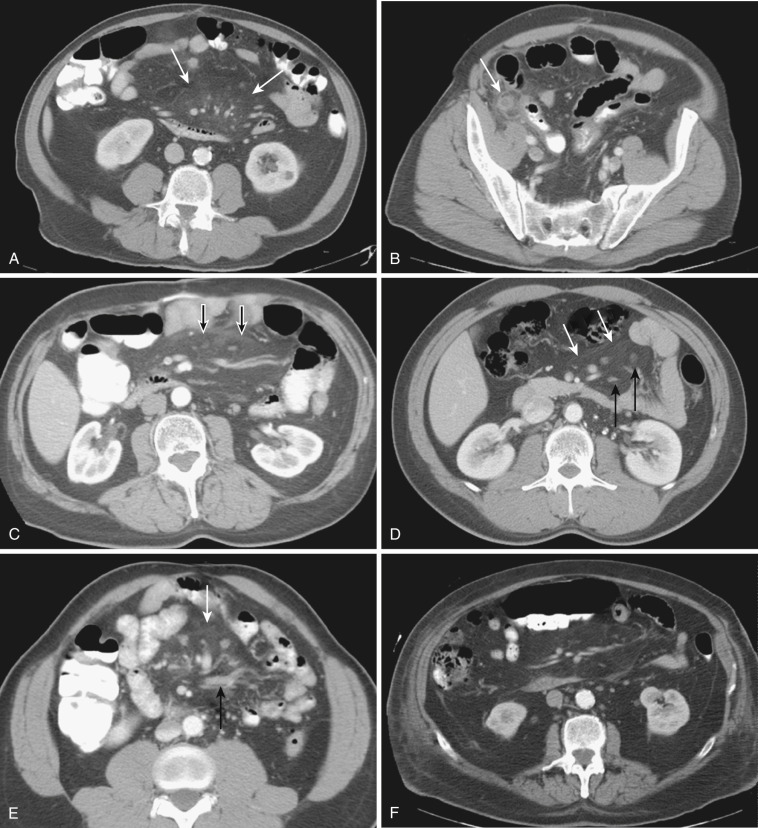
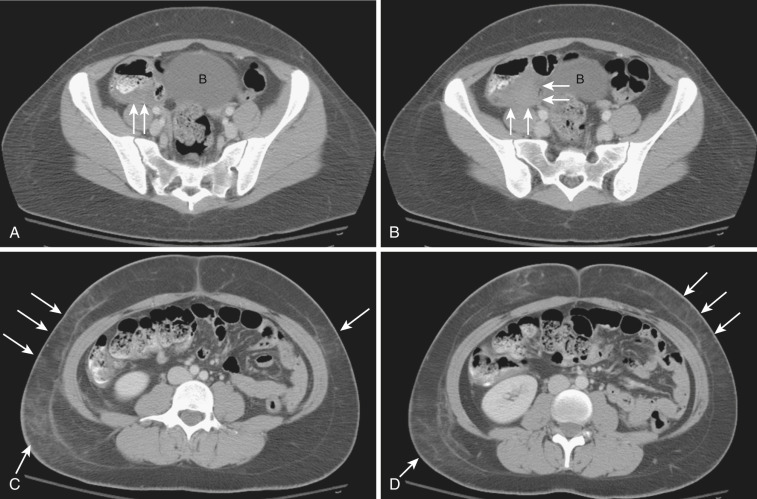
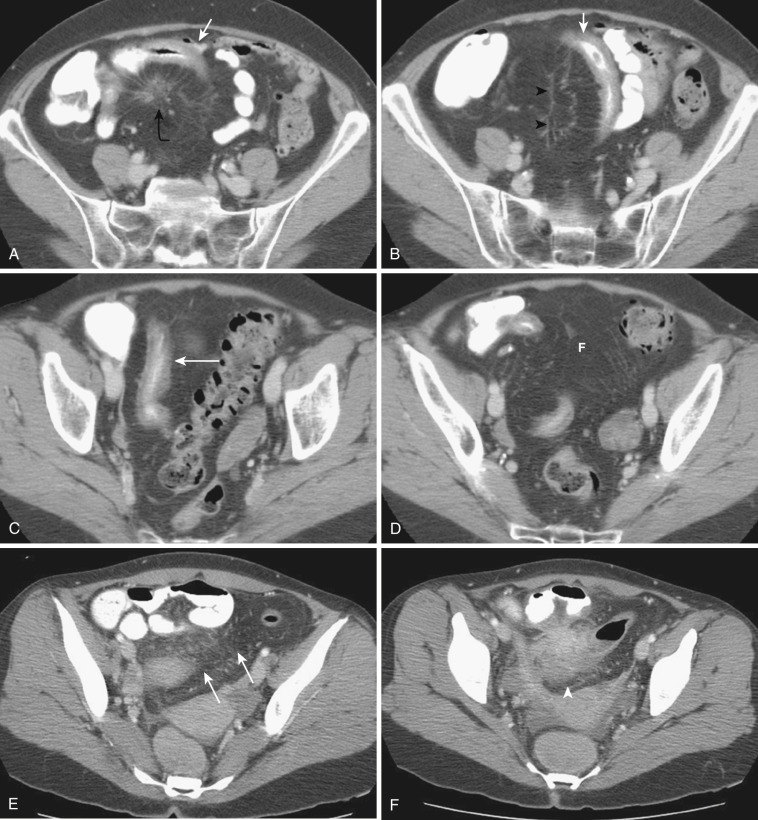
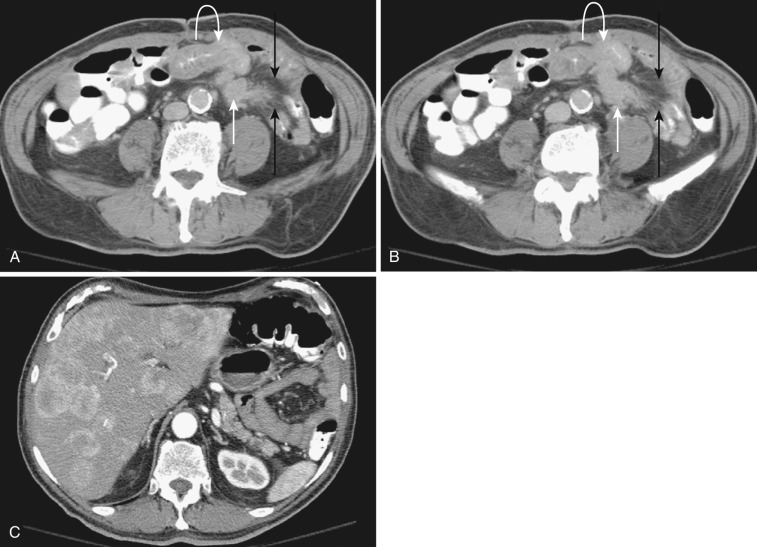
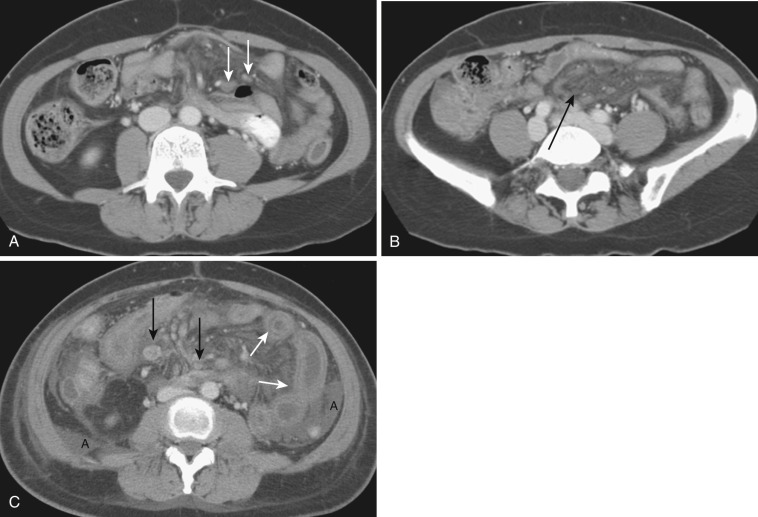
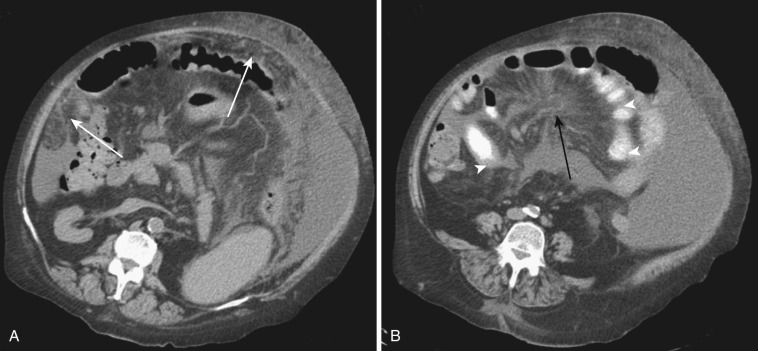
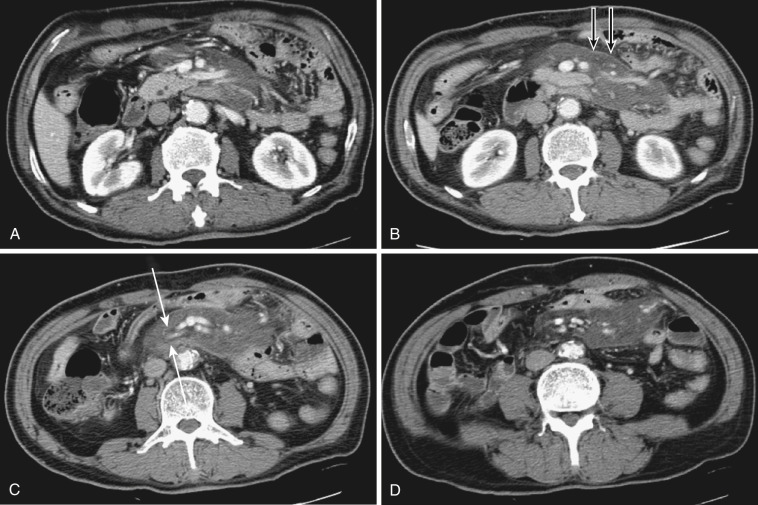
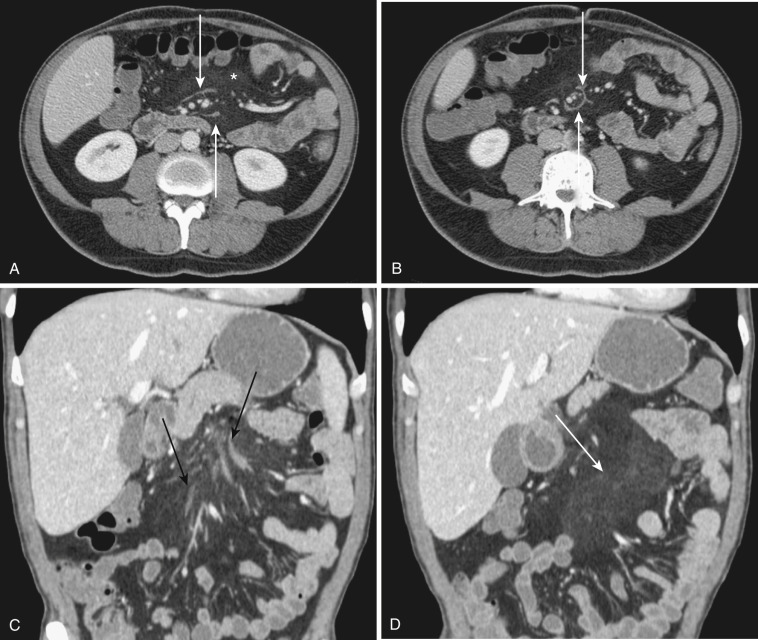
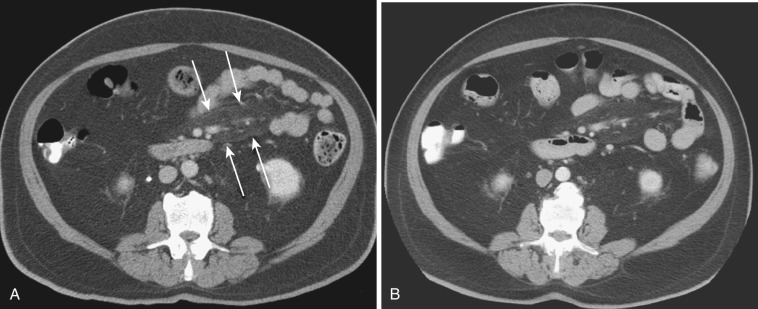
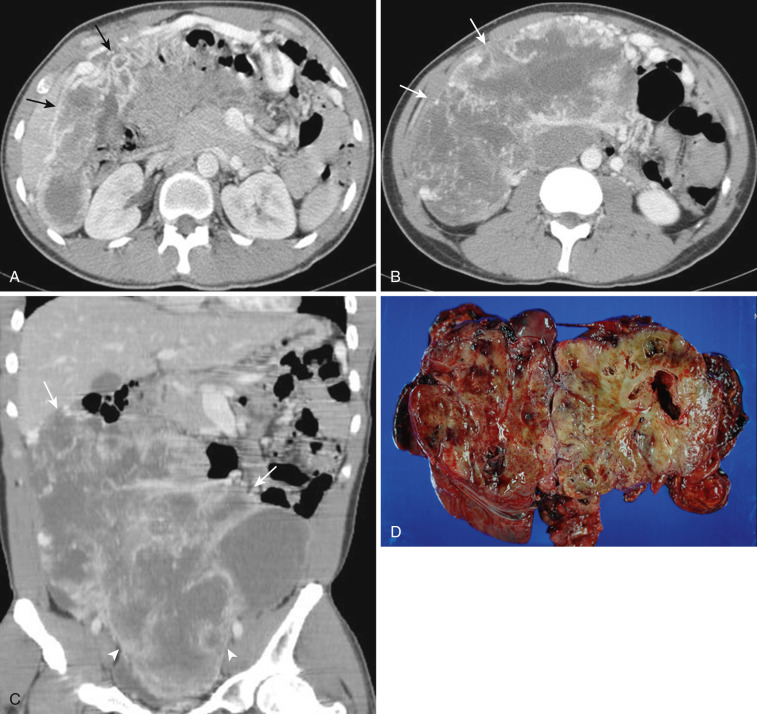
Depending upon the severity and cause of disease, the misty mesentery may show variable densities. From cases of lymphedema to edema, to inflammatory and neoplastic cell infiltration, to hemorrhage, and to fibrosis with calcifications, the density of misty mesentery becomes higher. In cases of sclerosing mesenteritis (see Fig. 47-14 ) and mesenteric involvement of carcinoid tumor (see Fig. 47-20 ), misty mesentery may manifest as a stellate or spiculated mass due to extensive fibrosis; often calcifications are associated.
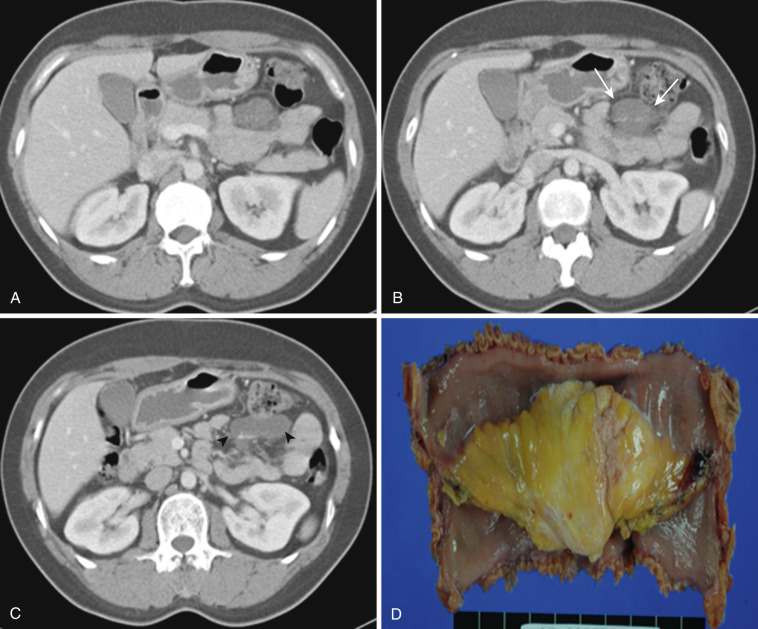
Mesenteric mass, either cystic or solid, is quite uncommon. Other than conglomerated massive lymphadenopathy of lymphoma and mesenteric involvement of carcinoid tumor, a solid mesenteric mass is rare. Even though carcinoid tumor arises primarily from bowel—most frequently from ileum—it is often difficult to recognize the primary bowel mass. Instead the spiculated or stellate mesenteric mass is the dominant finding, which is a distinctive form of sclerosing mesenteritis due to the tissue reaction to released hormones by the tumor (see Fig. 47-20 ). Primary mesenteric GIST (GI stromal tumor) ( Fig. 47-26 ), desmoid tumor associated with or without familial adenomatous polyposis ( Fig. 47-27 ), and inflammatory pseudotumor (see Fig. 47-15 ) are known entities. Other benign and malignant primary mesenchymal tumors or metastatic tumors are extremely rare ( Fig. 47-28 ).
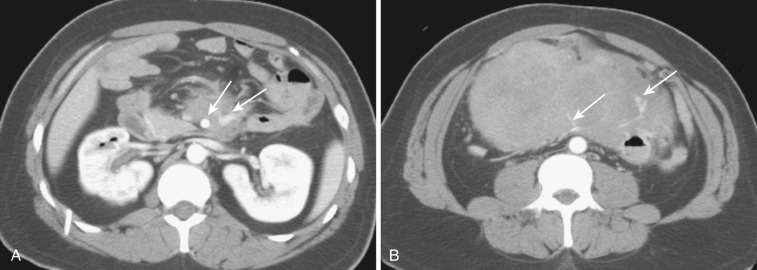
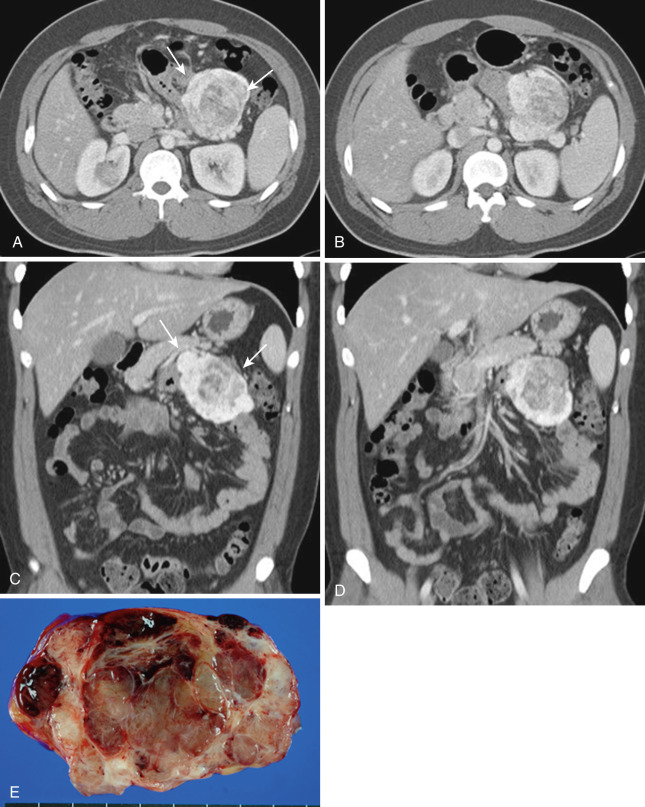
Also rare are cystic mesenteric lesions, commonly called mesenteric or omental cysts, which is rather descriptive terminology. A variety of different pathologic entities comprise such entities. Most common is lymphangioma ( Figs. 47-29 and 47-30 ). Other types include enteric cyst, enteric duplication cyst, mesothelial cyst, nonpancreatic pseudocyst, cystic mesothelioma, and cystic teratoma.
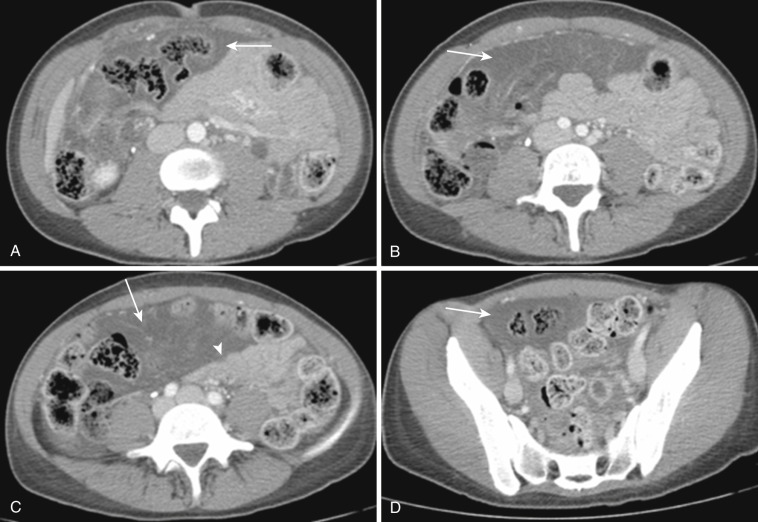
Normal mesenteric lymph nodes are routinely seen at CT and MRI. They are more dramatically visualized and better appreciated on coronal images and following contrast enhancement (see Fig. 47-7 ). It is difficult to define the criteria of a normal mesenteric lymph node. As in other areas of the body, the size, shape, number, and distribution of nodes should be considered. Abnormality is generally defined as any mesenteric lymph node that measures larger than 6 mm in short axis or when a group of three or more lymph nodes is clustered in one anatomic location.
A variety of diseases or conditions lead to mesenteric lymphadenopathy. Notably, lymphoma is the most common neoplastic cause ( Figs. 47-31 and 47-32 ). Non-Hodgkin's lymphoma in particular involves mesenteric nodes, usually along with paraaortic nodes. Hodgkin's lymphoma usually does not involve mesenteric nodes. GI tract malignancy frequently metastasizes to regional mesenteric lymph nodes. Genitourinary tract malignancy and extraabdominal malignancy seldom metastasize to mesenteric nodes. Inflammatory or infectious GI tract disease frequently cause regional mesenteric lymphadenopathy, but their size is usually less than 10 mm ( Fig. 47-33 ). In the case of primary mesenteric lymphadenitis, inflamed lymphadenopathy is the sole finding, without accompanying abnormal bowel changes, although an underlying infectious terminal ileitis is thought to be the cause ( Fig. 47-34 ). Other systemic diseases like systemic lupus erythematosus, systemic mastocytosis, and sarcoidosis may show mesenteric lymphadenopathy.
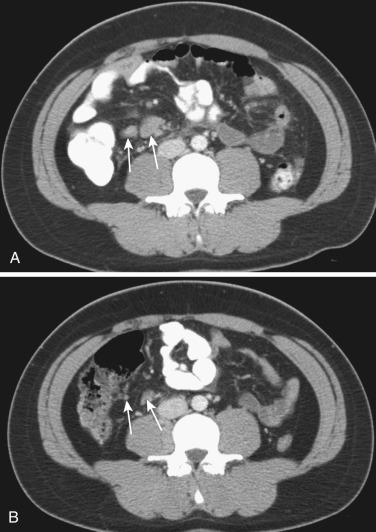
Hypodense lymphadenopathy with Whipple's disease has been overly emphasized; it is not a unique finding for Whipple's disease. Hypodense lymphadenopathy is more commonly seen in other benign and malignant diseases, notably in abdominal tuberculous or atypical tuberculous disease ( Figs. 47-35 and 47-36 ) and Kaposi's sarcoma. Furthermore, Whipple's disease is extremely rare. It is also worthwhile to recognize mesenteric cavitary lymph node syndrome as an entity characterized by cystic change in mesenteric lymph nodes and associated with celiac sprue.
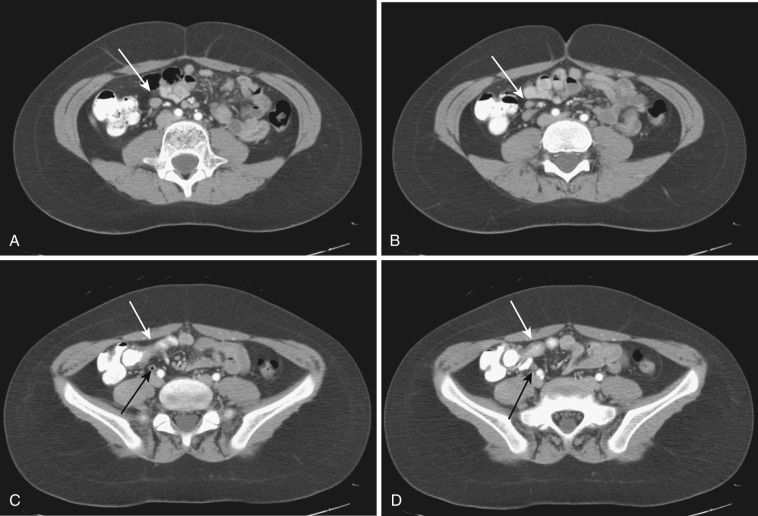
One commonly encounters cases of unexplainable mesenteric lymphadenopathy, most of them clinically insignificant and irrelevant. The best strategy to deal with concern is short-term follow-up study. If no changes arise in the short term, long-term follow-up can be done. If the lymphadenopathy still does not change, one can ignore it.
Mesenteric disease may result from changes of the mesenteric vessels. Arterial thrombosis or embolism or venous thrombosis cause ischemic mesenteric (bowel) disease ( Figs. 47-37 to 47-39 ; see Fig. 47-21 ). Mesenteric venous or arterial collaterals suggest current or past occlusion of mesenteric vessels or portal hypertension.
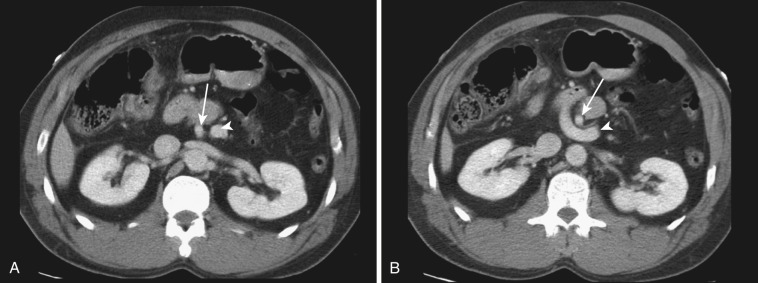
Alteration of mesenteric vessels in position, course, and shape can be important clues of certain bowel-related diseases. When whirling of mesenteric vessels is associated with bowel obstruction, volvulus is the most likely cause. In congenital nonrotation or malrotation of bowel, the relationship of the SMA and superior mesenteric vein (SMV) is reversed ( Fig. 47-40 ). Anterior displacement of the inferior mesenteric vein (IMV) by clustered small bowel loops is the sign of left paraduodenal hernia ( Fig. 47-41 ), whereas anterior displacement of the right colic vein by clustered small bowel loops is the sign of right paraduodenal hernia ( Fig. 47-42 ). When there is portal hypertension, multiple mesenteric varices develop. Omental varices sometimes mimic omental cake due to peritoneal carcinomatosis or peritonitis ( Fig. 47-43 ; see Fig. 47-22 ). Mesenteric venous congestion or dilatation of veins is seen in high-grade bowel obstruction, segmental misty mesentery, or active IBD. Mesenteric venous air is an important sign of necrotizing enteritis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here