Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Focal hepatic mass lesions can be divided into: (1) pseudolesions or pseudotumors, (2) nontumorous mass lesions, including cysts and inflammatory masses, (3) benign tumors, and (4) primary and secondary malignant tumors.
Ultrasonography plays a primary role in detection of focal hepatic mass lesions; for further characterization, computed tomography (CT) and magnetic resonance imaging (MRI) provide valuable information. This chapter discusses the CT and MRI features of hepatic mass lesions and their pathophysiology.
Various kinds of pseudolesions (focal masslike findings seen only on imaging) or pseudotumors (focal masslike parenchymal changes) are seen in the liver. As a first step in the imaging diagnosis of focal hepatic mass lesions, exclusion of these entities is important. Pseudolesions and pseudotumors are often associated with localized intrahepatic portal flow abnormalities.
In arterioportal (AP) shunt the hepatic arterial blood flows directly into the portal vein. AP communications are abundant through the peribiliary vascular plexus in the liver, and an AP shunt may form. However, as a result of biopsy or tumor, a direct fistula between the artery and portal vein can be created.
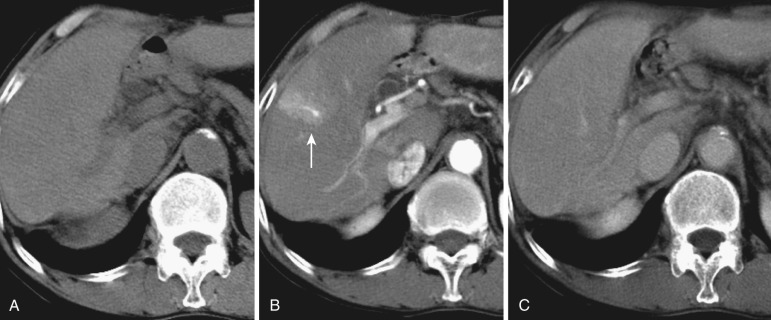
On dynamic CT and MRI, a wedge-shaped early enhancement is seen in the arterial phase, with occasional visualization of the portal venules in the center of the lesion; this area becomes isodense in the equilibrium phase ( Fig. 44-1 ). Coronal or sagittal multidetector CT images are occasionally valuable for demonstrating these features. Because AP shunt is usually not visualized on superparamagnetic iron oxide (SPIO)-enhanced and hepatobiliary-phase gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)–enhanced MRI, these methods are effective in differentiating AP shunt from small hypervascular tumors or other true lesions. In hypervascular hepatocellular carcinoma (HCC), corona enhancement due to drainage flow from the tumor is seen in the portal or equilibrium phase with washout from the tumor (discussed later). This hemodynamic pattern is different from that in AP shunt. The area with AP shunt often shows focal sparing in a fatty liver and rarely focal iron deposition. AP shunt is often associated with HCC, cavernous hemangioma, and other tumors.
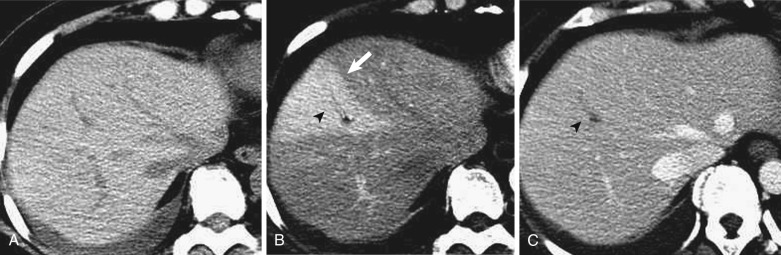
Intrahepatic portal vein obstruction leads to various imaging findings in the obstructed segment that may result in its cause being obscured. Because of compensatory blood flow from the hepatic artery to the distal portion of the portal vein through the peribiliary vascular plexus, the obstructed segment shows early enhancement on dynamic CT and MRI (segmental staining) ( Fig. 44-2 ). This segmental staining is often observed in HCC or other malignant tumors with portal vein invasion and makes it difficult to detect the underlying tumor itself. When Zahn's infarction occurs, the obstructed area shows hypodensity on precontrast CT, hypointensity on T1-weighted images, and hyperintensity on T2-weighted images. In a fatty liver this area often demonstrates focal sparing, and the causative disease can be overlooked.
Intrahepatic hepatic vein obstruction results in similar findings based on the segmental distribution of the hepatic veins.
Several kinds of direct venous inflow into the liver parenchyma from outside the main portal trunk have been analyzed by anatomic and imaging studies. These are called third inflow. The areas receiving third inflow are well demonstrated by CT during arterial portography (CTAP) as portal perfusion defects in an otherwise normal liver. According to CTAP-based analysis, third inflow includes flow from an aberrant right gastric vein (or pancreaticoduodenogastric vein), cystic veins, veins of Sappey, and aberrant left gastric vein. These veins usually connect directly to the intrahepatic portal venules. The areas receiving third inflow often show pseudolesions or pseudotumors. To differentiate these entities from true hepatic mass lesions, visualization of these veins by color Doppler ultrasonography or dynamic CT is useful ( Fig. 44-3 ).
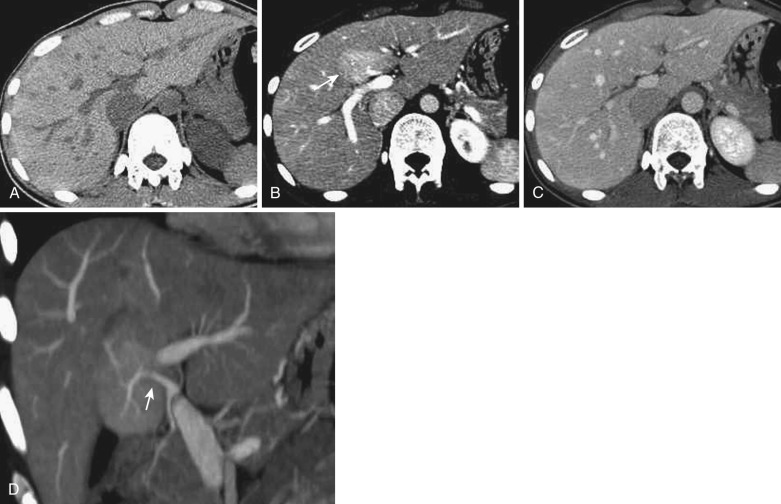
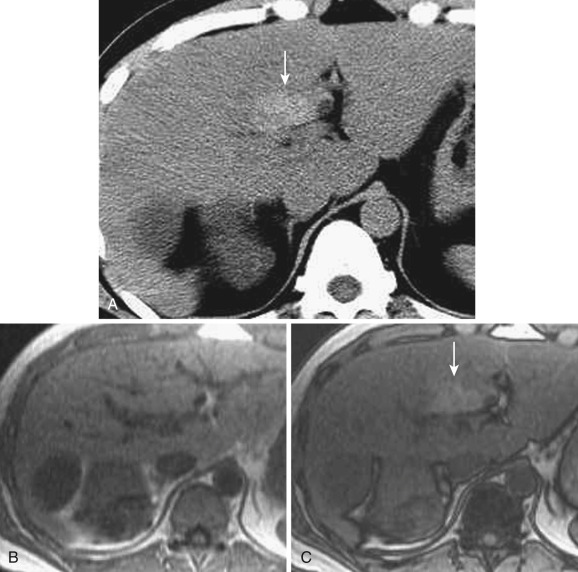
The cystic veins drain into the gallbladder fossa and communicate with the peripheral portion of the intrahepatic portal veins in segment IV or V. The drainage area often shows a tiny wedge-shaped area of early staining on dynamic CT and MRI because of earlier venous return relative to the surrounding liver ( Fig. 44-4 ). On nonenhanced and postcontrast CT and MRI, no definite abnormality is demonstrated. This staining can be better visualized when blood flow is increased by gallbladder cancer or cystitis. This inflow through the cystic veins frequently causes focal sparing in a fatty liver.
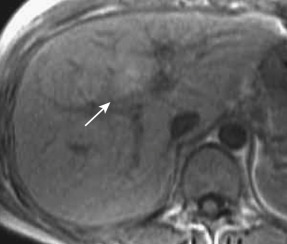
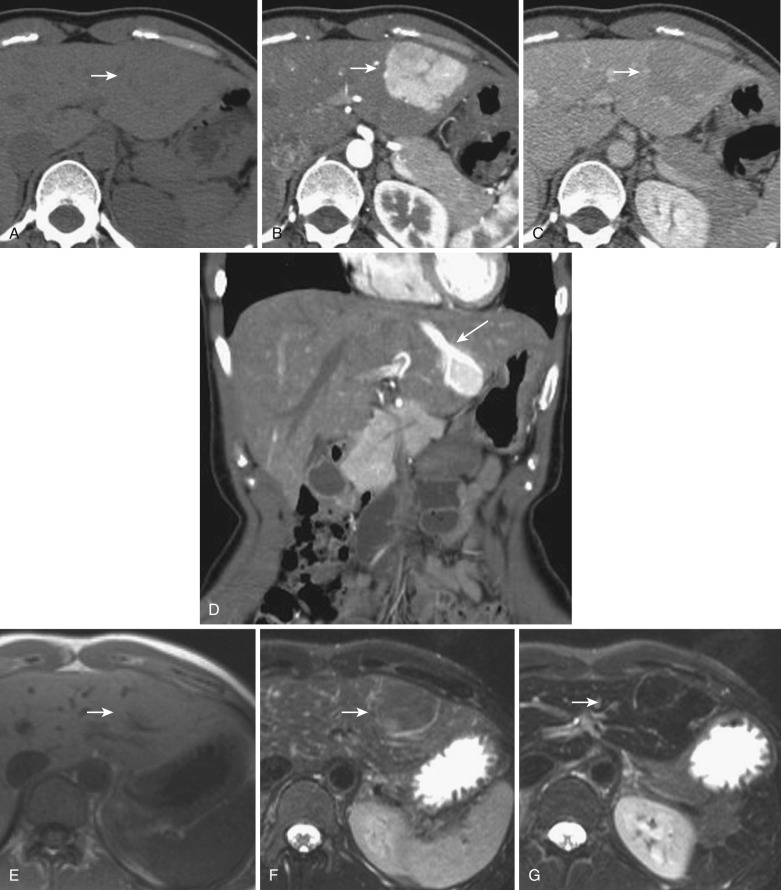
An aberrant right gastric vein accumulates blood from the gastric antrum, duodenum, and pancreas head; it is also referred to as the pancreaticoduodenogastric vein. The right gastric vein usually drains into the main portal trunk; however, it drains directly into the posterior aspect of segment IV of the liver in 6% to 14% of the general population and rarely into segment I, II, or III. The area drained by this vein occasionally demonstrates early enhancement on the arterial phase of dynamic CT and MRI (see Fig. 44-4 ) owing to relatively early venous return, focal sparing in a fatty liver ( Fig. 44-5 ), focal fatty liver ( Fig. 44-6 ), and hyperplastic change in a cirrhotic liver ( Fig. 44-7 ). On MRI this change can be seen in around 5% of cirrhotic patients. For purposes of differentiation, confirmation of the venous drainage by color Doppler is important. In addition, Gd-EOB-DTPA–enhanced MRI is also helpful for differentiation of these pseudolesions from HCC. On hepatobiliary-phase Gd-EOB-DTPA–enhanced MRI, this hyperplastic area is not well visualized; on the other hand, the majority of early well-differentiated HCC shows hypointensity. Focal sparing of iron in hemosiderotic liver in this area was recently reported.
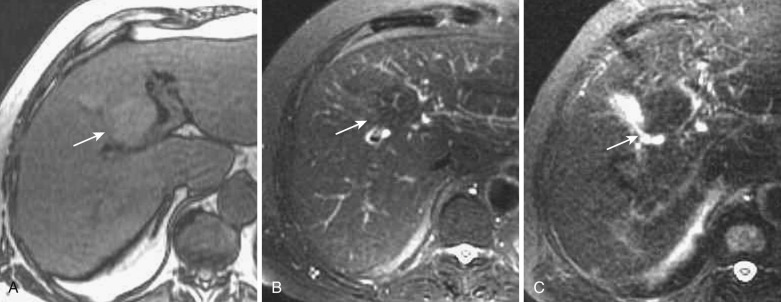
The inferior veins of Sappey flow into the anterior aspect of segment IV from the chest wall through the falciform ligament; they are also called paraumbilical veins. Focal fatty liver is often observed in this area ( Fig. 44-8 ). This area also demonstrates early enhancement or hypodensity on dynamic CT and MRI. These changes are not seen in patients with portal hypertension because of reversed flow in this venous system.
A hepatic segment with hepatic vein obstruction demonstrates the same imaging findings as a hepatic segment in an area with portal vein obstruction. This phenomenon is often observed in living donor liver transplantation. The drainage flow from hypervascular HCC demonstrates corona enhancement surrounding the tumor, mimicking extratumoral extension of the malignant neoplasm on dynamic CT or MRI (peritumoral pseudolesion). There may be a spared area in a fatty liver (peritumoral spared area) and rarely peritumoral focal fatty change.
Solitary liver cysts are occasionally encountered. Histologically a single layer of cuboidal epithelium is seen and the wall is composed of thin fibrous tissue. These cysts are frequently noted in older adults, implicating acquired factors in their pathogenesis. Although hepatic cysts are generally asymptomatic, compressive symptoms may develop if they become massive. Similar cysts may form diffusely throughout the liver in adult-type polycystic liver.
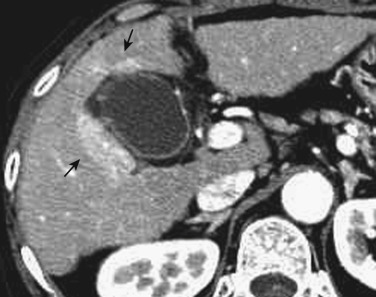
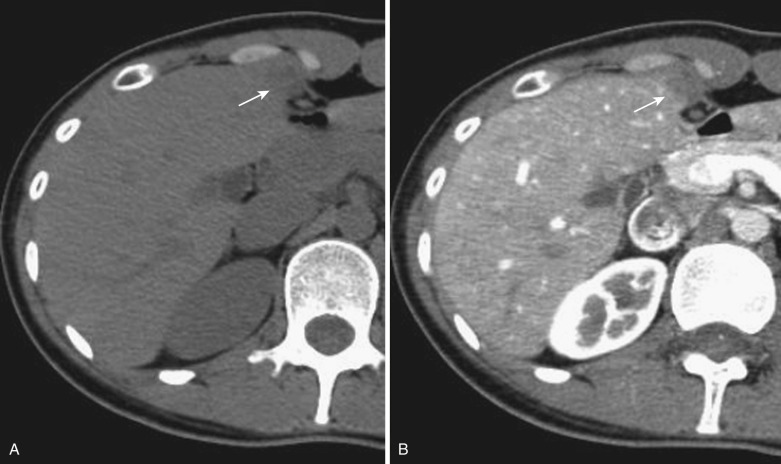
On CT a hepatic cyst is depicted as a smooth-rimmed hypodense mass with CT values near zero. They appear hypointense on T1-weighted images and extremely hyperintense on T2-weighted images. They show no enhancement at all on either postcontrast CT or MRI ( Fig. 44-9 ). When hemorrhage or inflammation occurs in the center of these cysts, the CT value increases and the signal intensity on MRI undergoes a variety of changes. Such lesions are referred to as complicated cysts (see Fig. 44-9 ).
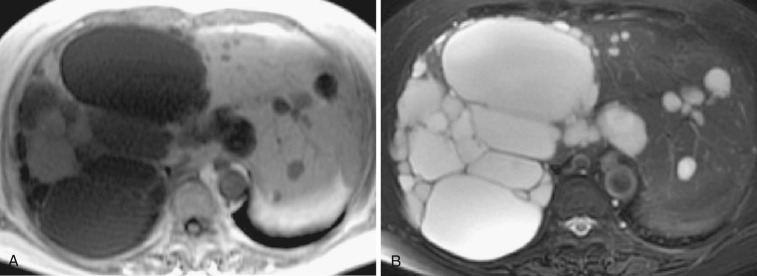
Polycystic liver disease can be seen in the pediatric population; when complicated by congenital hepatic fibrosis, it may be associated with portal hypertension and esophageal varices. Renal cysts are also present in approximately 70% of cases. Innumerable cysts are observed within the liver, with calcification and complicated cysts frequently noted. The imaging features of these cysts are basically the same as those of hepatic cysts (see Fig. 44-9 ).
Ciliated hepatic foregut cyst (CHFC) is a unilobular and well-demarcated cyst whose inner surface is ciliated and covered with mucin-producing cylindrical or cubic epithelium. Histologically they are the same as bronchogenic cysts. These cysts tend to occur on the anterior surface of the liver beneath the hepatic capsule. They contain mucinous fluid with various concentrations of proteins and fats. Although CHFC is typically benign, malignant transformation to squamous cell carcinoma has been reported in few cases.
The imaging findings of CHFC differ according to the concentration of mucin and the nature of the cyst's contents. On nonenhanced CT, CHFC is often depicted as an isodense nodule relative to the surrounding liver; it is sometimes hyperdense or slightly hypodense. On dynamic CT the cyst does not enhance at all. On T2-weighted MRI, the findings are the same as those of hepatic cysts. On T1-weighted MRI, hyperintensity due to the presence of high concentrations of mucin is noted in some cases ( Fig. 44-10 ).
Focal sparing in fatty liver shows relative hyperdensity on nonenhanced CT (see Fig. 33-5 ), hypointensity on T1-weighted images, and relative hyperintensity on opposed-phase T1-weighted images (see Fig. 44-5 ). This finding is usually related to disturbances of localized portal flow, and its configuration is occasionally wedge shaped.
Focal fatty liver is also strongly related to localized portal flow disturbances. Common locations and imaging features are as described earlier under the heading “Pseudolesion Due to Portal Vein Obstruction” (see Figs. 44-6 and 44-8 ). Focal fatty liver can mimic dysplastic nodules or well-differentiated HCC with fatty change in cirrhotic livers (see “ Hepatocellular Carcinoma ,” later), and the differentiation is often difficult when the change occurs in uncommon locations. Multiple focal nodular fatty infiltrations are rarely encountered and can mimic metastatic liver cancer, microabscesses, and biliary hamartomatosis ( Fig. 44-11 ). Opposed-phase MRI can identify the fatty change and is useful in differentiating among these entities (see Fig. 44-6 ).
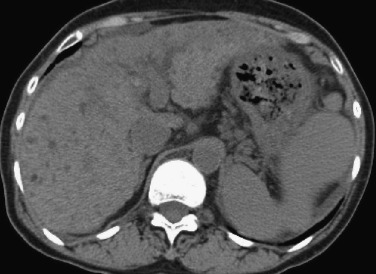
Focal iron deposition can be seen in areas with disturbed portal perfusion and results in segmental hypointensity on T2-weighted images.
Focal nodular hyperplasia (FNH) is defined as a tumorlike lesion characterized by a central fibrous scar with surrounding nodules of hyperplastic hepatocytes and small bile ductules in noncirrhotic livers. Most cases occur in women (the male-to-female ratio is 1 : 8). Because it is often associated with intrahepatic portal flow disturbance, FNH is now considered a reactive change to abnormal blood circulation. Unlike hepatic adenoma, the association with oral contraceptive use is still controversial. Clinically FNH is usually asymptomatic, with epigastric pain or hepatomegaly infrequently developing. Unlike in hepatic adenoma, intraperitoneal rupture is extremely rare. Grossly FNH is a well-demarcated solitary mass without a capsule, often located beneath the surface of the liver. However, it can be multiple and pedunculated from the liver. Internal bleeding and necrosis are not usually seen. In the central scar, feeding arteries, draining veins connected to the hepatic vein, and bile duct proliferation are seen. The feeding arteries distribute to the peripheral portion of the tumor through the central scar in a spoke-wheel pattern. Variable amounts of Kupffer cells are present in FNH.
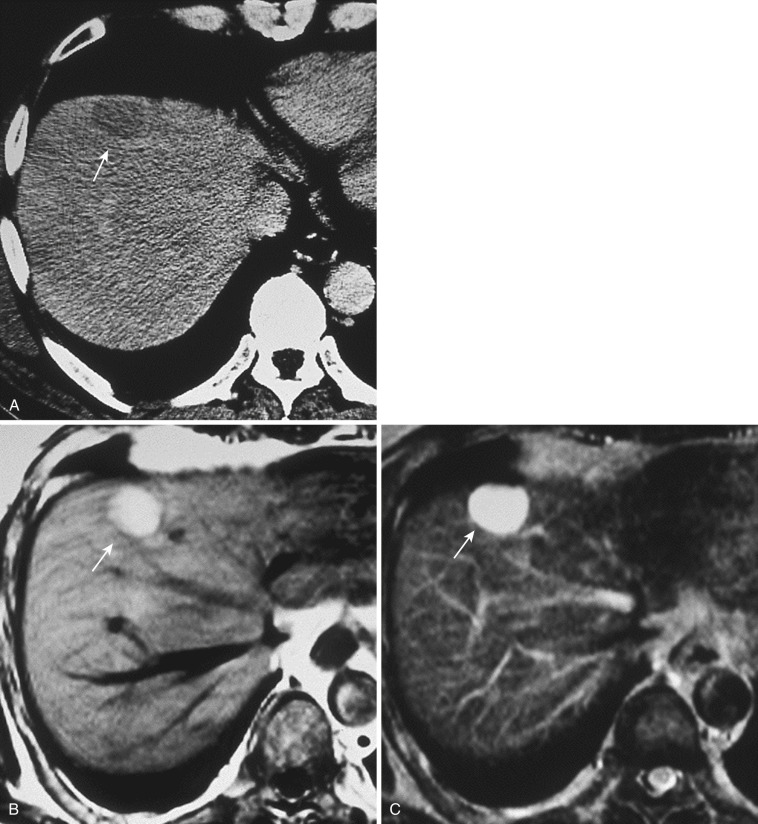
On imaging, FNH usually has a slightly elliptical shape. On nonenhanced CT it appears as a homogeneous hypodense mass with a central scar showing more marked hypodensity. However, in small lesions, visualization of the central scar is not definite on CT. The entire nodule is well and homogenously enhanced in the arterial phase of CT and MRI. The central scar is seen as more hypodense, but in small lesions it is depicted infrequently. If vessels radiating from the central scar to the periphery of the tumor are visualized, a near-definitive diagnosis of FNH can be made. In this spoke-wheel pattern, the feeding vessels penetrate the fibrous septum from the central scar and distribute to the tumor ( Figs. 44-12 and 44-13 ). This vasculature is most sensitively delineated by contrast-enhanced ultrasonography.
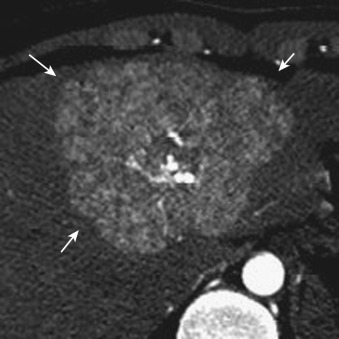
FNH in most cases shows hypointensity on T1- and hyperintensity on T2-weighted images. However, because the tissue composition of FNH resembles that of the surrounding liver, it can exhibit isointensity on T1- or T2-weighted images. The central scar is depicted as hypointense on T1- and hyperintense on T2-weighted images (see Fig. 44-12 ). The finding of hyperintensity on the latter is attributable to the presence of a rich vasculature within the scar tissue (vascular scar). On dynamic MRI, homogeneous early enhancement is seen, with persistent staining. The central scar shows delayed enhancement.
Both imaging and pathology studies have clarified that in FNH, the majority of the blood flow supplying the tumor from arteries refluxes to veins within the central scar and then enters the hepatic vein. The hemodynamics of FNH differs from that of hypervascular HCC (described later under “ Hepatocellular Carcinoma ”), and this difference is useful in the differential diagnosis. On dynamic CT and MRI of FNH, the margin of the stain is clear; hence washout of the contrast agent is not observed (see Figs. 44-12 and 44-13 ). In addition, direct draining from the tumor to the hepatic vein is occasionally depicted (see Fig. 44-12 ).
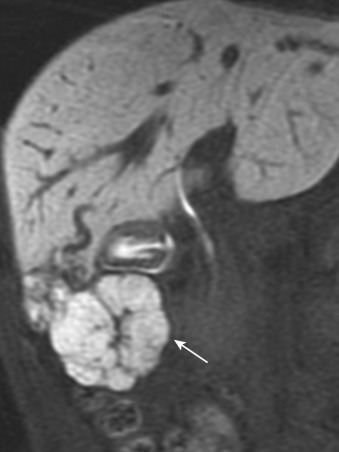
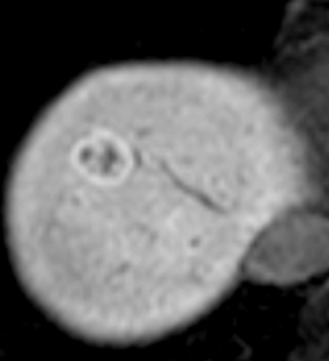
Because Kupffer cells are present in FNH, the signal intensity decreases on SPIO-enhanced MRI (see Fig. 44-12 ). The degree of uptake is variable depending on differences in the distribution density of Kupffer cells. Hepatobiliary agents such as mangafodipir trisodium (Mn-DPDP) and Gd-EOB-DTPA are useful in the characterization of FNH. Because the hepatocytes in FNH take up these agents, FNH shows hyperintensity on T1-weighted images enhanced by them ( Fig. 44-14 ). FNH often shows donut or ring-like peripheral hyperintensity on hepatobiliary-phase Gd-EOB-DTPA–enhanced MRI ( Fig. 44-15 ) because of lower expression of organic anion transporting polypeptide (OATP)1B3, which uptakes Gd-EOB-DTPA into hepatocytes in the hyperplastic hepatocyte surrounding the central scar.
Recently, telangiectatic FNH, which was formerly categorized as one of the subtypes of FNH, was categorized into the subtypes of hepatic adenoma (inflammatory adenoma).
FNH-like nodules are focal lesions occurring in cirrhotic livers that are morphologically similar to classic FNH in otherwise normal livers. They are sometimes misdiagnosed as HCC on imaging because both lesions show definite enhancement in the arterial phase of dynamic CT or MRI. These hypervascular lesions show early enhancement in the arterial phase of dynamic CT and MRI, with variable signal intensity on T1- and T2-weighted images. On Gd-EOB-DTPA and SPIO-enhanced MRI, uptake of these contrast mediums can be seen. Recently this kind of lesion has attracted particular attention in patients with alcoholic liver cirrhosis. Furthermore, recent immunohistochemical analysis revealed that around two thirds of these nodules associated with alcoholic cirrhosis could be categorized into inflammatory-type hepatic adenoma (named serum amyloid A [SAA]-positive neoplasm because the existence of hepatic adenoma in cirrhotic livers are not well accepted), different from the other one third demonstrating the immunohistochemical features of FNH. Interestingly the former shows hypointensity but the latter iso- or hyperintensity on hepatobiliary-phase Gd-EOB-DTPA–enhanced MRI.
Macroregenerative nodules are incidentally detected in patients with cirrhosis and Budd-Chiari syndrome. They are larger than the usual regenerative nodules in some cases. Histologically, macroregenerative nodules resemble the regenerative nodules seen in cirrhosis. They typically exhibit hypointensity on T2-weighted images and various signal intensities on T1-weighted images. On dynamic CT and MRI they generally do not enhance, although in Budd-Chiari syndrome they may be multiple and show enhancement ( Fig. 44-16 ).
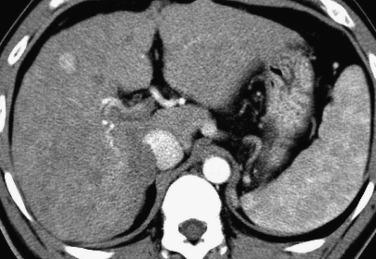
Nodular regenerative hyperplasia consists of multiple monoacinar regenerative nodules in the absence of fibrous septa, with increased intranodular portal supply. It is frequently associated with various nonhepatic chronic diseases, including myeloproliferative disorders (leukemia, polycythemia vera), lymphoproliferative disorders (lymphoma, plasma cell dyscrasia), and collagen diseases (rheumatoid arthritis, polyarteritis nodosa, lupus erythematosus). The pathogenesis of nodular regenerative hyperplasia remains unclear, but it may be similar to that of partial nodular transformation; the differences between these two entities are related to the cause and the distribution of the portal vein obliteration.
Imaging features of these nodules include hypointensity on T2-weighted images; in contrast, true hepatic tumors usually show hyperintensity on T2-weighted images. On T1-weighted images they typically demonstrate hyperintensity. These MRI findings are similar to those of the hyperplastic changes caused by aberrant venous drainage in cirrhotic livers and by dysplastic nodules or well-differentiated HCC. Gd-EOB-DTPA may show donut-type hyperintensity similar to FNH on hepatobiliary-phase Gd-EOB-DTPA–enhanced MRI ( Fig. 44-17 ).
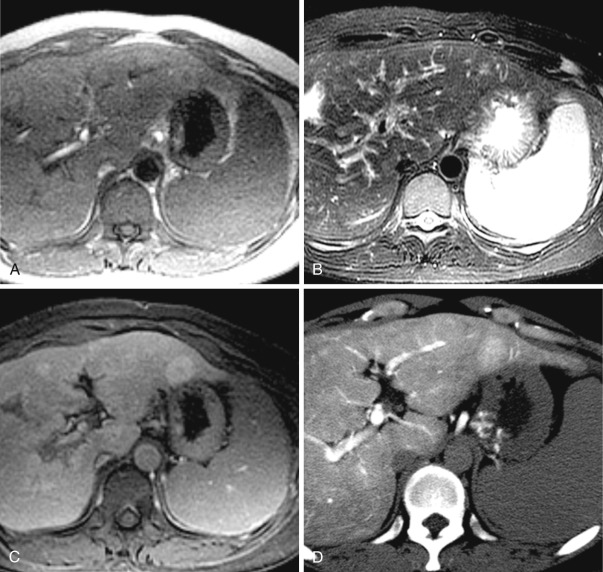
Peliosis hepatis consists of multiple small cystic lesions that communicate with sinusoids. Peliosis hepatis does not have endothelial cells in the cystic wall.
On nonenhanced CT, peliosis hepatis is hypodense. It shows enhancement relative to the background liver in the arterial phase of dynamic CT and becomes progressively isodense. Peliosis hepatis usually demonstrates hyperintensity on T2-weighted MRIs; on T1-weighted images it is hypointense compared with the background liver. On T1-weighted images after contrast enhancement, peliosis hepatis may exhibit a centrifugal enhancement pattern.
Biloma refers to a localized collection of bile that has escaped from the biliary tract, regardless of whether its location is intrahepatic or extrahepatic. Because the bile duct may rupture for a variety of reasons, including choledochal stones, bilomas may also form within the liver and subcapsular area. There has been a recent increase in the incidence of bilomas associated with bile duct injury after interventions such as transcatheter arterial embolization and radiofrequency ablation, as well as hepatic artery thrombosis following liver transplantation.
The imaging findings of biloma resemble those of hepatic cyst, with proximal bile duct dilatation frequently noted. If hepatobiliary scintigraphy reveals accumulation, a definitive diagnosis can be made. Hepatobiliary-phase Gd-EOB-DTPA–enhanced MRI may also help diagnose the presence of biloma.
Biliary hamartoma, also known as von Meyenburg's complex, is thought to be the result of a congenital defect in formation of the ductal plate. It is occasionally complicated by polycystic disease or peribiliary cysts. Biliary hamartoma is composed of bile ductule–like clusters surrounded by fibrous interstitium; it is not necessarily a rare condition, having been noted in 0.15% to 2.8% of cases in autopsy series. These nodules are usually small, in the range of 0.1 to 5 mm, but can occasionally measure up to 10 mm. In some cases they are limited to only one part of the liver; in other cases they are interspersed throughout.
On nonenhanced CT, biliary hamartoma is depicted as tiny hypodense nodules; they do not show water attenuation because of a partial volume effect. These nodules may be difficult to differentiate from hypovascular solid masses, particularly metastatic liver cancer, multiple microabscesses, and irregular fatty infiltration. On T2-weighted MRIs and MR cholangiopancreatography (MRCP), they characteristically show marked hyperintensity, reflecting their cystic nature ( Fig. 44-18 ).
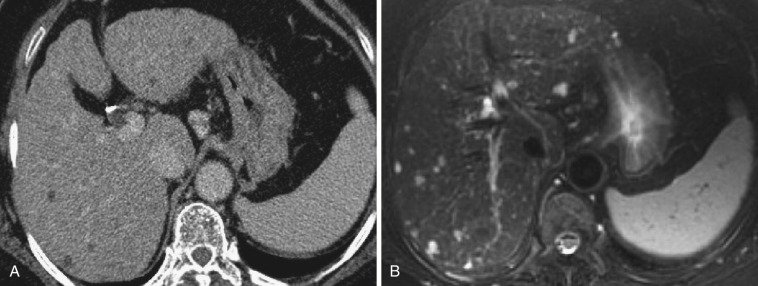
Peribiliary cysts are considered to be retention cysts of the peribiliary glands. They are frequently associated with polycystic liver and cirrhosis. In liver cirrhosis they gradually increase in size and number with disease progression.
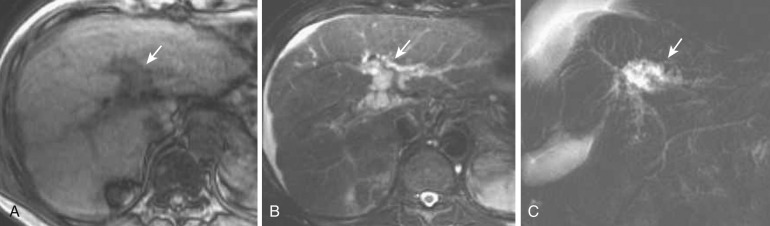
On ultrasonography they are depicted as round or tubular anechoic lesions situated along the larger portal tracts. On CT and MRI they are depicted as tubular lesions along the portal vein or as beaded communicating cystic lesions. However, in some cases, the findings resemble a periportal collar on CT and periportal abnormal signal intensity on MRI. Characteristically, lesions are present on both sides of the proximal intrahepatic portal vein. Peribiliary cysts can exhibit the “central dot” sign on enhanced CT, like that seen in Caroli's disease. MRCP is useful for obtaining the total picture of peribiliary cysts ( Fig. 44-19 ). On endoscopic retrograde cholangiopancreatography (ERCP), contrast medium does not flow into peribiliary cysts, which is useful in differentiating them from Caroli's disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here