Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radionuclide imaging today is an active and growing branch of medical diagnostics. Over the past decades, radionuclide imaging has advanced to become a cornerstone for the accurate diagnosis and appropriate management of a variety of medical conditions. Interestingly, however, it has its origins in the cardiovascular field: it was in the late 1920s when Herrmann L. Blumgart published his “Studies on the velocity of blood flow” in The Journal of Clinical Investigation , marking the birth of nuclear medicine [ ]. Through the following decades, the invention of the scintillation camera by Hal Anger [ ] and the commercial supply of readily available radiotracers have set the ground for its introduction in the clinical arena. The technique was further elaborated by the development of multiplane tomographic scanners, which have promoted its use for cardiac applications in daily clinical routine. Aside from 18 F-fluorodeoxyglucose positron emission tomography (FDG PET), the growth of nuclear medicine in the recent past has been mainly driven by the increasing use of single-photon emission computed tomography (SPECT) myocardial perfusion imaging studies. Today, over 10 million nuclear cardiology procedures are performed every year in the United States alone [ ]. However, cardiac SPECT has also raised public attention as a main contributor to the growing healthcare costs and increasing individual radiation exposure from diagnostic medical procedures that has been observed over the past two decades [ ]. This has prompted considerable efforts to limit radiation exposure, shorten acquisition protocols, and reduce costs, while at the same time improving diagnostic performance. The following sections provide an overview of the most important technical developments in cardiac SPECT imaging, including radionuclides, detector technology and camera design, and image reconstruction and processing. We place special emphasis on the most recent advances and developments that are currently becoming standard practice in many centers around the world or are likely to play an important role in the future.
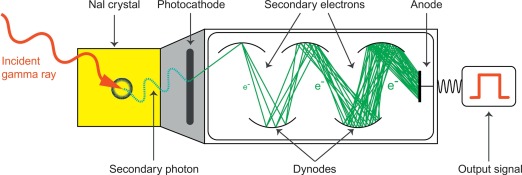
Gamma radiation refers to a form of electromagnetic radiation of very high frequency and high energy per photon, and consequently a deep penetration depth in human tissue (as opposed to relatively nonpenetrating, low-energy alpha and beta particles): the imperative necessity for all imaging purposes. The process of capturing emitted gamma rays from a radioactive source using a gamma camera is called scintigraphy ( Figure 3.1 ). The gamma camera contains one or multiple detectors made of an organic and optically clear crystal plane of very high density, a sensitive photomultiplier tube, and all the necessary electronics to process the photomultiplier tube output. To maximize electromagnetic interaction with the gamma rays, crystals made of elements with a high atomic number are implemented in the detectors, usually consisting of sodium iodide with thallium doping. When an emitted photon hits the crystal it causes the latter to scintillate, hence inducing an extremely short-lived (10 − 6 s) light impulse. In physical terms, the term scintillation describes the process of an electron in a crystal’s atom being dislocated (excited) by a gamma ray then returning to a stable energy state shortly afterward, thereby releasing a light impulse (scintillation). As the photons of this light impulse (secondary photons) reach the photomultiplier tube’s photocathode, the latter emits electrons as a result of the photoelectric effect. These electrons are then accelerated and directed by an electric potential so as to hit the first dynode of the tube. The impact of one single electron on the dynode releases a number of secondary electrons, thus creating a growing avalanche of secondary electrons that strike the next dynode: By this means, the electrical signal is being amplified to the extent of a measurable output signal representing the event of the initial photon hitting the photocathode ( Figure 3.1 ). Importantly, the output signal also inherits information about the energy of the original incident radiation on the scintillator. Thus, both intensity and energy of the radiation is being measured.
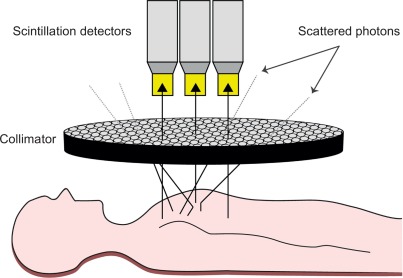
While this outlined principle permits the measurement of incident radiation, it does not allow for spatial allocation of the origin of gamma radiation per se because emitted gamma rays are subject to scattering within the human body, leading to stray gamma rays. To avoid misallocation, collimators consisting of parallel holes within a block of a material impermeable for gamma rays (i.e., mostly lead) are employed. The alignment of a collimator in front of the detector ensures that only parallel incident gamma rays strike the detector, thus allowing for two-dimensional allocation of the origin of the measured radiation ( Figure 3.2 ). The smaller and the longer the holes in the collimator, the fewer photons will get through to the scintillator, thereby increasing image resolution at the cost of lower count density and higher image noise. Conventional cardiac SPECT cameras are equipped with parallel-hole low-energy high-resolution collimators; however, some of the newer devices are mounted with pinhole collimators.
For the assessment of the heart and exact localization of myocardial perfusion defects, planar scintigraphy is not very well suited due to its two-dimensional nature. The advent of SPECT has therefore greatly improved the interpretation of myocardial perfusion scans: one or multiple scintillation detectors rotate in a step-wise fashion around the patient over an arc of at least 180°, enabling reconstruction of a tomographic data set of the heart using sophisticated reconstruction algorithms (see Section 3.5 ). The result is a three-dimensional image of the heart that allows the exact localization of perfusion defects within the myocardial wall ( Figure 3.3 ).
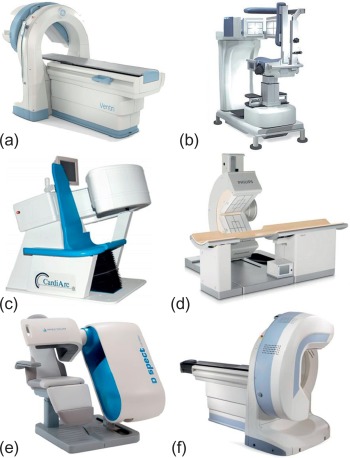
Since the introduction of the first SPECT camera, changes of camera designs were confined to optimizing the performance of sodium iodide detectors and to implementing multiple detectors in a single camera system. Furthermore, with the increasing demand for cardiac SPECT, manufacturers have released a number of devices that are specifically dedicated to cardiac imaging. These cameras are characterized by multiple small-footprint detector heads that center easily on the heart and allow imaging in supine or upright position, are compact and easy to fit in a small room, avoid claustrophobia, and improve patient comfort ( Figure 3.4 ). Despite their common purpose, however, devices from various vendors still offer many different features with regard to detector technology, collimator design, and software solutions (see later discussion). These advances have led to a substantial reduction of the time needed for image acquisition. Due to the step-wise fashion of tomographic image acquisition by rotation around the patient’s chest, the total time needed for completion of a 180° arc with a single detector head system used to be considerable. The advent of dual-head systems and, more recently, triple-head systems, however, has enabled nuclear cardiologists to perform an examination within 10–20 min.
A recent revolutionary milestone in technical innovation for cardiac SPECT has been the introduction of semiconductor detector technology using synthetic crystals composed of cadmium-zinc-telluride (CZT). CZT is artificially grown as a single crystal; it has a very high density and effective atomic number with a higher attenuation coefficient for photons than that of conventional sodium iodide crystals, allowing the application of thinner detectors. More importantly, however, the mechanism of function of CZT detectors differs fundamentally from a conventional sodium iodide detector: CZT detectors allow direct conversion of light into an electrical signal. When a photon hits the CZT detector, the impact generates directly an anion and a cation. Through application of an electric field, the charge carriers are swept to the cathode and anode of the system, inducing a current pulse that can be recorded. Thus, contrary to conventional detectors, CZT detectors avoid the need for bulky photomultiplier tubes, thereby reducing the size of the detector head and improving the theoretical in-plane resolution.
A CZT detector consists of the crystal and multiple underlying anodes, each reflecting a pixel. If, for example, a detector consists of 16 × 16 pixels, each with an edge length of about 2.5 mm, this results in a form factor that is strikingly smaller than a conventional scintillation detector ( Figure 3.5 ).
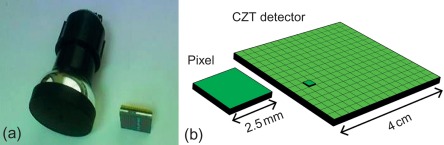
The intrinsic spatial resolution of current CZT detectors is approximately 4 mm, which is substantially better than the typical 10 mm achieved with conventional sodium iodide detectors [ ]. One of the reasons for this increase is that due to thinner crystals in CZT detectors, the charge carriers are detected by an anode that is only millimeters away from the original photon–crystal interaction. This allows a much more accurate spatial localization of the original incident than with a conventional detector where “triangulation” to the photon’s origin happens at the level of the photomultiplier several centimeters away from the crystal. Therefore, spatial resolution in a CZT detector is mostly only limited by pixel size, which in turn is restricted by how densely the electronics needed for recording power currents can be packed. Thus, as intrinsic resolution is mainly dependent on the size of a pixel, it can be expected to improve further with advancing miniaturization.
However, it has to be taken into account that superior intrinsic properties of a detector per se are meaningless without similar extrinsic properties that are set by the surrounding system design. For example, the most important extrinsic factor influencing the system trade-off between sensitivity and resolution is the collimator: a high-resolution collimator depicts only a very narrow column of activity from the patient, and therefore provides excellent spatial resolution at the expense of sensitivity. By contrast, a high sensitivity collimator accepts incoming photons from a wider range of angles, therefore increasing sensitivity at the expense of resolution. In novel camera systems, the high intrinsic resolution of CZT detectors allows the use of parallel-hole or multipinhole collimators with relatively large holes focused on the heart in order to improve count sensitivity.
Moreover, CZT detectors also show better energy discrimination than sodium iodide scintillation detectors, allowing a distinction between scattered and unscattered radiation more accurately, and thereby further improving image resolution [ ]. Accurate energy resolution is also a prerequisite for simultaneous imaging with more than one isotope; this is an area of ongoing research for CZT-based gamma cameras.
While all cameras have a specific limitation regarding the maximum count rates that can be measured, SPECT systems based on conventional scintillation detectors additionally show a nonlinear response with rising count rates leading to a roll-off and even to paralysis at high count rates (due to a detector “dead-time” that exceeds the rate of incoming photons). CZT detectors, by contrast show a linear count rate response up to the system maximum. The latter is limited only by computational power of the system. Hence, at present, the count rate performance of a CZT camera is twice as high as that of a conventional SPECT camera.
However, the most important advantage of CZT detectors arises from miniaturization of the detectors with inherent advantages for the geometry of the scanner. The small size of the CZT detectors allows for a detector geometry that rather resembles the design of a PET (positron emission tomography) more than a SPECT scanner. A large array of detectors is mounted on a detector arch and aligned in a curved geometry around the patient in order to face specifically the heart and thereby increase true counts from the area of interest. Instantaneous acquisition of cardiac activity from different projection angles becomes possible ( Figure 3.6 ).
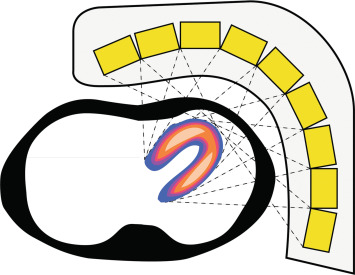
In summary, while the CZT detectors themselves constitute a milestone of technological development in terms of sensitivity, spatial and energy resolution, the true leap forward in innovation arises from the combination of such detectors with new collimator designs and modern iterative reconstruction algorithms in novel dedicated cardiac camera systems that are preferably equipped with a stationary array of multiple detectors. It is the combination of the above that has led to a 5–10-fold increase in system sensitivity and a twofold increase in image resolution and allows imaging of the heart within less than 5 min ( Figure 3.7 ), while at the same time yielding images with substantially better image quality at higher resolutions than state-of-the-art conventional SPECT cameras ( Figure 3.8 ).
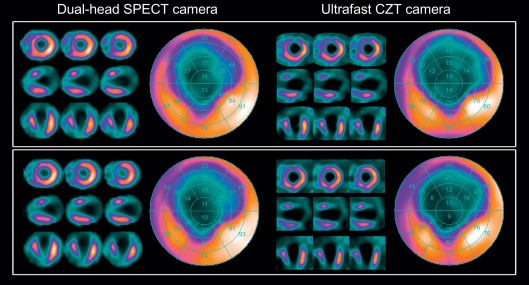
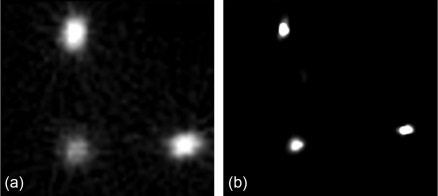
The clinical impact of shorter acquisition time lies not only in improved patient comfort and increased patient throughput and greater scanner efficiency. As an alternative to shortened acquisition time, this new technology can be used to reduce tracer activity and, consequently, effective radiation exposure (see also Section 3.6 ).
The ideal tracer for myocardial perfusion and viability SPECT imaging should have biochemical properties that allow it to be entirely and evenly distributed solely within the myocardium after high first-pass extraction from the blood, to be biologically inert, to be retained within the myocardium until imaging is completed and to be completely extracted afterward. Regarding physical aspects, it should emit gamma rays at a high energy, have a relatively short half-life, and be able to be easily and cost-effectively manufactured. While such an ideal tracer does not exist, the available radiopharmaceuticals today offer a variety of advantages and disadvantages, demanding from the nuclear physician a profound knowledge of the tracer to distinctively utilize the respective tracer for certain clinical questions. The following section provides an overview on the most common tracers along with a brief outlook on future trends.
Radioactive tracers are synthetic chemical compounds consisting of an endogenous or exogenous carrier molecule that partakes in human metabolism and in which one or more atoms have been replaced by a radioisotope through which its natural decay allows for imaging of the compound. Today’s most commonly used radioisotope for cardiac SPECT is the metastable 99m-Technetium ( 99m Tc), which is available in different commercially available compounds such as sestamibi (Cardiolite®, Du Pont™), tetrofosmin (Myoview®, GE Healthcare™), and teboroxime (CardioTec®, Squibb Diagnostics™). By contrast, 201-Thallium ( 201 Tl) acts as a potassium analogue and can therefore be used for imaging without the need of a biochemical carrier molecule as it partakes in myocardial metabolism, mostly via Na + /K + —adenosine triphosphate sarcolemmal membrane transport system. Although 201 Tl provides certain advantages over 99m Tc-labeled agents, such as lower gastrointestinal activity or redistribution properties that render additional resting injection potentially redundant, 99m Tc-based tracers are often preferred for their higher energy and their shorter half-life leading to higher count rates and better image quality at a lower radiation exposure. Table 3.1 provides a summary of the physical properties of the most commonly used radioisotopes.
| Emission energy (keV) | Radioisotope half-life (h) | First pass extraction (%) | Redistribution | |
|---|---|---|---|---|
| 201 Tl | 75 | 73 | 82–88 | Yes |
| 99m Tc-sestamibi | 140 | 6 | 55–68 | No |
| 99m Tc-tetrofosmin | 140 | 6 | 54 | No |
Due to their particular biochemical properties, the time-course of uptake and clearance of the presently available 99m Tc-compounds differs dramatically: sestamibi and tetrofosmin have a medium uptake rate and a very slow myocardial clearance allowing imaging for several hours after injection, whereas 99m Tc-teboroxime is a boronic acid adduct of technetium dioxime complex that is highly extracted at the myocardial first-pass and shows rapid blood clearance allowing imaging immediately after injection but only within a very limited time frame (circa 10–15 min). Hence, teboroxime has been mostly replaced by sestamibi and tetrofosmin due to effectiveness considerations in daily clinical routine. However, teboroxime may experience a future revival along with the technical advances in cardiac SPECT due to its higher and—more importantly—much more linear myocardial uptake even at high myocardial blood flows, rendering it a potentially ideal tracer for quantitative assessment of myocardial blood flow in combination with the newest generation of CZT scanners [ ]. The lipophilic neutral, 99m Tc-labeled compound 99m Tc-N-NOET shares many characteristics with teboroxime, including its high first pass extraction by the myocardium and an uptake that correlates with myocardial blood flow over a wide range of flow, but is not commercially available in Europe. Furthermore, there is ongoing research into the development of new SPECT tracers, noteworthy a group of 99m Tc-labeled agents that have crown ether functional groups (e.g., 99m Tc DBODC5, 99m Tc-N-MPO, or 99m Tc-15C5-PNP), which lead to a more rapid clearance from the liver, potentially enabling better delineation of the inferior wall of the left ventricle. Other research focuses on novel tracers with improved flow versus extraction relationship (e.g., 125 I-ZIROT). However, many of these tracers remain experimental and have not yet reached the clinical arena.
While the tracers mentioned above are used for myocardial perfusion and viability imaging, other tracers can be used to assess myocardial innervation. The 123-Iodine labeled meta-iodobenzylguanidine ( 123 I-MIGB) resembles the biochemical structure of noradrenaline and is thus taken up by presynaptic catecholamine transporters allowing imaging of the sympathetic innervation of the myocardium. Increased cardiac sympathetic innervation leads to reduced presynaptic uptake of the noradrenaline analogue 123 I-MIBG and has been associated with adverse left ventricular remodeling, progression of heart failure and increased risk of sudden cardiac death in heart failure patients [ ].
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here