Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Besides managing the injuries and illnesses from common disasters such as earthquakes and airplane crashes, emergency clinicians should also have competence in treating victims generated by terrorist attacks with chemical, nuclear, biologic, or high-energy explosive weapons. Conventional explosives remain the most common weapon used by terrorists; however, the risk from nuclear, biologic, and chemical agents may increase over time. The nomenclature for these weapons is not standardized. The military uses the acronym CBRNE, pronounced “see-burn-ee,” referring to c hemical, b iologic, r adiologic, n uclear, and e xplosive agents. This chapter uses weapons of mass destruction (WMD) because of its wide acceptance and familiarity.
The results of an attack with WMD, although admittedly of low probability, are potentially catastrophic. According to a World Health Organization (WHO) estimate, 50 kg of anthrax spores aerosolized above a city of five million people would result in 100,000 deaths, with an additional 150,000 people seriously infected. The cost of managing 100,000 cases of anthrax exposure is estimated at between $6.4 and $26.2 billion. These types of estimates have led to authorities establishing emergency preparedness as a priority.
Children are particularly vulnerable to these weapons. They are closer to the ground based on height and breathe at a faster rate than adults, increasing their relative exposure to aerosolized agents. Some chemicals, such as sarin, are heavier than air, so they tend to accumulate at the level where children are more likely to inhale them. Children have a greater surface area–to–volume ratio and their skin is thinner. This makes them more susceptible to agents that act on or through the skin. They have smaller fluid reserves and higher metabolic rates. Therefore, they are more vulnerable to dehydration from vomiting and diarrhea and suffer increased toxicity from a given exposure, such as to radioactive iodine ( 131 I).
The use of biologic and chemical agents as weapons dates to biblical times, although the threat from radiation and nuclear detonation is relatively new. Assyrians poisoned the wells of their enemies with rye ergot in the 6th century bc . During World War I, the Germans effectively used chlorine and mustard agent against the advancing Allied armies. The Japanese killed hundreds to thousands of Chinese citizens with bubonic plague during World War II by spraying towns with fleas infected with Yersinia pestis . In 2013, the Syrian government attacked cities within its own country using chemical nerve agents, resulting in an estimated 1300 deaths and 3600 injuries. United States government reports indicate that the Syrian government again used chlorine gas in May 2019.
WMD have been predominantly deployed by the military during times of conflict. Toward the end of the twentieth century, however, nonaffiliated terrorist groups began using WMD directed at civilians to achieve political ends. The Aum Shinrikyo used sarin gas in the 1995 Tokyo subway attack that killed 11 people. Terrorists initiated an anthrax attack using the United States mail in 2001 that resulted in 11 deaths. In 2018 and 2020, the fourth-generation nerve agent Novichok was reportedly used during a presumed assassination attempt, leading to four hospitalizations. As of early 2020, no one has used radiologic or nuclear devices in a successful mass terrorist attack; however, several highly radioactive sources have been stolen from American medical facilities, and a Russian dissident, Alexander Litvinenko, was assassinated with a radiologic agent ( 210 Po) in 2006 while living in London.
Many agents are potential candidates for weaponization, and some represent a substantial risk ( Box e15.1 ). Management strategies for patients exposed to WMD are frequently similar to strategies for hazardous materials exposure. However, several features associated with WMD make these events unique ( Box e15.2 ). Additional knowledge and skills are required in the evaluation and treatment of WMD victims. These plans represent only one small part of an overall comprehensive emergency management strategy for all hazards (see Chapter e14). Names of departments, bureaus, and agencies that can assist with planning and response to WMD events are listed in Table e15.1 .
Fear of unknown or unfamiliar
Lack of training for hospital personnel
Lack of equipment, including personal protective equipment (PPE) and diagnostic aids
Potential for mass casualties
Psychological casualties
Crime scene requiring evidence collection and interaction with law enforcement
Potential for ongoing morbidity and mortality (dynamic situation)
| Organization | Website | Telephone |
|---|---|---|
| Radiation Emergency Assistance Center/Training Site (REAC/TS) | orise.orau.gov/reacts/ | Daytime: 865-576-3131 Emergency: 865-576-1005 |
| State and local health departments | ||
|
www.astho.org/statepublichealth/ | (202) 371-9090 |
|
https://www.cdc.gov/publichealthgateway/healthdirectories/healthdepartments.html | |
| Centers for Disease Control and Prevention (CDC) | www.cdc.gov | 800-CDC-INFO |
| Federal Bureau of Investigation (FBI) | www.fbi.gov | |
| Federal Emergency Management Agency (FEMA) | www.fema.gov | 800-621-FEMA |
| U.S. Army Medical Research Institute of Chemical Defense | https://usamricd.apgea.army.mil/ |
Terrorists selecting radiation as a means to inflict casualties are unlikely to use nuclear weapons. These devices are heavily guarded, difficult to move because of their size and weight, and easy to detect. Although Russia acknowledges that 50 to 100 of its one-kiloton “suitcase” nuclear weapons are missing, the challenges associated with purchasing, moving, and detonating these devices are formidable. Sabotage at nuclear power stations is possible, but given tight security, multiple safety systems, and thick concrete housings surrounding the reactors, the threat is relatively low.
Simple radiologic devices, such as those used by hospitals for radiation therapy or commercial companies for industrial radiography, are more readily accessible. These sources are plentiful and often found in small, easily concealed containers. They do not detonate on their own and give no warning of their presence unless they are dispersed by a conventional explosive (radiologic dispersal device). Thefts of radiotherapy sources have occurred in the United States. Accidental dispersion from a stolen hospital therapy source in Brazil resulted in the screening of 112,000 people for contamination. A total of 249 people were found to be exposed—four of whom ultimately died. Placement of such a device at an information kiosk in a crowded mall during a busy holiday shopping season would silently expose countless persons to significant radiation.
Ionizing radiation, regardless of its type, causes injury at the cellular level, usually by damaging DNA. Rapidly dividing cells are the most sensitive. Patients have symptoms within hours to days, depending on the dose. Common syndromes associated with radiation exposure include cutaneous (burns), hematologic (bone marrow failure), gastrointestinal (e.g., vomiting and gastrointestinal bleeding), and at extreme doses, neurologic (seizure, coma, death). Reviews of medical treatment for radiologic casualties can be found elsewhere (Chapter 134).
A basic emergency department (ED) radiation protocol should address decontamination, triage, staff safety, personal protective equipment (PPE), and diagnostic procedures that emphasize radiation monitoring. Victims presenting to the ED will suffer from three types of exposure: irradiation, internal contamination, and external contamination. Irradiated victims have been exposed to a beam of radiation, similar to someone undergoing a chest x-ray examination. They are not radioactive and pose no threat to ED personnel.
Contaminated patients are more challenging, and early involvement of the radiation safety officer is critical. This individual evaluates the degree of the victim’s contamination and monitors radioactivity levels throughout the decontamination process. Internally contaminated patients present a therapeutic challenge because they have radioactive material inside their bodies (e.g., lungs and gastrointestinal tract) or incorporated into their cells. They should be placed in an isolation room where all secretions and body fluids can be collected. Various medications are available for administration to internally contaminated patients that can limit uptake or facilitate removal of certain radioactive elements. These medications include Prussian blue (Radiogardase) for cesium and thallium ingestions, diethylenetriaminepentaacetic acid (DTPA) for plutonium exposure, and potassium iodide for 131 I exposure for patients under 40 years of age within a few hours of exposure. Radiation Emergency Assistance Center/Training Site (REAC/TS; http://orise.orau.gov/reacts/ ) is always available at 865-576-3131 (emergency number: 865-576-1005) to provide assistance to health care providers.
Externally contaminated victims have radioactive material on their skin or clothing and are decontaminated by removal of clothing and washing with soap and water. Medical stabilization and lifesaving interventions take precedence over decontamination. Washing by protected personnel should continue until monitoring shows a level no more than double the background rate or when no further decrease is happening after multiple decontamination attempts. If wounds are present, they are decontaminated first, along with mucous membranes. It is imperative to remove foreign bodies. After the wounds are covered with a sterile, waterproof dressing, the remaining skin is washed. Hospitals should be prepared to decontaminate patients because historical data suggest that up to 80% of patients do not receive this intervention before arrival. Decontamination before hospital entry is crucial because these individuals can expose caregivers to radiation and contaminate the entire hospital through the ventilation system. Removal of clothing and covering of the head with a surgical cap can reduce contamination by 80% to permit stabilization in the decontamination unit, but complete decontamination should occur before exposure of unprotected staff if the patient’s medical condition permits.
Initial triage of radiation casualties is based on their overall condition, not on exposure. Even patients who have received a lethal dose of radiation do not die immediately as a consequence of the ionizing exposure. Therefore, a patient in acute distress from a myocardial infarction, sepsis, or a combined radiation-blast injury would be triaged ahead of a radiation victim with stable vital signs, regardless of the dose received. If a radiation casualty also suffers a severe injury or illness, immediate intervention is required. Most of the immediate morbidity and mortality associated with a radiologic dispersion device is related to traumatic injuries from the explosion and not the radiation exposure.
In addition to contaminated patients, the radiation safety officer is responsible for monitoring exposure to hospital staff. All personnel involved in the care of contaminated patients should wear dosimeters, which measure the amount of radiation received by the wearer. The safety officer tracks the amount of radiation received by each staff member and can remove a health care worker from the area if exposure exceeds Occupational Safety and Health Administration (OSHA) guidelines. Radiation monitoring is complex, and the radiation safety officer should be involved as early as possible. Hospitals should consider conducting disaster drills that include casualties suffering radiation injuries.
Although many radioactive elements are candidates for use in a terrorist attack, iodine-131 ( 131 I) and related isotopes deserve additional discussion because of heightened interest and ease of 131 I effective therapy. 131 I is found after a nuclear detonation or in reactor fuel rods. Although it is not impossible, the probability that terrorists could tap either of these sources is small. The use of 131 I in a radiologic dispersal device is unlikely because of its short half-life (eight days). The large number of childhood thyroid cancers that occurred after the accident at the Chernobyl nuclear power plant resulted, to a significant degree, from situations that will be unlikely to occur in the United States. These include delayed reporting of a breach in the reactor containment vessel preventing timely evacuation of all exposed populations; failure to effectively prevent ingestion of contaminated milk and vegetables; and presence of significant iodine deficiency in the exposed population.
The risk to children in communities surrounding the Fukushima nuclear power plant is an issue that will require long-term monitoring, though it is believed that exposures were not high enough to increase the incidence of long-term effects (cancer) beyond the background rate. Nonetheless, treatment of children aimed at preventing thyroid cancer after potential exposure to 131 I should be performed ( Table e15.2 ). Caveats for use of this table include increasing the amount of potassium iodide (KI) for adolescents approaching 70 kg to the adult dose (130 mg) and monitoring thyroid-stimulating hormone and free thyroxine (T 4 ) levels in neonates. Nonpregnant adults older than 40 years of age are unlikely to benefit from this intervention; therefore, current recommendations are to withhold KI unless doses over 5 Gy are received.
| Subpopulation | Predicted Exposure (cGy) | Potassium Iodide Dose (mg) | Number of 130-mg Tablets |
|---|---|---|---|
| Adults >40 years old | >500 | 130 | 1 |
| Adults 18 to 40 years old | ≥10 | 130 | 1 |
| Pregnant and lactating women | ≥5 | 130 | 1 |
| Children 3 to 18 years old | ≥5 | 65 | 1⁄2 |
| Children 1 month to 3 years old | ≥5 | 32 | 1⁄4 |
| Neonates, birth to 1 month old | ≥5 | 16 | 1⁄8 |
By convention, biologic weapons are divided into three groups: bacteria, viruses, and toxins. A characteristic shared by these agents is their ability to be dispersed as an aerosol. Because this is the most effective means to expose a large population, aerosol dispersal is the route that terrorists would most likely use to deploy such weapons. Victims, unaware of the exposure to a biologic weapon, commonly present to the ED with nonspecific influenza-like illness (ILI). Dermal contact and ingestion are also potential pathways for exposure, and some agents are harmful via these routes. People infected in the 2001 United States anthrax attacks were inoculated through aerosol and dermal exposures. It is logistically more difficult to produce large casualty numbers by nonrespiratory portals of entry, so agents spread primarily by injection or through the gastrointestinal tract are less likely candidates for wide deployment. If the goal is to disrupt the economy or to spread fear among the population, then almost any type of release will suffice, whether or not mortality occurs.
Patients exposed to biologic agents usually present with vague symptoms associated with an ILI. Unless a biologic attack is announced or suspected, the ED staff may not realize that they are treating victims. It is not always possible to distinguish natural occurrences from engineered outbreaks of diseases. Examples of non-terrorist-related occurrences of anthrax include cutaneous disease in intravenous heroin users in Europe, an outbreak of cutaneous anthrax in Bangladesh in 2010 with more than 400 cases, and isolated infections in drum makers after using contaminated animal hides. Because of the challenges in identifying the true etiology of acute events, personnel should consider the possibility, especially when warning signs are present ( Box e15.3 ). For example, large numbers of patients suddenly presenting with “the flu” outside of the influenza season should cause concern. For these reasons, health surveillance is paramount in identifying agents and potential sources. National monitoring programs such as BioWatch (proposed to be replaced by BioDetection 21 by 2025) monitor the ambient air for infectious agents. ED leadership should have a working relationship with local and state health departments as well as with local law enforcement and stay apprised of Centers for Disease Control and Prevention (CDC) and Department of Homeland Security guidelines.
Several infectious agents with potential for use as biologic weapons can spread in a hospital environment. Examples include Ebola and smallpox. Hospitals need protocols for PPE and patient isolation to ensure a safe environment. Such protocols are similar to those applied to other infectious diseases ( Box e15.4 ) in non-terrorist events (e.g., the 2014 Ebola outbreak). Implementation of such precautions is credited with halting the in-hospital spread of the Ebola virus in the 1995 Zaire outbreak. Decontamination is not a priority unless the exposure is acute. Standard precautions are usually sufficient, and special suits (e.g., levels A and B) are generally unnecessary, though Ebola precautions require covering all cutaneous surfaces. Table e15.3 shows the recommended isolation precautions for each infectious agent.
Isolate patient in single room with adjoining anteroom.
Have handwashing facilities and personal protective equipment (PPE) available in anteroom.
Use negative air pressure if possible.
Use strict barrier precautions: PPE, gowns, gloves, high-efficiency particulate air (HEPA) filter respirators, shoe covers, protective eyewear, N95 masks, or powered air purifying respirator (PAPR).
Alert hospital departments that generate aerosols: Laboratory (centrifuges), pathology (autopsies).
For some agents (i.e., Ebola) all skin surfaces must be covered by PPE.
| Infectious Agent | Recommended Isolation Precautions |
|---|---|
| Anthrax | Cutaneous infection: contact precautions Pulmonary infection: standard precautions Gastrointestinal infection: standard precautions |
| Botulism | Standard precautions |
| Plague | Standard and droplet precautions ∗ |
| Smallpox | Standard, contact, and airborne precautions |
| Tularemia | Standard precautions |
| Viral hemorrhagic fevers | Standard, contact, and airborne precautions |
∗ Some experts recommend airborne precautions due to potential for aersolization.
Whereas the CDC lists six Category A (high threat) agents (anthrax [Bacillus anthracis], botulism [ Clostridium botulinum toxin], plague [Yersinia pestis], smallpox [variola major], tularemia [Francisella tularensis], and viral hemorrhagic fevers [filoviruses (e.g., Ebola, Marburg)] and arenaviruses [e.g., Lassa, Machupo]), this chapter focuses on three biologic agents—anthrax, plague, and smallpox—that represent the greatest interest.
Bacillus anthracis, a gram-positive, spore-forming bacterium, is the causative agent of anthrax (“woolsorter’s disease”). The spores are extremely hardy and can survive for years in the environment. The disease is caused by exposure to the spores, not the bacilli, in their vegetative state. It is normally a disease of sheep, cattle, and horses and is rarely seen in developed countries because of animal and human vaccination programs. Disease in humans can occur when spores are inhaled, ingested, or inoculated into the skin. The spores germinate into bacilli inside macrophages. The bacteria then produce disease by releasing toxins (e.g., protective antigen, edema factor, and lethal factor) that cause edema and cell death.
Russia and the United States have developed anthrax into a biologic weapon. The effectiveness of this agent was clearly demonstrated by two events: an accidental release of spores from a biologic weapons facility in the former Soviet Union town of Sverdlovsk in 1979 and the intentional distribution of anthrax spores through the mail along the eastern seaboard of the United States in 2001. After the Sverdlovsk release, at least 66 adults died downwind from the compound during the next several weeks, and animal cases of anthrax were reported 30 miles away. The ability of non-state-sponsored terrorist groups to develop anthrax as a weapon is uncertain. The Japanese organization Aum Shinrikyo made several unsuccessful attempts to disperse anthrax throughout Tokyo. The individual believed responsible for the United States anthrax attack was not a foreign national. This is consistent with the fact that the strain of anthrax used in the attack (Ames strain) was developed by the United States government.
Inhalational anthrax is the most lethal form of the disease and is caused by inhalation of spores into the lungs. The mortality rate was thought to exceed 90%. However, data from the 2001 anthrax exposure call this figure into question (5 deaths in 11 cases). Although the actual mortality rate is unknown, and would depend on availability of intensive care resources, it is probably in the 50% range. The minimum number of spores required to produce disease is unknown. The original number quoted in the literature—1000 spores—appears high given the experience following the 2001 anthrax event.
After phagocytosis by macrophages, the spores germinate and are transported to the tracheobronchial lymph nodes, where the bacteria multiply. During the next 2 to 10 days, patients have an ILI with malaise, fever, and nonproductive cough. This initial phase can be delayed for more than one month. Within 24 to 48 hours of the initiation of symptoms, abrupt deterioration occurs with overwhelming sepsis, shock, hemorrhagic mediastinitis, dyspnea, and stridor. A chest radiograph obtained at this time may show a widened mediastinum and hilar adenopathy, but typical radiographic findings are not dramatic and are easy to miss ( Fig. e15.1 ). Computed tomography (CT) scanning of the chest is more sensitive and should be performed if the disease is suspected. Bloody pleural effusions can also occur, and examination of the lung fields frequently reveals consolidation. This can easily be confused with severe community acquired pneumonia ( Fig. e15.2 ). Death usually results within three days, and 50% of patients have hemorrhagic meningitis. Human-to-human transmission has not been reported with inhalational anthrax.
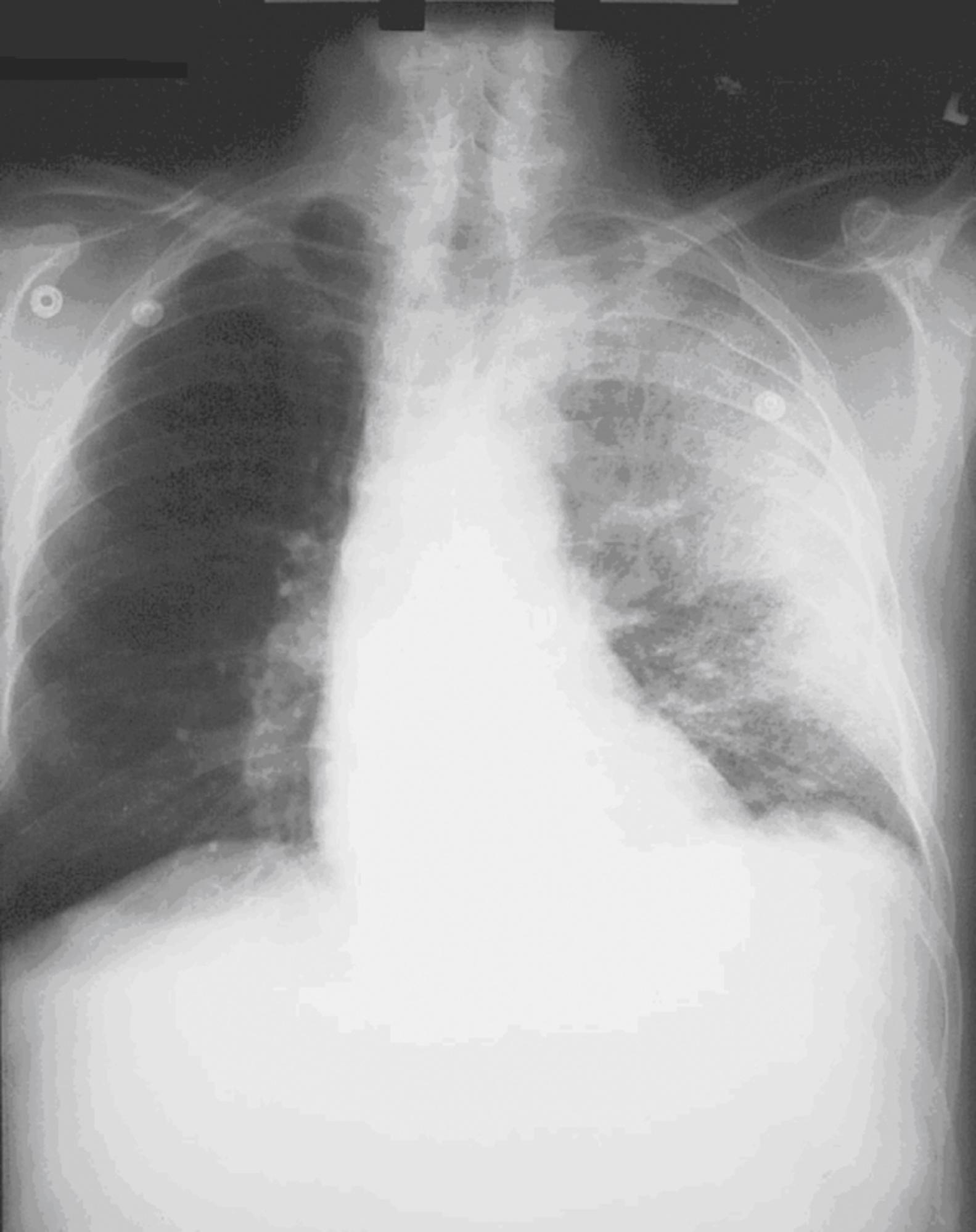

Initial diagnosis is generally made clinically on the basis of an influenza-like or septic illness; a suspicious chest radiograph or CT scan demonstrating hilar adenopathy, infiltrates, or pleural effusions; and a reason to consider anthrax in the first place (e.g., current outbreak or warning from authorities). Several clinical algorithms exist that attempt to separate patients with influenza from those with anthrax. Because these are based on a handful of anthrax cases, their usefulness is uncertain. Sputum culture, Gram stain, and blood cultures are not helpful until late in the course of the disease. Tests to confirm the diagnosis of inhalational anthrax include the polymerase chain reaction (PCR) for identification of anthrax markers in pleural fluid, serologic detection of immunoglobulin to protective antigen, and immunohistochemical testing of biopsy specimens. Recombinase polymerase amplification (RPA) assays for rapid identification of B. anthracis in a field setting have proven both sensitive and specific.
In addition to inhalational anthrax, cutaneous anthrax can occur in any area where large numbers of spores are released. This form of the disease occurs when spores are introduced into the skin, usually through open wounds or abrasions. The mortality rate is approximately 20% without treatment but decreases to 1% with treatment. After an incubation period of one to five days, a papule develops, progressing to form a large vesicle during the next several days. Severe edema occurs around the lesion and is associated with regional lymphadenitis. The lesions are not tender, and the patient may or may not be febrile ( Fig. e15.3 ). After approximately one week, the lesion ruptures, forming a black eschar (thus the name anthrax, from the Greek word for “coal”). In the next two or three weeks, either the eschar sloughs off and the illness resolves or the organism disseminates and the patient dies. Antibiotics do not halt the progression of local disease; they prevent dissemination and death. As with inhalational anthrax, the diagnosis is predominantly clinical. Confirmation is established by culture of the lesion, punch biopsy, or serologic testing. A total of 11 cutaneous anthrax cases occurred in the United States after the 2001 attack.

Cases of gastrointestinal anthrax and oropharyngeal anthrax are also possible after a terrorist attack. These rare manifestations usually occur with the ingestion of insufficiently cooked, contaminated meat. The mortality rate is approximately 50%. After ingestion, the spores are transported to regional lymphatic tissue, where symptoms develop after a two-to-five-day incubation period. Patients with oropharyngeal anthrax present with sore throat and neck swelling from cervical and submandibular lymphadenitis. The tonsils are also frequently involved, and symptoms are associated with fever and toxicity. Dysphagia and respiratory distress often follow. Gastrointestinal anthrax begins with nausea, vomiting, and fever associated with mesenteric lymphadenitis. Patients then experience severe abdominal pain, hematemesis, ascites, and bloody diarrhea, and may present with an acute abdomen.
Traditional treatment of anthrax infection has been with penicillin. However, weapons-grade anthrax is probably resistant to penicillin (although this was not the case with the 2001 United States attack). Current treatment recommendations reflect this fact ( Box e15.5 ). These consensus recommendations include fluoroquinolones and tetracycline for all children, regardless of age. Balancing the potential risks of such drugs against the consequences of infection by drug-resistant anthrax strains, the benefits justify the recommendations. Chest tube drainage of pleural effusions improved mortality in the United States anthrax attack, though numbers were limited. CDC recommendations also include antitoxin therapy, although a 2017 meta-analysis did not show improved mortality. Supportive therapy with vasopressor medications, and possibly tracheal intubation with mechanical ventilation, may be necessary.
Ciprofloxacin, 500 mg PO bid; or doxycycline, 100 mg PO bid; or amoxicillin, 500 mg PO tid.
Ciprofloxacin, 20 to 30 mg/kg/day PO divided bid (maximum 1 g); or doxycycline, 4.4 mg/kg/day PO divided bid (maximum 200 mg); or amoxicillin, 20 to 40 mg/kg/day PO divided tid (maximum 1500 mg).
All doses are given for 7 to 10 days.
For adults and children, consider the antibody-based therapies raxibacumab and anthrax immune globulin in addition to IV antibiotics.
Ciprofloxacin, 400 mg IV every 12 hours; or doxycycline, 100 mg IV every 12 hours; or penicillin G, 4 million units IV every 4 hours.
For patients without meningitis, add a second drug that inhibits protein synthesis, such as linezolid or clindamycin. For patients with meningitis, add a third drug to this regimen that penetrates the CNS, such as meropenem.
Ciprofloxacin, 20 mg/kg/day IV divided every 12 hours (maximum 800 mg); or doxycycline, 4.4 mg/kg/day IV divided every 12 hours (maximum 200 mg); or penicillin G, 250,000 to 400,000 units/kg/day IV divided every 4 hours (maximum 24 million units).
For patients without meningitis, add second drug that inhibits protein synthesis, such as linezolid or clindamycin. For patients with meningitis, add a third drug to this regimen that penetrates the CNS, such as meropenem.
All doses are given until toxicity resolves, then switch to oral form. Treat for 60 days or until the patient receives three doses of vaccine.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here