Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The thoracic aorta is conventionally described as consisting of three segments: the ascending aorta , aortic arch , and descending aorta . Conceptually, they may be regarded as the segmental supply to the heart, to the head, neck, and upper limbs, and to the thorax, respectively.
The ascending aorta arises from the aortic orifice, which in life is closed by the aortic valve. Just above its origin, it is in the midline of the chest, almost central within the heart, with the right atrium to its right, the right ventricular outflow tract anteriorly, the left part of the coronary groove and the transverse sinus on its left, and the left atrium posteriorly. It passes superiorly and to the left, with a gentle anterior convexity, and ends at the level of the second costal cartilage (manubriosternal junction) by becoming the aortic arch. The pulmonary trunk lies on its left throughout its course. The proximal 15 to 20 mm of the ascending aorta has a characteristic bulbous appearance at catheter angiography because of the three sinuses of Valsalva. At two of these sinuses, the ascending aorta gives rise to its only branches, the coronary arteries.
Although the ascending aorta is classically described as being 2.5 cm in diameter and 5 cm in length, these dimensions are highly variable in practice when measured on axial computed tomography (CT) images ( Fig. 50.1 ). The aortic diameter can exceed 30 mm at its origin in young patients with no clinical history or features of any cardiac disease. This is undoubtedly partly due to the oblique angle of measurement and pulsation artifact, but it may partly reflect the difference between an elastic living vessel filled with blood at high pressure and a rigid, empty cadaveric specimen. Dilation and elongation also occur with age and disease.
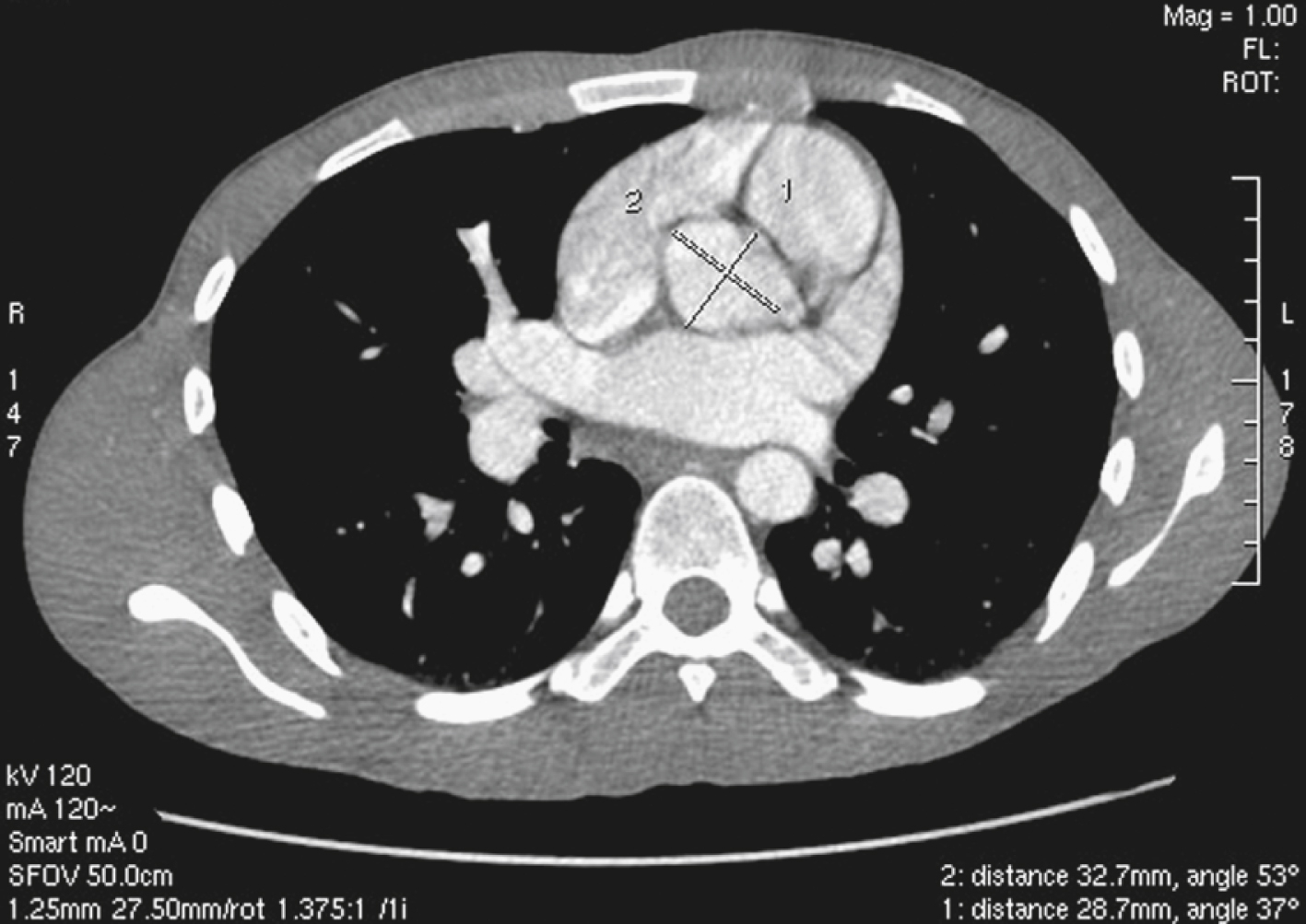
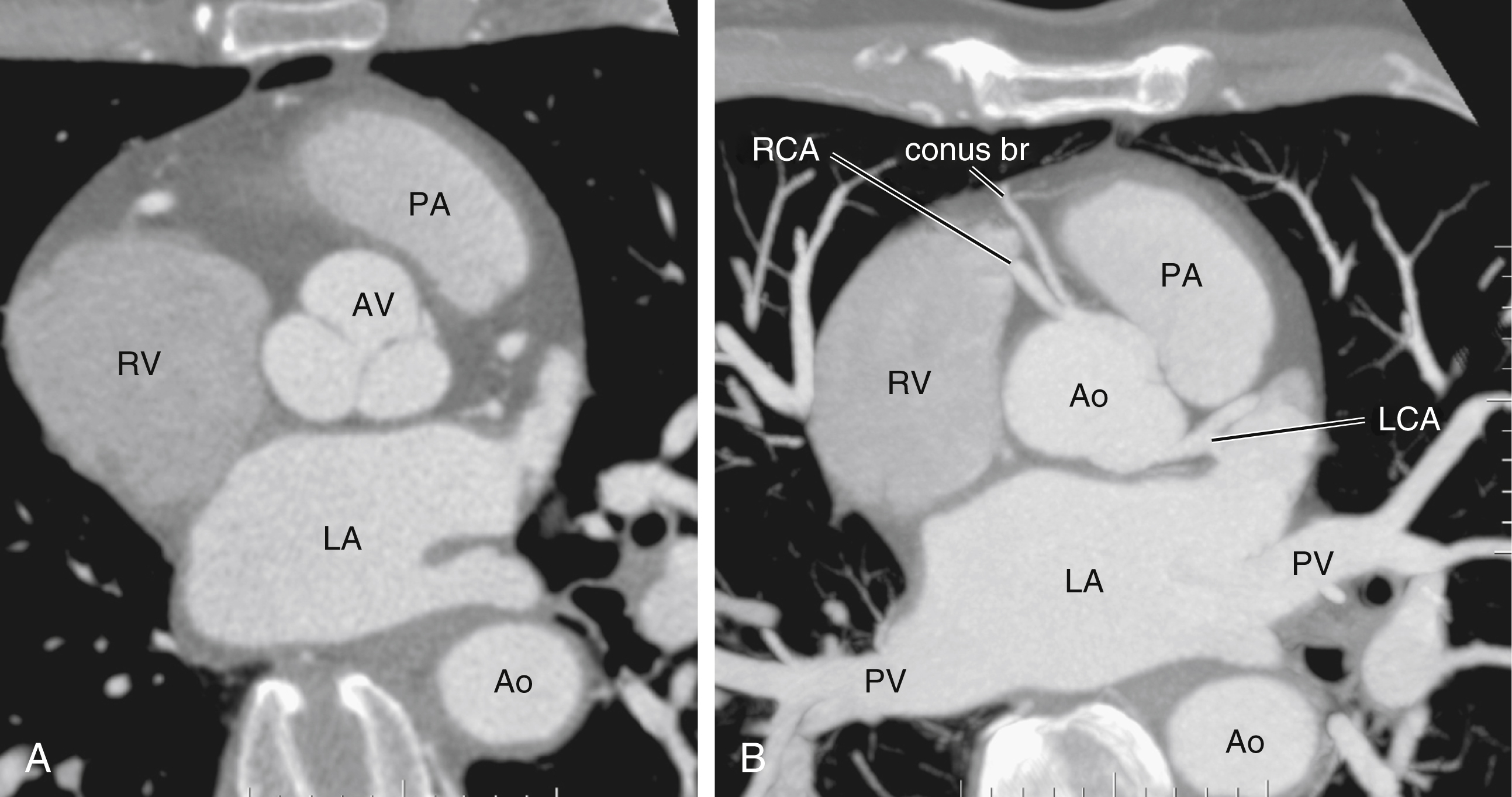
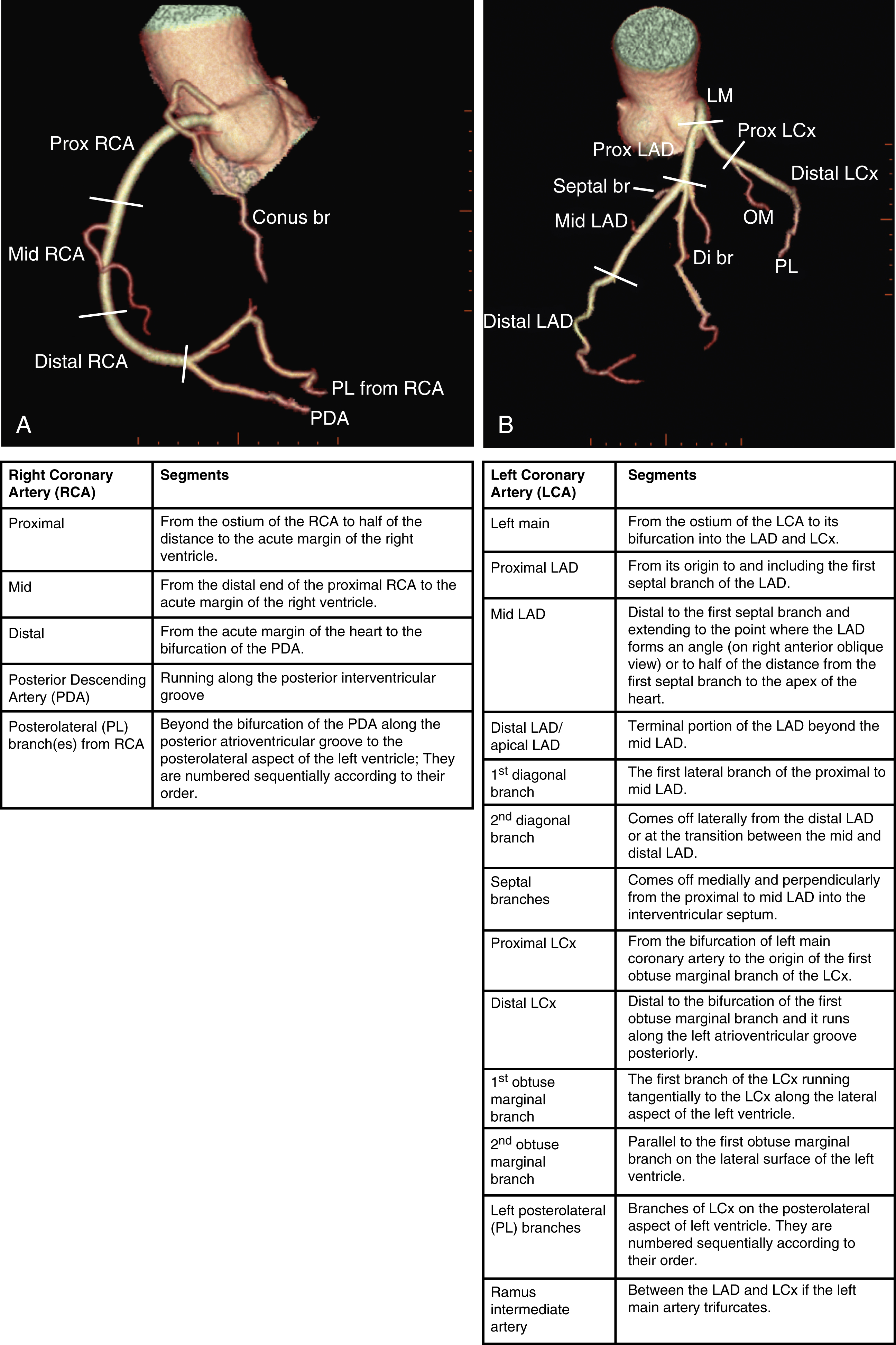
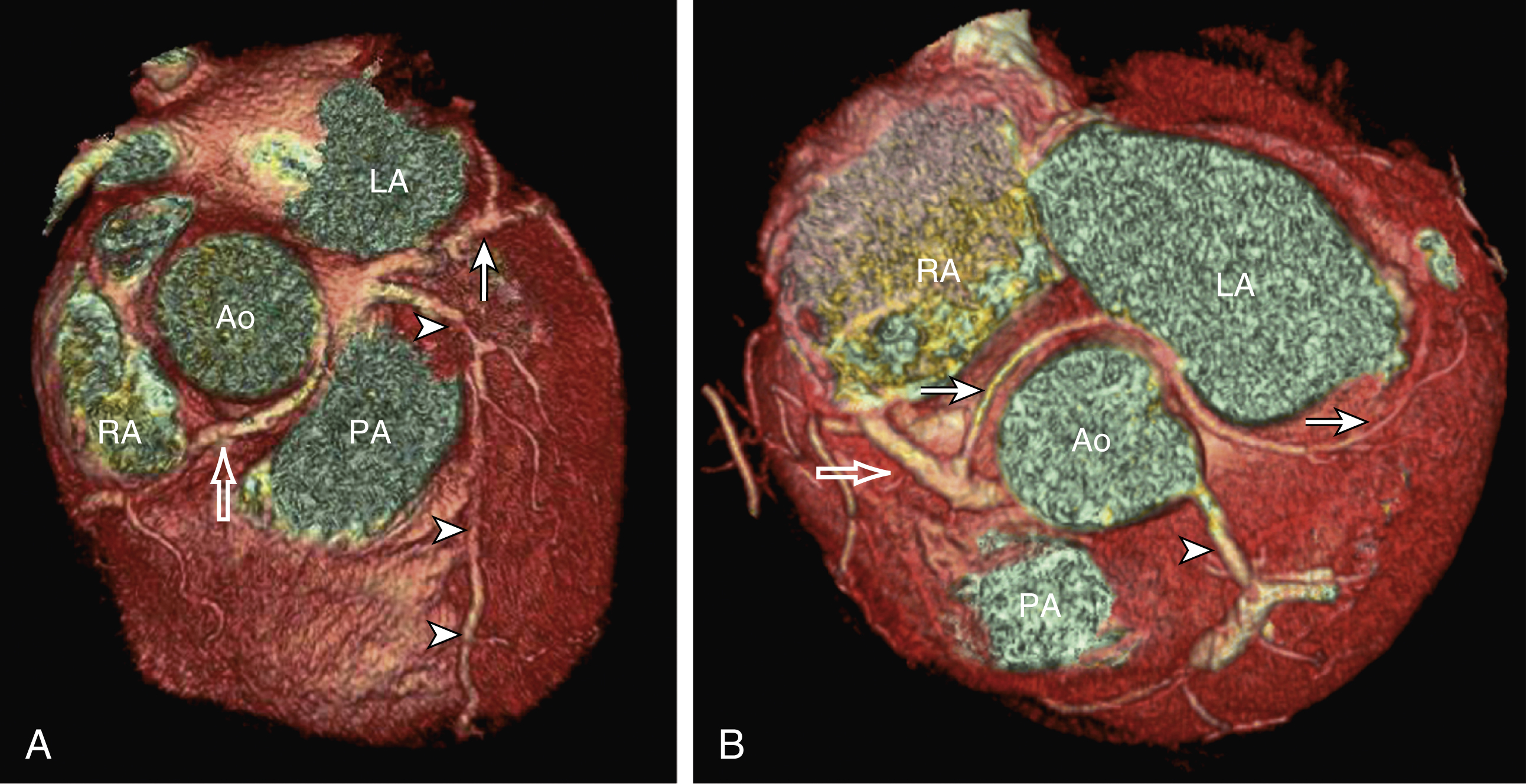
The heart receives blood supply from two major epicardial arteries, the left and right coronary arteries (LCA, RCA). These two coronary arteries originate from the aortic sinuses (coronary sinuses of Valsalva). There are normally three aortic sinuses: right anterior, left posterior, and right posterior. These sinuses are bordered by three leaflets of the aortic valve that form an inverted “Mercedes sign” on cross-sectional view of the aortic root ( Fig. 50.2A ). The LCA originates from the left posterior aortic sinus, whereas the RCA originates from the right anterior aortic sinus ( Fig. 50.2B ). The right posterior sinus is normally free of coronary arteries. Volume-rendered three-dimensional (3D) images of CT coronary angiography are used in this section to illustrate the courses of the coronary arteries.
The LCA courses lateral to the aorta and posterolateral to the pulmonary artery for a variable length (a few millimeters to 2 cm). It then bifurcates into two main branches, the left anterior descending (LAD) and the left circumflex (LCx) arteries. The segment of the LCA proximal to its bifurcation is commonly known as the left main coronary artery (LMCA). Occasionally the LCx has a separate origin from the LAD. In this situation the LMCA is nonexistent.
The LMCA supplies approximately 75% of the left ventricle. Severe disease (≥70%) in the LMCA puts the patient at high risk of cardiac death. The finding of severe LMCA disease should call for immediate attention from the treating cardiologist. Severe LMCA disease is normally treated by coronary artery bypass graft surgery. Percutaneous coronary intervention (PCI) of LMCA disease is normally reserved for those who are considered inoperable, high-risk patients, or those with protected left main disease (i.e., a patent bypass graft to the LAD or LCx). With the increasing use of drug-eluting stents in PCI, more LMCA disease is now being treated with PCI, particularly ostial or mid-LMCA disease. Distal LMCA disease that involves both the origins of the LAD and LCx is usually considered less favorable for PCI. So far, 10-year data for PCI to treat unprotected LMCA disease (i.e., the LAD or LCx is not grafted) show no significant difference in mortality rate or composite endpoint of death, myocardial infarction, or stroke between PCI and coronary artery bypass graft. Nonetheless, PCI of the LMCA is associated with an increased risk of target-vessel revascularization despite the use of drug-eluting stents.
Of the two main branches of the LCA, the LAD is usually the larger and more important one; it supplies the anteroseptum, anterior, anterolateral, and anteroapical walls of the left ventricle. The LAD runs along the interventricular groove on the anterior surface of the heart toward the apex of the heart ( Fig. 50.3A ). It gives off medial branches known as septal branches and lateral branches known as diagonal branches . Septal branches come off the LAD almost perpendicularly and supply the anterior two-thirds of the muscular interventricular septum. The first septal branch is usually the largest, and it supplies the atrioventricular (AV [His]) bundle and proximal bundle branches.
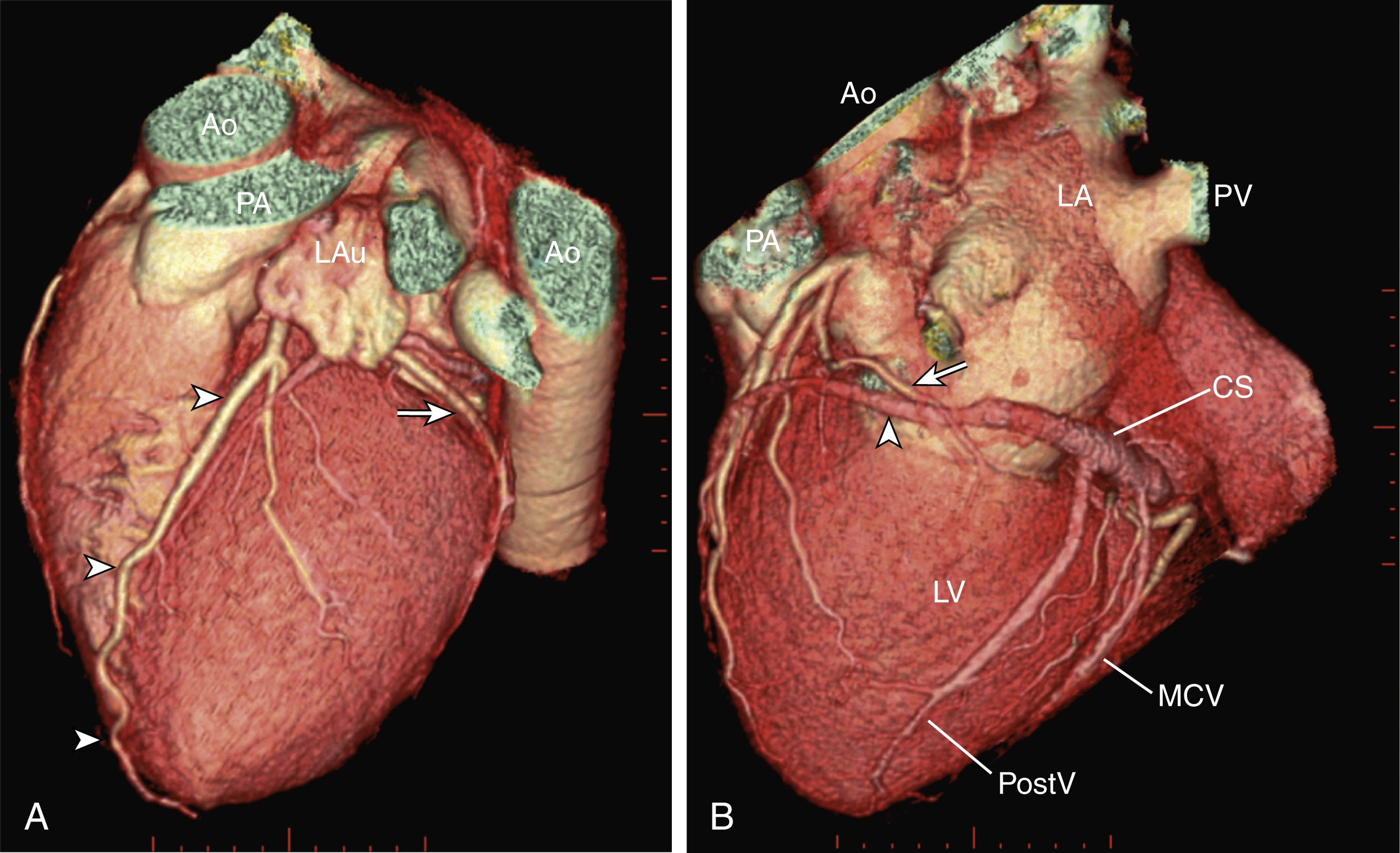
Lateral to the LAD, the first or second diagonal branches are sometimes of significant size and supply the high anterolateral wall of the left ventricle. Beyond the diagonal branches, the LAD reaches the apex of the heart. Sometimes the LAD wraps around the apex and continues its course along the interventricular groove onto the diaphragmatic surface of the heart for a variable length.
The LCx is usually the smaller branch of the LCA, supplying the lateral and posterior wall of the left ventricle. The LCx comes off the LMCA stem posterolaterally and runs beneath the left auricle. It runs along the left AV groove toward the posterior surface of the heart ( Fig 50.3B ). The main branches of the LCx are the obtuse marginal arteries that come off tangentially to the LCx and run along the lateral aspect of the left ventricle. Beyond the edge of the left ventricle, sometimes there are more branches coming off the LCx, supplying the posterolateral aspect of the left ventricle; these are the posterolateral branches of the LCx.
Occasionally there is a branch coming off the LMCA between the LAD and the LCx that is known as the ramus intermediate artery . The ramus intermediate artery can be of considerable size, supplying the anterolateral aspect of the left ventricle. Thus it functionally replaces the first diagonal branch of the LAD or first obtuse marginal branch of LCx.
The RCA comes off the right anterior sinus of Valsalva and runs downward in the right AV groove posterolaterally to the pulmonary artery and beneath the right atrial auricle ( Fig. 50.4A ). The RCA gives off branches to supply the right atrium, right ventricle, posteroseptum, and, to a variable degree, the inferior and posterior aspects of the left ventricle. The earliest atrial branch of the RCA is normally the sinoatrial (SA) nodal artery. The SA nodal artery comes off the RCA underneath the right auricle and runs posteriorly toward the left of the superior vena caval ostium to supply the SA node. There is considerable variation in the distribution of the SA nodal artery. In about 59% of hearts the SA nodal artery comes from the RCA. However, in about 38% of hearts the SA nodal artery is a continuation of a large left atrial branch of the LCx. In the remaining 3%, the SA node receives dual blood supply.
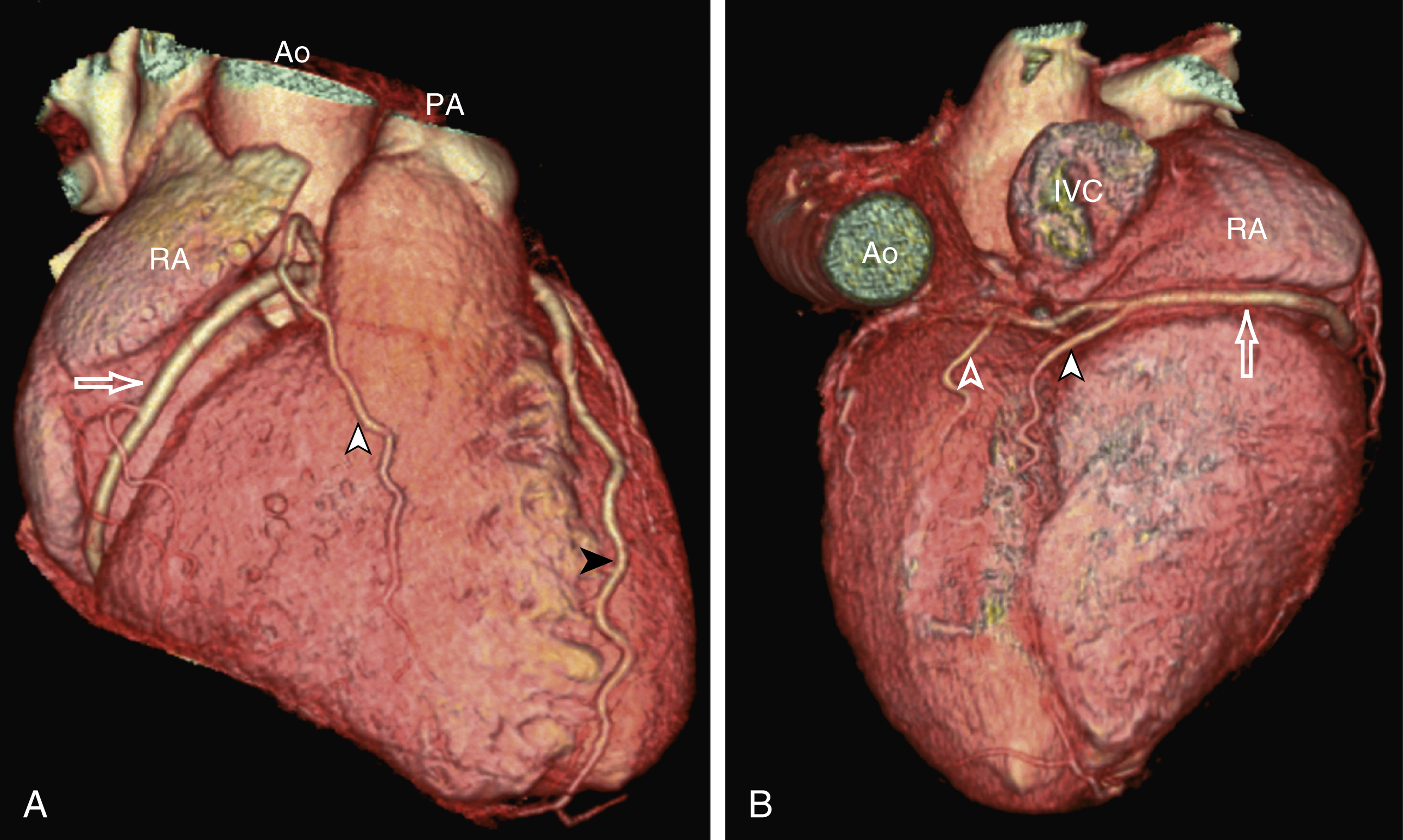
An early branch of the RCA that courses anteriorly and around the right ventricular infundibulum is the conus branch of the RCA. It may anastomose with an analogous branch from the LAD. In about 20% of hearts, the conus branch originates separately from the right anterior sinus of Valsalva instead of its parent artery, the RCA (see Fig. 50.2A ). As the RCA runs along the right AV groove toward the inferior border of the right ventricle, there is a branch coming off the RCA obliquely to supply the anterior surface of the right ventricle, known as the marginal branch of the RCA. Beyond the bifurcation of this marginal branch, the RCA wraps around the edge of the right ventricle and runs along the posterior AV groove to reach the crux of the heart, where the AV groove meets with the posterior interventricular groove. At the crux the RCA gives off a branch superiorly into the interatrial septum to supply the AV node in 90% of cases. Inferior to the crux along the posterior interventricular groove, the RCA gives off a branch known as the posterior descending artery (PDA) in about 85% of patients ( Fig. 50.4B ). The PDA supplies the posteroseptum and inferior wall of the right and left ventricles. A dominant RCA by definition supplies the PDA and continues beyond the crux along the left posterior AV groove to terminate into one or several posterolateral branches. In 10% of patients the PDA is a continuation of the left coronary system (predominantly the LCx, i.e., the left dominant coronary system). In about 5% to 10% of hearts, the RCA and LCx share the same blood supply to the diaphragmatic surface of the heart (i.e., a codominant coronary system).
In the right dominant system, occlusion of the proximal RCA in acute inferior myocardial infarction may lead to right-sided heart failure, hypotension, and AV nodal block (second- or third-degree AV block).
The definition of coronary segments is important, especially in reporting the results of CT coronary angiography. Sometimes it can be difficult to define a particular segment of the coronary artery tree or the branches of the main coronary segments. Specifying the exact location of the disease and the segment involved as precisely as possible is important in communicating with the treating physicians, especially in coronary arteries with multiple lesions. Several classifications are used to define coronary segments, such as the 26-segment Coronary Artery Surgery Study system and 15-segment American Heart Association system. Fig. 50.5 A and B illustrate the nomenclature of coronary arteries based on a modified 16-segment American Heart Association classification.
Coronary arteries with aberrant origins are known as anomalous coronary arteries (ACAs). The frequency of ACAs varies from 0.3% to 5.6% depending on the definition of ACA in each individual study. Studies with more stringent criteria only accept coronary arteries as anomalies if they originate from the opposite coronary sinuses of Valsalva. The less stringent studies may include coronary arteries with ectopic origins at their respective coronary sinuses. The most frequent forms of ACAs in descending order are an RCA originating from either the right or left sinus of Valsalva ( Fig. 50.6A ), an LCx arising from the right coronary sinus or RCA ( Fig. 50.6B ), and an LCA arising from the right sinus of Valsalva.
The clinical outcome of ACAs is not always benign. Just like any coronary artery, ACAs may undergo atherosclerosis and become the culprit artery for ischemic heart disease. Failure to adequately identify and assess ACAs may lead to misdiagnosis and failure to appropriately treat the culprit artery.
More significantly, ACAs are associated with sudden death. It has been reported that 12% to 31% of all cases of sudden death in young athletes or military recruits in the North American population may be associated with ACAs. Most sudden deaths associated with ACA occur during or after physical exercise. Although the exact mechanism of cardiac ischemia in such cases is unclear, it has been postulated that interarterial ACAs with a proximal course between the aorta and pulmonary arterial trunk (so-called malignant coronary anomaly) may be compressed during or after physical exercise (see Fig. 50.6A ). ACAs with an interarterial course can be corrected by surgical reimplantation of coronary arteries to their respective coronary sinuses of Valsalva. Therefore, early detection of malignant ACA may lead to prevention of sudden death in adolescents or young athletes with this condition.
The cardiac veins, in general, follow the distribution of the coronary artery system, running parallel and superficial to the coronary arteries and their branches. The great cardiac vein runs parallel but in opposite direction to the LAD in the anterior interventricular groove (see Fig. 50.3A ). The great cardiac vein continues its course in the left anterior AV groove along with the LCx artery (see Fig. 50.3A ). Throughout its course, the great cardiac vein receives its tributaries from the left ventricle and atrium. In the left posterior AV groove, the great cardiac vein becomes a larger venous structure known as the coronary sinus (see Fig. 50.3B ). The coronary sinus is about 3 to 5 mm in diameter and 2 to 5 cm in length and receives blood from most cardiac veins before it empties into the right atrium. One of its major tributaries is the middle cardiac vein, which runs superiorly in the posterior interventricular groove along the PDA (see Fig. 50.3B ). It empties into the coronary sinus at the crux of the heart. The lateral, posterolateral, and posterior cardiac veins of the left ventricle receive blood from their respective aspects of the left ventricle before emptying into the coronary sinus along the left AV groove. There are multiple anterior cardiac veins that receive blood from the right ventricle and drain into the small cardiac vein. The small cardiac vein runs along the right AV groove downward toward the crux, where it subsequently empties into the coronary sinus. Some of the anterior cardiac veins drain directly into the right atrium.
Identification of the coronary sinus and its tributaries are important in the electrophysiological study of the cardiac conduction system. The coronary sinus is a frequent site for placing a pacing catheter or electrode in an electrophysiologic study. In patients with heart failure and dyssynchrony between the right and left ventricles, cardiac resynchronization therapy with biventricular pacing can help to resynchronize the right and left ventricles, hence improving cardiac output, heart failure symptoms, and survival. Biventricular pacing involves placement of a pacing electrode into one of the anterolateral tributaries of the great cardiac vein or posterior vein of the left ventricle via the coronary sinus.
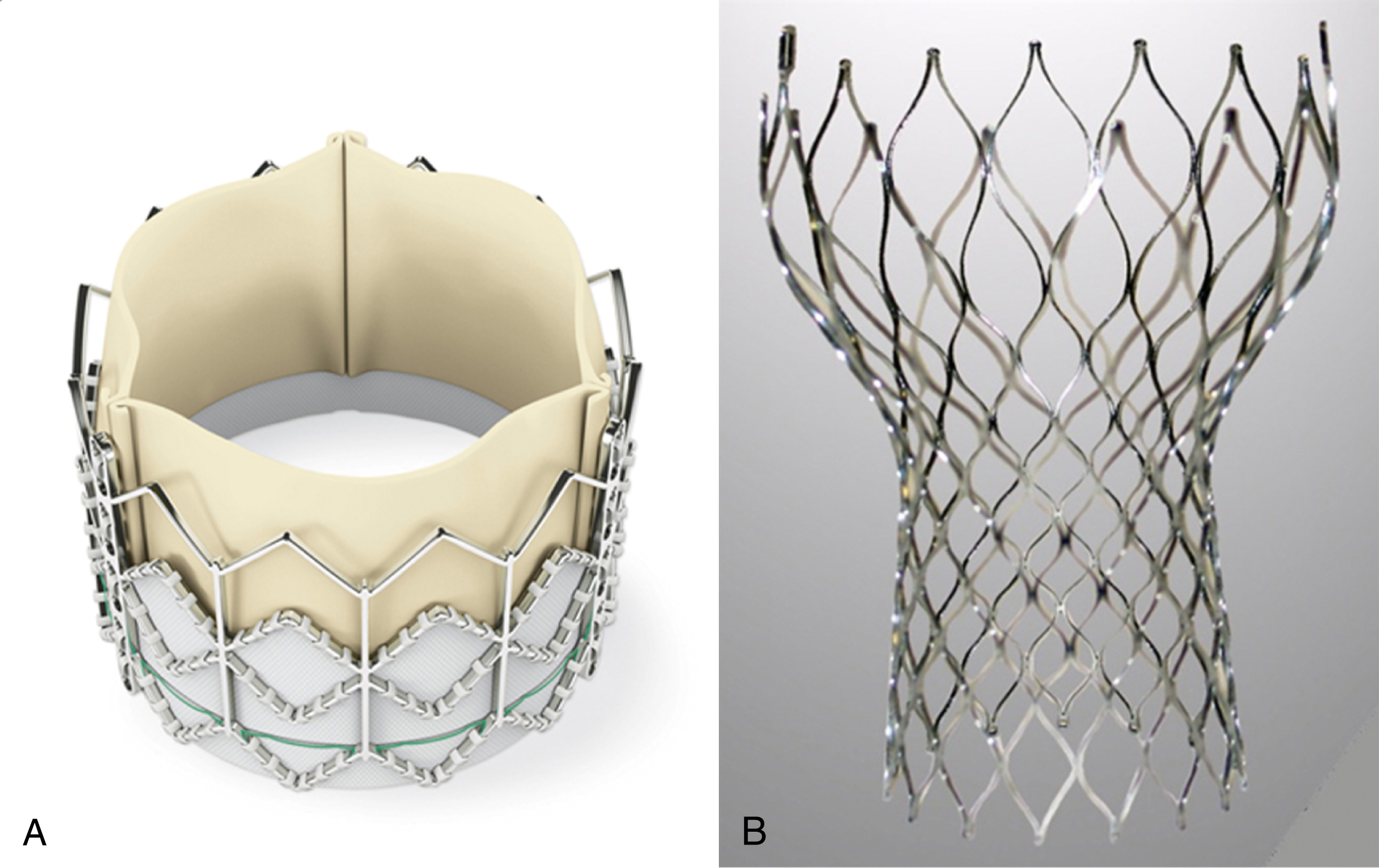
Aortic valve replacement has hitherto been performed via open heart surgery. Rapid advances in catheter-based treatment for valvular heart disease makes the less invasive catheter-based transcutaneous aortic valve implantation (TAVI) a reality. Currently there are several widely used TAVI systems, with two devices that have been used more extensively since the development of TAVI: a balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA) and a self-expandable CoreValve system (Medtronic Inc., Minneapolis, MN) ( Fig. e50.1 ). Both systems are bioprosthetic valves mounted on a catheter-based delivery system.
TAVI is currently indicated only for those patients with aortic stenosis with high surgical risks, such as age older than 80 years or for younger patients (>65 years) with severe comorbidities such as severe respiratory disease, liver cirrhosis, or previous cardiac surgery. The large multicenter Placement of Aortic Transcatheter Valves (PARTNER A) trial, comparing TAVI using the Edwards SAPIEN valve against surgical valve replacement, showed very promising results, with a noninferiority of TAVI in comparison with surgery in terms of its procedural success and mortality rates. In 30 days, TAVI had a lower mortality rate (3.4% vs. 6.5%), and at 1 year, TAVI’s mortality rate was as good as the surgical mortality rate (24.2% vs. 26.8%, respectively). TAVI has the advantages of shorter hospital stay and shorter recovery time. Nonetheless, TAVI was associated with a higher stroke rate (5.5% vs. 2.4% at 30 days and 8.3% vs. 4.3% at 1 year). In the PARTNER B trial, where TAVI was compared against best medical therapy for severe aortic stenosis in patients unfit for surgical aortic valve replacement, TAVI was associated with a 45% relative risk reduction in all-cause mortality rate at 1 year (30.7% vs. 50.7%). In view of the encouraging results of the PARTNER A and PARTNER B trials, it is expected that TAVI’s indications would be extended to include younger, lower-surgical-risk patients or patients not suitable for surgery across all age groups.
Accurate sizing of the aortic annulus and careful selection of patients are crucial to the success of TAVI. Various imaging modalities are currently in use to evaluate a patient’s aortic valve, aortic root, and suitability for TAVI. An echocardiogram is necessary to diagnose and determine the severity of aortic stenosis, aortic valve two-dimensional (2D) morphology (with or without concurrent aortic valve incompetence), degree of calcification, and aortic annular and sinotubular diameters. Similarly, fluoroscopic measurements of aortic annular diameter, aortic sinus diameter, diameter of sinotubular junction, height of the coronary ostium from the aortic annulus, and diameter of the ascending aorta are important measurements to obtain before acceptance of TAVI candidates but are now more conventionally being performed using multiplanar CT.
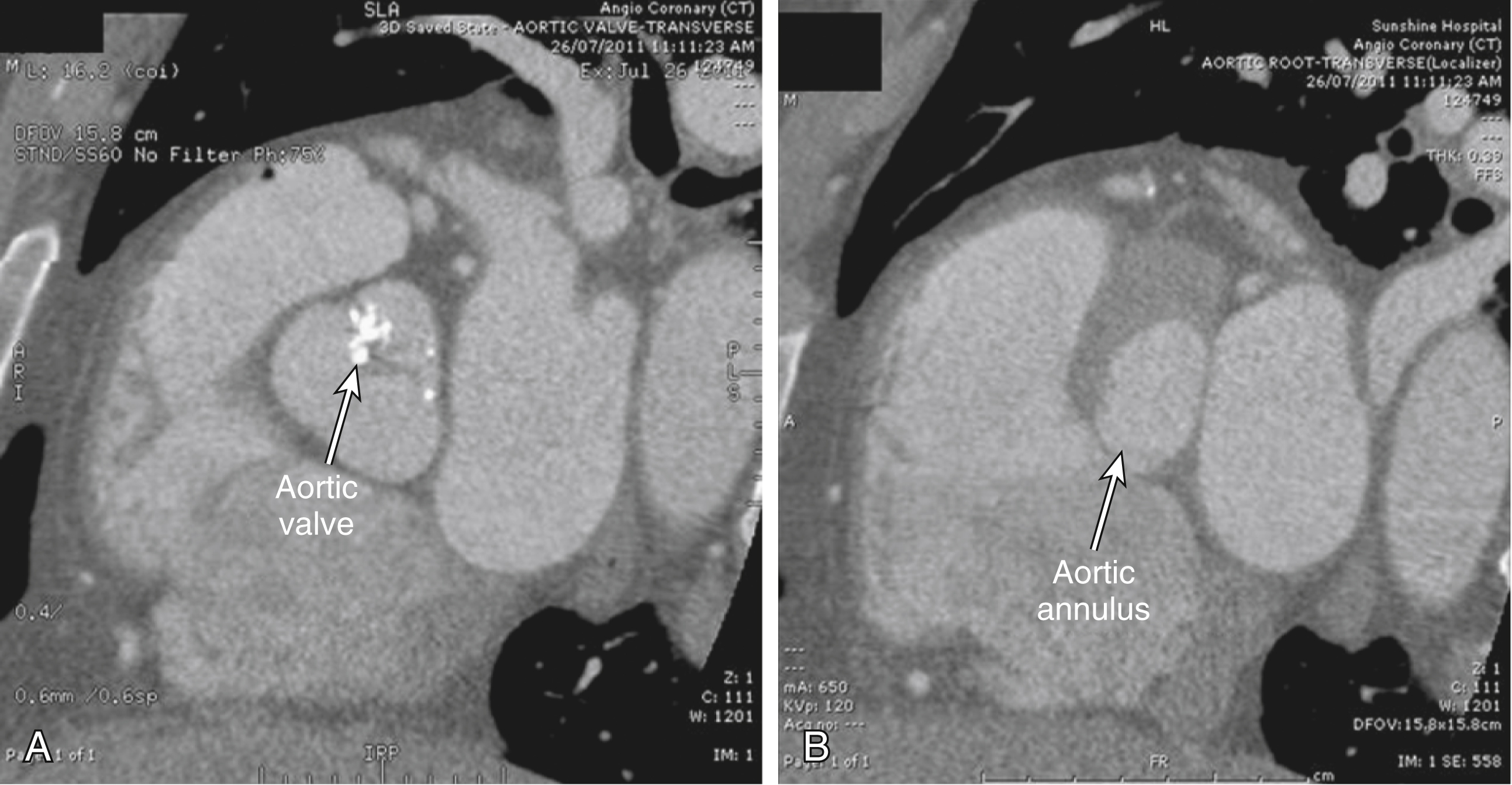
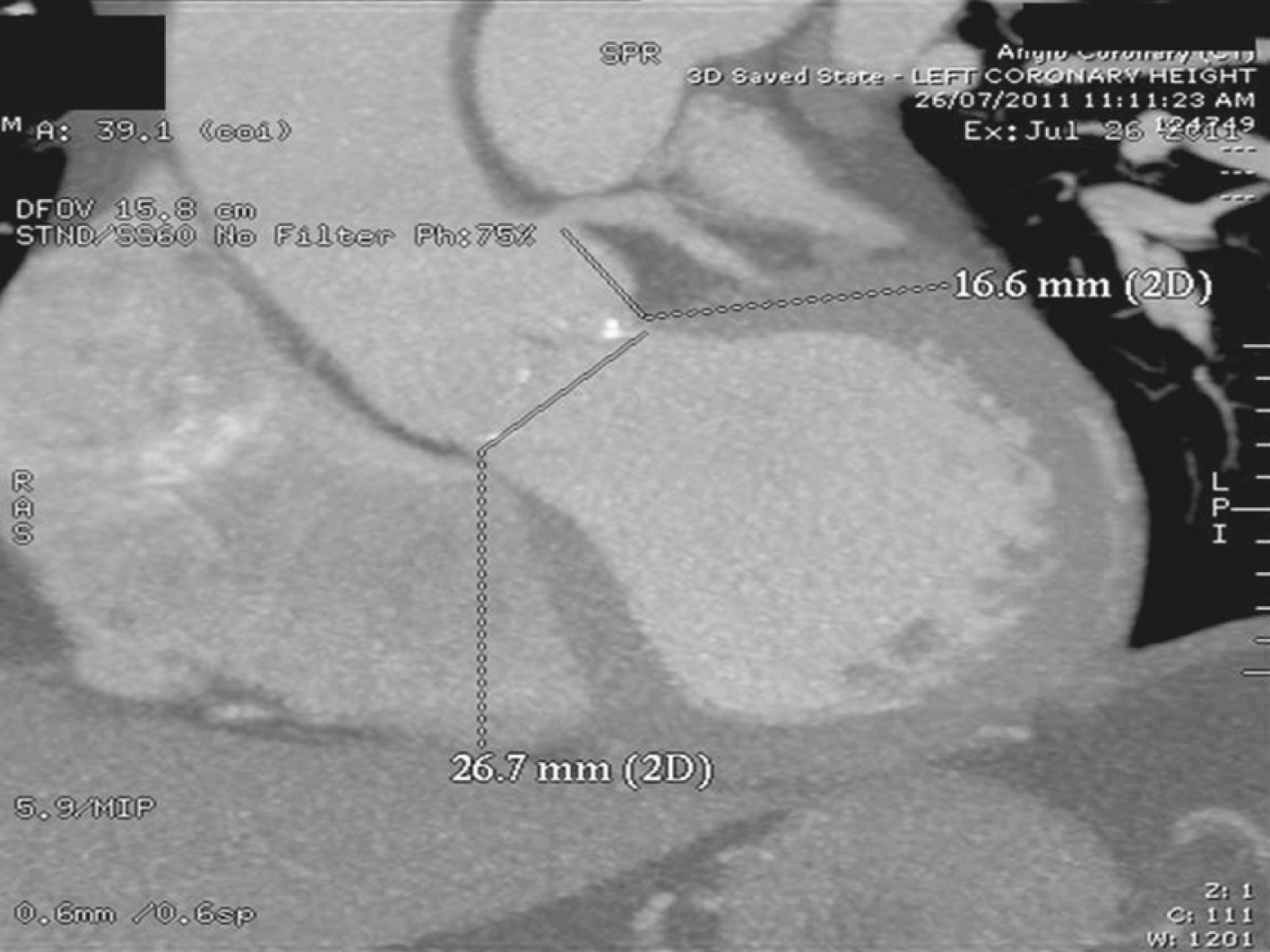
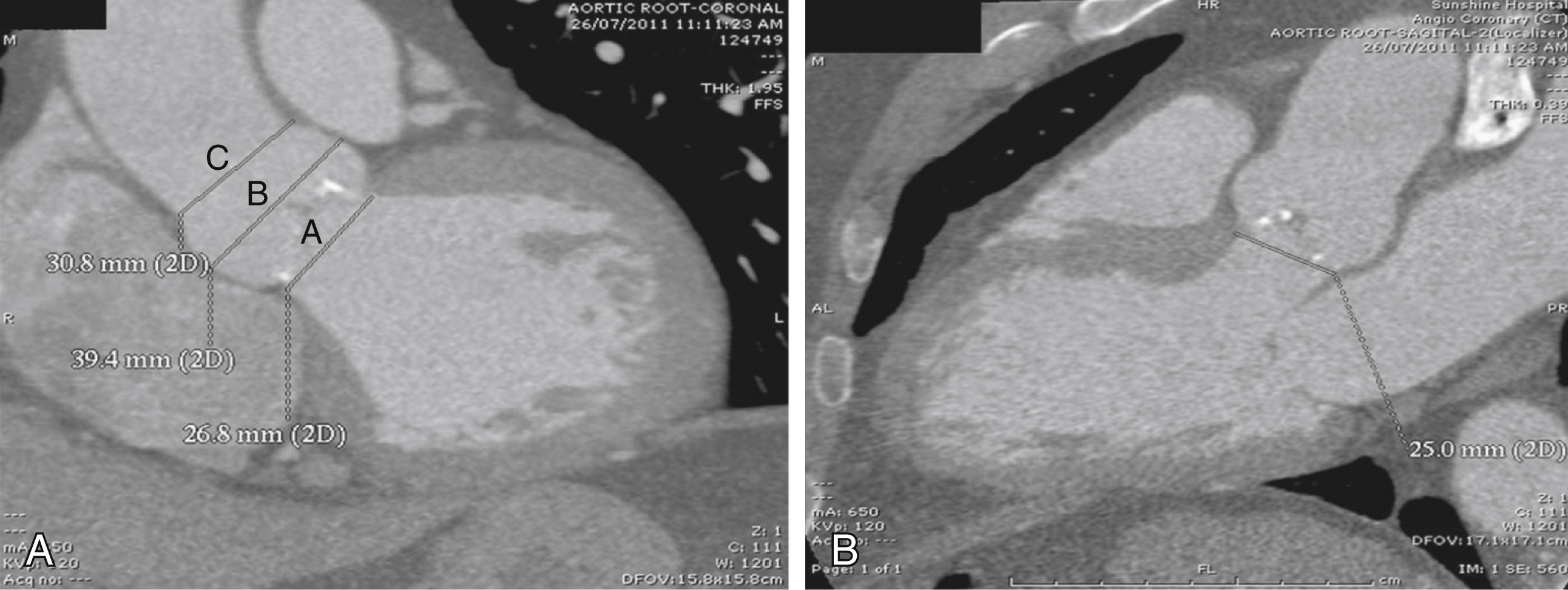
Because the aortic annulus is often oval rather than round, a proper assessment of the aortic annulus may require a multiimaging modality approach. Hence, 2D echocardiography (transthoracic and transesophageal) and fluoroscopy may be limited in showing the oval nature of the aortic annulus. Assessment with electrocardiogram-gated CT angiography of the aortic root has become accepted practice to visualize the annular shape and measure the size of the aortic annulus to reduce any chance for error or interreader variability ( Figs. 50.7 and 50.8 ). Common planes used to assess the aortic root are coronal ( Fig. 50.9A ), sagittal ( Fig. 50.9B ), and transverse planes of the aortic valve and aortic root (see Fig. 50.7 ). At present, a small aortic annulus (diameter <18 mm for Edwards SAPIEN valve; <20 mm for CoreValve) or a large aortic annulus (diameter >25 mm for Edwards SAPIEN valve; >27 mm for CoreValve), a dilated ascending aorta with a sinotubular diameter larger than 43 mm, or a low left coronary origin from the aortic annulus (height <14 mm) (see Fig. 50.8 ) may exclude patients from TAVI. These guidelines are continually changing as a variety of new valves become available.
A CT aortogram is also useful to evaluate the status of the aorta, iliofemoral, and subclavian arteries to determine whether the peripheral arteries can accommodate the delivery system of the TAVI device (i.e., access vessel has to be >6 mm). If peripheral access via the femoral or subclavian artery is unsuitable because of small size, tortuosity, or extensive atherosclerosis with concentric calcification, a transapical approach via a small thoracotomy is possible.
In a suitable patient, access is obtained via the femoral artery or subclavian artery with a 14-to 18-gauge vascular sheath (size of delivery system is expected to decrease with advances in catheter technology). The stenotic aortic valve is first crossed and predilated with a balloon saddled between the aortic valve leaflets. The heart is often paced at 160 to 180 beats/min to stop the balloon from being pushed out of its position. Once the stenotic valve is properly dilated, a prosthetic valve is positioned carefully to the ideal location and deployed under the guidance of a fluoroscopic aortogram. After deployment, a fluoroscopic aortogram is obtained to recheck the position of the prosthetic valve and assess for any aortic incompetence. If the valve is deployed too high, there is increased risk of aortic injury, device migration, paravalvular leak, and coronary occlusion. If the valve is deployed too low, there is increased risk of mitral valve dysfunction, heart block, and paravalvular leak.
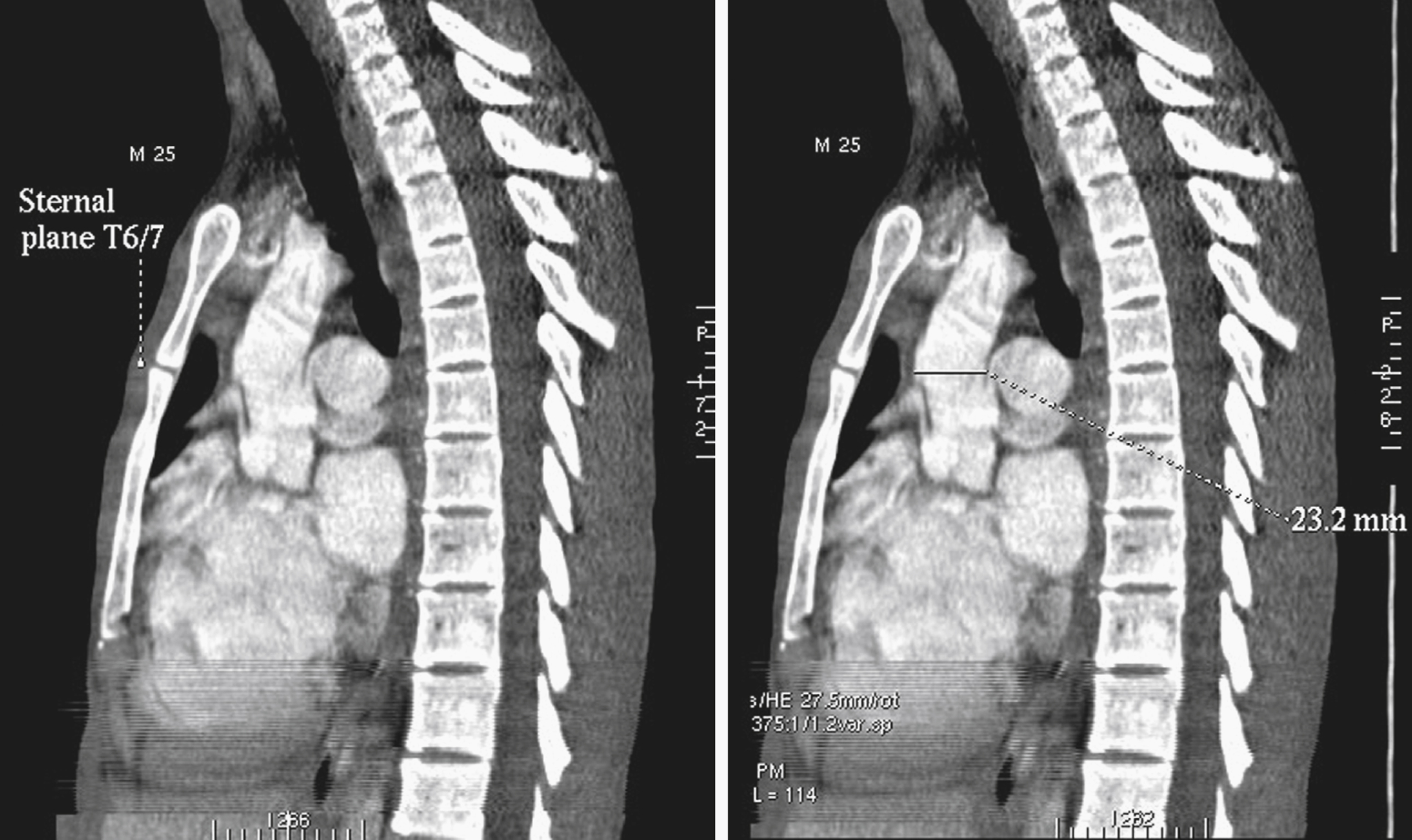
The aortic arch is the part of the aorta that lies in the superior mediastinum. Both its origin and termination are at the sternal plane , which is classically defined as the horizontal plane that passes from the manubriosternal junction to the T4/T5 intervertebral disk. It lies just above the level of the pulmonary trunk. At its origin, the arch is in the midline of the chest, with the thymic bed anteriorly, the superior vena cava to its right, a thin rim of fat and small lymph nodes to its left, and the trachea posteriorly. It arches superiorly over the right pulmonary artery, the aortopulmonary window, and the left main bronchus, in that order, toward the left side of the vertebral body of T4 in a direction that roughly parallels the proximal left pulmonary artery. About two-thirds of the way along its course, just beyond the origin of the left subclavian artery, are three important but radiologically occult relationships: the left vagus nerve, which crosses its left side; the recurrent laryngeal branch of the left vagus nerve, which hooks under it and then passes up along its right side; and the ligamentum arteriosum, which joins the undersurface of the arch to the superior surface of the left pulmonary artery. At its highest, a normal arch reaches midway up the manubrium, or about one vertebral body level above its origin. The arch typically ends left anterior to the body of T4.
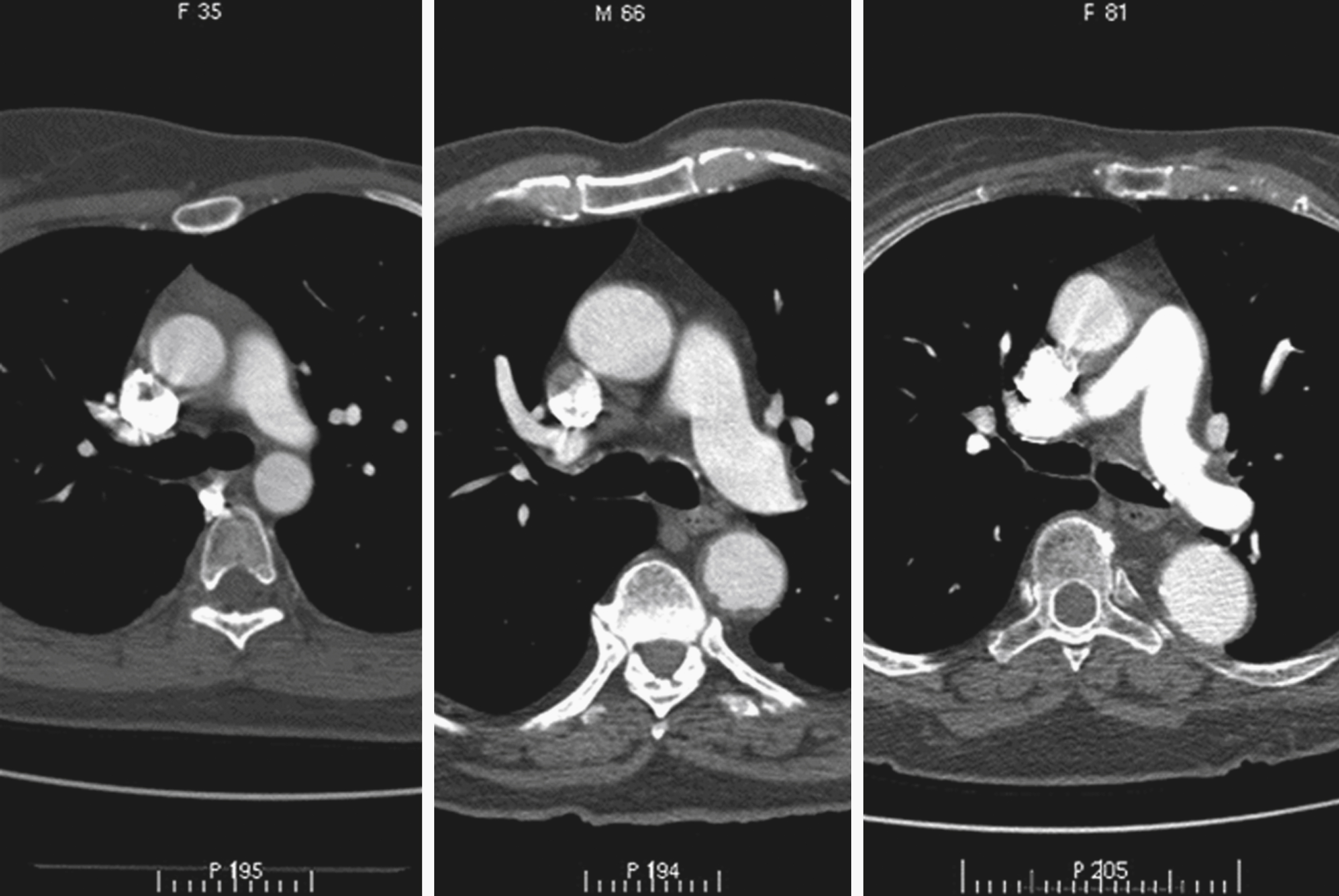
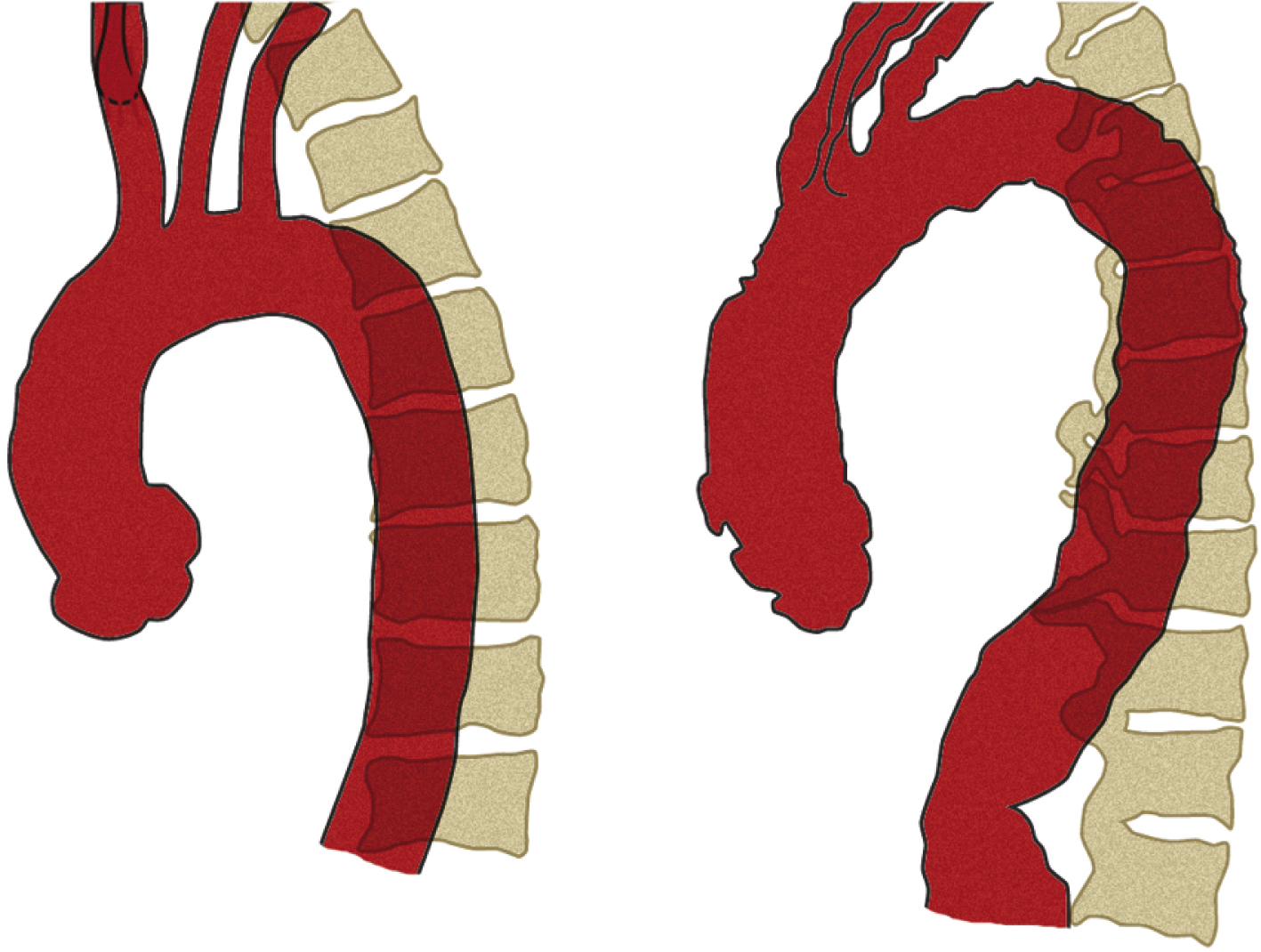
The diameter of the arch progressively decreases as it gives off its three major branches such that, barring significant aneurysmal dilation, its termination is some 25% to 30% narrower than its origin. The position of its termination is also highly variable and ranges from directly anterior to left posterior to the vertebral body. The latter position is more commonly seen in elderly patients, probably because of elongation of the vessel, which “pushes” the descending aorta posteriorly and leftward ( Fig. 50.10 ). The horizontal level of the sternal plane and the height to which the arch rises are also highly variable and dependent on body habitus ( Figs. 50.11 and 50.12 ).
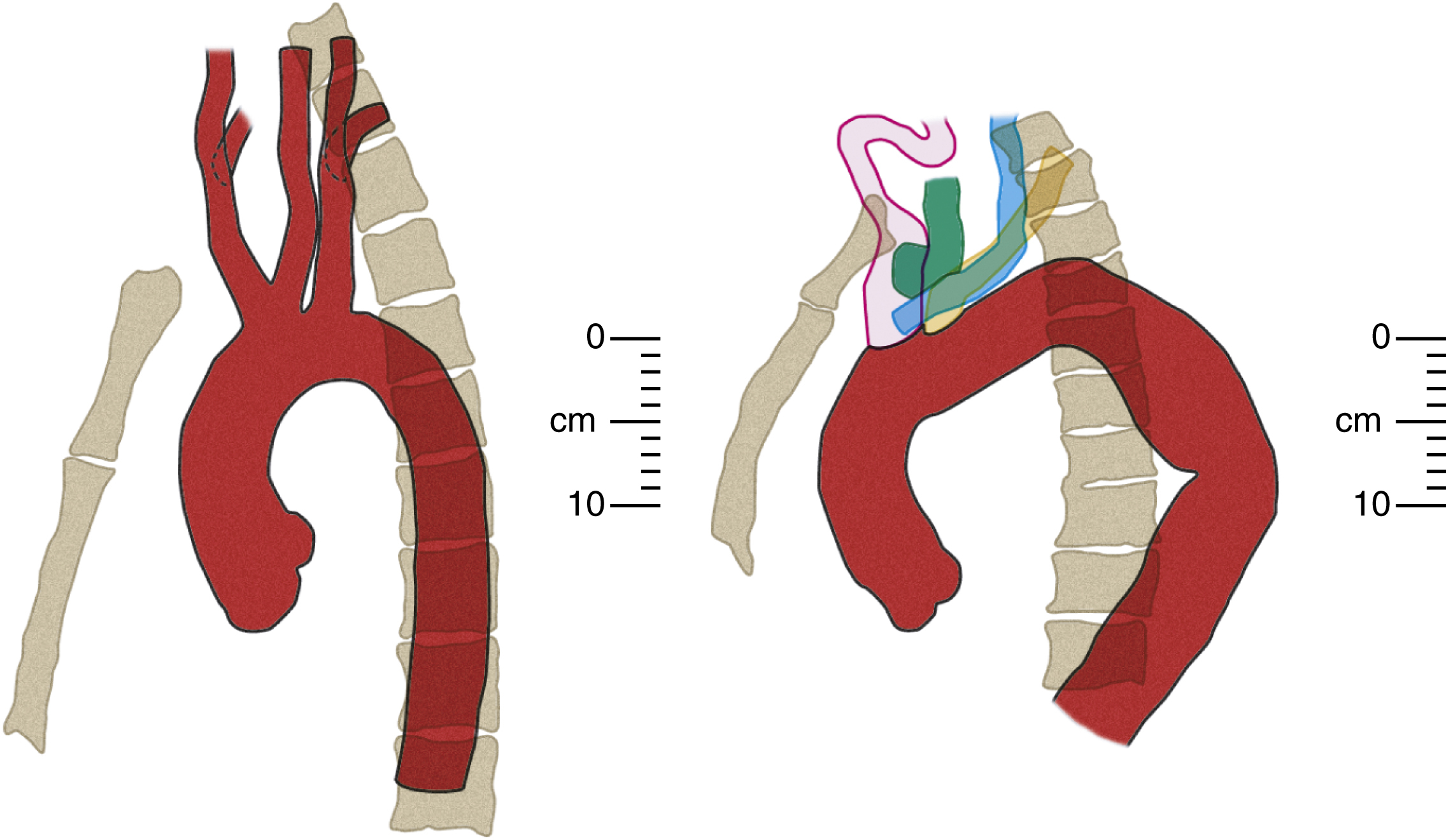
The curvature of the aortic arch and the angle of origin of its branches vary with age. In young patients, the origins of all three arch branches tend to be at almost the same horizontal level at the apex of the arch. In advanced age, the aorta is elongated with a more acute curvature, which has two effects on the arch: (1) it “pushes” the origins of the arch branches anteriorly, away from the vertebral column and (2) it raises a “new” and higher arch apex caudal (posterior) to the arch branches, which places their origins on a slope. Together, these effects increase the acuity of the angles of origin of the arch branches and cause them to be “bent backward” on the arch ( Fig. 50.13 ; also see Fig. 50.12 and Fig. 50.14 for more illustrations of the same phenomenon). Thus although selective cannulation of the arch branches in young patients is readily achieved with a catheter that has a tip angled at 45°, such as a Berenstein catheter, a reverse curve catheter such as a Simmons is often required in elderly patients.
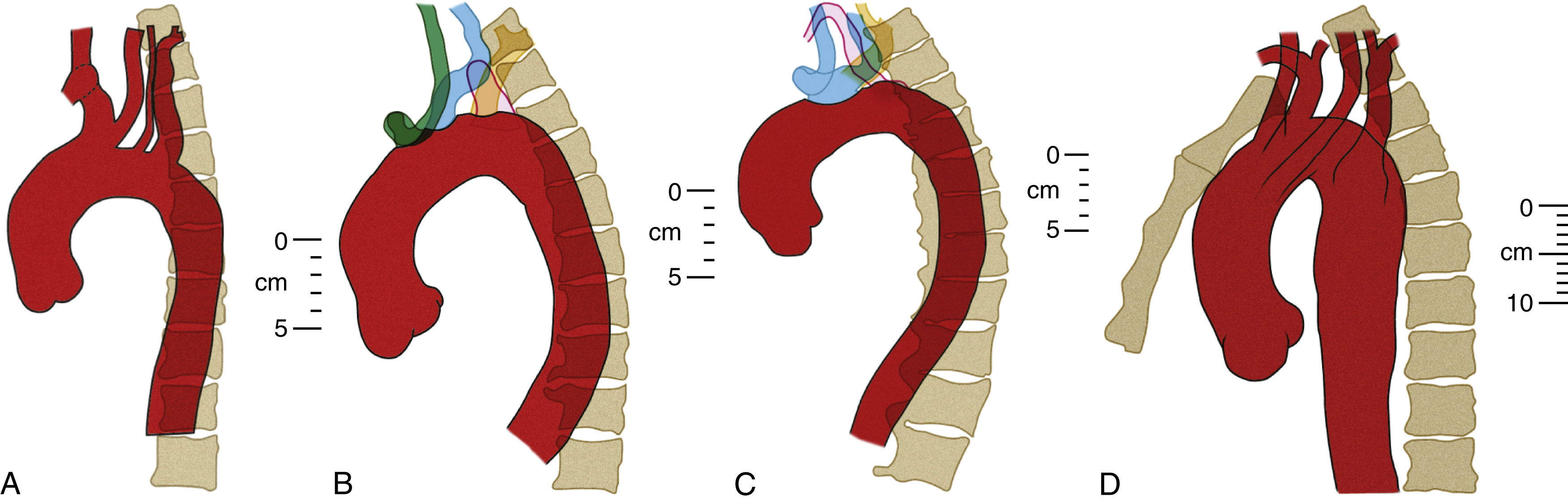
The aortic arch gives rise to the three great vessels of the head, neck, and upper limbs: the brachiocephalic (innominate) trunk, the left common carotid artery, and left subclavian artery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here