Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Opioids are an essential tool in a physician’s toolbox, but their addictive nature and lethality have also plagued humanity throughout history.
The development of OxyContin in 1996, combined with an advertising campaign and sponsorship of more than 20,000 educational programs, led to an acceleration in opioid prescription.
The first wave of the opioid epidemic began in 1999, with a large increase in prescription opioid overdose deaths. The second wave of the opioid epidemic began in 2010, as former prescription opioid users transitioned to heroin. The third wave of the opioid epidemic started in 2013, as fentanyl became more abundant in the illicit opioid supply.
The CDC guidelines for opioid prescription were released in 2016 and led to a decline in opioid use. However, clarification was released in 2019, emphasizing individualized approaches and the avoidance of hard limits, abrupt tapering, and sudden discontinuation of opioids.
10.3 million Americans misused opioids in 2018, 9.9 million of which misused prescription pain medications. This was associated with over 48,000 opioid-related overdose deaths that year alone.
Ultimately, pharmaceutical companies, drug distributors, pharmacies, physicians, nurses, and pharmacists share some responsibility for the opioid epidemic.
Current and future efforts to combat the opioid crisis are necessary, but all efforts must maintain a balance between preventing the abuse of opioids and making them available for legitimate medical purposes.
Pain physicians must understand the ongoing efforts to contend with the opioid epidemic and their roles in these efforts.
The opioid crisis has had a dramatic impact on medical care in the United States, affecting all medical providers, especially pain physicians and their patients. From 1999 to 2011, oxycodone use increased by 500%, with the quadrupling of opioid-related overdose deaths during the same period. , Despite formal recognition of the opioid crisis in 2011 and subsequently reduced opioid prescriptions, 10.3 million Americans still misused opioids in 2018, 9.9 million of which inappropriately used prescription pain medications. This was associated with over 48,000 opioid-related overdose deaths that year alone. Clearly, despite the efforts of numerous stakeholders in the United States government and medicine, combating the opioid epidemic is still necessary. As one of these stakeholder groups, pain physicians must not only understand ongoing efforts to contend with the opioid epidemic but also their specific roles in these efforts. Additionally, pain physicians have unique concerns about the initiation or limitation of prescription opioids, as well as public perception and backlash. In this context, this chapter reviews the history of the opioid crisis, current efforts, and the legal background that underlies these interventions.
Opium use has been recorded since at least 3400 B.C. by the Sumerians in lower Mesopotamia, and its addictive properties were likely appreciated soon after. While opium use spread throughout Asia and Europe during the following millennia, the first recorded “opium epidemic” occurred in China from the end of the 18th century to the beginning of the 20th century. This was sparked by the introduction of smoking opium, rather than ingestion and dramatic increases in production and trade by the British. By 1906, it is estimated that 13.4% of the Chinese population was addicted to opium.
The United States dealt with its own opioid epidemic in the late 19th and early 20th centuries. Morphine dependence increased after battlefield exposures during the civil war. This was exacerbated by the release of heroin in 1898, which was purported to carry no “danger of acquiring the habit.” Increasing opioid use in the United States and other Western countries, along with the unprecedented crisis in China, ultimately led to the formation of an international drug control system and widespread reforms, including the criminalization of non-clinical opioid use in the United States in 1915. , ,
The next wave of United States opioid use began in the 1960s–70s, as approximately 20% of service members returned from the Vietnam War addicted to heroin. , This was followed by a period of “opiophobia,” during which both patients and doctors limited prescription opioid use due to fears of iatrogenic opioid addiction. ,
The modern opioid crisis can be traced back to the publication of two now infamous scientific articles in the 1980s. The first was a letter to the editor published in the New England Journal of Medicine in 1980 by Porter and Jick, which stated the following:
Recently, we examined our current files to determine the incidence of narcotic addiction in 39,946 hospitalized medical patients who were monitored consecutively. Although there were 11,882 patients who received at least one narcotic preparation, there were only four cases of reasonably well-documented addiction in patients who had no history of addiction. Addiction was considered to be major in only one instance. The drugs implicated were meperidine in two patients, percodan in one, and hydromorphone in one. We conclude that despite the widespread use of narcotic drugs in hospitals, the development of addiction is rare in medical patients with no history of addiction.
The second was a retrospective review published in the International Association for the Study of Pain journal Pain in 1986 by Portenoy and Foley, which included 38 chronic pain patients managed with chronic opioid therapy. It concluded, “that opioid maintenance therapy can be a safe, salutary, and more humane alternative to the options of surgery or no treatment in patients with intractable non-malignant pain and no history of drug abuse.” These articles were widely presented and cited to support claims about the safety of chronic opioid therapy by physicians and the pharmaceutical industry. ,
As “opiophobia” was being assuaged by the medical literature, the pharmaceutical industry was concurrently innovating to create theoretically less addictive opioids, leading to the introduction of OxyContin, an extended-release formulation of oxycodone, in 1995 by Purdue Pharma. This medication, an extensive advertising campaign, and sponsorship of more than 20,000 medical educational programs were associated with an acceleration in opioid prescribing beginning in 1996. , Purdue Pharma also provided financial support to multiple professional societies that subsequently advocated for increased attention to the treatment of pain and opioid use for chronic non-cancer pain. , , For example, the American Pain Society introduced the “pain is the Fifth Vital Sign” campaign in 1995. Beyond providing education, this campaign impacted policy among the major hospital accrediting bodies: the Department of Veterans’ Affairs in 1999 and the Joint Commission in 2001. , These efforts were associated with a dramatic acceleration in opioid prescribing in the inpatient and outpatient setting in the following years ( Fig. 50.1 ).
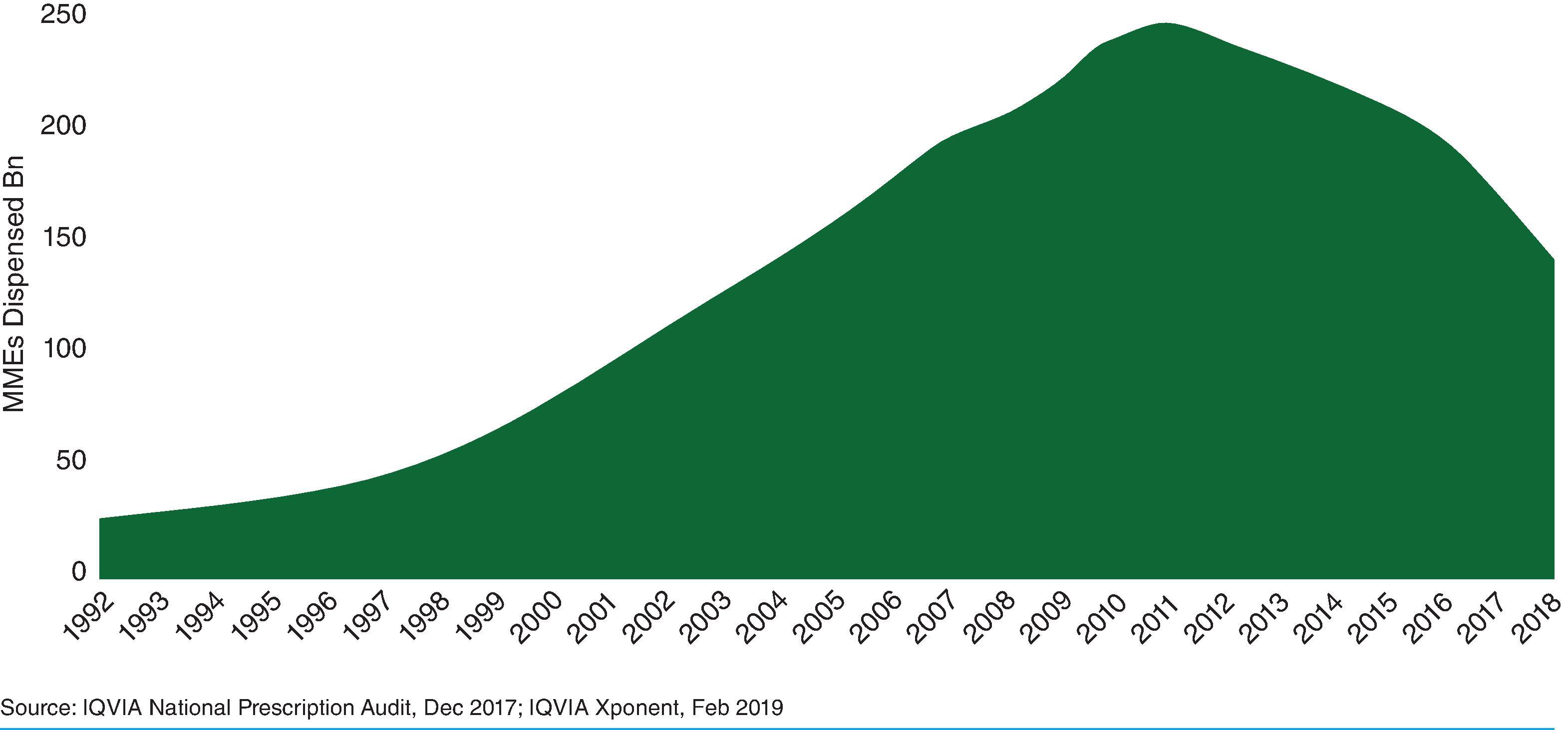
Well-meaning physicians, pharmacists, and patients who previously minimized opioids due to fears of dependence and addiction were now utilizing opioids more liberally to avoid undertreatment of pain and be concordant with contemporary guidelines. Not all parties were found to be well-meaning. However, exacerbating the growth in opioid prescriptions during this period without resulting in patient benefit. For example, Purdue Pharma paid an $8.3 billion settlement in 2020 as part of a plea deal on three felony counts of criminal wrongdoing. They are not the only pharmaceutical companies to face penalties, as settlements have been reached involving Johnson & Johnson, Mallinckrodt, and Teva Pharmaceuticals.
Physicians, pharmacists, physician assistants, nurses, organized crime, and street-level drug dealers have also been implicated in the spread of prescription opioids during this time. For example, one “pill mill” scheme in Arkansas involved recruiters who found patients to be inappropriately prescribed large amounts of opioids by a clinic. These patients would fill these prescriptions at the pharmacy involved in the scheme. The patients would then deliver the pills to drug dealers, and all parties involved received portions of the profits. Irregular, high-volume controlled substance prescription fills could theoretically be detected and reported by drug distribution companies, but this mechanism proved to be inadequate, leading to legal settlements involving the three largest drug distribution companies: AmerisourceBergen Corp., Cardinal Health Inc., and McKesson Corp.
The influx of prescription opioids in the market led to a large increase in opioid exposure in the United States. Between 1988 and 1994, the prescription opioid use rate in the past 30 days among medical and non-medical users aged 20 and over was 3.4%. Between 1999 and 2002, this number grew to 5.0%, and from 2003 to 2006, this rate peaked at 6.9% and persisted until at least 2012, with increased opioid usage increased opioid overdose deaths among medical and non-medical users during the first wave of the opioid crisis from 1999 to 2010 ( Fig. 50.2 ).
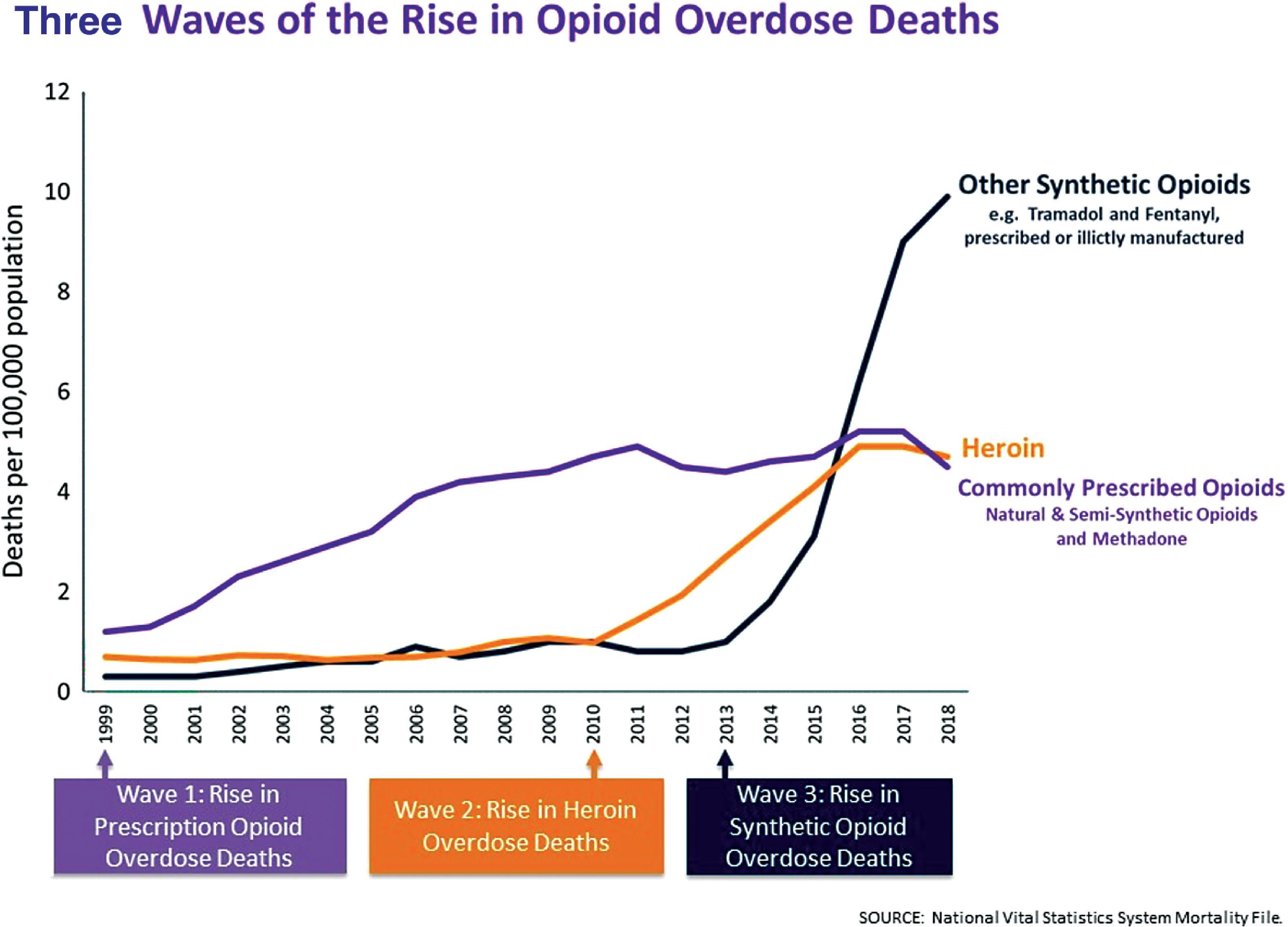
The second wave of the opioid crisis began in 2010, with a dramatic inflection in opioid overdose deaths involving heroin. The rise in heroin use and deaths has been attributed to several factors: increased availability of high-purity heroin, prescription drug abusers turning to heroin, and heroin use beginning at a younger age. As a measure of availability, heroin seizures at the Southwest border increased 352% from 2008 to 2015, according to the United States Drug Enforcement Agency. Additionally, as law enforcement focused on limiting the distribution of illicit prescription opioids, an unintended consequence was opioid users being shunted to cheaper and readily available heroin. Prescription pain pill abusers were 19 times more likely to start using heroin than the general public. Furthermore, the average age of heroin users dropped to as low as 21.4 years old, which was associated with higher rates of polysubstance abuse and alcohol binge drinking.
In 2011, the Obama administration formally addressed the prescription opioid epidemic and revealed a plan to combat the crisis. This plan detailed broad reforms involving many governmental agencies. Most notably, it initiated diverse education efforts, invested in prescription drug monitoring programs (PDMPs), encouraged appropriate drug disposal, and removed law enforcement barriers. Around this time, prescribed opioid overdose deaths stabilized, and morphine equivalents dispensed began to decline. , However, heroin use continued to grow.
In the setting of stable prescription opioid overdose deaths but increasing heroin associated deaths, the third wave of the opioid crisis began in 2013 with a rise in the use of fentanyl and other synthetic opioids. Increased law enforcement efforts made the production and shipment of heroin more difficult, which may have shifted drug production toward fentanyl. Fentanyl does not require harvesting poppies, and small shipments are profitable. Furthermore, fentanyl is found to be mixed with non-opioids such as cocaine and methamphetamine, suggesting that dealers may add fentanyl to non-opioids attempting to leverage its addictiveness. ,
As the opioid crisis persisted, several professional organizations, states, and federal agencies began releasing opioid prescribing guidelines, but these guidelines varied in their use of evidence, methods of addressing conflicts of interest, and ultimately their specific recommendations. This motivated the creation of the Centers for Disease Control and Prevention (CDC) Guidelines for Prescribing Opioids for Chronic Pain released in 2016, which intended to advise primary care clinicians on how to avoid problematic prescribing habits for adults with chronic, non-cancer pain. Although all pain medicine clinicians should be familiar with the full text of the CDC guidelines, the 12 primary recommendations are listed in Box 50.1 .
Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient. If opioids are used, they should be combined with nonpharmacologic therapy and non-opioid pharmacologic therapy, as appropriate.
Before starting opioid therapy for chronic pain, clinicians should establish treatment goals with all patients, including realistic goals for pain and function, and should consider how opioid therapy will be discontinued if the benefits do not outweigh the risks. Clinicians should continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety.
Before starting and periodically during opioid therapy, clinicians should discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy.
When starting opioid therapy for chronic pain, clinicians should prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) opioids.
When opioids are started, clinicians should prescribe the lowest effective dosage. Clinicians should use caution when prescribing opioids at any dosage, should carefully reassess evidence of individual benefits and risks when considering increasing dosage to ≥50 morphine milligram equivalents (MME)/day, and should avoid increasing dosage to ≥90 MME/day or carefully justify a decision to titrate dosage to ≥90 MME/day.
Long term opioid use often begins with the treatment of acute pain. When opioids are used for acute pain, clinicians should prescribe the lowest effective dose of immediate-release opioids and should prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than seven days will rarely be needed.
Clinicians should evaluate benefits and harms with patients within one to four weeks of starting opioid therapy for chronic pain or of dose escalation. Clinicians should evaluate the benefits and harms of continued therapy with patients every three months or more frequently. If benefits do not outweigh the harms of continued opioid therapy, clinicians should optimize other therapies and work with patients to taper opioids to lower dosages or taper and discontinue opioids.
Before starting and periodically during the continuation of opioid therapy, clinicians should evaluate risk factors for opioid-related harms. Clinicians should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase the risk for opioid overdose, such as a history of overdose, history of a substance use disorder, higher opioid dosages (≥50 MME/day), or concurrent benzodiazepine use, are present.
Clinicians should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. Clinicians should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every three months.
When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications and other controlled prescription drugs and illicit drugs.
Clinicians should avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible.
Clinicians should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone combined with behavioral therapies) for patients with opioid use disorder.
This guideline with explicit morphine milligram equivalent thresholds has had a dramatic impact on opioid prescriptions. High-dose opioid prescriptions, concurrent opioid, and benzodiazepine prescriptions, and overall opioid prescription rates all declined more rapidly after the publication of the guideline.
Before the end of his second term, President Obama signed two additional pieces of legislation into law to further combat the opioid crisis, the Comprehensive Addiction and Recovery Act (CARA) of 2016, followed by the 21 st Century Cures Act. , CARA dedicated new funding toward the opioid epidemic and focused on prevention, overdose reversal, treatment, recovery, law enforcement, and criminal justice reform. The 21 st Century Cures Act supplemented this work with grant funding for substance abuse and mental health treatment.
In January 2017, Donald Trump was inaugurated as president of the United States. By October 2017, President Trump declared the opioid crisis a public health emergency, which remained designated as an emergency until October 2018. The emergency status led to reduced paperwork and wait times for several governmental efforts to address the crisis. However, the overall impact of this emergency declaration is a matter of debate.
In November 2017, the President’s Commission on Combating Drug Addiction and the Opioid Crisis released its final report with 56 recommendations. This was followed in March 2018 by the release of President Donald J. Trump’s initiative to stop opioid abuse and reduce drug supply and demand. These two reports outline a variety of priorities and continue to impact actions by the administration. For example, they advocate increased funding of opioid-related government activities, a mass media prevention campaign, increasing access to treatment for substance use disorder, drug court rather than prison, legislation to assist former felons in finding work, and expanding law enforcement resources. , A progress update published in May 2019 by the Trump administration touted examples of its progress toward the goals of these reports: $6 billion in funding over two years to fight the opioid crisis, a large ad campaign, a reduction in opioids dispensed, increased arrests and seizures by law enforcement, increased use of medication-assisted treatment (MAT), increased naloxone prescription, and several other reported measures of success.
Another effort by the Trump administration to combat the opioid crisis, the Prescription Interdiction and Litigation Task Force, was announced in February 2018. This task force aims to support local jurisdictions with the filing of lawsuits against prescription drug producers and distributors. For example, the task force filed a statement of interest in a federal court case that combined over 600 lawsuits lodged by disparate government entities against opioid manufacturers.
In addition to efforts to reduce opioid use, another development in the opioid crisis is a “swing of the opioid pendulum” back toward rational but slightly more liberal opioid prescribing. Though the CDC guideline was in many respects a success, it also led to the creation of some inflexible policies and practices that were inconsistent with the original guidelines. For example, misapplication of the guidelines affected the care of patients with cancer, sickle cell crises, and opioid use disorders. , The guidelines were also used to justify rapid tapers or complete discontinuation of opioids in some patients. , Such practices are not only difficult for patients but can also be associated with an increased risk of overdose death in high risk populations. These concerns prompted several of the authors of the CDC guideline to provide further guidance regarding the proper application of the guidelines in April 2019. , Individualized approaches were emphasized, and hard limits, abrupt tapering, and sudden discontinuation of opioids were discouraged. ,
At the time of writing, there is room for optimism in the current state of the opioid crisis. Prescription opioid dispensing has been declining every year since its peak in 2011, and it declined by 17% in 2018 alone. Deaths from opioid-related overdoses may have also plateaued since the peak of 48,580 deaths in November 2017, although deaths from synthetic opioids continue to rise. Overall life expectancy rose in 2018 for the first time since 2014. The CDC attributed this partially to decreased unintentional injuries, which includes opioid-related overdose deaths. Part of the decline in opioid-related overdose deaths may be due to increased use of MAT for opioid use disorder. From 2014 to 2018, MAT prescriptions increased 47%, from 11 to 16.2 million. Opioid misuse among youth in the United States is also trending down. For example, 2.7% of 12 th -graders reported illicit prescription opioid use, the lowest percentage ever recorded since surveying began in 1991. Despite these encouraging trends, the opioid crisis remains a significant problem. More people die from opioid-related overdoses than car accidents in the United States, and the number of opioid-related overdose deaths has remained essentially unchanged from November 2017 to August 2019. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here