Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Molecular feedback mechanisms lend robustness and the ability of living cells to respond and adapt to their environment. As a means to ward off cancer, for example, the expression of proto-oncogenes in response to growth factors is normally followed by the expression of tumor suppressor genes. Myc and p53—a proto-oncogene and a tumor suppressor gene, respectively—are good examples of genes with concomitant expressions; and, in fact, several molecular pathways (direct and indirect) by which Myc upregulates p53, as well as pathways by which p53 downregulates Myc, have been identified (see for a review). Thus, negative feedback loops (nFBLs) between Myc and p53 exist (and we have analyzed and modeled how these nFBLs coordinate cell proliferation and differentiation ). In this chapter, we investigate the nFBL between two genes, Kras and Ink4a, that are implicated in the early stages of pancreatic ductal adenocarcinoma (PDAC) development. Oncogenic mutations in Kras and loss or deficiency of Ink4a are exhibited by an overwhelming majority of PDAC patients and in various other human cancers .
The significance of the Kras-Ink4a nFBL may be related to its role in determining thresholds of Kras activation. Note that individuals with oncogenic Kras mutations can remain normal and healthy, perhaps because a certain threshold of Kras activity has not been reached. To explore this idea, we discuss the possible factors determining this threshold in the section Threshold of Kras Activation . At the single molecule level, Kras switches between inactive and active protein conformations; but, at the level of protein population (in a single cell), Kras activity behaves more like a rheostat rather than a switch . We shall discuss how positive feedback from Kras effector signaling pathways to Kras itself can influence the value of Kras activation threshold. A few of these effector pathways are considered in the section Kras Effector Pathways in PDAC Development , with discussion of their potential control points and the effects of their perturbations on the cell cycle and apoptosis—processes that ultimately determine the rate of tumor growth.
In our review of Myc-p53 interactions , we indicated pathways by which Myc downregulates p53 in abnormal conditions (as in various cancers). The mutual downregulation between Myc and p53 constitutes a positive feedback loop (pFBL) that weakens the normal nFBL between them. A similar pFBL can occur between Kras and Ink4a. In the section Negative versus Positive Feedback Loops between Kras and Ink4a , we discuss a case where Kras indirectly downregulates Ink4a, thus forming a pFBL between Kras and Ink4a. This pFBL creates a potentially unstable situation where Ink4a is suppressed and a runaway Kras activation may ensue.
In the section Ink4a against microRNAs in the Control of Kras , we give examples of Kras-mediated pathways that affect the expressions of microRNAs (miRs) whose targets feed back to Kras. Although these feedbacks are all pFBLs, they are different in details: one set is characterized by mutual upregulation between Kras and a miR, while the other set involves mutual antagonism between Kras and a miR. This section illustrates how the nFBL between Kras and Ink4a may play a role in suppressing the deregulatory effects of the miR-mediated pFBLs that can amplify Kras activity.
In the last section, Concluding Remarks , we give a summary of the complexity and confounding features of these Kras pathways, and the role that mathematical and computational modeling can play to increase our understanding of the system.
Activating mutations in the KRAS gene (e.g., KrasG12D) may also be found in normal healthy individuals and are therefore not reliable markers for PDAC . It is believed that Kras proteins must be activated beyond a certain threshold before initiating cell transformation. We define a “threshold” to mean a particular level of an input signal below which there is no (or insignificant) response and above which there is a significant response (a binary switch would have a zero or full response below or above the threshold, respectively). As we shall see in this section, there can be different thresholds of Kras activation—one viewed at the single-protein level where switching between structural conformations occurs, and another at the protein population level where Kras effector pathways are involved, particularly those that positively feed back to Kras.
A property of a Ras (Kras, Hras, Nras) protein that makes it an appropriate decision element for signal transmission is the ability to act as a binary switch between different structural conformations depending on whether it is bound to GDP (guanosine diphosphate) or GTP (guanosine triphosphate) (see Figure 12.1 and Refs ). GTP-bound Ras is said to be active because it can interact with proteins involved at the head of Ras effector pathways. GDP-bound Ras has significantly reduced ability for such interactions and is therefore referred to as inactive. Guanine nucleotide exchange factors (GEFs) catalyze Ras activation by stimulating the exchange between the GDP bound to Ras and cytosolic GTP. On the other hand, GTPase-activating proteins (GAPs) inactivate Ras by catalyzing the hydrolysis of bound GTP to GDP. Ras itself has an intrinsic GAP activity. The detailed mechanism and kinetics of these processes have been and are being investigated (for example, see Refs ).
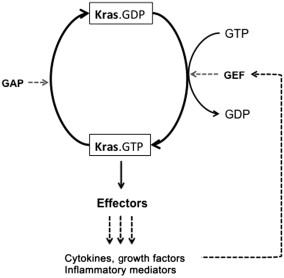
In a population of Ras proteins (in a single cell), because of the variable ratios between active and inactive Ras proteins, the activity of the ensemble of Ras proteins is expected to behave more like a rheostat —one in which the overall Kras population activity is monotonously regulated and without noticeable switching features. Activating mutations in the Kras gene have been found to drastically change the ratio of GDP-bound to GTP-bound Ras. Under basal resting conditions, it is estimated that the percentage of GTP-bound Ras is less than 5% for wild-type Ras, compared to over 50% for Ras with oncogenic mutations . Among the possible reasons for the increased levels of GTP-bound Ras mutants, insensitivity to GAPs and significant reduction of the intrinsic GTPase activity of Kras have been shown to be the primary reasons .
As depicted in Figure 12.1 , Kras effector pathways can induce cells to produce cytokines, growth factors, and inflammatory mediators that drive Kras activation in autocrine or paracrine manner in a cell population . For example, it has been shown by various groups that the NF-κB pathway is involved in the pFBL that drives Kras activity to prolonged and sustained levels (this loop involves an NF-κB target, Cox-2, which is an enzyme that induces several mediators of inflammation such as PGE2).
The positive feedback to the KRas GTPase cycle (see Figure 12.1 ) generates an interesting threshold on Kras activity. We have shown earlier that a positive feedback to a cyclic reaction can create a threshold that depends on the total level of the proteins involved in the cycle (in the present case, the total of inactive and active Kras proteins). Our previous results are summarized in Figure 12.2 . In Figure 12.2 (B), the steady state level of active Ras ( R a,ss ) is plotted against total Ras protein ( R total ). It was shown that below a certain level of R total (this level is indicated by R ∗ in Figure 12.2 (B)), active Ras always goes to zero at steady state (the “s” above the zero horizontal line for R total less than R ∗ in the figure indicates that the zero steady state is stable—in the sense that any perturbation out of the zero state always returns to zero eventually; in other words, there could be transient Ras activities, but these transients eventually go to zero). This zero steady state still exists beyond R ∗ but is now unstable (shown by the dashed line in Figure 12.2 (B) and labeled “u”) in the sense that a small perturbation will always increase until a positive value of the steady state is reached; this locus of positive steady states is shown by the solid diagonal line to the right of R ∗. As shown, R a,ss increases as R total increases beyond R ∗. We emphasize that as long as R total is below R ∗, the inactive state is the only state that exists at steady state.
![Figure 12.2, (A) A positive feedback loop (dashed curve) coupled with the R i – R a cycle. This loop means that the rate of production of R a is a function of R a as well as R i . ( R a = active Ras, R i = inactive Ras); (B) The steady state of R a ( R a,ss ) as a function of R total (= R i + R a ) for the case when the kinetics of the forward and reverse reactions is mass action (see Ref. [21] for more details). Figure 12.2, (A) A positive feedback loop (dashed curve) coupled with the R i – R a cycle. This loop means that the rate of production of R a is a function of R a as well as R i . ( R a = active Ras, R i = inactive Ras); (B) The steady state of R a ( R a,ss ) as a function of R total (= R i + R a ) for the case when the kinetics of the forward and reverse reactions is mass action (see Ref. [21] for more details).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheSignificanceoftheFeedbackLoopsbetweenKrasandInk4ainPancreaticCancer/1_3s20B9780124081031000121.jpg)
The parameter that controls the Kras activation threshold is R total ( Figure 12.2 (B)), the total Ras protein, and attention should be given to studies on the regulation of total Ras protein levels . For a better understanding of Kras activity thresholds, the details of the pFBLs between Kras and its effector pathways (dashed curves in Figures 12.1 and 12.2 (A)) would have to be elucidated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here