Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The coronavirus disease 2019 (COVID-19) continues to have a devastating impact on virtually all aspects of human life, from the healthcare system to the economy, social relations, and mental health.
Key mechanisms that may have a role in the pathophysiology of multiorgan injury secondary to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) include direct viral toxicity, endothelial cell damage and thromboinflammation, dysregulation of the immune response, and dysregulation of the renin–angiotensin–aldosterone system.
Omicron variant carries high transmissibility but milder symptoms than earlier variants. Moreover, the Omicron variant is more likely to infect the upper respiratory tract and less able to cause lung infection.
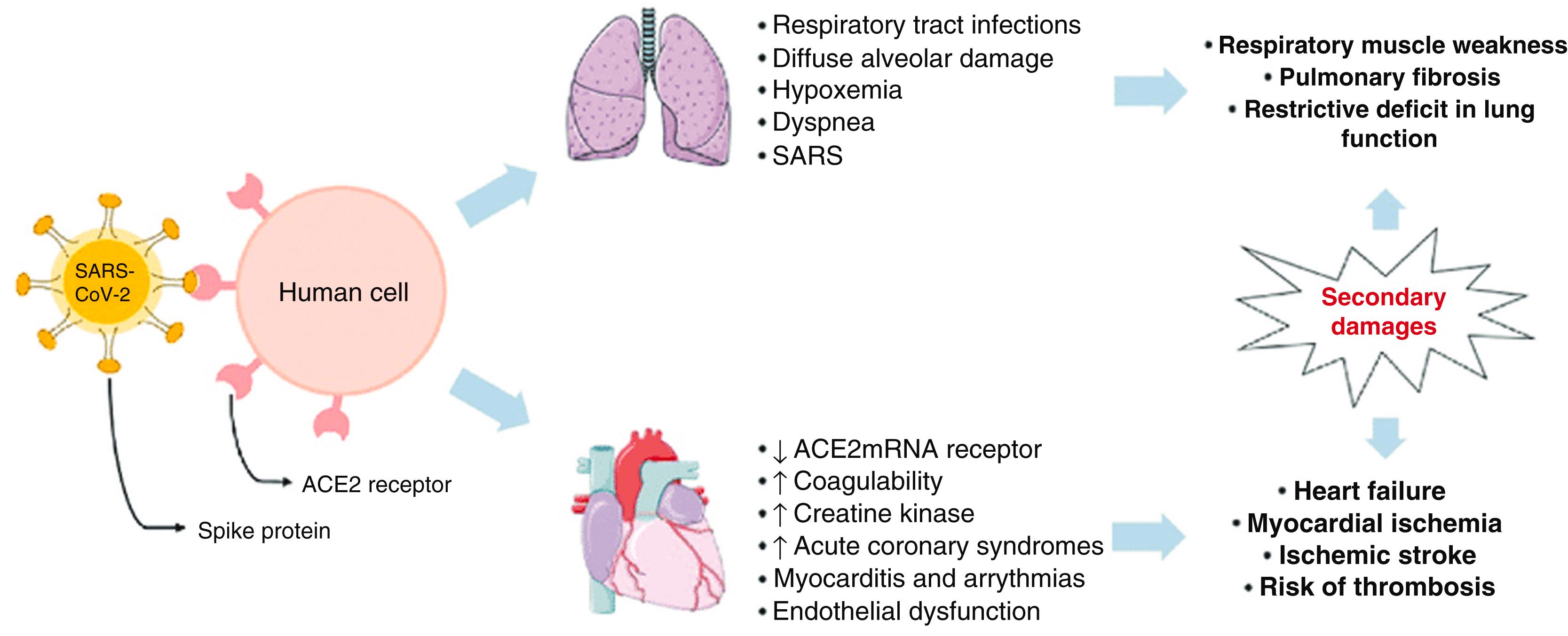
SARS-CoV-2 can cause both direct and indirect cardiovascular sequelae including myocardial injury, acute coronary syndromes, cardiomyopathy, acute cor pulmonale, arrhythmias, cardiogenic shock, and thrombotic complications.
Impairment in lung diffusion capacity is a primary abnormality in patients with COVID-19, followed by restrictive ventilatory dysfunction.
Postacute sequelae of SARS-CoV-2 infection describes the long-term symptoms that might be experienced weeks to months after primary infection with SARS-CoV-2.
Anesthesiologists must familiarize themselves with the expansive organ system manifestations described in both acute and chronic COVID-19 disease.
In 2019, Wuhan, China, reported the outbreak of a novel coronavirus associated with severe viral pneumonia. The virus, which has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), proceeded to cause a worldwide pandemic with over 630 million infections and 6.6 million deaths as of early November 2022. The disease caused by the SARS-CoV-2 virus was named coronavirus disease 2019 (COVID-19) on February 11, 2020 by the World Health Organization and continues to have a devastating impact on virtually all aspects of human life, from the healthcare system to the economy, social relations, and mental health.
COVID-19 is associated with an array of clinical manifestations that vary in severity from asymptomatic presentation to acute respiratory distress syndrome (ARDS) and total organ failure. , Although SARS-CoV-2 infection is frequently asymptomatic, 20% of patients require hospital admission. Infection fatality risk increases with age and has been reported as high as 14.2% in patients 75 years or older within a New York City cohort during the spring of 2020. The delta variant has enhanced infection concerns beyond the elderly community, as global increases in cases have been reported in young adults and children. The delta variant is estimated to be more than twice as transmissible and may cause more severe infection, even though rarely among otherwise healthy young adults and children. The Omicron variant was first reported in November 2021 and posted a serious threat to public health owing to its contagious and vaccine-escape mutations. Omicron-infected patients exhibit milder symptoms than those infected by the earlier variants. Moreover, the Omicron variant is more likely to infect the upper respiratory tract and less able to cause lung infection. However, it is possible that the pathogenicity of the Omicron variant may be underestimated because of rising levels of herd immunity via previous infections and vaccinations. Notably, the proportion of young patients was higher among those infected with Omicron, which may result in reduced pathogenicity. In addition, the impact of Omicron is not attenuated by reduced pathogenicity, the healthcare system is still under enormous pressure because of the high transmissibility of the Omicron variant.
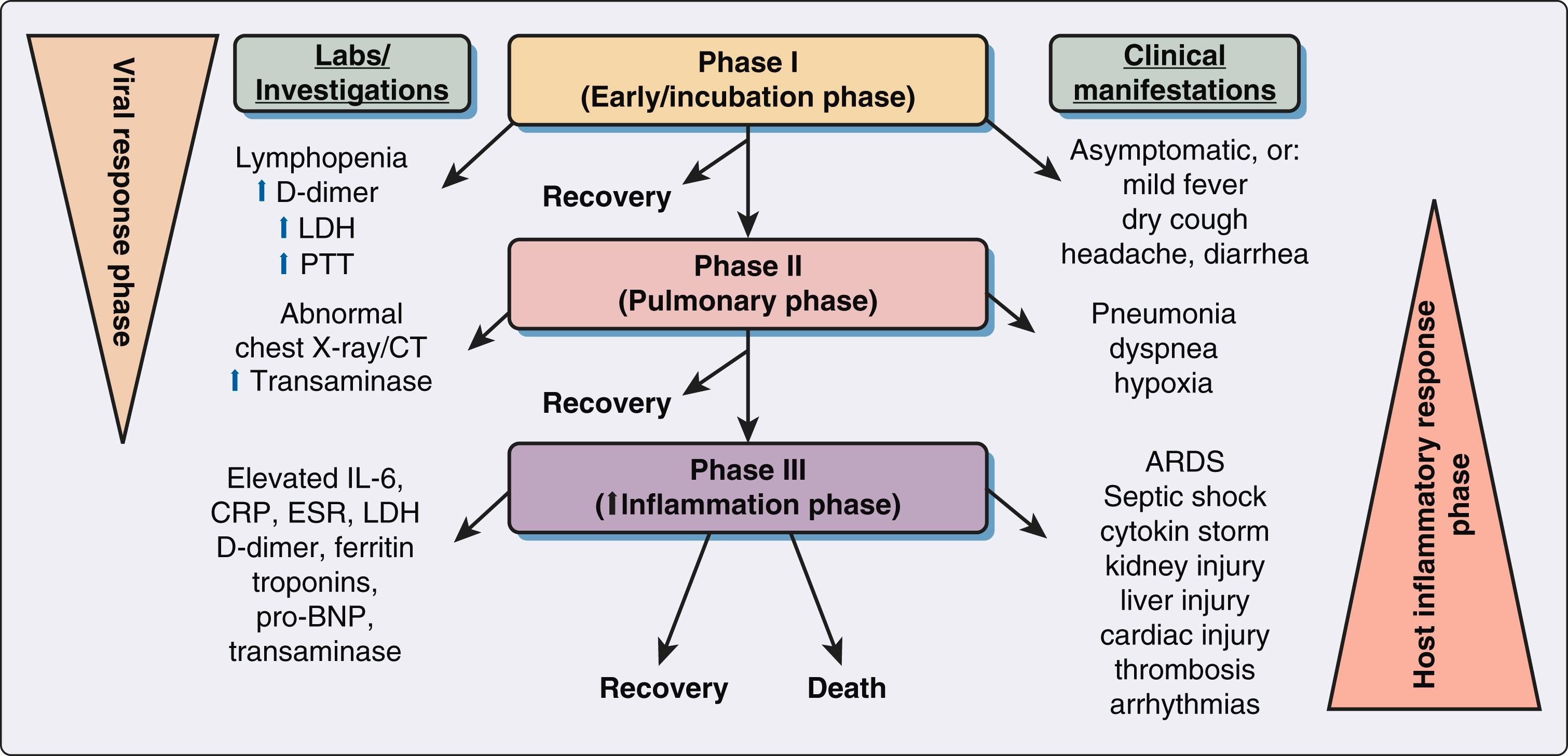
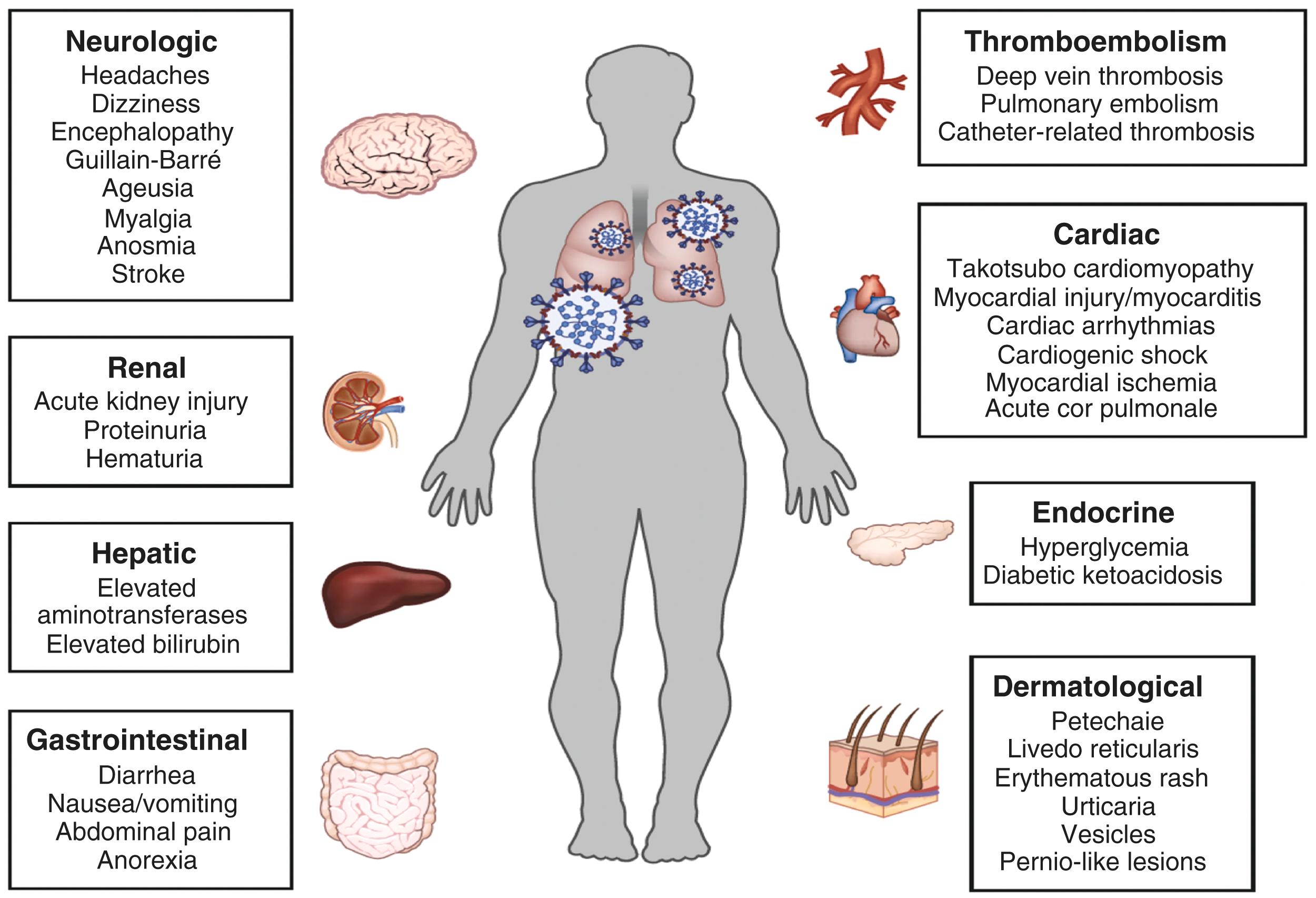
A person infected with SARS-CoV-2 will initially experience fever, sore throat, dry cough, headache, fatigue, restlessness, myalgia, anosmia, and dysgeusia. Symptoms may progress from mild to moderate pneumonia followed by hypoxia and potentially severe complications such as ARDS, systemic inflammatory response syndrome, multiorgan failure, and/or shock if left untreated. , The COVID-19 disease course can be divided into three phases: early phase, pulmonary phase, and increased inflammatory phase ( Fig. 4.1 ). Mechanisms that favor progression of COVID-19 from milder to more severe stages are believed to be largely dependent on the effectiveness of the early immune response. Lung inflammation and subsequent respiratory failure due to pneumonia is the major cause of in-hospital deaths. However, the cardiovascular and renal systems are frequently involved in severe clinical cases, and emerging literature suggests that the hematologic, gastrointestinal and hepatobiliary, endocrinological, neurologic, ophthalmologic, and dermatologic systems can all be affected, thus making COVID-19 a systemic disease ( Fig. 4.2 ). This pathology may reflect either extrapulmonary dissemination with replication of SARS-CoV-2 or widespread immunopathological sequelae of the disease.
SARS-CoV-2 appears to employ mechanisms for receptor recognition similar to those used by prior virulent coronaviruses such as SARS-CoV, the pathogen responsible for the SARS epidemic of 2003. SASR-CoV-2 wide type or variants before Omicron mainly infect lung epithelial cells, which are TMPRSS2 high expressed cells, and enter host cells by plasma membrane route. The coronavirus spike protein facilitates entry of the virus into target cells. The spike subunit of SARS-CoV-2 engages angiotensin-converting enzyme 2 (ACE2) as an entry receptor ( Fig. 4.3 ). In addition, cell entry requires priming of the spike protein by the cellular serine protease transmembrane serine protease 2 (TMPRSS2) or other proteases. Coexpression of ACE2 and TMPRSS2 on the cell surface is required for the completion of this entry process, and the efficiency with which the virus binds to ACE2 is a key determinant of transmissibility. Recent studies have demonstrated higher affinity of binding of SARS-CoV-2 to ACE2 in comparison to SARS-CoV, which may partially explain the increased transmissibility of SARS-CoV-2.
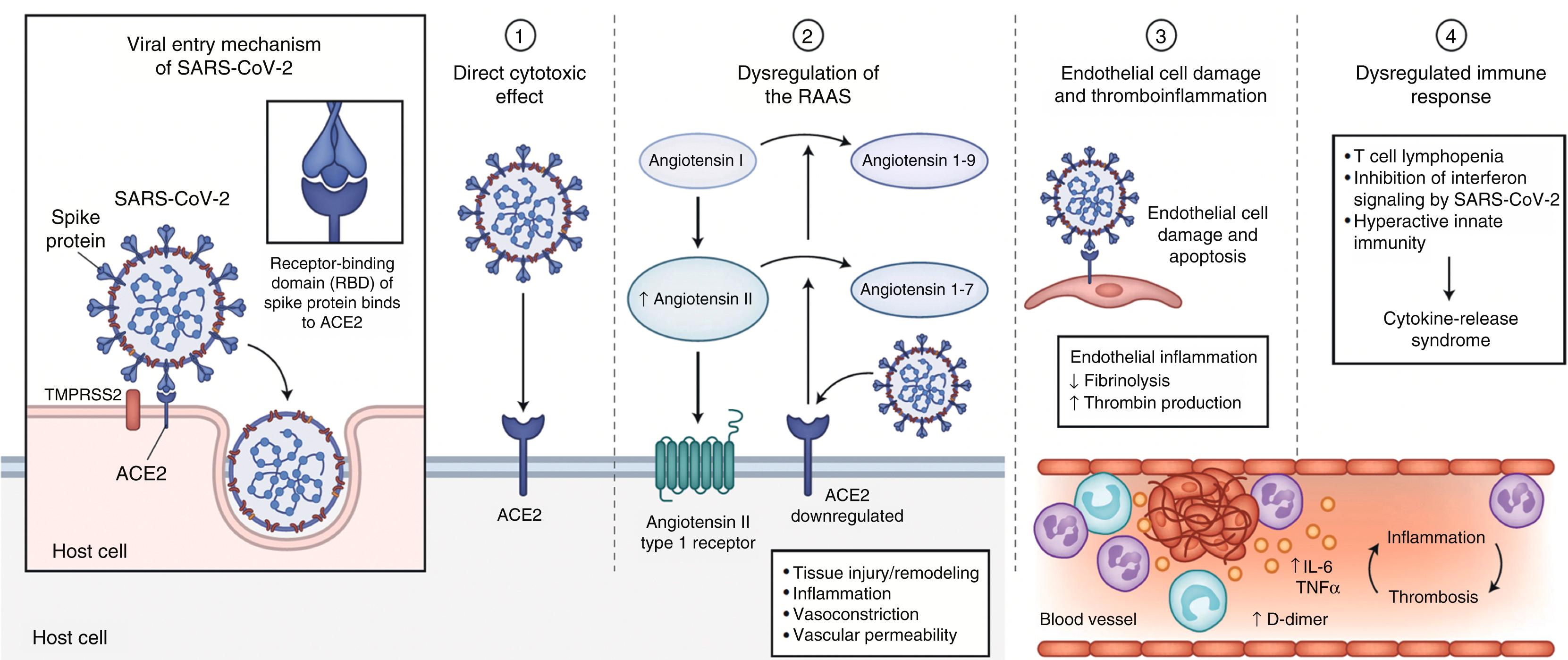
In contrast, SASR-CoV-2 Omicron variant mainly infects the upper airway epithelial cells, which are TMPRSS2 low-expressed cells, and enter host cells by the endosomal route. In the endosomal entry route, a virus first binds to ACE2 and the virus–ACE2 complex is internalized via endocytosis into the endosomes, where S protein is cleaved by Cathepsin L. Then the viral and endosomal membranes are fused together to form a pore and release the viral genome . Key mechanisms that may have a role in the pathophysiology of multiorgan injury secondary to infection with SARS-CoV-2 include direct viral toxicity, endothelial cell damage and thromboinflammation, dysregulation of the immune response, and dysregulation of the renin–angiotensin–aldosterone system (RAAS) ( Fig. 4.3 ). The relative importance of these mechanisms in the pathophysiology of COVID-19 is currently not fully understood. While some of these mechanisms, including the ACE2-mediated viral entry and subsequent tissue damage as well as the dysregulation of the RAAS, may be unique to COVID-19, the immune pathogenesis caused by the systemic release of cytokines and microcirculation dysfunction may also occur secondary to sepsis.
SARS-CoV-2 is transmitted mainly through direct or indirect respiratory tract exposure. Given the high expression of ACE2 in multiple epithelial cell types of the airway including alveolar epithelial type II cells in the lung parenchyma, SARS-CoV-2 has tropism for the respiratory tract. Live SARS-CoV-2 virus and viral subgenomic mRNA isolated from the upper airway can be detected by real-time polymerase chain reaction (RT-PCR). Later in the disease course, viral replication may occur in the lower respiratory tract, which manifests in severe cases as pneumonia and ARDS.
Studies have isolated viral RNA from fecal samples at high titers. It has been less commonly isolated from urine and blood. , Histopathological studies have confirmed organotropism of SARS-CoV-2 beyond the respiratory tract, including tropism to renal, myocardial, neurologic, pharyngeal, and gastrointestinal tissues. In addition, single-cell RNA sequencing studies have further verified expression of ACE2 and TMPRSS2 in lung alveolar epithelial type II cells, nasal goblet secretory cells, cholangiocytes, colonocytes, esophageal keratinocytes, gastrointestinal epithelial cells, pancreatic β-cells, and renal proximal tubules and podocytes. , These findings suggest that direct viral tissue damage may be at least partially responsible for the multiple organ injury.
Endothelial cell damage through ACE2-mediated entry of SARS-CoV-2 with subsequent inflammation and the generation of a prothrombotic milieu is another proposed pathophysiologic mechanism of COVID-19. ACE2 expression has been demonstrated in the arterial and venous endothelium of several organs, and histopathological studies have found microscopic evidence of SARS-CoV-2 viral particles in endothelial cells of the kidneys and lungs. Infection-mediated endothelial injury (characterized by elevated levels of von Willebrand factor) and endothelialitis (marked by the presence of activated neutrophils and macrophages) found in multiple vascular beds (including the lungs, kidney, heart, small intestine, and liver of patients with COVID-19) can trigger excessive thrombin production, inhibit fibrinolysis, activate complement pathways, initiate thromboinflammation, and ultimately lead to microthrombi deposition and microvascular dysfunction.
Platelet–neutrophil cross-communication and activation of macrophages in this setting can also facilitate a variety of proinflammatory effects such as cytokine release, the formation of neutrophil extracellular traps (NETs), and fibrin and/or microthrombus formation. NETs further damage the endothelium and activate both extrinsic coagulation pathways and intrinsic coagulation pathways. They were detected at higher levels in patients hospitalized with COVID-19 in a study from the United States, and a ‘pro-NETotic state’ was positively correlated with severe illness. Hypoxia-mediated hyperviscosity and upregulation of the hypoxia-inducible factor 1 signaling pathway subsequent to acute lung injury may also contribute to the prothrombotic state. Finally, direct coronavirus-mediated effects may also lead to an imbalance of pro- and anticoagulant pathways. Small case reports and case series have demonstrated the presence of fibrinous exudates and microthrombi in histopathological examinations in patients with COVID-19.
The presentation of severe COVID-19 is characterized by a dysregulated immune response and cytokine release syndrome due to overactivation of innate immunity in the setting of T-cell lymphodepletion. Prior preclinical and human studies with pathogenic human coronaviruses have proposed rapid viral replication, antagonism of interferon signaling, and activation of neutrophils and monocyte-macrophages as mediators of hyperinflammation. Elevation of serum inflammatory markers such as C-reactive protein (CRP), ferritin, erythrocyte sedimentation rate, D-dimer, fibrinogen, and lactate dehydrogenase is predictive of subsequent critical illness and mortality in patients with COVID-19. Higher levels of the cytokine interleukin-6 (IL-6) in the serum have also been linked to a worse prognosis and have been found to correlate with fibrinogen levels in patients with COVID-19. Clinical trials for treating COVID-19 by targeting the IL-6 signaling pathway are underway with the hope of mitigating the deleterious effects linked to the activation of this pathway.
Infection may stimulate a severe immune reaction called “cytokine storm,” in which the body dissipates too many cytokines in the blood very quickly and uncontrollably. As a result, the production of neutrophils, proinflammatory cytokines (IL-1b, IL-6, tissue necrosis factor [TNF] α, etc.), and chemokines (chemokine ligand [CCL] 1, C-X-C motif chemokine ligand 10 [CXCL] 10, CCL3, etc.) exceeds the levels of antiinflammatory cytokines in the body, which in turn leads to multiorgan damage. A majority of patients are reported to exhibit diffuse bilateral pneumonia surrounded with ground-glass opacity either progressing or coexisting with consolidation. Histological examinations have also evidenced that the lower respiratory tract holds a higher overall viral load than the upper respiratory tract. In addition, pathological findings in the infected lungs have indicated the appearance of proteinaceous exudates in lung tissues as well as in bronchial lavage fluids, development of pulmonary edema, bilateral diffuse alveolar damage, interstitial thickening, and the infiltration of T cells or inflammatory monocytes, etc., in comparison to a healthy lung. Moreover, COVID-19 patients have been marked with relatively low levels of lymphocytic T cells (CD4+ and CD8+) and natural killer (NK) cells, i.e., overall low levels of lymphocyte counts in the blood profile. Two of the most prominent reasons for low lymphocytes are hypokalemia and hypophosphatemia, metabolic derangements induced by the impact of SARS-CoV-2 on patients’ ACE-angiotensin-II (ACE-Ang-II) that prevents the degradation of intact ACE-Ang-II within the system. As a result, aldosterone production triggers frequent vomiting, diarrhea, and urination as part of multiple organ injury. People with comorbidities such as diabetes, hypertension, hypothyroidism, chronic lung diseases, malignancy, and obesity are at a significantly higher risk of severe COVID-19 infection due to altered immune response.
Maladaptive functions of the RAAS constitute another plausible pathophysiological mechanism of SARS-CoV-2 infection–related tissue damage. The RAAS is composed of a cascade of regulatory peptides that participate in key physiological processes of the body, including fluid and electrolyte balance, blood pressure (BP) regulation, vascular permeability, and tissue growth. ACE2 has emerged as a potent counterregulator of the RAAS pathway. ACE2 cleaves angiotensin I into inactive angiotensin 1–9 and cleaves angiotensin II into angiotensin 1–7 that has vasodilatory, antiproliferative, and antifibrotic properties. While the pathophysiology of SARS-CoV-2 may not be limited exclusively to ACE2-related pathways, these findings may have implications for the organ-specific clinical manifestations of COVID-19. Recent findings suggest that bradykinin and the kinin–kallikrein system may also play a prominent role in COVID-19 via a newly described bradykinin storm. Bradykinin storm could be the result of the SARS-CoV-2–mediated reduction in ACE2 availability and the downregulation of des-Arg -bradykinin degradation. This is associated with BP dysfunction as well as inflammation. An accumulation of bradykinin has been detected in bronchoalveolar lavage fluid from COVID-19 patients, and this has been correlated to symptoms associated with COVID-19.
After the outbreak of COVID-19, the initial reports suggested that male sex, older age, and cardiovascular risk factors, such as hypertension, diabetes, and obesity, were common in COVID-19 patients. This has been confirmed in most subsequent studies, which have shown an association between the severity of COVID-19 and cardiovascular risk factors as well as related organ damage. Roughly 40% to 50% of hospitalized COVID-19 patients had underlying cardiovascular or cerebrovascular diseases, and an association has been found between the severity of COVID-19 infection and acute cardiovascular damage. Those with concomitant cardiac diseases (heart failure, atrial fibrillation, coronary artery disease) had a much poorer prognosis in terms of mortality, thromboembolic events, and septic shock rates when compared with patients without a history of cardiac disease. In the Yale COVID-19 Cardiovascular Registry, more than 40% of patients hospitalized with COVID-19 presented with a cardiovascular disease such as coronary artery disease, heart failure, and atrial fibrillation. As in previous studies, hypertension, type 2 diabetes, and dyslipidemia were common cardiovascular risk factors, and in-hospital cardiovascular events were common. Atrial fibrillation was detected in 19% of the patients, myocardial infarction (MI) in 17%, and acute decompensated heart failure in 14%. Overall, 18% of the patients died in the hospital and 39% experienced a major adverse cardiovascular event following admission. Hypertension was the most frequent concomitant comorbidity in COVID-19 (prevalence of 56.6%) followed by obesity (41.7%) and diabetes (33.8%). An independent association between hypertension and the risk of COVID-19 (OR: 2.29, P <0.001) has recently been validated in a meta-analysis of six studies that included 1558 patients.
COVID-19 causes severe ARDS by binding and entering target epithelial lung cells through the peptide ACE2. As treatment with the renin-angiotensin-aldosterone system blockers has been shown to upregulate ACE2 at the cell surface, early concerns arose regarding the use of ACE inhibitors or angiotensin receptor blockers (ARBs) as these drugs might increase the risk or severity of COVID-19 infection. However, a recent study and meta-analysis have demonstrated that ACE inhibitors and ARBs were not associated with increased risks of organ failure in COVID-19, , thus reinforcing the safety of using these drugs to protect patients in a number of important cardiovascular diseases. This is clinically crucial as the discontinuation of RASS blockers is accompanied by a marked increase of cardiovascular complications and mortality in many of these disease states. The protective nature of these drugs may include their reported antiinflammatory effects as excessive inflammatory reaction and a pronounced increase of multiple inflammatory markers have been shown to adversely affect the course of COVID-19.
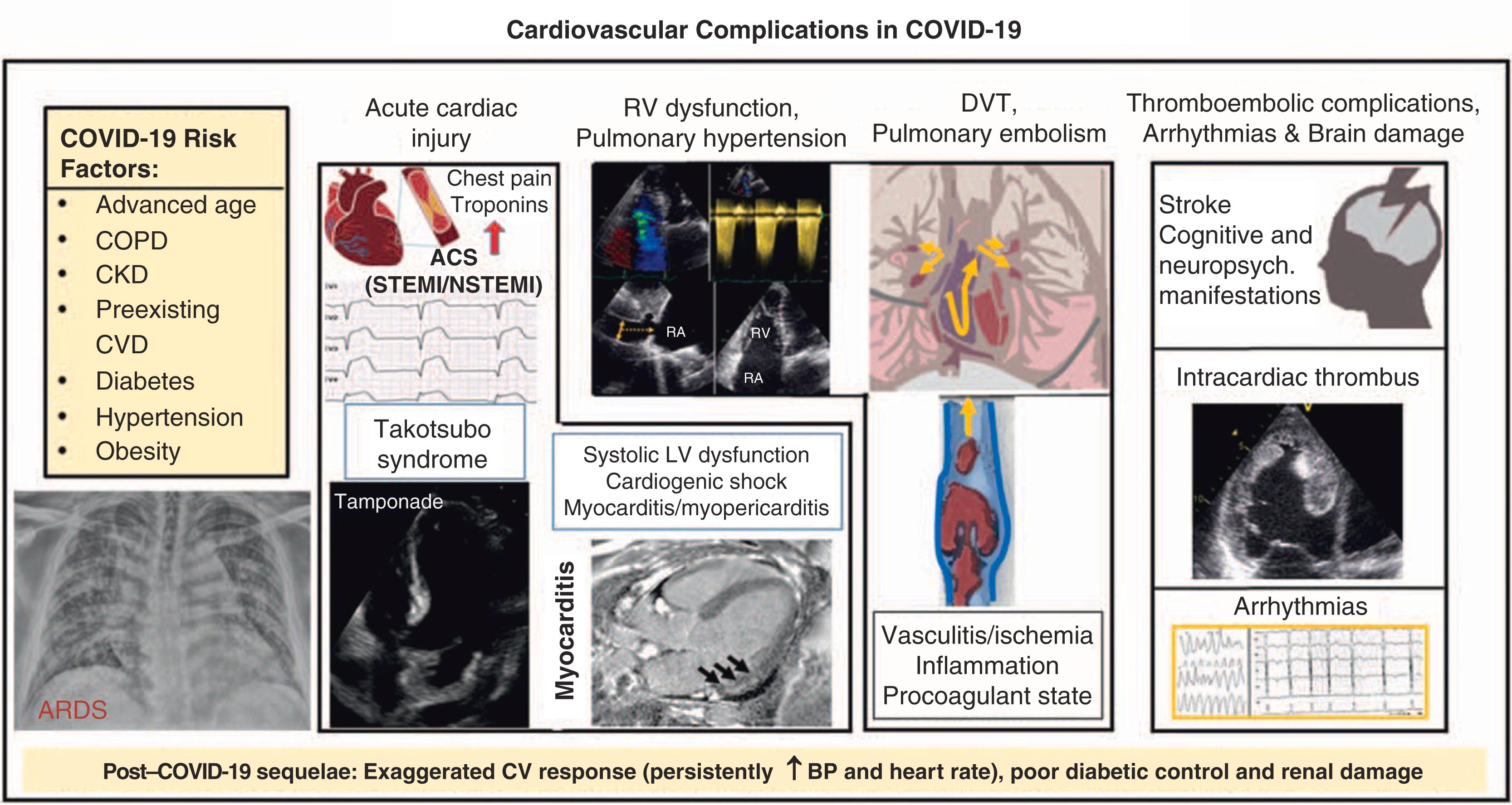
SARS-CoV-2 can cause both direct and indirect cardiovascular sequelae including myocardial injury, acute coronary syndrome (ACS), cardiomyopathy, acute cor pulmonale, arrhythmias, cardiogenic shock, and thrombotic complications. Myocardial injury with elevation of cardiac biomarkers above the 99th percentile of the upper reference limit occurred in 20% to 30% of hospitalized patients with COVID-19. Preexisting cardiovascular disease increased this rate to 55%. A greater frequency and magnitude of troponin elevations in hospitalized patients have been associated with disease severity and poorer outcomes. Biventricular cardiomyopathy has been reported in 7% to 33% of critically ill patients with COVID-19. Isolated right ventricular (RV) failure with and without confirmed pulmonary embolism has also been reported. A study of 138 patients in Wuhan, China, demonstrated that cardiac arrhythmias, including new-onset atrial fibrillation, heart block, and ventricular arrhythmias, are prevalent, occurring in 17% of hospitalized patients and 44% of patients in the intensive care unit (ICU) setting. In a multicenter New York City cohort, 6% of 4250 patients with COVID-19 had prolonged QTc (corrected QT; >500 ms) at the time of admission. Among 393 patients with COVID-19 from a separate cohort from New York City, atrial arrhythmias were more common among patients who required mechanical ventilation than among those who did not (17.7% versus 1.9%). Reports from Lombardi, Italy, showed an increase of nearly 60% in the rate of out-of-hospital cardiac arrest during the 2020 COVID-19 pandemic relative to a similar period in 2019, which suggested the etiology to be either COVID-19 or another untreated pathology related to patients’ reluctance to seek care ( Fig. 4.4 ). Acute cardiac injury in COVID-19 is associated with poor prognosis. Shi et al. showed that patients with acute cardiac injury had a significantly higher risk of in-hospital mortality compared to patients without cardiac injury from the time of symptom onset (hazard ratio 4.26, 95% CI, 1.92–9.49) or hospital admission (hazard ratio 3.41, 95% CI, 1.62–7.16). Box 4.1 summarizes COVID-19 cardiac manifestations’ clinical presentations, COVID-19 specific considerations, and general considerations.
Myocardial ischemia and MI (type 1 and 2)
Myocarditis
Arrhythmia: new-onset atrial fibrillation and flutter, sinus tachycardia, sinus bradycardia, QTc prolongation (often drug induced), torsades de pointes, sudden cardiac death, pulseless electrical activity
Cardiomyopathy: biventricular, isolated right or left ventricular dysfunction
Cardiogenic shock
Do not routinely discontinue ACE inhibitors or ARBs in patients already on them at home; assess on a case-by-case basis
Perform an electrocardiogram or telemetry monitoring for patients at medium to high risk for torsades de pointes who are being treated with QTc-prolonging drugs
Carefully consider the utility of diagnostic modalities, including cardiac imaging, invasive hemodynamic assessments, and endomyocardial biopsies, to minimize the risk of viral transmission
Primary percutaneous coronary intervention remains the preferred approach for most patients with STEMI; consider fibrinolytic therapy in select patients, especially if personal protective equipment is not available
Utilize noninvasive hemodynamic assessments, and measurement of lactate, troponin, and beta-natriuretic peptide concentrations, with sparing use of routine echocardiography for guidance about fluid resuscitation, vasoactive agents, and mechanical circulatory support
Minimize invasive hemodynamic monitoring, but can consider in select patients with mixed vasodilatory and cardiogenic shock
Consider point-of-care ultrasound to assess regional wall motion abnormalities to help distinguish type 1 MI from myocarditis
Early catheterization and revascularization is recommended for high-risk patients with NSTEACS (e.g., GRACE score >140)
Consider medical therapy for low-risk patients with NSTEACS, particularly if the suspicion for type 1 MI is low
Monitor and correct electrolyte abnormalities to mitigate arrhythmia risk
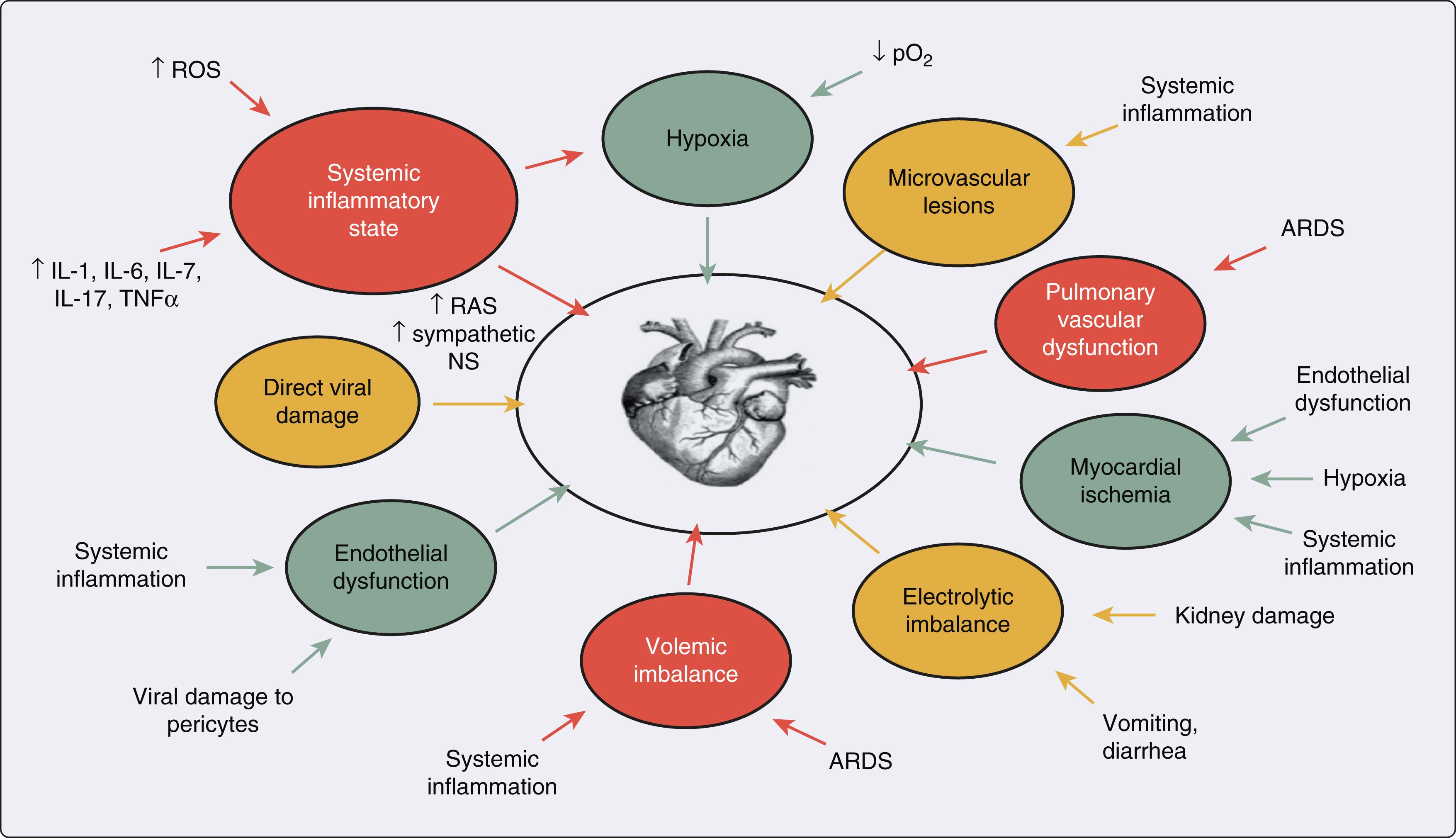
The pathophysiology underlying cardiovascular manifestations is likely multifactorial. ACE2 has high expression in cardiovascular tissues, including cardiac myocytes, fibroblasts, endothelial cells, and smooth muscle cells, which supports a possible mechanism of direct viral injury. Patients with preexisting cardiovascular disease may have higher levels of ACE2, which would potentially predispose them to more severe COVID-19. Myocarditis is a presumed etiology of cardiac dysfunction, and the development of myocarditis may relate to viral load. While isolation of the virus from myocardial tissue has been reported in a few autopsy studies, other pathological reports have described inflammatory infiltrates without myocardial evidence of SARS-CoV-2. In addition, the finding of direct viral infection of the endothelium and accompanying inflammation as reported in a patient with circulatory failure and MI lead to the possibility of virus-mediated endothelial cell damage as an underlying mechanism. Systemic inflammatory response syndrome (cytokine storm) is another putative mechanism of myocardial injury. Lastly, isolated RV dysfunction may occur as a result of elevated pulmonary vascular pressures secondary to ARDS, pulmonary thromboembolism, or virus-mediated injury to vascular endothelial and smooth muscle tissue.
Other potential etiologies of myocardial damage not specific to COVID-19 include severe ischemia or MI in patients with preexisting coronary artery disease, stress-mediated myocardial dysfunction, tachycardia-induced cardiomyopathy, and myocardial stunning after resuscitation or prolonged hypotension. While patients with viral infections are at risk for MI in general, this risk may be amplified in patients with COVID-19 given its propensity to create hypercoagulability and thus potentially increase thrombotically mediated MI. Moreover, distinguishing the presentation of atherosclerotic plaque rupture MI (type 1 MI) from myonecrosis due to supply–demand mismatch (type 2 MI) in the setting of severe hypoxia, hemodynamic instability, and myocarditis can be challenging. This was especially evident in a recent case series of 18 patients with COVID-19 who developed ST segment elevation on electrocardiogram with 10 of these patients diagnosed with noncoronary myocardial injury ( Fig. 4.5 ).
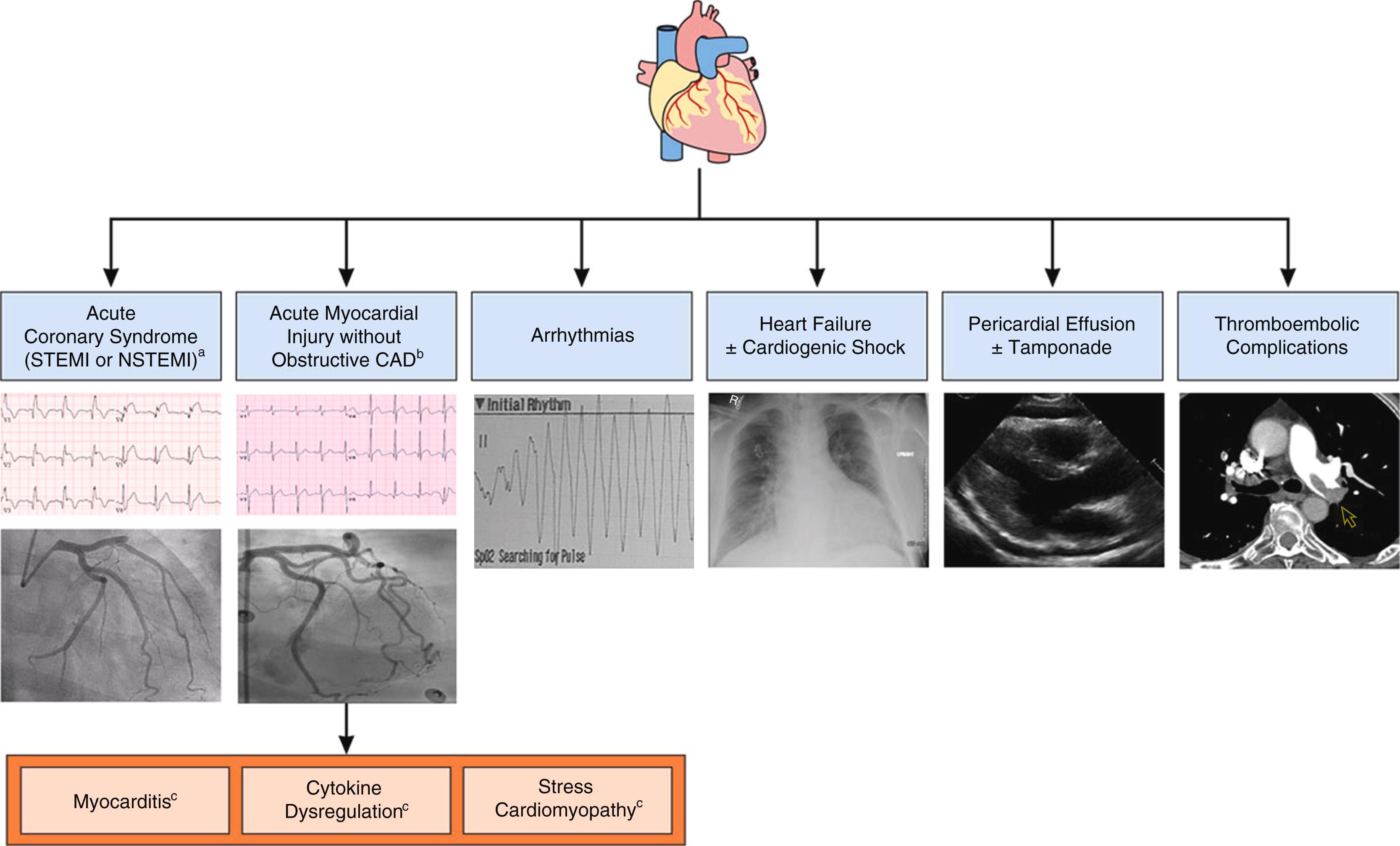
Acute COVID-19 cardiovascular syndrome (ACovCS) includes acute cardiac injury, thromboembolic complications, atrial and ventricular arrhythmias, hemodynamic instability, and sudden cardiac death ( Fig. 4.6 ). The clinical presentation of acute cardiac injury may be chest pain, shortness of breath, syncope/near syncope, tachycardia, elevated myocardial troponins and natriuretic peptides, regional wall motion abnormalities or global left ventricular (LV) dysfunction on echocardiography, ST segment depression or elevation, or T-wave abnormalities on electrocardiogram (ECG). Cardiac injury in COVID-19 patients may occur both with and without acute obstruction of the coronary arteries (irrespective of a preceding atherosclerotic coronary artery disease), which portends poor prognosis. It is believed that virally induced thrombi may result in ACSs (NSTEMI or STEMI), although other causes have been described including type 2 MI related to tachycardia, hypotension, and hypoxemia; microvascular damage secondary to diffuse microembolism; cardiotoxic effects of an inflammation-induced cytokine storm; and stress-induced cardiomyopathy (also termed Takotsubo syndrome) ( Fig. 4.7 ). , Acute heart failure syndromes (systolic RV and LV dysfunction with or without cardiogenic shock), myopericarditis, and myocarditis ( Fig. 4.8 ) are also common and may be induced by direct viral invasion of the myocardium (primary cardiomyocyte injury) or by an enhanced inflammatory response (cytokine-mediated injury). Recently, severe cases of acute perimyocarditis with subsequent cardiac tamponade in patients with COVID-19 infection have been reported. However, the prevalence of myocarditis in COVID-19 is extremely rare, and larger retrospective multicenter cohort studies have found it to be 1% or less.
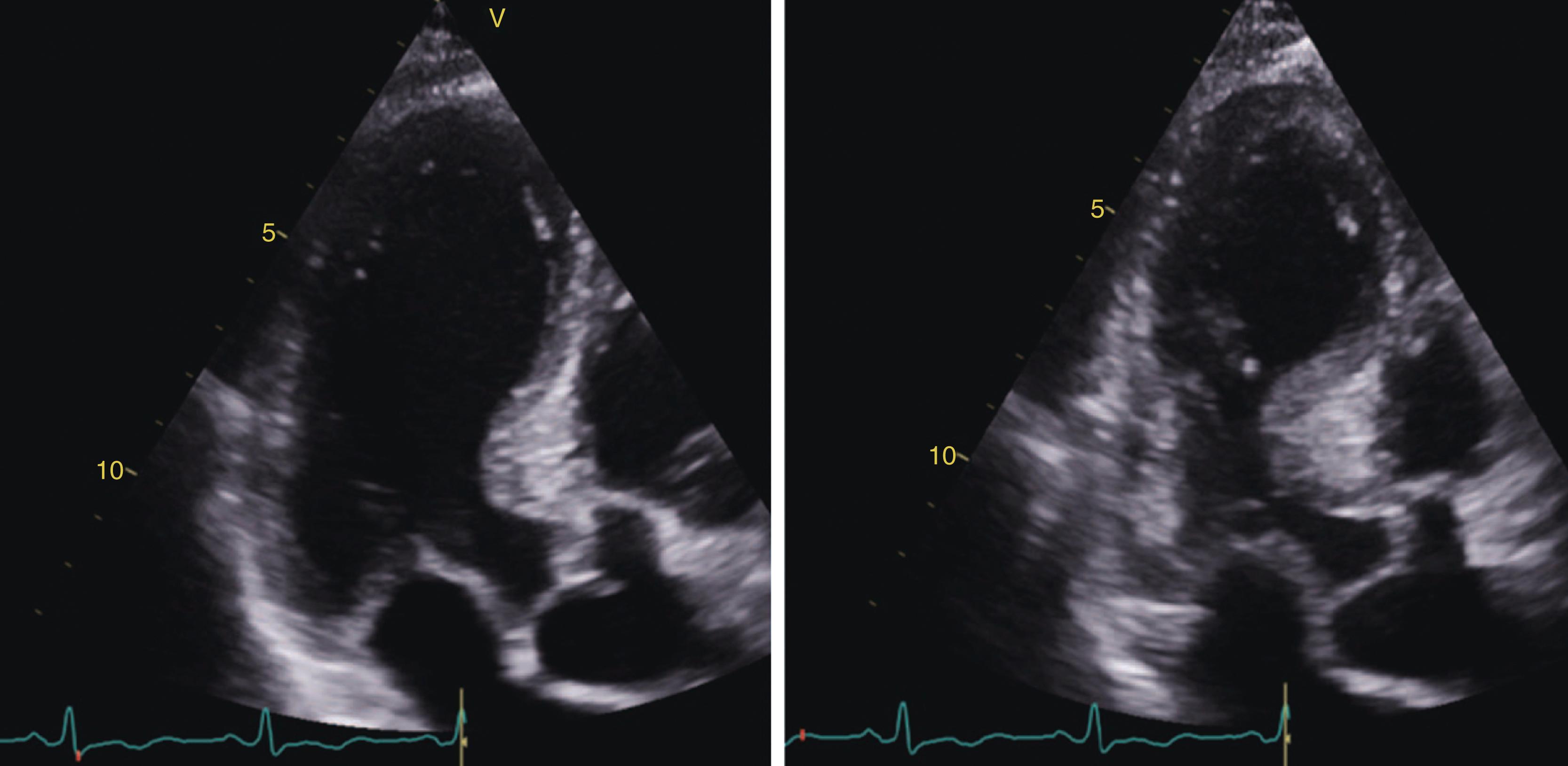
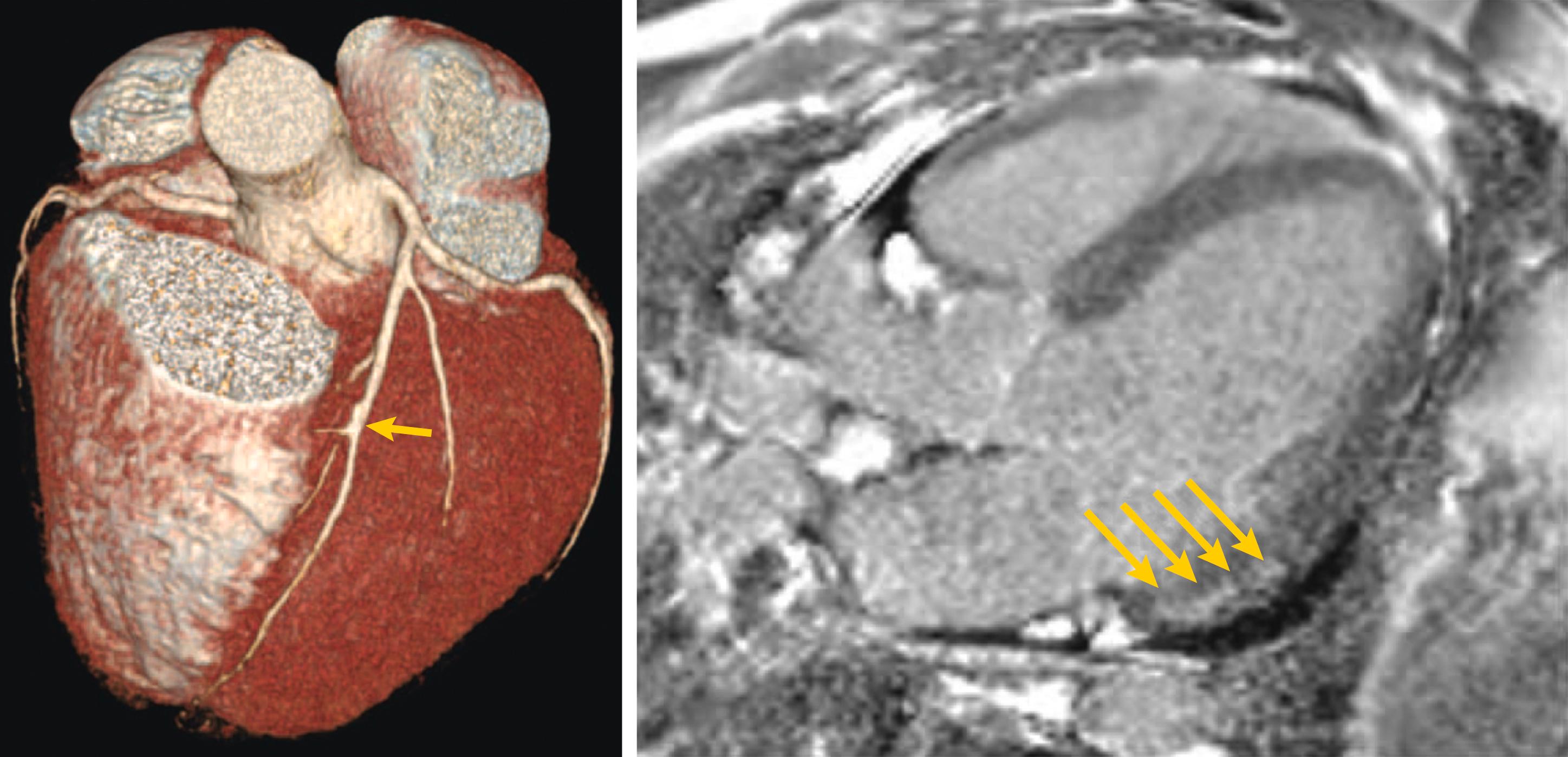
COVID-19 may also affect the RV, but it has been less extensively studied in comparison to the LV. , RV dysfunction was the most common abnormality seen in a multicenter international cohort of over 300 hospitalized patients with COVID-19 at a rate of approximately 26%. Use of speckle tracking echocardiography found reduced basal longitudinal strain to be present in 52% of patients in one single-center study; patients who were obese, black or had hypertension and diabetes were more likely to present with reduced basal longitudinal strain. Another echocardiographic study of COVID-19 patients found that the most prevalent abnormality was reduced longitudinal strain in more than one basal LV segment (71%). This pattern reminded of a “reverse Takotsubo” morphology that is not typical for other viral myocarditis presentations. Although the tricuspid annular plane systolic excursion (TAPSE) was found to be preserved in hospitalized COVID-19 patients, a cohort of COVID-19 ARDS patients from the United Kingdom demonstrated a specific phenotype of RV radial dysfunction with sparing of longitudinal function. Notably, in these patients, the RV–pulmonary artery coupling as reflected by the fractional area change divided by RV systolic pressure (RV afterload) was an important marker of disease severity. In addition, RV longitudinal strain has been identified as a powerful predictor of mortality in COVID-19 patients.
Limited data are available on the prevalence of spontaneous coronary dissection in COVID-19 patients. Spontaneous dissection is linked to risk factors that are also highly prevalent in COVID-19 including systemic inflammatory response, hemodynamic stress, and hypertension. There is also lack of evidence pertaining to COVID-19–related native and prosthetic valve endocarditis. It is important to highlight that COVID-19 can cause decompensation of an underlying heart failure. This may lead to a mixed shock and require specific therapeutic approaches. Differentiating COVID-19–related acute lung injury from patients with acute pulmonary edema and preexisting heart failure is crucial as continuous positive airway pressure is contraindicated in heart failure patients and beneficial in lung injury patients. Finally, it is important to note that the clinical presentation of some fulminant myocarditis, such as giant cell myocarditis, may resemble COVID-19 myocarditis.
Detectable troponin elevation carries prognostic value in acute COVID-19 infection. Shi and colleagues reported higher mortality in those with troponin elevation from a single-center cohort in Wuhan, China, finding a threefold to fourfold increased risk of death. Later, Lombardi and colleagues validated these findings in a multicenter cohort in Italy with over 600 patients, with a more attenuated hazard ratio of 1.7. In one of the most diverse cohorts studied with over 2000 patients admitted to a New York City hospital system, Smilowitz and colleagues illustrated that the risk of death was twofold higher among patients with troponin elevation. Importantly, the degree of troponin elevation was associated with more severe critical illness (defined as ICU admission, need for mechanical ventilation, discharge to hospice, or death). While these seminal studies defined troponin elevation as greater than the 99 th percentile of the upper limit of normal, Qin and colleagues illustrated that troponin elevation in COVID-19 infection was associated with mortality even at thresholds 19% to 50% lower than those traditionally used in clinical settings.
Moreover, risk for mortality and adverse outcomes appears to be continuous with the degree of troponin elevation; higher troponin continues to amplify risk, providing clinicians with not only a qualitative risk assessment for patients, but a quantitative assessment as well. As such, measuring troponin for hospitalized COVID-19 patients has been integrated into routine clinical practice and management algorithms. For hospitals, it serves to forecast trajectory and identify patients that may require more intensive resources in times of scarcity. Several societal guidelines including the World Health Organization and the Chinese Clinical Guidance for COVID-19 recommend measuring troponin for all admitted patients, while others including the American College of Cardiology (ACC) recommend testing when clinically indicated.
The clinical presentation of other forms of fulminant myocarditis may resemble COVID myocarditis. An endomyocardial biopsy might be considered in patients presenting with life-threatening arrhythmias and cardiogenic shock; however, in the presence of COVID-19, such an approach may not be feasible due to patient instability, procedural risk, and risk of healthcare staff exposure, especially if the biopsy results would not change clinical management. Patterns of delayed myocardial enhancement consistent with acute myocarditis have been described in contrast-enhanced ECG-gated multidetector computed tomography (CT) in non–COVID-19 cases. This rapid, noninvasive testing may be useful for the assessment of myocardial injury in patients with COVID-19 and warrants further investigation.
Given the extremely contagious nature of COVID-19, a priority is placed on minimizing COVID-19 exposure for healthcare staff and non-COVID patients while successfully managing the care of COVID patients. This goal can be facilitated by limiting testing and patient transfer for diagnostic procedures, especially for procedures that do not directly influence patient management. Consideration is also given to limit testing that requires terminal room cleaning necessitated by patient transfer, as that process can add significant delays to diagnostic testing for other patients. Such strategies may result in increased uncertainty about the diagnosis but are unlikely to increase adverse short-term outcomes in patients without fulminant presentations. For example, if a type 1 MI can be excluded on clinical grounds for a patient noted to have acutely elevated troponins, then a biopsy is unlikely to change immediate clinical management no matter the ultimate pathology. This strategy is aligned with recent ACC recommendations. An exception to that approach may be warranted in a COVID-19 patient with hemodynamic or electrophysiological instability who undergoes coronary angiography to exclude obstructive coronary artery disease. As the patient is already in the catheterization laboratory, incremental infectious risk of patient transport is reduced. A biopsy may be useful to gain insight into the mechanism of the acute myocardial injury, including the possibility of giant cell myocarditis.
In accordance with these guiding principles, the majority of patients with an abnormal troponin in the setting of COVID-19 infection can be followed up with expectant management until recovery from the acute viral syndrome. Patients with COVID-19 and myocardial injury who are hemodynamically and electrophysiologically stable with mild to moderate elevations of troponin should not routinely undergo an echocardiogram, angiography, or cardiac imaging. These diagnostic studies can be delayed until recovery from COVID-19 or avoided altogether unless the patient clinically deteriorates and develops hemodynamic instability, shock, ventricular arrhythmias, or concerning troponin levels ( Fig. 4.9 ). Point-of-care cardiac ultrasonography (POCUS) may be a viable alternative to the aforementioned modalities as it can be performed at bedside without the aerosolization risk of transesophageal echocardiography (TEE). Determination of ejection fraction and cardiac tamponade could help identify higher-risk patients and support earlier initiation of guideline-directed medical therapy once the patient is stable. The European and American Societies of Cardiology recommend POCUS for COVID-19 patients and consider bedside critical care echocardiography as an effective option to screen for cardiovascular complications in the COVID-19 infection. However, unnecessary or repeated imaging not vital to clinical decision-making should be avoided in accordance with the American Society of Echocardiography COVID-19 statement. Patients discovered to have newly diagnosed depressed LV systolic function (low LV ejection fraction) without elevated troponin are more likely to have preexisting cardiomyopathy than myocarditis. Although the lack of definitive diagnostic studies may temporarily increase diagnostic uncertainty, it reduces the risk of COVID-19 transmission to staff and seems unlikely to compromise short-term (<60 days) patient outcomes in clinically stable patients.
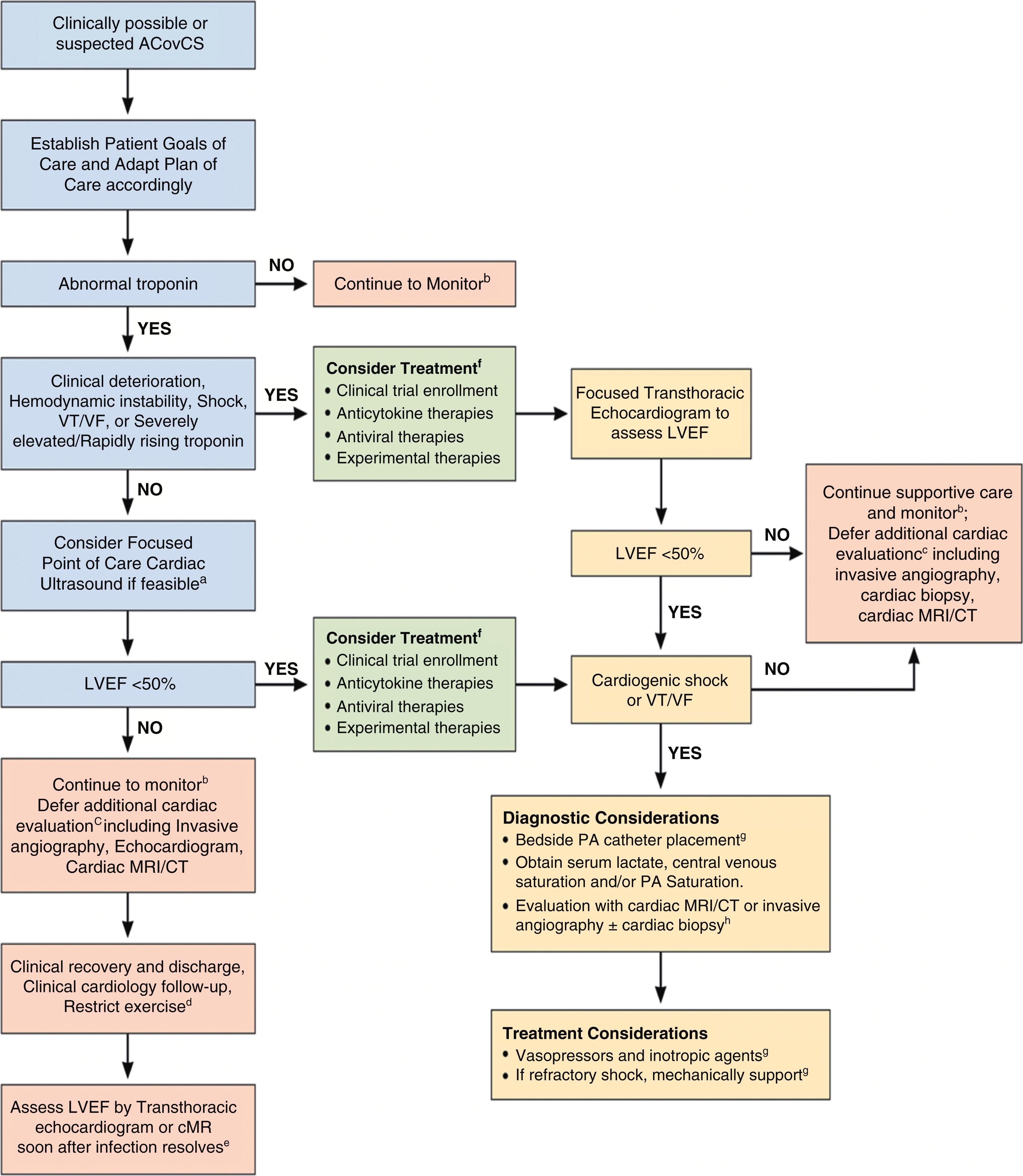
There are no comprehensive expert recommendations and only limited data to inform our clinical decision-making for the pharmacotherapy of ACovCS. Because most small case series and studies of viral myocarditis in general involve fulminant and complicated presentations, significant publication bias exists in the literature. Published experiences with COVID-19–associated myocardial injury are even more limited, including retrospective small case series and individual case reports. Thus, the best practices for treating the acute myocardial injury in ACovCS currently need to be extrapolated from prior non–COVID-19 experiences and the available limited quality COVID-19 data. In general, treatment of ACovCS should be completed with a multidisciplinary team that includes infectious disease consultation to help guide therapy selection. Several experimental therapies attempting to limit SARS-CoV-2 replication or the immune response have been proposed with multiple clinical trials currently underway. At present, there are no therapies with rigorous clinically supported efficacy for COVID-19 in general or for ACovCS specifically. In select cases with refractory shock or ventricular arrhythmias caused by ACovCS, mechanical support can be considered if available at the treating facility. Case reports have described successful rescue of patients with cardiogenic shock with the use of veno-arterial and veno-arterial-veno extracorporeal membrane oxygenation (ECMO). If cardiogenic shock is suspected secondary to myocarditis, expert consultation with an advanced heart failure team should be strongly considered ( Fig. 4.9 ).
The primary infection of the respiratory system through SARS-CoV-2, particularly in type 2 pneumocytes, is manifested by the progression of systemic inflammation and overactivation of immune cells, resulting in increased levels of proinflammatory cytokines (IL-6, IL-7, IL-22 and CXCL10). Thus, inflammation generated in the lungs is characterized through blood mediators. Zhao et al. suggested that the excessive immune response plays an important role in pathogenesis, where the abnormal increases in CRP, IL-6, and neutrophils contributed to acute lung damage and are associated with significant pathophysiology and fatal events. Another important process is the activation of coagulation with formation of thrombi in the lungs and other organs, indicating the damage caused by endothelial dysfunction from the interruption of pulmonary vasoregulation, generating ventilation perfusion mismatch ( Fig. 4.10 ).
Regarding pulmonary function, Mo et al. evaluated 110 noncritical middle-aged patients diagnosed with COVID-19 and performed pulmonary function testing on the day of discharge or one day prior to discharge. This group reported important abnormalities in diffusing capacity for carbon monoxide (DLCO) in 47.2% of the cases (DLCO was 64.79% of predicted for severe pneumonia cases) and total lung capacity (TLC) in 25% (TLC was 79.16% of predicted for severe pneumonia cases). Less frequent abnormalities were noted in forced expiratory volume in the first second (FEV1) (13.6% of the cases), forced vital capacity (FVC) (10%), FEV1/FVC (4.5%), and small airway function (7.3%). In addition, the percent-predicted value for TLC in severe cases of pneumonia due to COVID-19 was lower than that reported for mild illness, suggesting greater lung volume impairment in severe cases. These findings indicate impairment in lung diffusion capacity is a primary abnormality in patients with COVID-19, followed by restrictive ventilatory dysfunction. Moreover, viral infections increase the risk of pulmonary fibrosis, which can be one of the serious complications following recovery from COVID-19. The prevention of pulmonary fibrosis due to COVID-19 is an issue that needs to be urgently addressed. It is also important to emphasize that pulmonary function testing should be considered as a clinical follow-up examination for patients recovering from COVID-19, noting that patients with impaired lung function from multiple pathologies can benefit from pulmonary rehabilitation programs.
There is emerging evidence suggesting that the respiratory muscles may also be affected by SARS-CoV-2 infection. A postmortem study by Shi et al. examined respiratory muscles and found that ACE-2 is expressed in the myofiber membrane of the human diaphragm, and evidence of viral infiltration of SARS-CoV-2 was observed in the diaphragm myofibers of patients with COVID-19. This potential COVID-19–related acute respiratory muscle myopathy is concerning as COVID-19–related ARDS may also reduce respiratory system compliance. The combined effects from COVID-19 infection may shift the balance between the pressure demands of breathing (alveolar ventilation) and the pressure generating capacity of the respiratory muscles increasing the risk of respiratory failure, thus requiring mechanical ventilation. However, mechanical ventilation alone has been shown to induce rapid atrophy and profound weakness of the respiratory muscles. In addition, COVID-19 patients that are of advanced age, obese, and have preexisting lung disease are at an increased risk of severe respiratory complications associated with respiratory muscle weakness and difficulty weaning from mechanical ventilation. These factors contributing to respiratory muscle weakness likely contribute to poor outcomes following COVID-19 infection, especially in high-risk patients and may explain the persistent dyspnea and fatigue in patients recovering from COVID-19.
Severe COVID-19 is characterized by high rates of thromboembolic complications, and procoagulant markers such as D-dimers are often substantially increased even in the absence of clinically relevant macrothrombi. COVID-19–associated hypercoagulability has gained significant attention, and a large number of clinical trials aimed at improving outcomes with antithrombotic therapies have started. A recent meta-analysis from 66 studies in COVID-19 patients showed an estimated prevalence of venous thromboembolism (VTE) of 14.1%. The VTE event rates in COVID-19 are considerably higher than those previously reported in acutely ill, non–COVID-19 patients admitted to the ICU. In addition to VTE, increased prevalence of arterial thrombotic events such as myocardial and cerebral infarction (up to 3%, 95% CI, 2%–5%) has been reported in the ICU setting. Furthermore, remarkably high numbers of thrombosis in extracorporeal circuits (up to 8%) have been observed. Autopsy studies not only showed a high incidence of pulmonary macroemboli but also revealed severe endothelial injury, increased angiogenesis, microemboli, and occlusion of alveolar capillaries in patients who died from COVID-19. On the basis of clinical course and observed coagulation parameters, three stages of clinical COVID-19 coagulopathy have been proposed ( Fig. 4.11 ). Stage 1 is characterized by mild symptoms without the need for oxygen supply or other respiratory support, mild systemic inflammation and mildly systemic coagulopathy. In stage 2, patients develop more severe symptoms and often require additional oxygen supply. This phase is characterized by progressive pulmonary inflammation and local coagulopathy with increased incidence of microthrombi. In stage 3, the patient’s condition deteriorates further and requires critical organ support such as high-flow oxygen therapy, mechanical ventilatory support, or circulatory support including ECMO. This stage is characterized by a strong proinflammatory reaction and development of overt local and systemic coagulopathy with high D-dimer and fibrinogen concentrations, prolonged prothrombin time (PT), reduced platelet counts, and a very high incidence of pulmonary embolism and deep venous thrombosis ( Fig. 4.11 ).
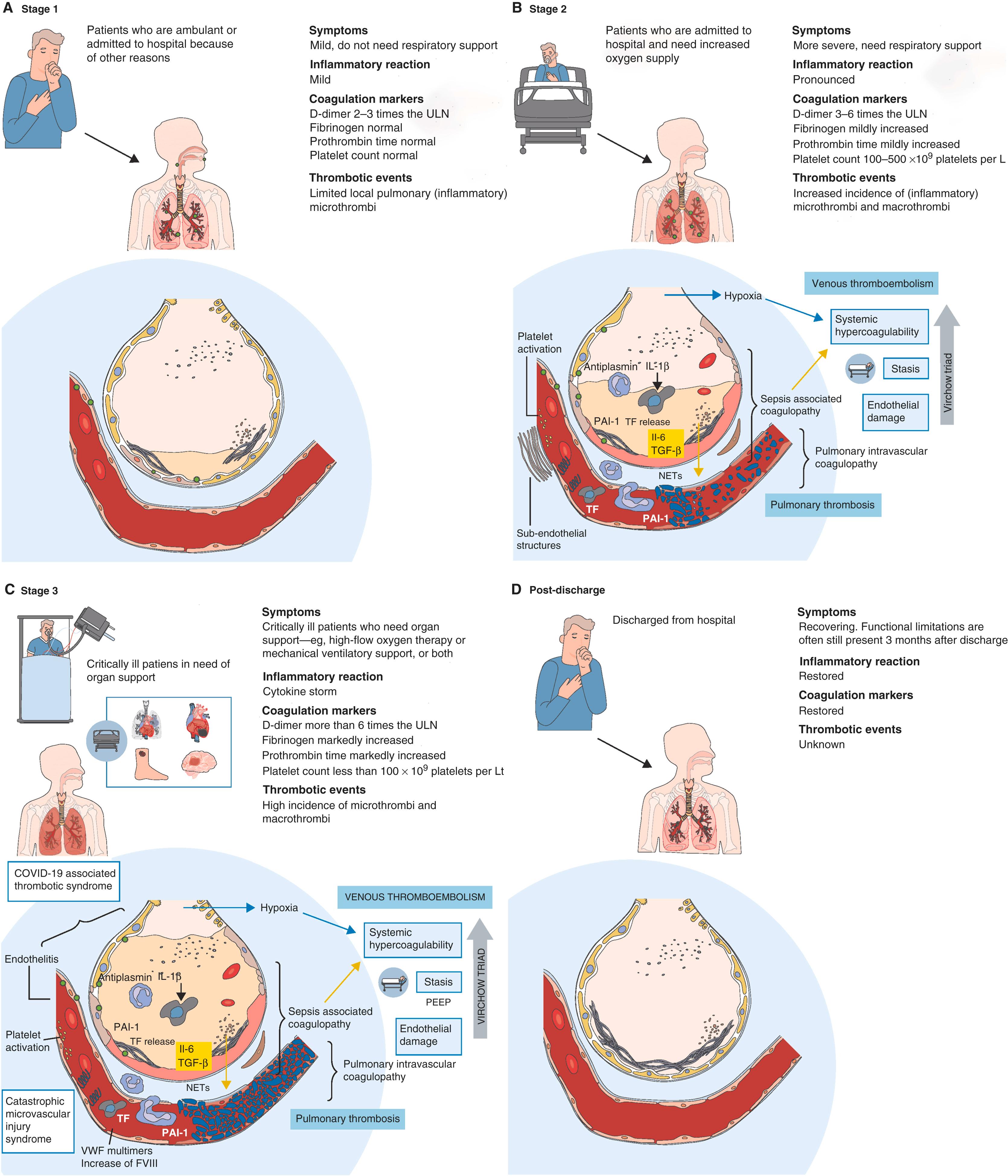
Uncontrolled inflammation, along with hypoxia and direct viral-mediated effects, likely contributes to the high rates of thrombotic complications in COVID-19. The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation. Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu. This effect is similar and perhaps more severe when compared to other viral illnesses. In addition to the macrothrombotic events, the development of in situ thrombosis in small vessels of the pulmonary vasculature (pulmonary intravascular coagulopathy) is an area that requires further study. Autopsy studies of patients who died due to COVID-19 have shown high rates of microvascular and macrovascular thromboses, especially in the pulmonary circulation. A postmortem series of seven patients from Germany showed that alveolar capillary microthrombi were nine times more common in people who died of COVID-19 than in those who died of influenza. Microthrombi and microangiopathic pathologies associated with foci of hemorrhage were also noted on autopsies of 10 African American patients with severe COVID-19 from New Orleans, Louisiana, USA.
In propensity score-matched comparisons, there were no statistically significant differences in mortality, time to mechanical ventilation, or length of hospital stay in patients that received chronic anticoagulation therapy and those that did not. In addition, several small case series described major bleeding complications in patients with COVID-19 who received intermediate dosed thromboprophylaxis. In the ICU stage, therapeutic dose heparin does not improve clinical outcomes or mortality in critically ill patients with COVID-19 who require organ support and may cause harm; multiple clinical trials are currently investigating this issue. For non-ICU COVID-19 patients, clinical trials with therapeutic dose anticoagulation are underway. On March 25, 2021, the United Kingdom National Institute for Health and Care Excellence adapted the COVID-19 rapid guidelines to consider a therapeutic dose of low-molecular-weight heparin in patients with COVID-19 who are likely to be in hospital for at least 2 days, need supplemental oxygen, and have not required high-flow oxygen or other organ support. Currently, an absence of high-quality, randomized trials has precluded firm conclusions, and recommendations are weak with limited evidence. Because of the high thrombogenicity of COVID-19 in more severe stages, all guidelines agree that low-molecular-weight heparin thromboprophylaxis should be administered to all patients admitted to the hospital with COVID-19. However, the appropriate thromboprophylaxis approach and the proper dose for hospitalized patients remains a topic of debate. The only preprint paper to date in ICU patients suggests that a therapeutic dose of low-molecular-weight heparin or unfractionated heparin does not improve clinical outcomes and might be associated with increased risk of bleeding complications. Thromboprophylaxis with intermediate-dose, low-molecular-weight heparin is advised in some guidelines for critically ill or non-ICU patients with additional thrombotic risk factors.
Acute kidney injury (AKI) is a frequent complication of COVID-19 and is associated with mortality. In China, the reported incidence of AKI in hospitalized patients with COVID-19 ranged from 0.5% to 29% and occurred within a median of 7 to 14 days after admission. Studies from the United States have reported much higher rates of AKI. In a study of nearly 5500 patients admitted with COVID-19 in a New York City hospital system, AKI occurred in 37%, with 14% of the patients requiring dialysis. About one-third were diagnosed with AKI within 24 hours of admission in this study. Of 257 patients admitted to ICUs in another study from New York City, 31% received renal replacement therapy (RRT). Furthermore, hematuria has been reported in nearly half of patients with COVID-19, 92 and proteinuria has been reported in up to 87% of critically ill patients with COVID-19. Hyperkalemia and acidosis are common electrolyte abnormalities associated with the high cell turnover seen in patients with COVID-19, even among patients without AKI. COVID-19 is also increasingly reported among patients with end-stage renal disease and recipients of kidney transplants with higher associated mortality rates. Box 4.2 summarizes COVID-19 renal manifestations’ clinical presentations, COVID-19–specific considerations, and general considerations.
AKI
Electrolyte abnormalities (hyperkalemia, hyponatremia, and hypernatremia, among others)
Proteinuria
Hematuria
Metabolic acidosis
Clotting of extracorporeal circuits used for RRT
Evaluate urine analysis and protein-to-creatinine ratio at admission, given the association of proteinuria and hematuria with outcomes
Consider empiric low-dose systemic anticoagulation during the initiation and day-to-day management of extracorporeal circuits for RRT
Consider colocalization of patients who require RRT and use shared RRT protocols
Consider acute peritoneal dialysis in select patients to minimize personnel requirements
Individualize fluid balance strategies guided by markers of volume status (serum lactate, urinary electrolytes, and hemodynamic measures) and of pulmonary, myocardial, and renal function
Consider continuous RRT in critically ill patients with severe AKI and/or serious or life-threatening metabolic complications that do not respond to medical therapy
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here