Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access video content for this chapter online at Elsevier eBooks+
Access video content for this chapter online at Elsevier eBooks+
Lymphedema is estimated to affect over 30 million patients in the US, with breast cancer being the most frequent cause. Incidence of breast cancer-related lymphedema (BCRL) can exceed 30% of patients undergoing axillary dissection and adjuvant radiotherapy. Few clinical guidelines exist for the management of lymphedema, thus for many years treatment was limited to conservative interventions such as physical therapy, compression, and massage. These therapies can be burdensome and expensive, with a shortage of certified lymphedema therapists to aid patients. Such barriers to effective management have led to high rates of patient dissatisfaction with available modalities.
Although conservative management remains the first-line treatment option in most patients, novel surgical techniques have evolved over the last several decades. These interventions serve as an adjunct to non-surgical treatments. Reductive techniques, such as liposuction or direct excision, reduce the hypertrophic tissue present in the intermediate and later stages of lymphedema, while physiologic techniques, such as vascularized autologous lymph node transplant (VLNT) or lymphovenous bypass (LVB), improve or restore the physiologic network of lymphatic drainage and have the potential to mitigate disease progression.
Many surgical options exist, yet no standardized algorithm for surgical treatment has been established. In this chapter, the authors describe their approach to the management of BCRL, highlighting key non-surgical and surgical treatment modalities.
Lymphedema can be caused by either an anatomic inability for the lymph-conducting pathways to transport lymphatic fluid or from external damage to the lymphatic system, such as iatrogenic removal of lymph nodes. In BCRL, lymphedema occurs due to a mechanical disruption of outflow and a rise in lymphatic pressure, caused by the loss of lymph nodes and upstream vessels. This creates a backflow of lymphatic fluid, leading to lymphatic dilation, valve incompetence, and further dilatation. The end-result is dermal backflow, characterized by the reverse flow of lymphatic fluid toward the subdermal lymphatic system. The subdermal plexus is a high capacitance, low resistance system. This combination allows a large amount of reversed lymphatic fluid to collect within the subcutaneous space. Damage to the lymphatic vessels from higher pressures causes the vessel walls to undergo fibrosis, which encourages fibrinoid thrombi to accumulate within the lumen, obliterating any remaining patent channels. Histologically, in the initial stage of lymphedema, both endothelial cells and smooth muscle cells at the proximal level of the lymphatic trunks become damaged. As the damage progresses, cellular degeneration and occlusion of the lymphatics travels distally.
In the first report of surgical treatment of lymphedema, in 1912 Charles et al . described a massive resection of scrotal and skin and subcutaneous tissues, with the resulting wound closed using split-thickness skin grafts. This reductive technique relies on the principle of excising scarred adipose and connective tissue. Almost two decades later, Sistrunk et al . described an excisional technique to treat upper extremity lymphedema secondary to mastectomy. In 1967 Thompson et al . reported a similar treatment of BCRL; however, these techniques were highly invasive, and postoperative complications such as prolonged pain, poor healing, infection, and unwanted lymphocutaneous fistulas were commonly reported.
To reduce patient morbidity, other reductive techniques were explored. Liposuction was first described for the treatment of lymphedema by Brorson et al ., who reported favorable results and recommended this as the surgical method of choice. This technique is effective in removing hypertrophic fatty tissue, but leaves behind excess skin. Additionally, there is a potential risk of damaging any residual lymphatic vessels that may remain in the extremity, leading to further resistance to treatment in the future. Maintaining the reduced volume requires lifelong adherence to compression garments. Nonetheless, liposuction can be a viable and effective option for chronic, non-pitting lymphedema in compliant patients.
With the rising popularity of flap-based reconstructions in the mid-20th century, the first flap for treatment of lower limb lymphedema was described by Sir Harold Gilles in 1950. Gilles transferred a flap of skin and subcutaneous tissue from the non-affected upper extremity to the affected groin in a two-stage operation. This concept of flap interposition was an early precursor of lymph node transplant, using distant adipose tissue to restore functional anatomy. Baumeister and Siuda et al . went on to report an approach to upper limb lymphedema in which healthy lymphatic vessels from the medial thigh area were used as a composite graft. However, the morbidity of the donor site was significant, thus the technique did not gain popularity. Campisi et al . later described a vein interposition graft between the proximal and distal lymphatic vessel bundles to bypass the obstructed area, forming a lymphaticolymphatic bypass. In this procedure, multiple lymphatic vessels are inserted into the distal cut end of a vein graft and secured by sutures, and lymphatic vessels in the supraclavicular area are anastomosed to the other end of the graft. This was the precursor to microsurgical interventions that would soon dominate the surgical landscape of lymphedema. The concept of a lymphovenous shunt operation, in which the cut end of a lymphatic vessel is anastomosed to the side of a vein, was first described in a rat model. Yamada et al . successfully applied this technique to treat patients with lower limb lymphedema, and many others have since reproduced their findings.
In recent decades, technologic advances in microsurgical equipment allowed for the development of lymphatic supermicrosurgery. This technique anastomoses vessels with a diameter of 0.3–0.8 mm. Koshima et al . reported the first case of supermicrosurgical LVB by direct anastomosis of small lymphatic vessels directly to the venous capillary bed, with effective egress of lymphatic fluid. Transplantation of healthy lymphatic tissue to diseased areas has also been refined with the aid of microsurgical advancements. Becker et al . reported treating upper limb lymphedema by transplanting composite soft tissue including inguinal lymph nodes to the axilla or elbow region. This is effective in addressing extremity lymphedema including BCRL.
Diagnosis of BCRL was historically made based on clinical examination. Patients present months to years following their cancer treatment with symptoms of ipsilateral arm swelling, fluid shifts, fatty enlargement, and recurrent infections ( Fig. 47.1 ). Advances in diagnostic imaging now provide more detailed information regarding the functional and morphologic features of each patient’s lymphatic system, allowing for tailored therapies.
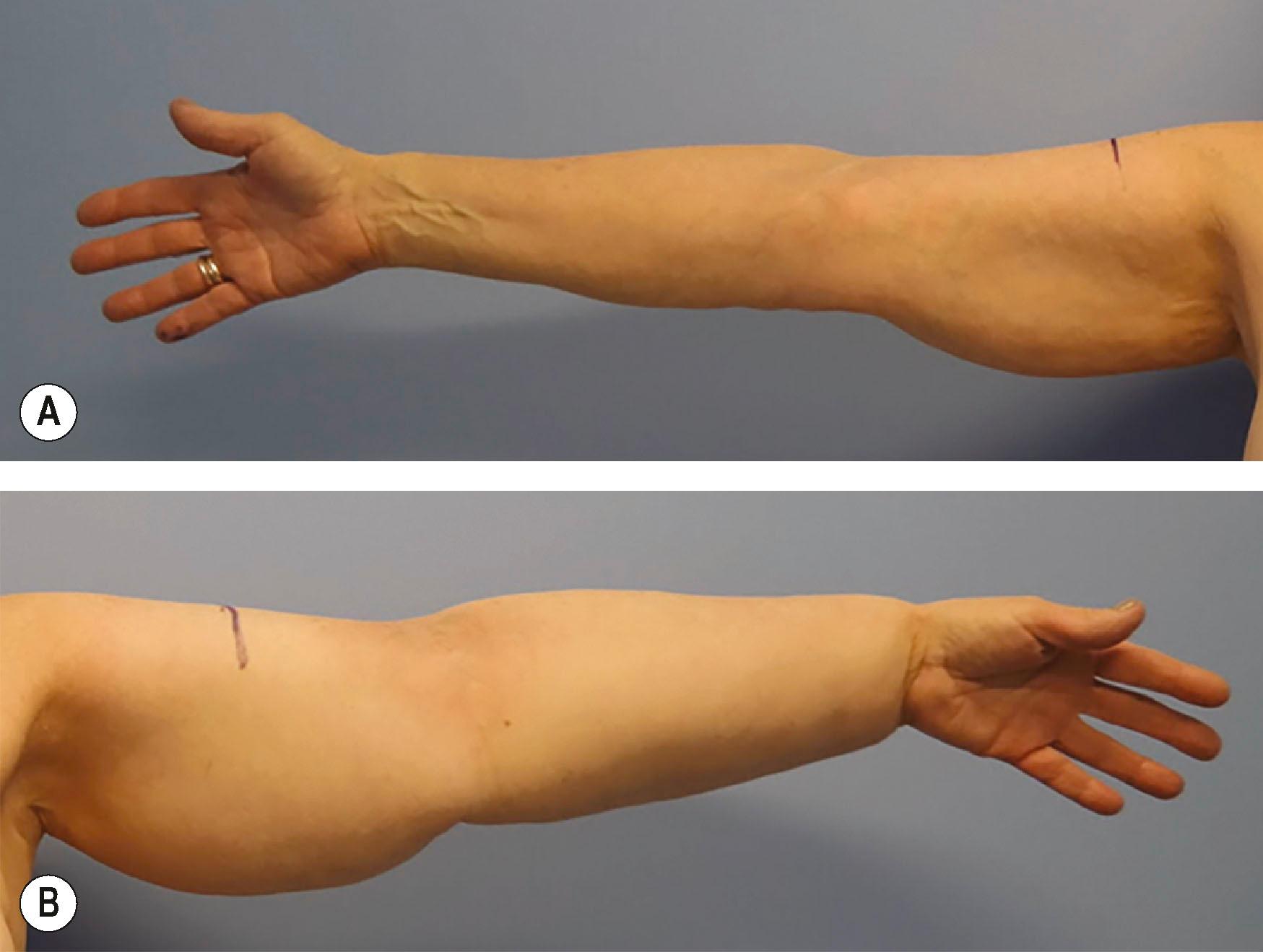
The clinical evaluation should begin with a detailed history of the breast cancer treatment course. Specific attention should be given to prior surgical interventions, axillary lymph node biopsy or dissection, and adjuvant radiotherapy. It is also important to interrogate the course of the patient’s lymphedema symptoms, differentiate between pitting and non-pitting edema, identify the presence of recurrent inflammatory events such as cellulitis, and quantify the extent of tissue involvement over time. Non-pitting edema is indicative of advanced lymphedema, in which the affected tissue has already undergone fibrosis. In addition to the clinical history, lymphedema stage may be assessed according to the International Society of Lymphology (ISL) criteria. Stage I consists of early edema that improves with limb elevation, stage II is pitting edema that does not resolve with elevation, and stage III describes non-pitting edema in the setting of fibrotic subcutaneous tissue and overlying skin changes.
Physical examination should include limb circumference and/or volumetric measurements, noting the presence or absence of pitting edema, testing for a Stemmer sign (inability to pinch the overlying skin on the dorsum of the hand), evaluation for scars in the axilla, and noting overlying skin changes or ulceration. Photographs of limb size and appearance are important for documenting progression.
Volumetric assessment can be determined using circumferential measurements at predetermined anatomic levels bilaterally, comparing the affected extremity to the unaffected side. Limb circumference measurements are taken at frequent intervals (4 cm) using the truncated cone formula to estimate limb volumes; these measurements can be used to track patients’ outcomes with treatments over time. Additional volumetric assessment modalities include water displacement measurements and 3D imaging systems, which have been shown to provide comparable volumetric assessments in a more time-efficient manner. A 10% difference in volume between the affected and unaffected limbs is consistent with a clinical diagnosis of lymphedema. Volumetric measurements determine the volume of the whole limb and cannot distinguish between different tissue types. Bioimpedance analysis, however, can provide a more accurate reflection of volumetric changes within lymphatic volume. This technology examines resistance to electrical currents through different tissues to determine the volume composition of various fluid compartments within the body, differentiating between extracellular fluid, which is analogous to lymphedematous fluid volume, and intracellular tissue.
Along with physical evaluation, it is imperative that providers assess the impact of BCRL on patients’ psychosocial wellbeing. Studies have shown that breast cancer survivors with BCRL have body image disturbances, depression, and overall lower physical and mental quality of life compared with breast cancer survivors without BCRL. The goals of both non-surgical and surgical therapy are ultimately aimed at improving individuals’ symptoms and overall quality of life.
Non-surgical treatment modalities are a cornerstone of lymphedema management. The interventions for patients with a diagnosis of lymphedema prioritize reducing edema, preventing infection, and maintaining extremity function.
Therapy programs are individualized based on lymphedema stage and origin, as well as patient functional status and self-care ability. Before beginning any targeted therapy, patient education on dermatological care, diet, exercise, and weight control is essential. The mainstays of non-surgical treatment are complete decongestive therapy (CDT), compression therapy, and advanced pneumatic compression pumps, which are most effective in symptom reduction in early-stage lymphedema.
CDT remains the gold standard of non-surgical lymphedema treatment. CDT encompasses two phases: reductive (phase 1) and maintenance (phase 2). The first phase is an intensive regimen guided by a certified lymphedema therapist (CLT) who aids with manual lymphatic drainage (MLD), short-stretch bandaging for gradient compression, and skin hygiene. MLD is a hands-on massage technique that applies gentle pressure to reorient fluid to proximal areas with properly functioning lymphatics ( Fig. 47.2 ). The maintenance phase involves the lifelong use of compression garments, in addition to continuing exercises and skin care. For maximal efficacy, garments must be well-fitting and must be worn throughout the day, with wrapping or a nighttime garment used at night. Garments can vary in pressure between 20 and 60 mmHg, although 30 mmHg is typically sufficient to increase the interstitial pressure and decrease extracellular fluid accumulation in the upper extremity.
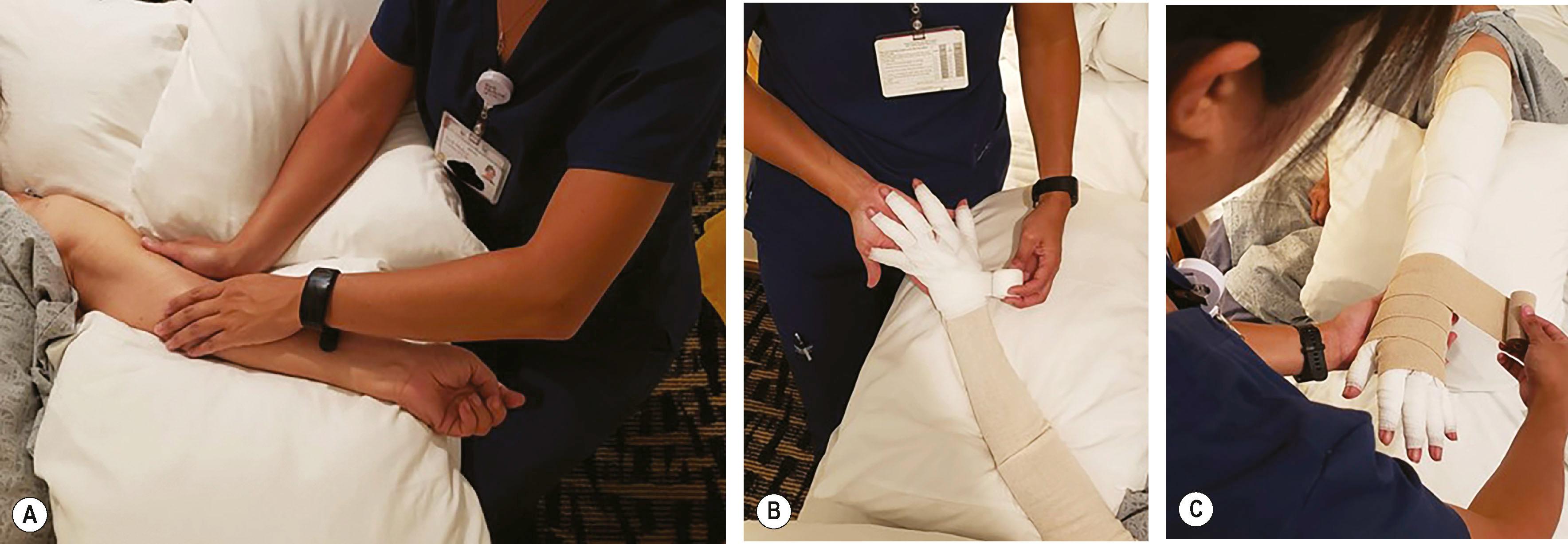
Volume reduction occurs primarily during the first phase. Once limb volume stabilizes, therapy transitions to the maintenance phase. CDT is safe and effective at achieving maximal reduction in upper extremity volume; however, it can be expensive and time-consuming, therefore leading to low patient compliance over time. Only a minority of patients sustain the minimum volume attained, and CDT has not been shown to significantly impact long-term volume reduction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here