Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The practice of hepatopathology requires a clear understanding of liver anatomy and physiology, as a prelude to understanding the expression of pathological processes in the liver. As an anatomical entity, the liver is deceptively simple. It is large, representing about 2% of the total body mass of an adult human and occupying most of the right upper quadrant of the abdomen. It has a roughly triangular profile, with incomplete clefts helping to define the different ‘lobes’ of the liver. It has only one point of vascular inflow, the porta hepatis. Blood exits through several venous orifices into the inferior vena cava, which traverses a deep groove in the dorsum of the liver. There are no ‘moving parts’ of the liver, with the exception of daily secretion of several litres of bile into the common hepatic duct, which exits from the porta hepatis.
Belying its macroscopic simplicity, the liver is home to biosynthetic and biodegradative metabolic pathways of unequalled complexity, generating enough metabolic heat to be a prime source of core homeostatic temperature maintenance. This chapter considers the embryology, macroanatomy and microanatomy of the liver and its basic response to injury, concluding with the appearance of ‘normal’ liver in biopsy and autopsy specimens.
In human embryos the liver first appears at the end of the third week of development. The liver bud, or hepatic diverticulum , arises as a hollow midline outgrowth of endodermal tissue from the ventral wall of the future duodenum. The connective tissue framework of the liver into which the endodermal bud grows is of mesenchymal origin and develops from two sources: (1) the septum transversum , a transverse sheet of mesenchymal cells that incompletely separates the pericardial and peritoneal cavities and is the primordium for both the diaphragm and the liver, and (2) cells derived from the mesenchymal lining of the associated coelomic cavity, which actively invade the septum transversum. The confluence of endodermal cells from the hepatic diverticulum growing into the mesenchymal primordium creates the solid organ destined to become the liver ( Fig. 1.1 ).
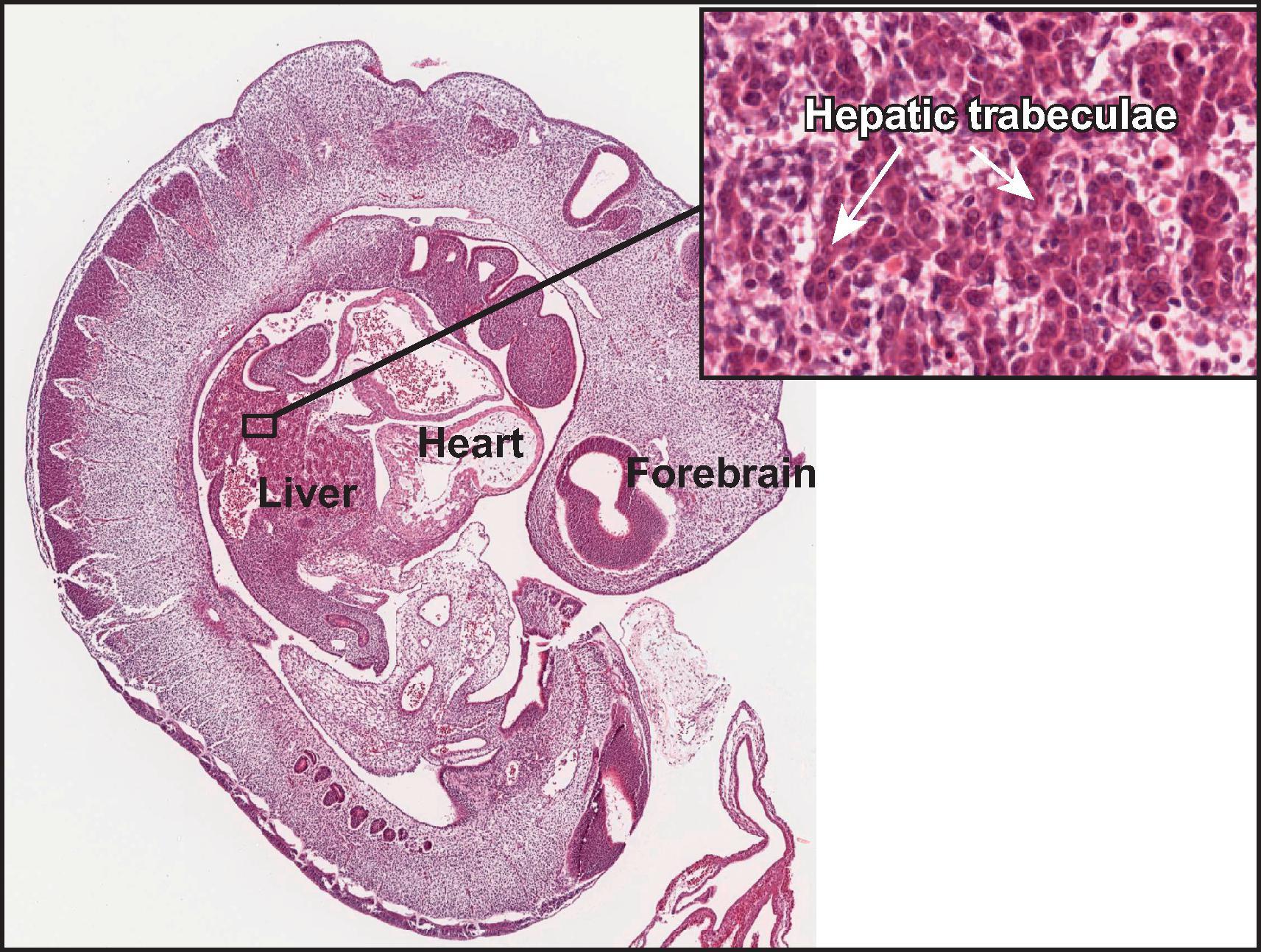
During the fourth week, buds of epithelial cells within the mesenchymal stroma extend radially out from the hepatic diverticulum. Between the epithelial cords, a plexus of vascular hepatic sinusoids develops. As the epithelial buds grow into the septum transversum, they break up into thick, anastomosing epithelial sheets that meet and enmesh vessels of the hepatic sinusoidal plexus, forming the primitive hepatic sinusoids ( Fig. 1.2A ). The intimate relation between hepatocytes and sinusoidal capillaries, so characteristic of the adult organ, is therefore already anticipated in the 4-week-old embryo. The hepatic diverticulum remains as a tether between the developing liver primordium and the duodenum, ultimately becoming the extrahepatic biliary tree.
Once established, the liver grows rapidly during the fetal period, to become the largest single visceral organ (by mass) for the remainder of gestation. It bulges into the peritoneal cavity on each side of the midline, as right and left lobes, which are initially symmetrical. It also grows ventrally and caudally into the mesenchyme of the anterior abdominal wall, extending down to the umbilical ring. Associated with these changes, the stomach and duodenum, which were initially in broad contact with the septum transversum, draw away from it, thus producing a midsagittal sheet of mesoderm, the ventral mesogastrium or future lesser omentum. As the duodenum withdraws from the septum transversum, the stalk of the original hepatic diverticulum is also drawn out to form, within the lesser omentum, the epithelial elements of the extrahepatic bile ducts. Simultaneously, the cephalad aspect of the liver becomes partly freed from its originally broad contact with the septum transversum by extensions of the peritoneal cavity and its visceral and parietal mesothelial surfaces so that, in the adult, direct contact of the liver with the diaphragm persists only as the ‘bare area’ of the liver. This is bounded by the attachments of peritoneal reflections, which form the coronary and falciform ligaments.
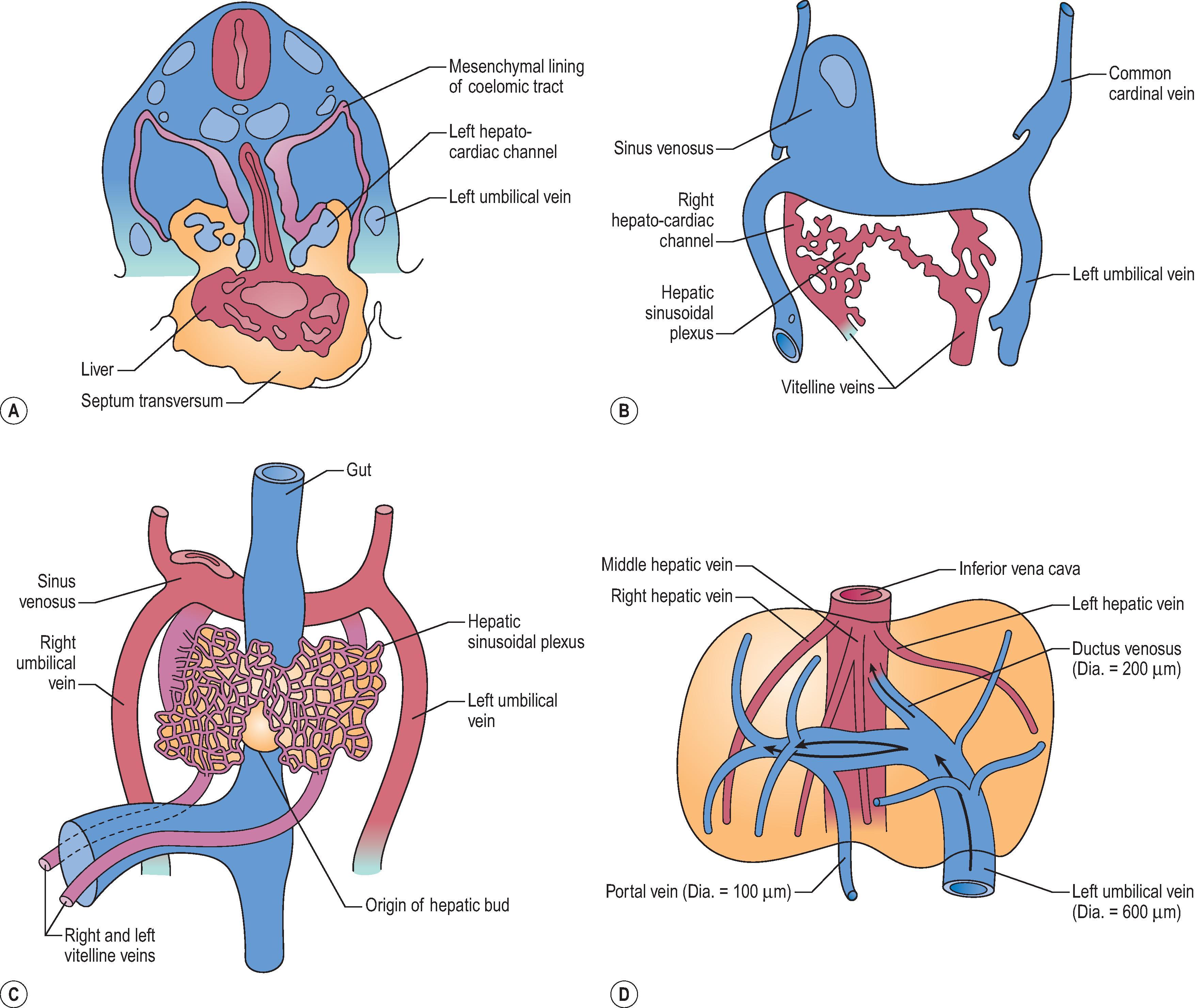
By the fifth week of development (in embryos of 5 mm), the liver parenchyma consists of anastomosing sheets of liver cells, each sheet being several cells in thickness. Coursing between the liver cells is the vascular ‘sinusoidal plexus’. Initially, the afferent hepatic blood supply is through the symmetrically arranged vitelline veins returning from the abdominal region of the embryo. Blood is also received from the laterally placed right and left umbilical veins, which run in the body wall and carry oxygenated blood from the placenta directly to the paired horns of the sinus venosus of the cardiac primordium. Both the vitelline and the umbilical sources of blood enter into the hepatic sinusoidal plexus through a developing branching vascular network. Blood draining from the sinusoidal plexus passes through symmetrical right and left hepatocardiac channels, to enter the sinus venosus through this same network ( Fig. 1.2B ). This network, along with the mesenchyme through which it passes, constitutes the early portal tract system within the liver parenchyma.
Once these vascular connections are made ( Fig. 1.2C ), the circulatory pattern within the liver changes rapidly. The left umbilical vein becomes the principal source of blood entering the liver, partly because it comes to carry all the blood returning from the placenta when the right umbilical vein withers and disappears (generating the ‘double-artery/single-vein’ umbilical cord of the term fetus), and partly because the initial volume of blood returning from the gut in the vitelline veins is small. The definitive vascular pattern of the fetal liver is already established by the seventh week in embryos, about 17 mm long ( Fig. 1.2D ). The originally paired vitelline veins have given way to a single portal vein that, on entering the liver, divides into right and left branches. Blood in the left umbilical vein traverses a venous extension in the falciform ligament and has a choice of three routes: (1) through the liver in branches that enter the sinusoidal plexus of the left half of the liver; (2) through the sinusoidal plexus of the right half of the liver, by retrograde flow through its connection with the left branch of the portal vein; and (3) through the ductus venosus traversing the short space between the porta hepatis to the inferior vena cava, to enter directly into the systemic venous circulation. By these routes, the converging bloodstreams from the definitive portal vein and indirectly from the umbilical vein enter the rapidly enlarging hepatic primordium through the porta hepatis. Intrahepatic mesenchyme condenses around the intrahepatic branching portal venous system, making up the ramifying portal tracts of the liver (see later discussion). Formal branches of the portal vein become identifiable at about the fifth week of gestation; draining hepatic veins form during the sixth week of gestation and acquire their adult configuration in the seventh week of development.
The hepatic artery is derived from the coeliac axis. Arterial sprouts appear much later than the portal venous and hepatic venous system in the embryo, beginning to grow only after 10 weeks of development. These arterial sprouts extend into the hepatic primordium from the porta hepatis along the mesenchyme of the portal tract system, spreading to the organ periphery as the fetal liver enlarges. The hepatic arterial system continues to proliferate and grow after birth, reaching an adult form only at 15 years of age. In the adult, about four arteries supply the largest intrahepatic bile ducts. At the level of the terminal portal tracts, there is a uniform 1:1 pairing of hepatic arteries and terminal bile ducts, and approximately two artery/bile duct pairs per single portal vein. The most terminal portions of the portal tree lose their portal veins, leaving only residual hepatic artery/bile duct dyads, which themselves disappear into the parenchyma. ,
To complete this discussion, the rapid changes in hepatic circulation at birth must be considered. A sphincteric mechanism closes the ductus venosus at its proximal end, blood flow ceases in the umbilical vein, and the left side of the liver receives blood that now flows from right to left through the left branch of the portal vein. The closed segment of the umbilical vein between the umbilicus and the liver regresses to form the ligamentum teres ; the ductus venosus undergoes fibrosis and becomes the ligamentum venosum .
Primitive hepatocytes are derived exclusively from the endodermal outgrowths of the hepatic diverticulum. Hepatocellular synthesis of alpha fetoprotein (AFP) begins at the earliest stage of liver differentiation, about 25–30 days after conception, and continues until birth. Glycogen granules are present in fetal hepatocytes at 8 weeks; the maximal glycogen reserve is achieved at birth, but the rapid onset of glycogenolysis over 2–3 days postpartum depletes the storage to approximately 10% of term levels. Hepatocellular haemosiderin deposits appear early in development, predominantly in periportal hepatocytes, and become more marked as hepatic haematopoiesis decreases (see later). Hepatocellular bile acid synthesis begins at about 5–9 weeks and bile secretion at about 12 weeks. Canalicular transport and hepatic excretory function, however, are still immature at birth and for 4–6 weeks postpartum, and therefore exchange of biliary solutes across the placenta (especially bilirubin) is important in the fetus. Within the sinusoids, endothelial cells, Kupffer cells and hepatic stellate cells (HSCs) appear at 10–12 weeks. When the adult liver is ultimately formed, hepatocytes constitute 80% of the cells in the normal liver. Of the remaining 20%, bile duct epithelial cells (cholangiocytes) comprise 1–3%, sinusoidal endothelial cells 10%, Kupffer cells 4%, and lymphocytes 5%.
The extrahepatic and intrahepatic biliary system is best understood if the liver is regarded as an exocrine gland. The endodermal cells of the hepatic primordium give rise not only to the epithelial parenchyma—the future hepatocytes—but also to the epithelial lining of the intrahepatic bile duct system. The extrahepatic bile ducts are derived from the caudal portion of the hepatic diverticulum, the portion that does not invade the septum transversum but remains as a stalk connecting the foregut to the developing liver. The caudal part of this tethering diverticulum forms a secondary bud, constituting the epithelial primordium of the cystic duct and gallbladder. The epithelial lining of the extrahepatic bile ducts is continuous at its caudal end with the duodenal epithelium and at the cephalic end with the primitive hepatic sheets.
The intrahepatic ducts develop from the limiting plate of hepatoblasts that surround the mesenchyme of the primordial portal tracts. This has been known since the 1920s and was confirmed using immunohistochemical methods and monoclonal antibodies to (cyto) keratins and cell surface markers. , Specifically, normal adult hepatocytes express keratins 8 and 18 (K8 and K18), whereas intrahepatic bile ducts express K7 and K19. During the first 7–8 weeks of embryonic development, no intrahepatic bile ducts are evident, and the primordial epithelial cells express K8, K18 and K19. At about 9–10 weeks (27–30 mm embryos), primitive hepatocytes (hepatoblasts) surrounding large portal tracts near the liver hilum express these cytokeratins more intensely and form a layer of cells that ensheaths the mesenchyme of the primitive portal tracts to form the so-called ductal plate ( Fig. 1.3 ). This is followed by the development from the hepatoblast parenchyma of a second but discontinuous layer of epithelial cells which show a similar phenotypic change, and thus a network of segmentally double-layered bile duct precursor structures is formed, , with asymmetric expression of the hepatoblast phenotype in the cell layer abutting the parenchyma, and of cholangiocytes in the cell layer abutting portal tract mesenchyme. This process is recapitulated centrifugally along the length of the portal tract tree, from the hilum outward to the periphery.
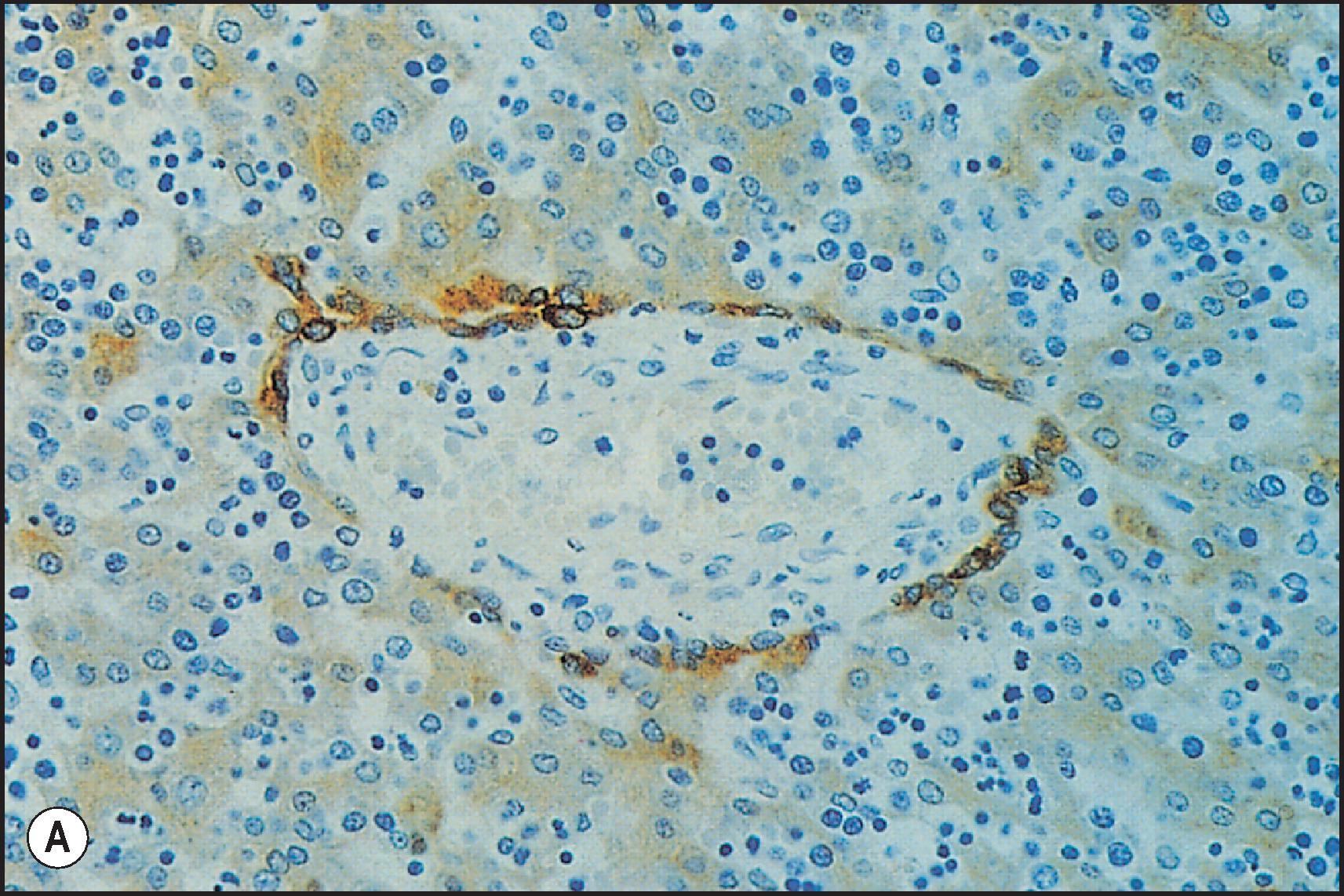
Ductal plate cells not differentiating into cholangiocytes instead differentiate into periportal hepatocytes (forming the ‘limiting plate’, hepatocytes abutting portal tracts). In turn, as the terminal bile ducts mature within the portal tract mesenchyme, cholangiocyte epithelial tethers remain as a connection with the hepatic parenchyma. While traversing the portal tract mesenchyme, these structures are bile ductules , circumferentially lined by bile duct epithelial cells (cholangiocytes) derived from the ductal plate. As these channels impale the parenchymal interface, they become hemilunar, with half the circumference as bile duct epithelial cells and the other half as hepatocytes. These are the canals of Hering , which may penetrate the parenchyma for up to one-third the zonal distance to the terminal hepatic vein or may skirt the portal tract interface as a residual short canal ( Fig. 1.4 ). The canals of Hering are thought to harbour resident stem cells throughout life, serving as a source for a robust proliferative ‘ductular reaction’ response after liver injury. ,
The developmental remodelling of the ductal plate into the maturing biliary tree also requires invading mesenchymal tissue to separate cholangiocyte structures in the ductal plate from the liver parenchyma. The resultant tubular structures become incorporated into the mesenchyme surrounding the portal vein branches. The developing hepatic arterial system, growing centrifugally into the portal tract system, appears to be the organizing element for the remodelling of the ductal plate into the formal intrahepatic biliary tree. This coordination of bile duct formation and hepatic artery formation explains the pairing of hepatic arteries and terminal bile ducts observed in the adult human liver.
Bile canaliculi between hepatocytes are first seen in human embryos at the sixth week, long before bile production begins at 12 weeks. They develop from membrane foldings between junctional complexes and appear as intercellular spaces within sheets of presumptive hepatocytes, thereby constituting an ‘apical’ luminal channel between hepatocytes. The bile canaliculi drain centripetally from the perivenous zonal region toward the periportal zone, discharging their fluid into the hemilunar canals of Hering, where the hepatocyte:bile duct epithelial cell connection occurs. At the level of hepatocytes, and in addition to the fluid pressure generated by active secretion of biliary solutes and fluid, contraction of the hepatocellular subapical pericanalicular actin network provides a contractile mechanical force for propulsion of newly formed bile downstream.
The entire process of intrahepatic bile duct development may not be complete at
40 weeks’ gestation, and full expression of K7 is not found until about 1 month postpartum. Thus the intrahepatic bile duct system is still immature at birth. Indeed, the liver doubles in size in the first year of life and continues to grow incrementally until adulthood, ultimately arriving at a mass 10 times that at birth. Formation of the intrahepatic biliary tree is therefore not fully complete until many years after birth. Failure of remodelling and resorption during fetal liver development produces the ductal plate malformation , , which is significant in the production of various congenital malformations of the intrahepatic biliary tree. The extensive information now available about the molecular signalling of liver development provides critical insights into such diseases as biliary atresia and Alagille syndrome (see Chapter 3 ).
Despite their common ancestry, mature hepatocytes and cholangiocytes of ductal epithelium in the adult liver are considered as distinct cell types. The epithelium of the terminal twigs of the biliary tree—the canals of Hering—includes typical hepatocytes and typical ductal cells, but no forms intermediate between the two. However, the adult liver retains multiple stem cell niches, including hepatic stem/progenitor cells in or near canals of Hering and biliary tree stem/progenitor cells within peribiliary glands along the larger intrahepatic bile ducts. As noted above, when there is significant injury to either hepatocytes or cholangiocytes at the interface between portal tracts and parenchyma, a profound ductular reaction can occur. On repair of the injury, hepatocytes and cholangiocytes will return to expressing their normal respective keratins (K8, K18 for hepatocytes; K7, K19 for cholangiocytes).
The limiting plate merits further comment. , , Referring back to embryological development, once the ductal plate has involuted, only canals of Hering remain at the portal tract–parenchymal interface as elements containing bile duct epithelial cells. The remainder of the interface is rimmed by mature hepatocytes, directly abutting the portal tract mesenchyme and representing the limiting plate. When liver injury occurs at the interface, involving destruction of hepatocytes and influx of inflammatory cells, the limiting plate is compromised or destroyed; this process is termed ‘interface hepatitis’. The canal of Hering–bile ductular compartment proliferates in response, giving rise to what has recently been termed ‘invasive ductular reaction’, which, in entering into the parenchymal space, can help re-establish the drainage of bile from hepatocyte canaliculi to canals of Hering and the biliary tree.
Hepatic haematopoiesis is a feature of the embryonic and fetal liver of mammals, including humans. The yolk sac is the initial site of haematopoiesis from primitive progenitor cells. Colonization of the liver by definitive erythroid-myeloid progenitor cells begins at about 6 weeks (10 mm embryo). , Foci of haematopoietic cells appear extravascularly alongside the sheets of hepatocytes, and by the 12th week the liver is the main site of haematopoiesis, having superseded the yolk sac, with much lower contributions from skin and kidney. Hepatic haematopoietic activity begins to subside in the fifth month of gestation, when the bone marrow becomes haematopoietic, and has normally ceased within a few weeks after birth. Parenchymal haematopoiesis is largely erythropoietic; haematopoiesis within portal tracts tends more toward granulocytes, megakaryocytes and monocytes.
As previously noted, the first morphological indication of the development of the liver is an endodermal proliferation in the ventral part of the foregut, just caudad to the cardiac mesoderm and septum transversum, at the 18th postfertilization day in the human embryo. Ventral foregut endoderm is marked by the expression of the homeobox transcription factor gene, HHEX , and over the ensuing days is subdivided into hepatic (HNF1β)-expressing or pancreatic homeobox factor 1 (PDX1)-expressing progenitor domains, a process called specification. Beginning about the 23rd day of gestation, the cardiogenic mesoderm provides an FGF signal that is important for the proliferation of the precursor endodermal cells ; both FGF1 and FGF2 appear to be involved. These precursor cells proliferate and invade as hepatoblasts into the surrounding septum transversum, forming the liver bud proper. Expression of metalloproteinases both by mesenchymal cells of the septum transversum and by hepatoblasts is required to enable hepatoblast migration into the septum transversum. Liver bud outgrowth occurs through about the 56th day of gestation, without further differentiation of the hepatoblasts. Beginning about days 56–58 in the fetal liver, terminal differentiation of hepatoblasts begins, continuing for the remainder of pregnancy as the mature structure of the liver is laid down.
Many of the molecules and receptors involved in regulation of the hepatoblasts and subsequent hepatocyte and cholangiolar differentiation have now been identified. It has become increasingly apparent that cellular interactions with nonparenchymal cells play a key role in early hepatic development. , , The sheets of hepatoblasts that invade the septum transversum in the developing mouse liver express the transcription factors HNF1β and HNF4α, while the surrounding mesenchyme expresses GATA4 ; the migratory properties of the hepatoblasts appear to require a homeobox gene PROX1 . GATA6 also appears to be essential in the formation of the early liver bud, as do FoxA1 and FoxA2 (under the induction of HNF-1β). Some of these factors (e.g. GATA4) appear to be important in early stimulation of hepatocyte-specific gene expression, including AFP, transthyretin and albumin; this occurs before morphological change toward a hepatocyte or a cholangiocyte phenotype.
Expression of bone morphogenic protein (BMP) by the septum transversum activates the expression of GATA4. , β-Catenin and Wnt signalling are critical for induction and control of liver development. Studies utilizing embryonic stem cells and RNA technology have also reinforced the role of FoxA2 in hepatocytic differentiation. The myriad of transcription factors and signalling molecules identified thus far that may be involved in early liver induction are summarized elsewhere. Vasculogenic cells (angioblasts) are also critical for these earliest stages of organogenesis, before blood vessel formation. In the mouse embryo, angioblasts were found as a loose necklace of cells interceding between the thickening, hepatically specified endoderm and the mesenchyme of the septum transversum. This mesenchymal–epithelial interaction precedes the emergence of the liver bud and persists throughout further liver development. Lastly, mesothelial cells of the septum transversum act as the multipotential mesenchymal progenitor cells that produce HSCs, fibroblasts around blood vessels and vascular smooth muscle cells.
During later fetal liver development there is continued expansion of the parenchymal cell mass. This involves both stimulatory signals and protection from tumour necrosis factor alpha (TNF-α)-mediated apoptosis; these phenomena involve the AP-1 transcription factor c-Jun, hepatoma-derived growth factor (HDGF), the Wnt signalling pathway, the nuclear factor-κB (NFκB) pathway and the hepatocyte growth factor (HGF)–c-met pathway, among others. , In the final step of differentiation, PROX1 and HNF4α direct hepatoblasts to a hepatocyte phenotype, whereas HNF6, SOX9 and HNF1β guide the hepatoblast to a cholangiocyte phenotype. Signalling of the Jagged-1/Notch pathway is important for creating the proper balance in the numbers of hepatocytes and cholangiocytes. , , Development and maintenance of hepatocytic differentiation and function are under the control of HNF4α.
The differentiation events occuring in the ductal plate are also patterned with portal vein and hepatic arterial development, implicating a complex system of molecular signals between these cellular compartments and their supporting mesenchyme. These signals include expression by cholangiocyte precursors of the SRY-related HMB box transcription factor 9 (SOX9), hepatocyte nuclear factor 6 (HNF6) or HNF1β growth factors and vascular endothelial growth factor A (VEGF-A), , with SOX4 also playing a role. Key downstream molecular signals guiding cholangiocyte differentiation then include the Delta ligand:Notch receptor pathway, Wnt, transcription growth factor β (TGFβ), Hippo–Yap and fibroblast growth factor (FGF) signalling pathways. , – Maturation of primitive bile ductular structures into mature bile ducts is then promoted by LKB1, a tumor suppressor encoded by the STK11 gene.
The liver lies almost completely under the protection of the rib cage, projecting below it and coming into contact with the anterior abdominal wall only below the right costal margin and the xiphisternum. The liver is moulded to the undersurface of the diaphragm, the muscular part of which separates it on each side from the corresponding lung and pleural sac. The liver is separated by the central tendon of the diaphragm from the pericardium and the heart. The anterior dome of the liver and its medial, ventral and lateral aspects are covered by the Glisson capsule , the connective tissue sheath of the liver with its glistening peritoneal surface. The posterior surface of the liver is the least accessible, and its relationships are of some clinical importance. It includes the following, from right to left:
The ‘bare area’, which is surrounded by the reflections of peritoneum that form the superior and inferior layers of the coronary ligaments. It lies in direct contact with the diaphragm, except where the inferior vena cava (IVC), the right adrenal gland and the upper part of the right kidney intervene.
The caudate lobe, which lies between the IVC on the right and, on the left, the fissure of the ligamentum venosum and the attachment of the lesser omentum. The caudate lobe projects into the right side of the superior recess of the lesser sac and is covered by peritoneum; behind it lies the right crus of the diaphragm, between the IVC and the aorta.
A small area on the left posterior surface, covered by peritoneum and apposed to the abdominal oesophagus.
The traditional division of hepatic anatomy into right and left lobes (delineated by the midline falciform ligament) and caudate and quadrate lobes is of purely topographical significance. A more useful and important subdivision is made on the basis of the branching pattern of the hepatic artery, portal vein and bile ducts. As these are followed into the liver from the porta hepatis, each branches in corresponding fashion, accompanied by a branching tree of connective tissue, derived from the original mesenchyme of the developing liver. On this basis of vascular anatomy, the liver is divided into right and left ‘physiological’ lobes of about equal size. The plane of separation between these two ‘hemilivers’ corresponds, on the visceral surface of the liver, to a line extending from the left side of the sulcus for the IVC superiorly, to the middle of the fossa for the gallbladder inferiorly. This parasagittal plane lies approximately 2–3 cm right of the midline. Each lobe has been further subdivided into portobiliary-arterial segments.
Within each hemiliver, the primary branches of the portal vein divide to supply two main portal segments, each of which is further divided horizontally into superior and inferior segments. According to this scheme, there are thus eight segments, or nine if the dorsal bulge of the liver between the groove of the IVC and midline—the caudate lobe—is separately designated. Using the Couinaud system for designating segments ( Fig. 1.5 ), the numerical assignments are caudate lobe (I); left lobe: to the left of the left hepatic vein and falciform ligament, superior (II) and inferior (III); between the left and middle hepatic veins, superior (Iva) and inferior (IVb); and right lobe: medio-inferior (V), latero-inferior (VI), latero-superior (VII) and medio-superior (VIII). Because segment IVb lies between the falciform ligament medially and the gallbladder fossa and groove for the IVC laterally, this region also is designated the quadrate lobe. Numerology aside, the caudate lobe stands at the watershed between right and left vascular and ductal territories; its right portion in particular may be served by right or left vessels and ducts, although its left part is almost invariably supplied by the transverse portion of the left branch of the portal vein.
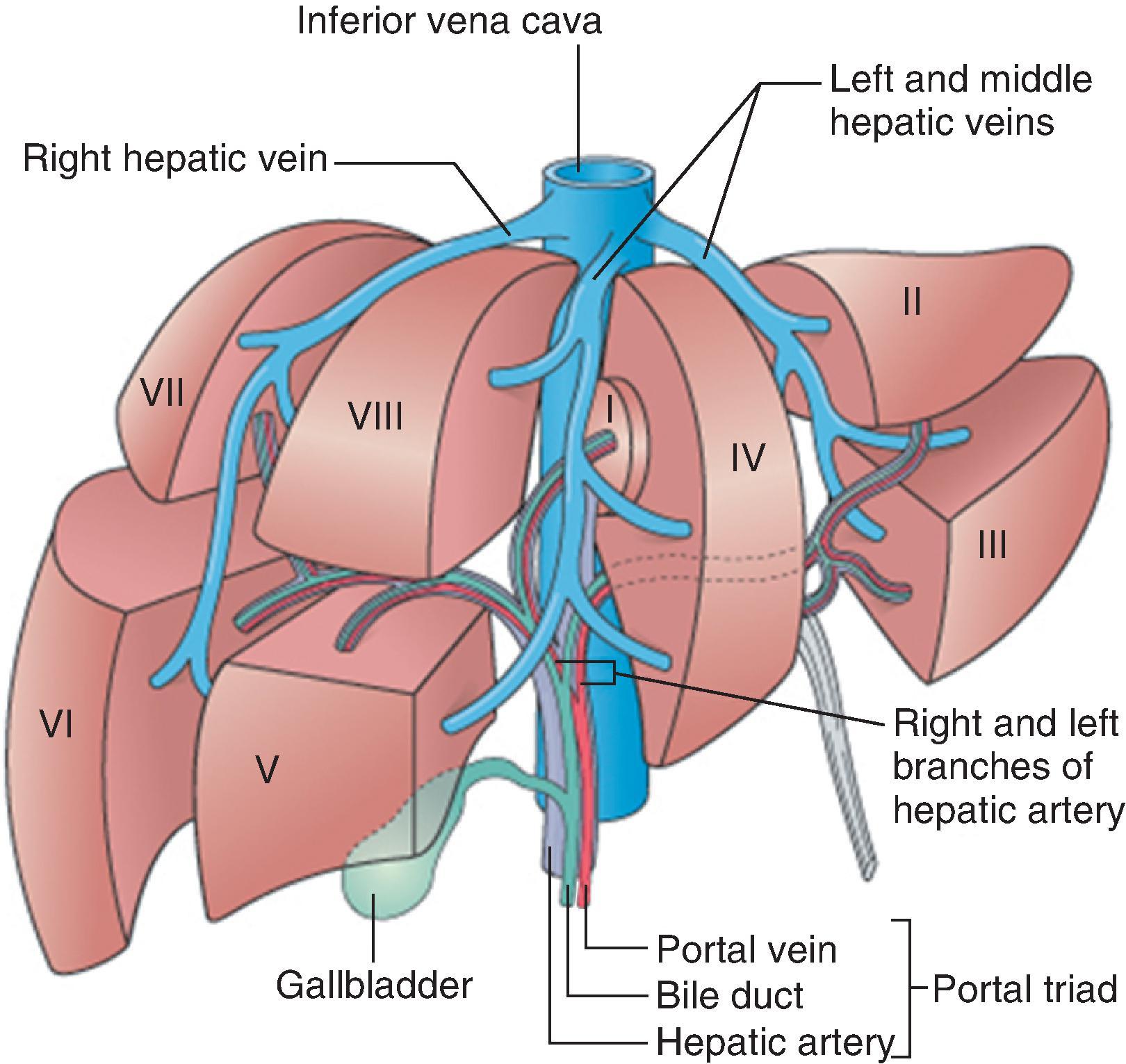
Although the Couinaud system of liver segments does not strictly relate to embryologic development, the nine designated segments represent separate areas of portal venous, hepatic arterial, biliary and hepatic venous supply-and-drainage. While there is substantial opportunity for vascular anastomoses between the different hepatic segments, there are no major intrahepatic vascular connections between the right and left hepatic arteries or portal vein systems. The virtually independent vascular supply for each segment has been shown by studies in living humans, using computed tomography (CT), magnetic resonance imaging (MRI) and ultrasonography (US), together with intravenous (IV) contrast injections, which allow ready recognition of the liver’s major vascular structures. Preoperative imaging of the hepatic vascular anatomy, especially of hepatic arterial anatomy, thus underpins the surgeon’s ability to perform a partial hepatectomy and achieve haemostasis for the residual liver, despite the absence of defining connective tissue septa between segments.
While the branches of the hepatic artery and portal vein and the tributaries of the hepatic ducts run together and serve segments of liver, the hepatic veins run independently and are intersegmental. As with the portal vein, they lack valves. The three major hepatic veins—the right, middle and left (the middle and left often forming a common trunk)—enter the upper end of the retrohepatic segment of the IVC. The terminal portion of each hepatic vein frequently is at least partially exposed above the posterior surface of the liver, where the veins are vulnerable to being severed by blunt abdominal trauma. In addition to these major hepatic veins, several accessory hepatic veins (about five per liver) open into the lower part of the hepatic segment of the IVC. Because the caudate lobe regularly drains directly into the IVC, it may escape injury from venous outflow block.
The definition of the fundamental structural and functional ‘unit’ of the liver has been a goal since the first description of liver lobules by Weppler in 1665 (cited by Bloch ). Over the years, several concepts of the basic structural organization of the liver have been operative. The hexagonal ‘lobule’ described by Kiernan in 1833 remains the standard by which hepatic microarchitecture is named ( Fig. 1.6 ). ‘Portal tracts,’ containing a portal vein, hepatic artery and bile duct, constitute the periphery of the hexagonal lobule, occupying three of the six apices of the hexagon. The effluent hepatic vein is at the centre of the lobule, thus its name ‘central vein’. To this day, regions of the parenchyma are referred to as ‘periportal’ or ‘pericentral’.
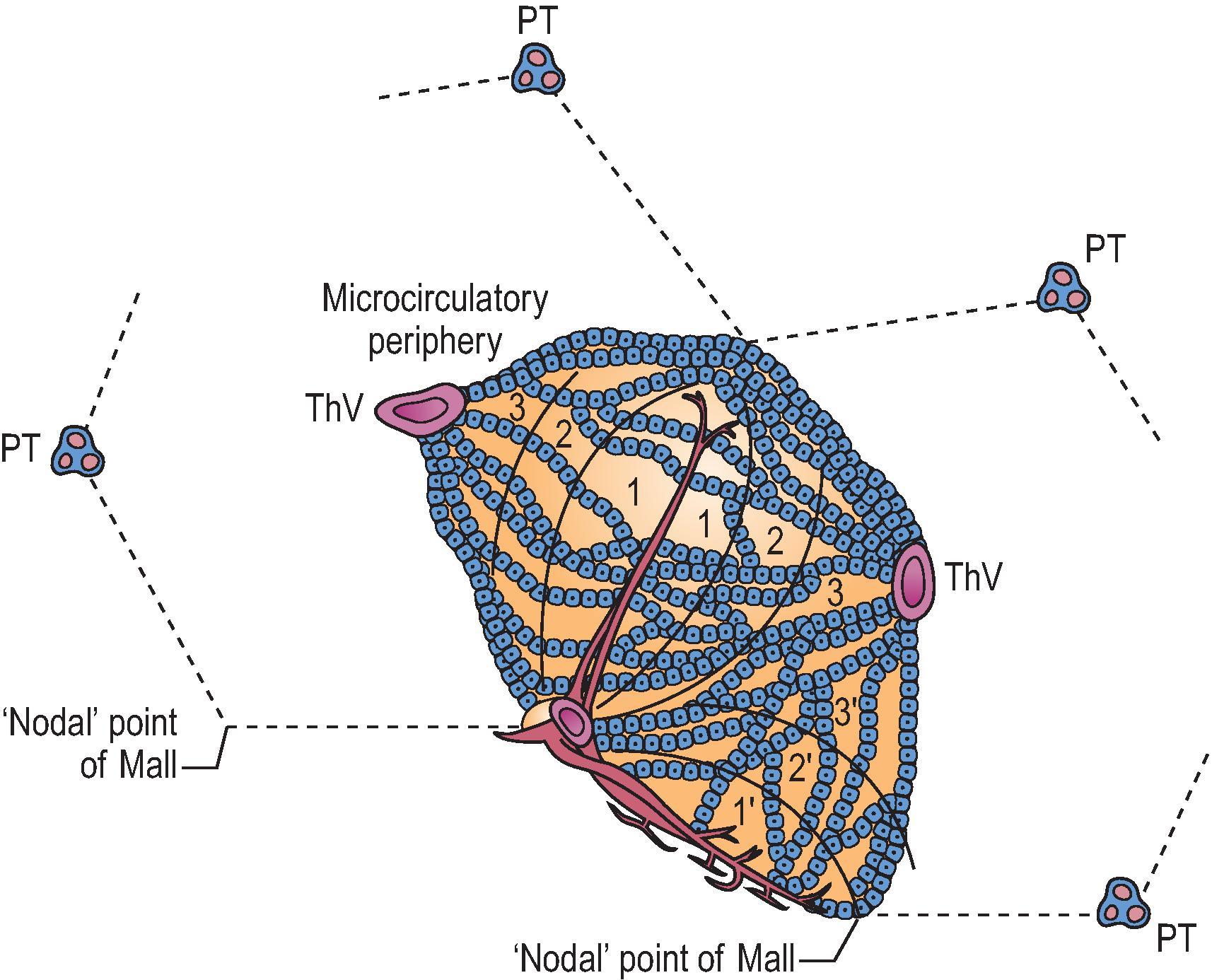
More useful are subdivisions of the classic hexagonal lobule into smaller functional physiological units. The most robust concept is the ‘liver acinus’ (see Fig. 1.6 ), defined by Rappaport et al. in 1954. In this formulation the portal tract is at one point of the base of a triangle and the effluent vein is at the sharp apex of the triangle. The vein is regarded as the ‘terminal hepatic vein’. In the idealized hexagonal lobule, there are six acinar units. However, actual liver microanatomy is not ideal, and there are ever-variable relationships between portal tracts and terminal hepatic veins across the two- and three-dimensional (2D and 3D) anatomy of the liver parenchyma. Regardless, a key element of the Rappaport ‘acinus’ is inlet venules derived from portal veins of various sizes, which penetrate the parenchyma and traverse the base of the triangle. Blood emanating from these venules perfuses the parenchyma across a broad zonal front, converging at the apex of the terminal hepatic vein. The most proximal zone, defined by the blood flow, is zone 1 , with a midzonal zone 2 and a terminal zone 3 recapitulating the ‘pericentral zone’ of the hexagonal lobule. Importantly, zone 1 is not ‘periportal’; it is an ellipsoid region of higher-oxygenated parenchyma, with its long axis being the base of the isosceles triangle (remembering that acini emanate symmetrically on both sides from the penetrating venule). Likewise, the inlet venule is orthogonal to a line drawn between parallel terminal hepatic veins coursing through the parenchyma. In three dimensions the perfect geometry is an interlocking system of triangular acini that can be viewed as contributing to the larger hexagonal lobules. Meticulous 3D reconstruction of the liver (in this case the rat) demonstrates that the actual microanatomy of lobules-and-acini exhibits a much more varied geometry.
Other formulations for liver microanatomy have been proposed. In the ‘primary hepatic lobule’ model, Matsumoto et al. regarded the ‘vascular septum’ emanating from the portal tract (the ‘penetrating venule’ of the liver acinus) as being at the origin of the unit’s blood flow. Later, the ‘cholehepaton’ was viewed as similar to the nephron and synthesized concepts of a ‘choleon’ and ‘hepatic microcirculatory unit’. A group of hepatocytes, again a triangle with the terminal hepatic vein at its apex, is drained by a single bile ductule–canal of Hering at the base of the triangle. In this way a countercurrent cholehepaton unit is described, with blood moving through sinusoids from the base to the apex of the triangle and bile moving from apex to base in return.
Most of these concepts represent different ways of looking at an organ of deceptive structural complexity and with a multitude of functions. However, a basic difference between the liver acinus and the other ‘lobule-based’ concepts makes them mutually exclusive. Early editions of this text stated that the concept of the ‘liver acinus’ was proving to be of the greatest value to the pathologist in the interpretation of disordered structure and function. It represented the structural and functional liver unit concept that allowed for an explanation of important histopathological features, such as portal-central bridging, hepatic necrosis and fibrosis. However, the concept of the liver acinus was based on vascular injection of coloured gelatin-based infusion fluids. The meticulous vascular reconstruction of the liver performed by Matsumoto et al. , provides critical insight into the curvilinear perfusion of ‘primary hepatic lobule’, as will be detailed later. Hence, among devoted hepatic anatomists, this last concept has gradually gained attention, and several other concepts (e.g. choleon, hepatic microcirculatory unit, choleohepaton, single sinusoid–hepatic functional unit) can be considered as variants of or existing within the primary hepatic lobule.
Given all these considerations, the terminology of hepatic microanatomy is somewhat fluid and certainly mixed. Nevertheless, the practice of liver histopathology exists mostly in the two worlds of the classic ‘lobule’ and Rappaport ‘acinus’. We thus ask the reader to understand the use of these mixed terminologies in subsequent chapters.
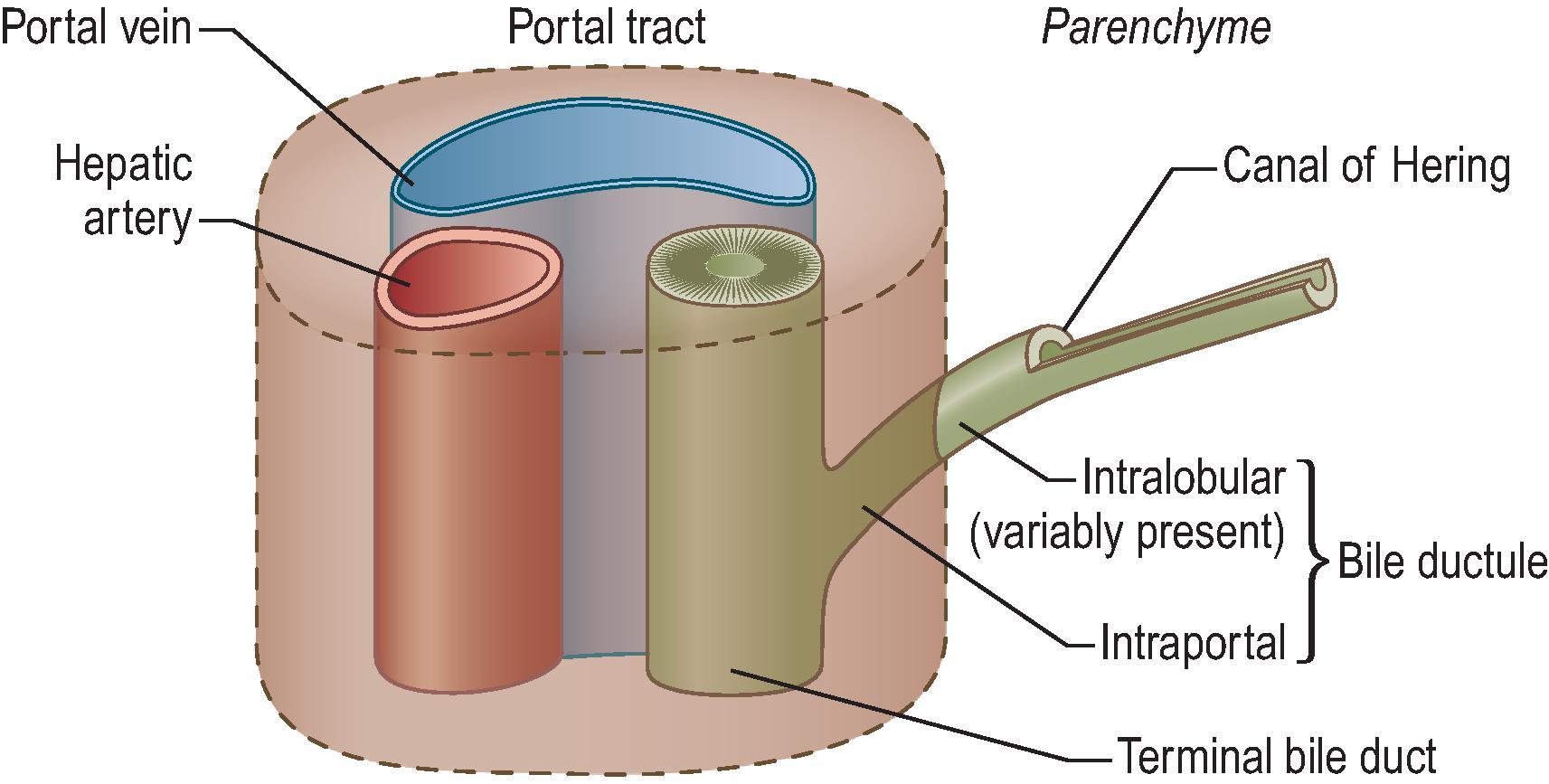
The portal tract derives its name from the ramifications of the portal venous system through the liver. The portal system also provides the tracts along which the hepatic artery system and biliary tree travel, giving rise to the alternate term ‘portal triad’. However, as the terminal portal tracts reach their own terminus, the portal vein system ends, ironically leaving a final portal ‘dyad’ lacking a terminal portal vein but containing hepatic artery–bile duct pairs before these also end after a short distance. In the periphery of the normal adult liver sampled by percutaneous liver biopsy, portal ‘triads’ containing portal vein, hepatic artery and bile duct profiles constitute only 70% of portal tracts. Careful histological examination also reveals an additional 30% of diminutive ‘portal dyads’ containing only hepatic artery and bile duct profiles. Lastly, the portal system ramifies through approximately 17–20 orders of branches to supply the entire corpus of the liver. The subdivisions are not strictly dichotomous, however, in that one branch may ultimately have fewer subbranches than another. Thus the liver ultimately has an irregular lobular organization at the microscopic level, consistent with the observations from 3D reconstruction.
Beginning at the porta hepatis, the portal vein divides into successive generations of distributing or conducting veins, so called because they do not directly give off inlet venules. The vein diameters remain in the macroscopic range, introducing negligible vascular resistance. According to their diminishing position in the hierarchy of branching, they may be classified as interlobar , segmental and interlobular . The portal venous system branches in a nondichotomous manner, such that smaller portal tracts may branch off conducting portal tracts without the latter losing their conducting capability.
These further branches of the portal system are those that produce the smallest portal veins, which do distribute their blood into the sinusoids. These succeeding branches are (1) the preterminal portal venules, microscopically found in portal tracts of triangular cross-section, and (2) the terminal portal venules, which taper to about 20–30 µm in diameter and are surrounded by scanty connective tissue in portal tracts of circular rather than triangular cross section. As denoted in Fig. 1.6 , from both the preterminal and terminal portal venules arise side branches, designated inlet venules , which have an endothelial lining with a basement membrane and scanty adventitial fibrous connective tissue but no smooth muscle in their walls. They pass through the periportal connective tissue sheath and enter into the parenchyma to open into the sinusoids. Although these inlets were reported to be guarded by sphincters composed of sinusoidal lining cells—the afferent or inlet sphincters, this concept has not been supported in more recent years. Some early branching of the smallest conducting veins may produce more than one portal vein within some portal tracts, and these supply only those sinusoids which abut the portal tract.
According to the anatomical studies of Matsumoto et al. , describing the primary hepatic lobule, the conducting portal system and the terminal portal branches can both give rise directly to penetrating inlet venules. The inflow-front for portal venous blood thus has a sickle shape. This is at variance with the concept of the acinus, in which portal blood flow exhibits radial symmetry only around the inlet venule arising from terminal portal vein branches. Moreover, the inlet venules do not run the entire length of the distance between two portal tracts, but rather taper off into a sinusoidal configuration near the middle of the interportal distance. In other words, the vascular watershed areas are not located at the nodal points of Mall (see Fig. 1.6 ), but at the midpoint of the interportal distance (midseptum M of Zou et al. ). Zonal gradients of metabolic activities and of enzyme histochemical staining patterns also conform to the sickle-zone pattern, not with the acinus diagram. The sickle-zone pattern has been confirmed by others. It is also important to note that the septal venules are not orthogonal to the portal tract, but in three dimensions follow a somewhat arcuate course into the parenchyma.
The importance of keeping this more sophisticated anatomical perspective becomes clear when examining the damaged liver. For example, according to the acinar concept, with severe hepatic congestion involving destruction of hepatocytes in acinar zone 3 and potentially in zone 2, the surviving parenchyma of acinar zone 1 would appear clover shaped, with the portal tract at its centre, but still damaged. However, the primary hepatic lobular concept predicts that immediate periportal damage does not actually occur in vascular insufficiency or chronic venous congestion. Instead, the ischaemic hepatic damage occurs in sickle-shaped zones standing in relief with preserved viable parenchyma.
The hepatic artery supplies branches throughout the portal tract system. In the largest interlobar portal tracts, there are typically four hepatic arteries accompanying the interlobar bile ducts. The hepatic artery/bile duct ratio drops until, in the most distal portal tracts, hepatic arteries are paired in close proximity with interlobular bile ducts in a 1:1 ratio. Even at this level, there are typically two hepatic artery–bile duct pairs per portal vein in the peripheral portal tracts sampled by percutaneous liver biopsy.
The terminal blood flow of the arteries is by three routes: into a plexus around the portal vein, into a plexus around a bile duct, and into terminal hepatic arterioles. The perivenous plexus is characteristically distributed around interlobar, segmental and interlobular portal vein branches within the portal area; it drains into hepatic sinusoids. Arterioportal anastomoses between perivenous arterioles and terminal portal venules can be observed in mammalian liver, but the frequency of these in normal human livers is uncertain. , By the level of the terminal portal veins, the arteriolar plexus is absent.
A peribiliary arterial-derived plexus supplies all the intrahepatic bile ducts. Around the larger ducts the peribiliary plexus is two layered, with a rich inner, subepithelial layer of fine capillaries and an outer, periductular venous network which receives blood from the inner layer. The smallest, terminal bile ducts have only a single layer of fine capillaries. Ultrastructural studies have shown that the capillaries are lined by fenestrated endothelium. The peribiliary plexus drains principally into hepatic sinusoids. The peribiliary plexus develops in parallel with the development of the intrahepatic bile ducts, spreading from the hepatic hilum to the peripheral area of the liver and becoming fully developed with the full maturation of the biliary system. In addition to providing the vascular supply to bile ducts, the peribiliary vascular plexus is thought to be involved in the reabsorption of bile constituents (including bile acids) taken up apically by bile duct epithelial cells and secreted across their basal plasma membrane into the portal tract interstitium, constituting a ‘cholehepatic’ circulation. The cholehepatic reuptake of biliary substances may play a key adaptive role during times of downstream bile duct obstruction, because these solutes may be ‘dumped’ into the systemic circulation for disposal by the kidneys. Conversely, cholehepatic circulation of medications such as sulindac may compete with normal biliary secretion of bile acids, thereby inducing functional cholestasis.
Terminal hepatic arterioles have an internal elastic lamina and a layer of smooth muscle cells, and they open into periportal sinusoids via arteriosinus twigs. Although some mammalian species exhibit hepatic arterioles that penetrate deeply into the parenchyma before entering sinusoids near to the hepatic veins, reports of such penetrating arterioles in humans have been disputed. Regardless, isolated parenchymal arterioles may sometimes be seen in liver biopsies. Ekataksin has suggested that these vessels supply isolated vascular beds in the parenchyma. Perhaps more importantly, isolated arteries that traverse the hepatic parenchyma to feed the hepatic capsule and hepatic veins may play a principal role in the development of subcapsular haemorrhage and in arterial collateral formation following transcatheter arterial chemoembolization for liver cancers.
Having perfused the parenchyma through the sinusoids, blood enters the terminal hepatic venules (‘central veins’ of classic lobules). Scanning electron microscopy (SEM) has clearly demonstrated in the walls of terminal hepatic veins the fenestrations through which the sinusoids open, and the astute observer can see these sinusoidal openings into terminal hepatic veins in histological sections. The terminal vein branches drain into sublobular veins, which in turn form larger hepatic vein branches, whose macroanatomy is described earlier. The venous anatomy does not strictly parallel the distribution of the portal system, because hepatic veins traverse between portal system-defined lobules as the venous system exits the liver. This is understandable; ultimately the hepatic veins need to exit through the dorsum of the liver, whereas the portal system enters the liver ventrally. The hepatic venous system also does not ramify as extensively as the portal system, so there is a slight preponderance of terminal portal tracts to terminal hepatic veins throughout the liver.
Total hepatic blood flow in normal adults under resting conditions is between 1500 and 1900 mL/minute, or approximately 25% of cardiac output. Of this, about two-thirds are supplied by the portal vein and the remainder by the hepatic artery. Because of variations in splanchnic blood flow, portal vein blood flow increases after feeding and decreases during exercise and sleep. The hepatic arterial buffer response (HABR) counteracts changes in portal vein blood flow, resulting in a decrease in hepatic arterial blood flow during periods of higher splanchnic blood flow to the liver and an increase in hepatic arterial blood flow when portal vein flow is reduced. , Direct external regulation of hepatic blood flow is mediated primarily through the hepatic artery, influenced by such vascular mediators as angiotensin II. Hepatocyte secretion of adenosine also stimulates the HABR. During times of reduced portal venous blood flow, as during acute occlusion or in cirrhosis, the HABR also helps maintain hepatic perfusion so as to support its core metabolic functions, although it cannot fully compensate for lost portal perfusion. The HABR may be counterproductive in selected clinical situations. One example is downregulation of hepatic arterial blood flow in living-related liver transplantation, where vigorous portal vein blood flow in the adult graft may lead to sluggish hepatic arterial blood flow and predispose to hepatic artery thrombosis. A second example is the potential need for co-injection of mesenteric circulation vasodilators to preserve splanchnic perfusion of the liver during hepatic arterial chemoembolization of hepatic tumours, so as to counteract the HABR downregulation of hepatic arterial blood flow during the procedure.
Intrinsic regulation of blood flow within the liver is quite complex. In keeping with the HABR physiologic mechanism described earlier, hepatic arterial resistance sites are located at the presinusoidal level. This concept is further supported by ultrastructural studies that document occasional strictures at branching sites of the most distal hepatic arterial channels. In contrast, portal venous resistance sites are located at the sinusoidal or postsinusoidal level. On the basis of detailed morphologic studies, a sinusoidal ‘inlet sphincter’ was proposed to regulate entry of portal venous blood, regulated by endothelin-1 (ET-1) and mediated by the contractile capabilities of perisinusoidal HSCs. ,
There also is heterogeneity in the blood flow through the sinusoids. In the upstream zone the sinusoids form an interconnecting polygonal network. Downstream, however, the sinusoids become organized as convergent parallel channels which drain into the terminal hepatic venule, a convergent architecture that is evident histologically at medium power. In this downstream region, short intersinusoidal channels connect adjacent parallel sinusoids. In the setting of this sinusoidal structural heterogeneity, modulation of blood flow through individual sinusoids also is modulated and variable in the sinusoids themselves. , The sinusoidal endothelial cells respond to a variety of vasoactive substances, including ET-1 and nitric oxide (NO). By contracting or swelling, they may vary the diameter of the sinusoid lumen. Vascular resistance is also increased by ET-1 responsive contraction of sinusoidal endothelial fenestrae. ET-1 responsive contractile hepatic stellate cells (HSCs) along the length of the sinusoid may also restrict sinusoidal blood flow. , Where the lumen is narrowed, blood flow may be impeded by leukocytes that transiently plug the vessel, a feature that is more common in the narrower, more tortuous periportal sinusoids. It seems likely that flow through some sinusoids may be intermittent, whereas others have relatively constant rates of blood flow.
Lastly, blood entering the hepatic venules is thought to pass through efferent or outlet sphincters which, like the inlet sphincters, are composed of sinusoidal endothelial cells. However, endothelin-1-stimulated contraction of postsinusoidal smooth muscle sphincters in sublobular veins may also play a key role in the regulation of sinusoidal blood flow.
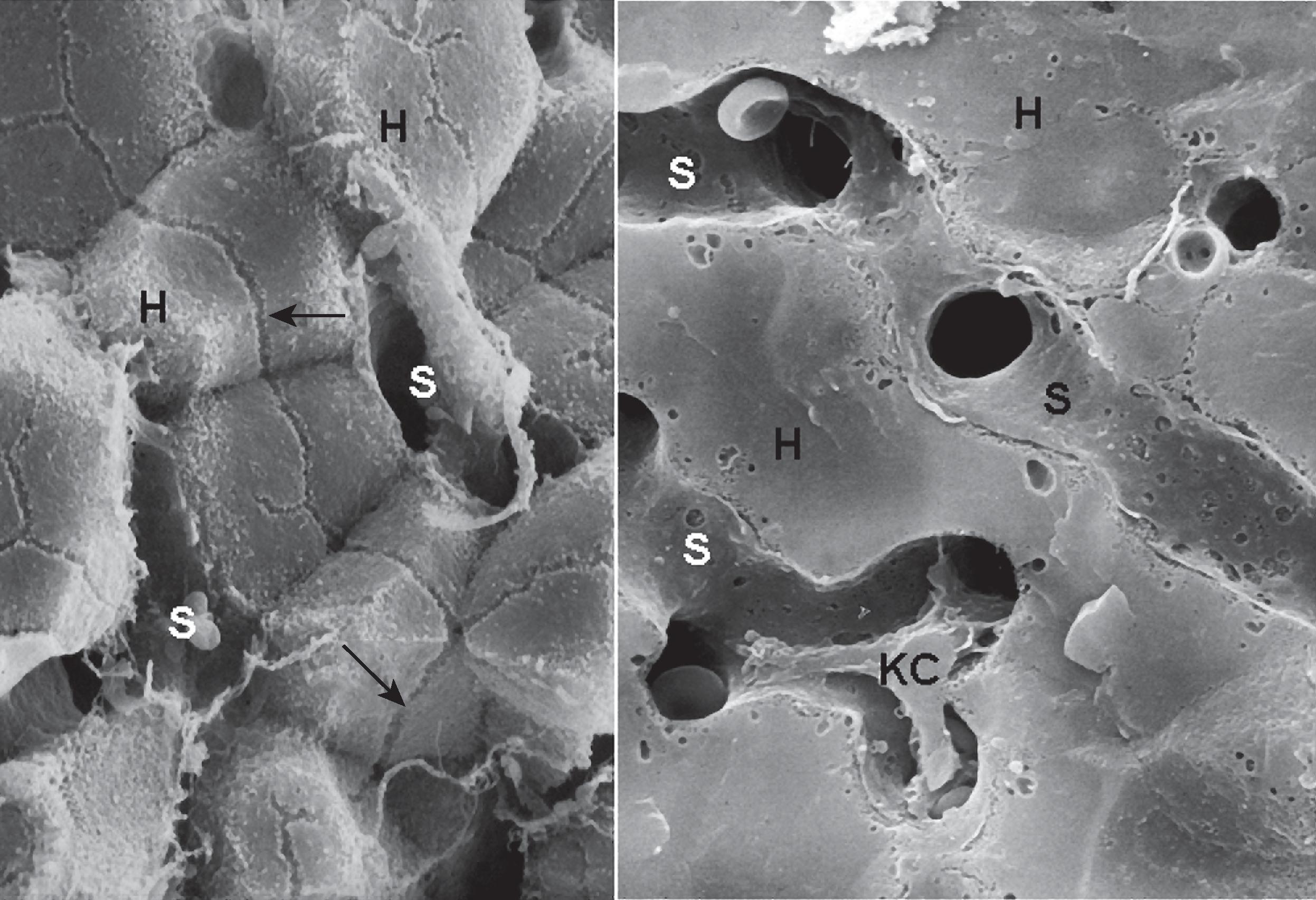
Hepatocytes are polyhedral cells approximately 20 to 30 µm in diameter, with a volume of approximately 5000 µm 3 . Their organization into anastomosing plates of the parenchyma is best illustrated by SEM ( Fig. 1.7 ). The liver cell plates anastomose extensively in the periportal region but become more simplified and radially oriented as they approach the terminal hepatic vein.
As with other epithelial cells, the hepatocyte is highly polarized ( Fig. 1.8 ). Within its plasma membrane, three specialized regions, or domains, are recognized: sinusoidal , which faces the sinusoid and the perisinusoidal space; lateral , facing the intercellular space between hepatocytes; and canalicular , bounding that specific part of the intercellular space constituting the bile canaliculus. , Using a more generic terminology, the sinusoidal and lateral domains constitute the basolateral plasma membrane of the hepatocyte, and the canalicular domain is the apical domain. This polarity is largely maintained by the tight junctions formed between adjacent hepatocytes, which delineate the basolateral domain from the canalicular domain and create a barrier between fluid in the intercellular space and bile in the canaliculus. , In addition to tight junctions, there are also gap junctions and desmosomes in the lateral domain, and it is across lateral-domain gap junctions that intercellular communication takes place. Stereological studies in the rat have shown that the basolateral, canalicular and lateral domains constitute approximately 70%, 15% and 15%, respectively, of the total cell surface area.
The domains of the hepatocyte plasma membrane are not simply topographical entities. They are specialized to serve different basolateral and apical functions of the hepatocyte. Molecular differences include composition of the lipids in the plasma membrane, the complement of membrane proteins, function of endocytic and exocytic compartments and relationship to the cytoskeleton. Beyond direct regulation of membrane protein function, hepatocytes can dynamically regulate actual membrane lipid content and the concentration of specific proteins in each domain, providing a powerful additional mechanism for functional control of plasma membrane function. ,
The sinusoidal surface of the hepatocyte faces the perisinusoidal space of Disse, the tissue space between hepatocytes and the endothelial sinusoidal lining cells. The hepatocyte sinusoidal surface is covered with abundant microvilli, each measuring 0.5 µm long but not evident, even as a brush or striated border, on optical microscopy. Microvilli may protrude through the fenestrae of the endothelial cells and into the sinusoidal lumen. The surface specialization is related here, as elsewhere, to absorptive and secretory activity; it obviously increases the surface area, but by a factor smaller than might be expected: approximately sixfold, compared with the 40- to 50-fold increase in surface area imparted by the microvilli of the small intestinal absorptive enterocyte. In the plasma membrane between the bases of the microvilli are small, surface indentations or pits. Some of these represent secretory vacuoles discharging fluid into the plasma by a process of exocytosis. Others are clathrin-coated pits involved in selective receptor-mediated endocytosis, termed ‘caveolae’, invaginated membrane microdomains enriched in cholesterol and sphingolipids and the cholesterol-binding protein caveolin, and responsible for selective membrane trafficking into the interior of the cell.
Hepatocytes along the limiting plate surrounding portal tracts have a surface which abuts the adjacent portal tract mesenchyme. These hepatocytes are irregularly covered with microvilli and may be moulded around connective tissue fibres, producing irregular indentations. The space of Mall is conceptualized as the fluid space between hepatocytes along the limiting plate in the periportal area and portal tract fibrous tissue; it is in continuity with the perisinusoidal space of Disse.
The lateral surface of the hepatocyte extends from the margin of the sinusoidal surface to the bile canaliculus and is specialized for cell attachment and cell–cell communication. Although simple in contour, the lateral surface is not entirely flat; microvilli may extend into it from the sinusoidal surface and protrude into narrow extensions of the space of Disse, and occasional folds (plicae) and round-mouthed openings may represent pinocytic vesicles. There are also knob-like protrusions and corresponding indentations; when fitted into one another, these would form the ‘press-stud’ or ‘snap-fastener’ type of intercellular attachment long familiar in transmission electron microscopy (TEM).
The lateral domain also has specialized areas called ‘gap junctions’ or, more accurately, ‘communicating junctions’. These are seen on TEM as patches of close approximation of the two membranes and in freeze-fracture preparations as irregularly shaped aggregates of particles on the inner leaflet (P face). The gap between the two membranes is 2–4 nm wide and is bridged by the intramembrane particles (of protein or lipid), which project like ‘bobbins’ from the external surface of each of the two membranes. Since each ‘bobbin’ is perforated by a central pore and apposed ‘bobbins’ are in contact, communications are established which provide for the transfer of ions or metabolites (or both) between hepatocytes.
The ‘bile canaliculus’ is defined as an intercellular space formed by the apposition of the edges of gutter-like hemicanals delimited by tight junctions, on the facing surfaces of adjacent hepatocytes ( Fig. 1.9A, B ). Canalicular diameter varies from 0.5 to 1.0 µm in the perivenular area and from 1 to 2.5 µm in the periportal zone, in accordance with flow of bile from the centrilobular region of the lobule toward the portal tract. The canalicular surface is unevenly covered by microvilli, which are more abundant along a ‘marginal ridge’ at each edge of the hemicanaliculus. In experimental biliary obstruction the canaliculi become dilated and the microvilli disappear, except along the marginal ridges. Intracellular subapical microfilaments are concentrated around the canaliculi, forming distinct, organelle-free pericanalicular sheaths and extending into the microvilli. This pericanalicular microfilament web is contractile, enabling hepatocytes to propel secreted bile along the canalicular channel. The presence of contractile elements in the pericanalicular zone can be demonstrated by indirect immunofluorescence ( Fig. 1.9C ). The presence of adenosine triphosphatase (ATPase) can be demonstrated histochemically; CD10 is a reliable immunostain for identifying the bile canaliculus ( Fig. 1.9D ). Brown-tinted accumulations of lipofuscin may also outline the canalicular pole of the hepatocyte, reflecting the presence of pericanalicular lysosomes, more evident in the adult liver because of the aging process. When present, lysosomal lipofuscin deposits are more prominent in perivenular hepatocytes.
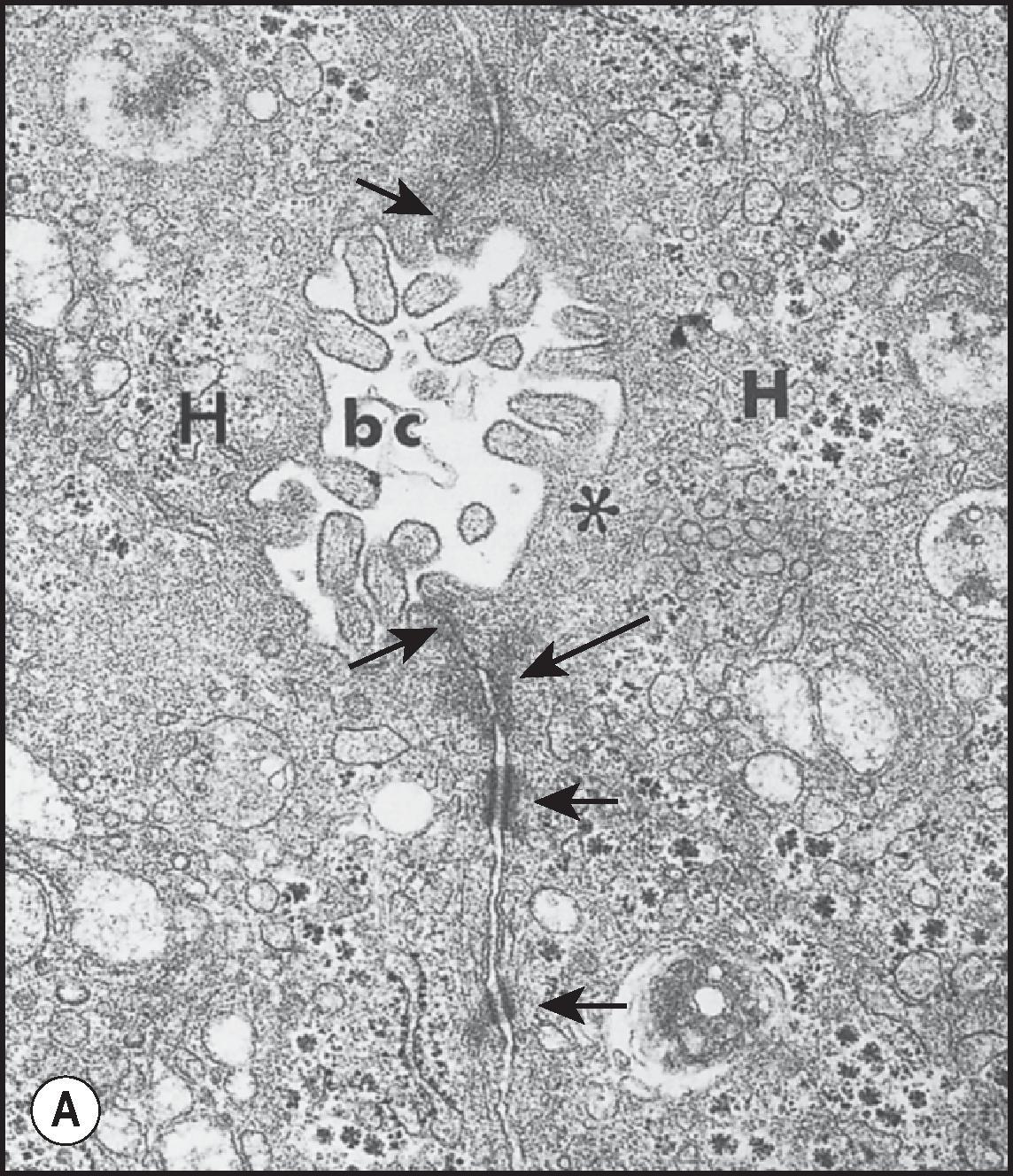
The canalicular surface is isolated from the rest of the intercellular surface by junctional complexes (see Fig. 1.9A ): desmosomes, intermediate junctions, tight junctions and gap junctions. The ‘tight junctions’ constitute a permeability barrier to macromolecules between the bile canaliculus and the rest of the intercellular space. ‘Tightness’, however, is a relative term; there seems to be a positive correlation between degrees of tightness and the number of strands forming the junction. On this basis, the canalicular tight junctions are comparable to those elsewhere in the body (e.g. rete testis, vasa efferentia) regarded as only ‘moderately tight’. Intriguingly, tight junction proteins are also involved in key regulatory functions at nonjunctional locations, such as hepatocyte differentiation, proliferation and migration.
The hepatocyte nucleus shows characteristics expected in the nucleus of a cell actively engaged in protein synthesis: large, occupying 5–10% of the volume of the cell; spherical, with one or more prominent nucleoli; and with scattered chromatin. The nuclear membrane is double layered and contains many pores ( Fig. 1.10 ). At birth, all but a few hepatocytes are mononuclear and of uniform size. In the adult liver there is considerable variation in both number and size of nuclei. About 25% of the adult hepatocytes are binucleate; the two nuclei are similar in size and staining properties. Hepatocyte nuclei fall into various sizes, with volumes in the ratio 1:2:4:8. This variation reflects polyploidy, with the DNA content increasing correspondingly. At birth in humans, almost all hepatocytes are diploid (and mononucleate). From the eighth year, when more than 90% of hepatocytes are diploid, the number of tetraploid nuclei (i.e. those with twice the normal DNA content) increases, to reach about 15% in children of 15 years and 40% by middle adulthood. , Tetraploid cells are thought to arise by mitosis of cells with two diploid nuclei. The DNA content of each nucleus doubles, but the chromosomes are then arranged on a single mitotic spindle, so that division produces two daughter cells, each with a single tetraploid nucleus. The significance of polyploidy in hepatocytes is unknown. Since cell size is proportional to cell ploidy, polyploidy does not provide an increased amount of genetic material per unit volume of cytoplasm.

Hepatocyte mitotic division provides for intrauterine and postnatal growth of the liver, which continues well into childhood. By adulthood the liver has a very low mitotic index, with estimates ranging from 1 mitosis per 10,000–20,000 cells to up to 2.2 mitoses per 1000 cells. Nevertheless, a high percentage of hepatocyte nuclei are euchromatic, indicating that transcription of most of the genome is occurring continuously. Almost all the DNA is in the extended configuration, and minimal heterochromatin is observed.
Conversely, hepatocytes engaged in protein biosynthesis have a large nucleolus (sometimes several) that can be recognized on light microscopy. The nucleolus is where ribosomal genes are located and where ribosome biogenesis occurs. Electron microscopy (EM) reveals the nucleolus to contain three main components: rounded fibrillar centres composed of thin, loosely distributed fibrils; a dense fibrillar component containing tightly packed fibrils that surround the fibrillar centres; and the granular component, constituted by granules which embed both fibrillar components. Ribosomal genes exist in an extended, ready-to-be-transcribed configuration within the fibrillar centres and partly in the dense fibrillar component. Although the precise location of ribosomal gene transcription remains unclear, newly transcribed RNA molecules undergo early processing and maturation in the dense fibrillar component and are assembled into preribosomes in the granular component. Protein-rich ribosomal subunits then exit the nucleus through pores in the double-membrane nuclear envelope.
The endoplasmic reticulum (ER) is a network of parallel, flattened sacs or cisternae on whose cytoplasmic surfaces may be attached polyribosomes to constitute the rough ER (RER) ( Fig. 1.11 ). Clusters of RER are scattered randomly throughout the hepatocyte cytoplasm and constitute approximately 60% of the ER. The remaining 40% is the smooth ER (SER), lacking a ribosomal coating. The SER also forms anastomosing networks of tubules and vesicles of varying diameters which are continuous with the cisternae of the RER. The outer nuclear membrane also has attached ribosomes and is continuous with the membrane of the RER. The SER is often found in the region of the Golgi apparatus and communicates with it. The SER frequently has a close topographical relationship with glycogen ( Fig. 1.12 ). The ER occupies 15% of the total cell volume, and its surface area—approximately 60,000 µm 2 per hepatocyte—is more than 35 times the area of the plasma membrane. There is also zonality in the distribution of the ER; the surface area of SER in the centrilobular area is twice that in the periportal zone. ,
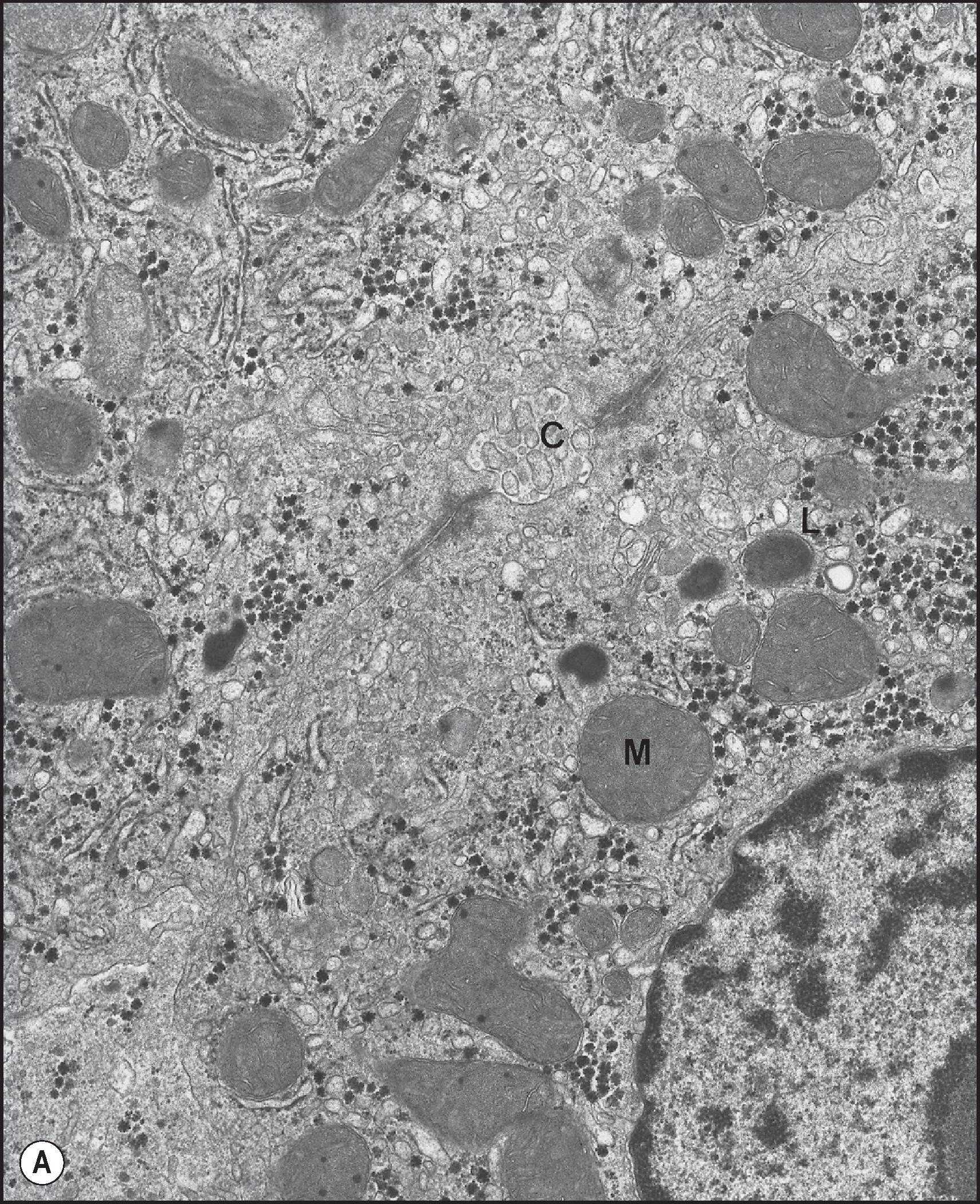
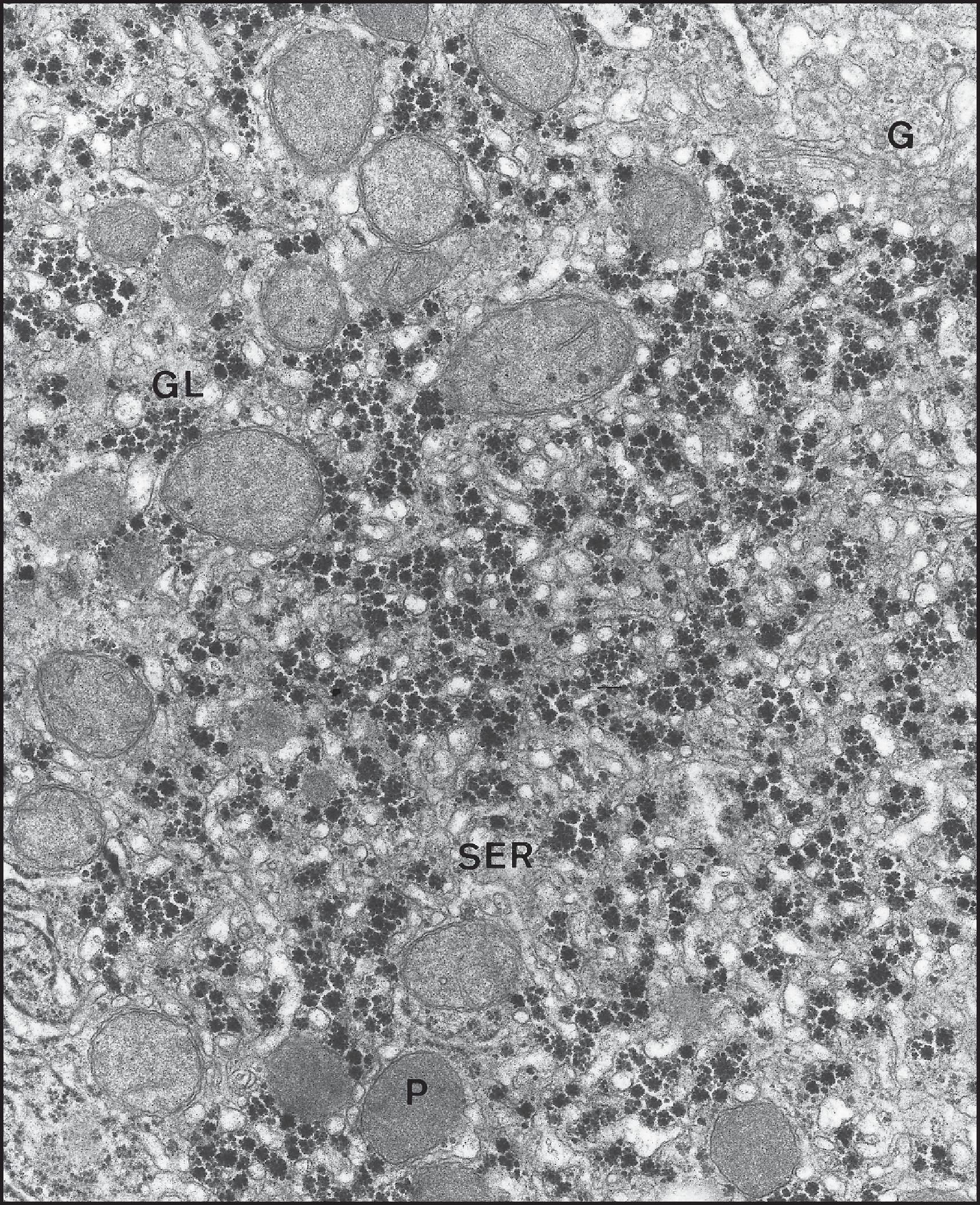
The cell functions associated with the ER include (1) protein synthesis, both of secretory proteins and some of the protein constituents of the cell and organelle membranes; (2) metabolism of fatty acids, phospholipids and triglycerides; (3) production and metabolism of cholesterol and possibly production of bile acids; (4) xenobiotic metabolism; (5) ascorbic acid synthesis; and (6) haem degradation. In the case of protein synthesis, polypeptides synthesized by RER ribosomes are retained in the membrane or are ejected into the lumen of the RER for folding and post-translational modification. The protein products pass through the SER to the Golgi apparatus for packaging and insertion into membranes throughout the cell (in the case of membrane proteins) or for exocytotic secretion across the basolateral or apical membranes of the hepatocyte.
The cytochrome P-450 system is localized in the ER membrane; this is the system whereby the liver cell metabolizes and detoxifies xenobiotics. This enzyme system can be reversibly induced by certain xenobiotics, such as phenobarbital, and this is accompanied by the synthesis and hypertrophy of the ER; the mechanisms involved in new membrane production are not clear. The preponderance of SER in the centrilobular region of the lobule and the presence of haem in cytochrome P-450 enzymes explain the darker hue of the centrilobular region, which can be observed by visual inspection of cut-liver sections.
Glucose 6-phosphatase is localized on the ER, playing a key role in dephosphorylation of intracellular glucose 6-phosphate before release of glucose into the circulation by hepatocytes. As a correlate, the SER proliferates during synthesis of glycogen and thus is available for hepatocyte glycogenolysis when hepatocellular release of glucose is required for metabolic needs elsewhere.
Each hepatocyte contains as many as 50 Golgi zones (which may not be separate but rather form a tridimensional continuity) situated most frequently beside the nucleus or in the vicinity of the bile canaliculus. Each complex appears as a stack of four to six curved, flattened parallel sacs, often with dilated bulbous ends containing electron-dense material. The convex or cis surface is directed toward the ER, and small vesicles in the cis -Golgi transfer synthesized proteins from the ER. The concave or trans surface is the origin of secretory vesicles. Vesicles break off from the ends of the sacs and carry the contained secretory proteins, including lipoproteins, for discharge at the sinusoidal surface or less often at the canalicular surface. Membrane proteins destined for insertion into any of the plasma membrane domains also are routed through the Golgi complex. The complex and its associated cytoplasm constitute approximately 2–4% of the cell volume. In addition to its role in the secretion of proteins, the Golgi complex has a large complement of glycosylating enzymes, important in the glycosylation of secretory proteins and in the synthesis and recycling of membrane glycoprotein receptors. The Golgi complex is capable of rapid and reversible structural reorganization into a tubuloglomerular network while maintaining its biosynthetic capabilities. With the SER, RER, lysosomes, other intermediate organelle compartments and even the nuclear and mitochondrial envelope membranes, the Golgi is an integral part of the complex intracellular organelle network involving vesicular trafficking that enables uptake, sorting, degradation, biosynthesis, trafficking and secretion of cellular proteins and lipids. , ,
The existence of lysosomes was first predicted by De Duve on the basis of biochemical studies on liver homogenates. He subsequently identified them as the ‘peribiliary dense bodies’ found in electron micrographs of liver and established them as a new species of cell organelle. Their functions in health and disease have been reviewed , and are of particular importance to pathologists because of their involvement in several storage diseases (see Chapter 3 ).
Lysosomes present a variety of appearances in electron micrographs of liver and may be part of the intracellular membranous network known as the ‘GERL’ (Golgi-SER-lysosome). The GERL is involved with endocytosis and exocytosis, serving as a site for sorting of secretory proteins for secretion and for trafficking of endocytosed proteins to lysosomes for degradation. Indeed, the GERL is the site where the lysosomal enzyme acid phosphatase makes its first appearance, most likely playing a role in formation of lysosomes.
Several classes of lysosomes can be identified within the hepatocyte cytoplasm: (1) primary lysosomes , small in size, are considered to be functionally in a resting phase; (2) secondary lysosomes are functionally activated and delimited by a single membrane (see Fig. 1.11 ); (3) autophagic vacuoles contain parts of the degrading cytoplasmic organelles and are often delimited by a double membrane; and (4) residual bodies are larger than primary and secondary lysosomes and are usually more numerous in older individuals. The residual bodies contain the residues of nondigested material or pigments such as lipofuscins (considered undigestible permanent residues). Lipofuscin granules are the most numerous lysosomal bodies present in human hepatocytes.
Lysosomes are frequently found near the plasma membrane proximal to the bile canaliculus and can discharge their contents into the biliary space. The lysosomes in periportal hepatocytes are often larger and more positive for acid phosphatase than those in centrilobular hepatocytes. ,
Lysosomal pleomorphism reflects a variety of functions. First, although the liver cell is long-lived, there is evidence for turnover of its cytoplasm and organelles. Cytoplasmic constituents may be incorporated within and digested by the primary lysosome, forming an autophagic vacuole, then forming a secondary lysosome. Autophagic vacuoles therefore show fragments of organelles or cell inclusions in various stages of digestion. Second, lysosomes also incorporate lipofuscin pigment, which may accumulate undigested over long periods, forming residual bodies; material of exogenous origin, including iron, stored as ferritin, which accumulates in large quantities in iron overload states; and copper, which accumulates in copper-overload conditions and cholestasis. Third, coated vesicles and multivesicular bodies result from receptor-mediated endocytosis. In a complicated sequence of intracellular events, ligand–receptor complexes in clathrin-coated pits on the basolateral cell plasma membrane are internalized to form endocytic vesicles, or endosomes . Soluble ligands which are internalized in this way include insulin, low-density lipoproteins, transferrin, immunoglobulin A (IgA) and asialoglycoproteins. Fusion of endosomes occurs to form multivesicular bodies. Some of these vesicles are responsible for transcytosis or intracellular transport from the basolateral domain to the canalicular domain. Others fuse with primary lysosomes, and their contents undergo partial degradation before being exocytosed at the canalicular or basolateral domain. Still other vesicles undergo complete degradation and become increasingly electron dense with the formation of dense bodies. , Microtubules appear to have an important role in sorting the pathways along which endocytic vesicles move within the hepatocyte.
Peroxisomes are single membrane-bound ovoid granules 0.2–1.0 µm in diameter (see Fig. 1.12 ). They were first described as ‘microbodies’ by Rouiller and Bernhard in 1956. The properties of peroxisomes in liver have been reviewed elsewhere. Each hepatocyte may contain 300–600 peroxisomes, and they comprise 1.5–2% of cell volume. Peroxisomes may be more numerous in perivenular hepatocytes, but they are generally homogeneously distributed within the hepatic lobule. , There is morphological heterogeneity between species; in the rat, peroxisomes contain a paracrystalline striated core or nucleoid in which urate oxidase is concentrated; human peroxisomes lack a core. Peroxisomes contain oxidases that use molecular oxygen to oxidize a number of substrates with the production of hydrogen peroxide (thus the name of the organelle), which in turn is hydrolysed by peroxisomal catalase. Approximately 20% of the oxygen consumption of the liver is used in peroxisomal activity. The energy produced by this oxidation is dissipated as heat. An alcohol overload may be metabolized in the liver by peroxisomal catalase. Drugs, such as clofibrate, which lower blood lipids cause a proliferation of peroxisomes, an increase that has been causally linked to the hypolipidaemic action. Alterations in hepatocyte perixosomes have been reported in bacterial infections, viral hepatitis, Wilson disease and alcoholic liver diseases (ALDs). , Various metabolic disorders have been described in which there is either an absence of peroxisomes or a deficiency of peroxisomal enzymes , (see Chapter 3 ). Peroxisomes have become of particular interest owing to their critical role in hepatic lipid metabolism and their contribution to hepatocellular function or dysfunction in nonalcoholic fatty liver disease (NAFLD).
Mitochondria are large organelles (1.5 µm in diameter and up to 4 µm long) that number approximately 1000–2000 per hepatocyte and constitute about 20% of the cytoplasmic volume of hepatocytes. Mitochondria are the site of oxidative phosphorylation of adenosine diphosphate (ADP) to adenosine triphosphate (ATP), constituting the source of cellular aerobic metabolism. Longer (up to 4 µm) and larger (up to 1.5 µm in diameter) mitochondria are more numerous in periportal hepatocytes. Mitochondria are bounded by an outer and an inner membrane, each 5–7 nm thick (see Fig. 1.11 ). The outer membrane possesses special pores that allow the passage of molecules smaller than approximately 2000 daltons. The inner membrane’s surface area is greatly increased by the presence of numerous cristae, which fold within the mitochondrial matrix. The space between inner and outer membranes presents a low-density matrix and ranges from about 7 to 10 nm in thickness. Mitochondria have a relatively low-density matrix in which lamellar or tubular cristae and a variable amount of small, dense granules can be observed; these granules may represent a concentration of calcium ions (Ca 2+ ), a small circular DNA and ribosomes. The dense granules have a diameter of 20 to 50 nm. In addition, filaments of circular mitochondrial DNA about 3–5 nm in width and granules approximately 12 nm in diameter containing mitochondrial RNA are also present. The DNA codes for some of the mitochondrial proteins are synthesized in ribosomes within the organelle, but most of the mitochondrial protein is encoded by nuclear DNA. Mitochondria are self-replicating and have a half-life of approximately 10 days. Mitochondria may fuse and are remarkably mobile organelles, moving about in the cytoplasm and closely associated with microtubules.
Mutations in the mitochondrial genome account for various mitochondrial myopathies and may also play a role in development of NAFLD. Indeed, it has become apparent that specific biochemical abnormalities of mitochondria may play an important role in the pathogenesis of certain liver diseases and that genetic defects in mitochondrial proteins and enzyme systems may be the underlying cause of other liver and metabolic diseases. Because mitochondria possess a distinct and unique extranuclear genome, a new class of maternally, or mitochondrially, inherited diseases have emerged (see Chapter 3 ).
The structural compartmentation of mitochondria provides for topographical localization of various enzyme systems, the details of which form almost a science in themselves. It need only be said here that the outer membrane is relatively unimportant as a locus for enzymes; it keeps the inner membrane together and contains porin , a transport protein that forms channels permeable to molecules of less than 2000 daltons. The inner membrane and crystal lamellae support the respiratory chain enzymes involved in oxidative phosphorylation, which generates ATP. The matrix contains most of the components of the citric acid cycle and the enzymes involved in β-oxidation of fatty acids and in the urea cycle. Mitochondria are randomly distributed within individual hepatocytes but are larger and more numerous in periportal cells, corresponding with the greater levels of oxidative respiration and metabolism in the periportal region.
The cytoplasm of any cell is a highly concentrated matrix of proteins and microfilaments, within which organic and inorganic solutes diffuse. , Movement of larger components through the matrix, especially membrane-bound vesicles, involves directed transport along the cytoskeletal fibres, the composition of which is described in the next section. A hepatocyte is extremely rich in non-membrane-bound cytoplasmic inclusions, including glycogen granules, lipid droplets and various pigments. Glycogen granules are the most abundant and on EM may occur in the monoparticulate form (β particles, 15–30 nm in size) or more frequently as aggregates of smaller particles arranged to form ‘rosettes’ (α particles; see Fig. 1.12 ). Glycogen granules are dispersed in the cytoplasm but are often associated with the SER. Intranuclear glycogen usually appears as β particles. Glycogen is depleted during fasting, disappearing first from periportal hepatocytes and then from perivenular cells. On refeeding the sequence reverses. In this manner, hepatocytes constitute a major metabolic energy reserve during fasting, thus supporting systemic glucose homeostasis.
Lipid inclusions appear as empty vacuoles in histological sections or as osmiophilic droplets on TEM and usually are not surrounded by membranes. Lipid droplets consist of triglycerides in their interior and are coated with a monolayer of phospholipids. Small lipid droplets have a high surface/volume ratio and are accessible to cytoplasmic lipases, which may degrade the retained triglyceride quickly. Large lipid droplets have a low surface/volume ratio and may reside in hepatocytes long after the metabolic processes in their deposition have subsided.
A variable number of iron-containing granules is often present within the hepatocyte cytoplasm, depending heavily on the iron status of the host. These are usually in the form of ferritin particles. With an approximately spherical shape, the iron-containing protein ferritin consists of a protein shell (apoferritin) 11 nm in diameter and an iron-containing central core approximately 5 nm in diameter. Hepatocyte iron deposits may also occur as single membrane-bound lysosomal bodies (residual bodies), forming aggregates of iron-containing electron-dense particles (siderosomes, haemosiderin granules). In addition to hepatocytes, liver endothelial cells and Kupffer cells also accumulate intracellular iron under conditions of iron overload.
The major components of the cytoskeleton of most eukaryotic cells comprise 6-nm-diameter microfilaments , 8–10-nm-diameter intermediate filaments (IFs) and 20-nm-diameter microtubules ; the definitive review of their structure and function in the hepatocyte remains that of Feldmann. These are structurally, chemically and functionally distinct, linear macromolecules coursing through the cytoplasm. IFs are relatively stable macromolecules capable of modulation measured in minutes and longer intervals. Microfilaments and microtubules are highly dynamic structures capable of rapid polymerization and depolymerization on a second-to-second basis and thus rapid adaptation in response to functional demands. For all these macromolecules, polymerization and depolymerization of their constituent molecules are under the influence of various intracellular factors, including free Ca 2+ ions, high-energy compounds and associated proteins. In addition, various accessory proteins modulate these components and link them to one another, to cell organelles and to the cell membrane; these are part of a microtrabecular lattice, or cytomatrix. These structures interact to regulate internal organization, cell shape, movement, division, secretion, metabolism and intercellular communication.
Microfilaments are double-stranded molecules of polymerized fibrous (F) actin; the monomeric form of the protein is globular (G) actin; and these two forms exist in equilibrium in the cell. The microfilaments are present in bundles and form a 3D intracellular meshwork. There is extensive intracellular binding and cross-linking with other intracellular proteins, such as myosin, lamin and spectrin. The filaments are mainly located at the cell periphery; they attach to the plasma membrane and extend into microvilli. They are particularly concentrated in the pericanalicular zone, forming a pericanalicular web, and attach to the junctional complexes which limit the canaliculus. Four main functions are postulated for the contractile microfilaments of the hepatocyte: (i) translocation of intracellular vesicles implicated in bile secretion, especially by insertion and removal of canalicular plasma membrane transport proteins; (ii) coordinated contraction, producing peristaltic movement in the canaliculus ; (iii) with microtubules, transmembrane control over the topography of intrinsic proteins in the phospholipid bilayer of the cell membrane, thus influencing the protein mosaic and functional differentiation of a particular membrane domain ; and (iv) possible modulation of the structure and tightness of the ‘tight junction’, thus regulating the permeability of the paracellular pathway. , The functional roles for microfilaments involve cell membrane motility, endo- and exocytosis, secretion and vesicle transfer.
Microtubules are a family of unbranched rigid tubules of variable length that are structurally similar in all cells. These polymers are composed of two subunits of tubulin, α and β. Polymerization and growth take place from organizing centres, including centrioles. Microtubules are part of the mitotic apparatus and are therefore important in cell division. They are also present in cell cilia. As with the microfilaments, microtubules attach to and cross-link a number of proteins. Microtubules are involved in the blood-bound secretion of several liver cell products, including lipoprotein, albumin, retinol-binding proteins (RBPs), secretory component, fibrinogen and other glycoproteins. , As a cytoskeletal framework, microtubules play a role in the intracellular translocation of vesicles containing IgA and horseradish peroxidase.
IFs are a family of self-assembling protein fibres. They are structurally similar in all cells, with component polypeptides arranged in an α-helical coiled-coil arrangement which confers tensile strength. These filaments comprise the central scaffold of cells, imparting structural stability to the 3D intracellular architecture. Unlike the microtubules and microfilaments, which are specifically composed of their respective subunit classes, IFs are strikingly heterogeneous in subunit composition and antigenicity. IFs are grouped into five general types —keratin, desmin, vimentin, glial fibrillary acidic protein (GFAP) and neurofilaments—the distribution and expression of which are highly specific to cell type.
Keratins (or cytokeratins) are the IFs of epithelial cells and are present in hepatocytes and, in greater amounts, in bile duct epithelium. In hepatocytes, keratins are located just inside the plasma membrane and are particularly condensed as a pericanalicular sheath that extends into desmosomes. They are linked to desmosomes on the lateral plasma membrane of hepatocytes, providing scaffolding for the bile canalicular region. Keratins also attach to other components of the cytoskeleton, which includes serving as anchors for the contractile activities of microfilaments. Keratins also attach to organelles such as the RER and vesicles.
Keratins function as ‘the mechanical integrators of cellular space’, since their firm attachment to desmosomes (which in turn attach to desmosomes on adjacent hepatocytes) provides an integrated continuity of stable 3D architecture across a multicellular region. In the hepatocyte, keratins maintain structural polarity, provide a scaffolding for the bile canaliculus and provide a framework for the distribution of actin and endocytotic vesicles along the plasma membrane. , In disease states the hepatocellular keratin network, in particular, may become highly disrupted, as discussed later.
Sinusoids form a complex vascular network, transporting mixed, nutrient-rich venous and oxygen-rich arterial blood from the terminal portal vein to the hepatic vein branches. The sinusoids have an average diameter of about 5–10 µm ( Fig. 1.13 ), but they may distend to about 30 µm. As noted earlier, periportal sinusoids (zone 1) are more tortuous than the perivenular ones (zone 3). These are special vascular channels that pass between each hepatocyte plate, maximizing contact of blood and its contents with the parenchymal elements of the liver ( Fig. 1.14A ). Sinusoids differ from other capillaries in several features because they possess (1) a fenestrated endothelium, without basal lamina; (2) resident macrophages, the Kupffer cells, which bulge into the sinusoidal lumen; (3) special intraluminal resident lymphocytes; and (4) HSCs, considered pericytes, lying in the space of Disse subjacent to the sinusoidal endothelium, which store vitamin A and can transform into myofibroblasts. The space of Disse also contains sparse, loose extracellular matrix (ECM) in normal conditions and a few nerve endings, all elements in close contact with microvilli of the hepatocyte plasma membrane. The sinusoids can become capillarized in the chronically diseased liver ( Fig. 1.14B ), as discussed later.
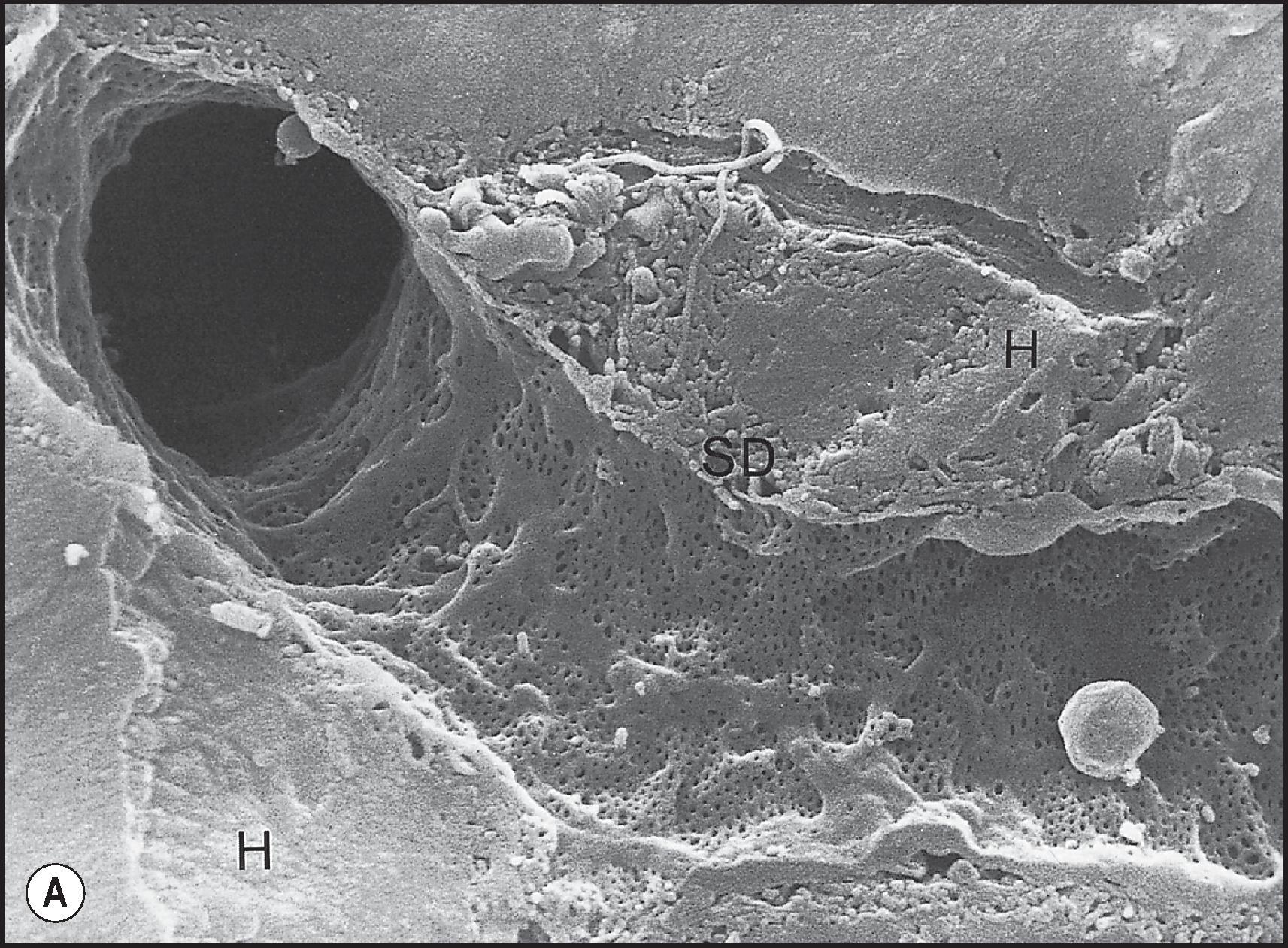
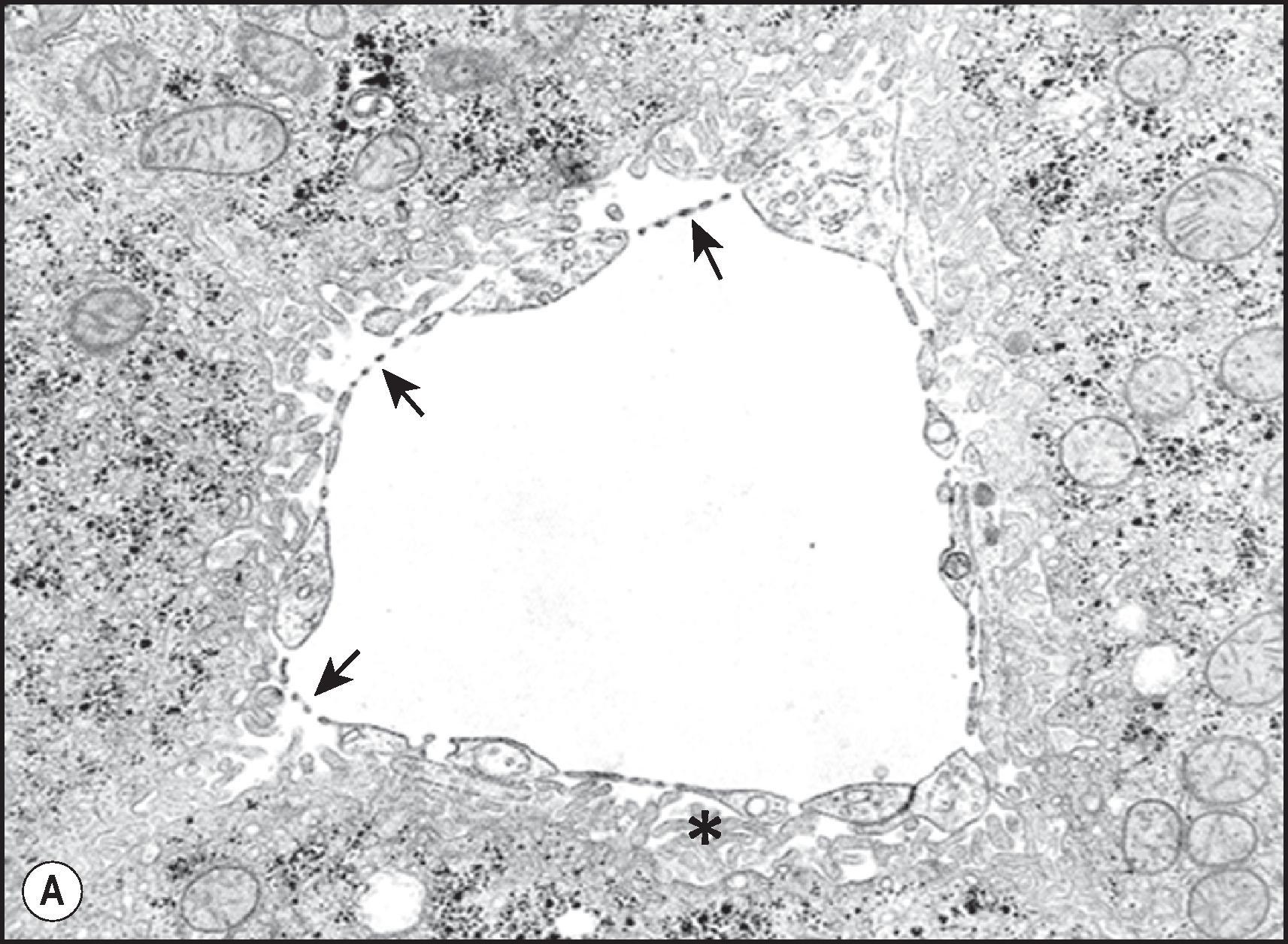
Four distinct types of sinusoidal cells can be identified ( Fig. 1.15 ), each with its own characteristic morphology, topography and population dynamics. All sinusoidal cells represent about 6% of the lobular parenchyma volume (2.5%, 2.0% and 1.4% for endothelial, Kupffer and stellate cells, respectively) and 26.5% of all the liver plasma membranes. The sinusoidal lumens occupy approximately 10% of the lobular parenchymal volume. The unique characteristics of sinusoids and sinusoidal cells in the normal liver explain their major role in facilitating exchange between blood and hepatocytes, in intercellular communication and in ECM deposition, inflammation and liver immunity. All these functions have major consequences in liver pathology.
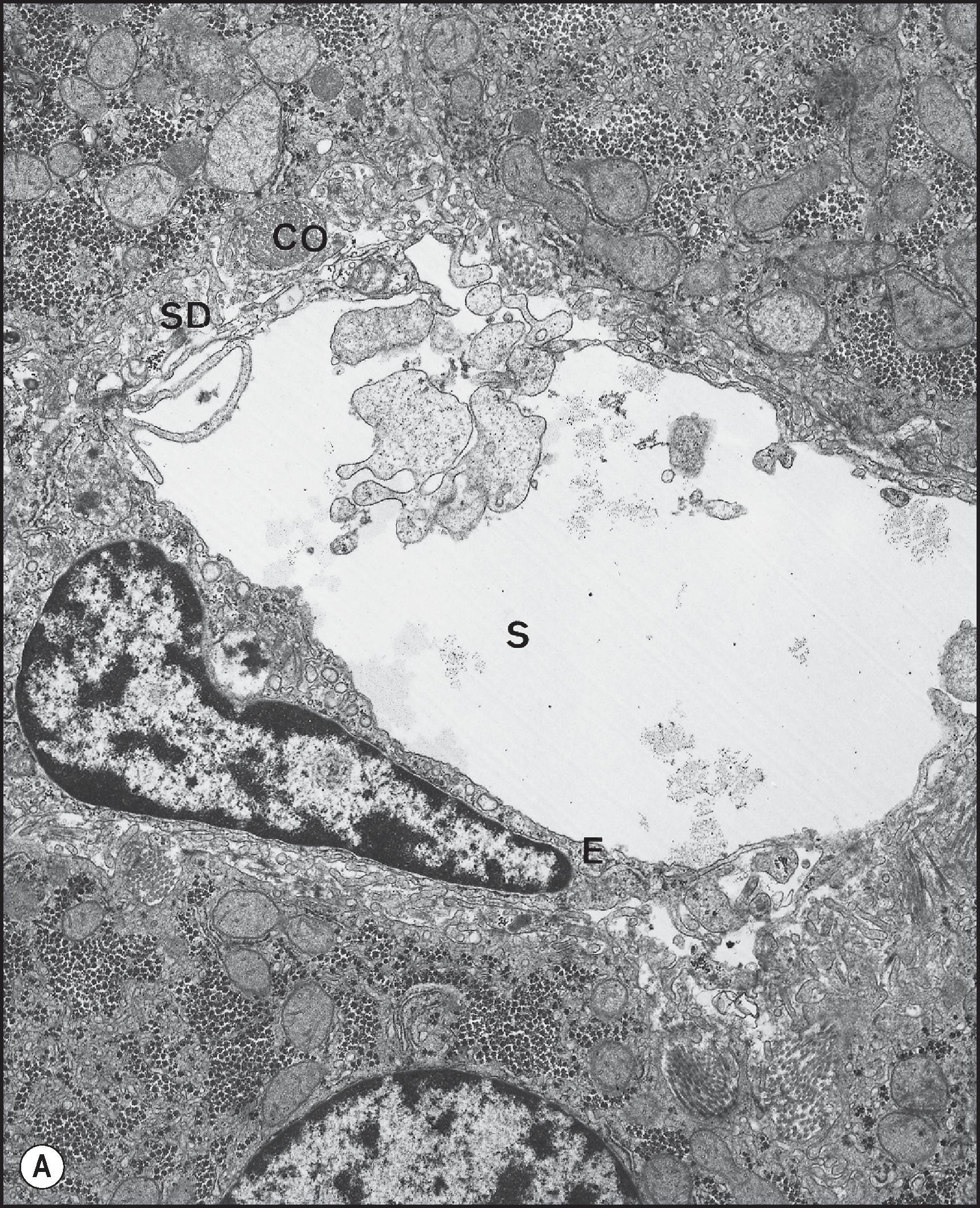
Liver sinusoidal endothelial cells (LSECs) originate from bone marrow and form a highly dynamic barrier between the vascular space and underlying hepatocytes. There is no underlying basal lamina of the sinusoidal endothelium; instead, LSECs rest on a delicate mesh of ECM that supports the LSECs but does not block fluid or solute movement. The main characteristics of LSECs are (1) their smooth, thin, attenuated cytoplasmic sheet, about 150–170 nm in maximum thickness ; (2) the numerous holes (fenestrae) that perforate the cytoplasmic sheet, facilitating exchange between blood and hepatocytes; and (3) their numerous intracytoplasmic pinocytotic vesicles, indicating a high capacity for endocytosis (see Figs 1.13B and 1.15A ). The cytoplasmic extensions of LSECs may overlap, but there are no intercellular junctions (e.g. endothelial adherence, tight) otherwise found in vascular endothelial cells. Under appropriate stimuli, inflammatory cells can undergo ready diapedesis (migration) from the vascular compartment into the space of Disse.
Fenestrae are open transcellular pores without a diaphragm. They are so abundant that on SEM the greater part of the cell has a net-like appearance, forming a tenuous barrier, reinforced where adjacent endothelial cells overlap (see Fig. 1.13B ). The fenestrae are dynamic structures that may vary greatly in size but generally fall into two size categories: small fenestrae (0.1–0.2 µm in diameter) grouped in clusters, forming ‘sieve plates’, and large fenestrae (up to 1 µm in diameter), which are more numerous at the distal end of the sinusoid. Thus, endothelial cell porosity is higher in the perivenular zone than in the periportal zone. Fenestrae are delineated by fibrillar cytoskeleton elements which could be actin microfilaments; however the exact function of this actin ring surrounding fenestrae and its role in fenestrae dynamics remain under study. Recently, method of STED (stimulated emission depletion) super-resolution microscopy provided the ability to observe, quantify fenestrae, study their molecular composition and analyse LSEC live dynamics. , In vitro studies have been shown that actin-binding drugs, which stabilize or disassemble actin microfilaments, promote an increase in fenestrae formation. There is evidence that fenestrae are labile, dynamic structures (2% to 20% of the SEC surface area) whose diameter may change in response to endogenous mediators (e.g. serotonin) and exogenous agents such as alcohol.
This unique porous structure allows the endothelial cells to filter coarsely the sinusoidal blood. Solutes, including macromolecules, pass freely through the fenestrae from the lumen into the space of Disse and come into contact with the basolateral plasma membrane of hepatocytes. Conversely, macromolecules secreted by hepatocytes pass freely into the vascular space. However, large particles (e.g. intact chylomicrons) and formed blood elements are excluded. With the fenestrated, nondiaphragmatic structure of LSECs and the absence of an abluminal basal lamina, the liver sinusoid is the most porous of all endothelial barriers.
The underlying ECM also modulates the fenestrae of the LSECs, especially under disease conditions. For example, at the earliest stage of liver fibrosis, when ECM is initially deposited in the space of Disse, a capillarization of sinusoids is observed, corresponding to loss of endothelial fenestrae and formation of a continuous basement membrane (see Fig. 1.14B ). Such phenotypic changes occur also with aging. This decreased porosity impairs the passage of triglycerides from plasma to hepatocytes for degradation, leading to increased risk of systemic atherosclerosis. Thus, alterations in the number or diameter of endothelial fenestrae in liver diseases have important implications for hepatic microcirculation and function.
In addition, fenestrae play a role in the formation of hepatic lymph, representing half of the total lymph generated in the body. Filtered plasma entering the space of Disse flows in a retrograde manner back to the portal tracts, entering there into lymphatic channels for drainage through the hepatic hilum en route to the thoracic duct.
Another striking feature of LSECs is their high endocytotic activity. , This process appears to be used for uptake and lysosomal degradation of plasma macromolecular solutes, rather than providing an alternative route for their transport from the sinusoidal lumen to the space of Disse. A large number of endogenous compounds may be endocytosed; some are effete molecules and are cleared from the circulation, and others are modified and do appear to undergo transcytosis to hepatocytes, perhaps in a more selective fashion than macromolecular solutes passing only through the fenestrae. This includes removal and degradation of soluble immune complexes, as well as removal of potentially dangerous macromolecules. LSECs have also been shown to store and metabolize serum immunoglobulins and to remove hyaluronic acid (HA)/chondroitin sulphate proteoglycans from the circulation. Numerous scavenger receptors have been identified (mannose receptor, Fc-γ receptor IIb2, other endocytosis receptors). Thus the LSECs are highly effective scavenger cells. LSEC endocytic uptake of solutes may also stimulate intercellular signalling to hepatocytes, playing a major role in homeostasis and immunity.
The LSECs have a predominantly anaerobic metabolism, reflected by a low number of mitochondria and production of high levels of lactate. They have limited but profound biosynthetic activity, producing nitrous oxide (NO), endothelins, prostaglandins and possibly cytokines such as interleukin-1 (IL-1) and IL-6, all of which have potent effects on vascular tone and the functions of nearby cells. Through both the generation of NO and the influence of their secreted cytokines on the contractile function of underlying stellate cells (see later), LSECs play a key role in the regulation of sinusoidal blood flow. LSECs are subject to damage by some hepatotoxins. For example, LSECs are the initial target of some hepatotoxic drugs (e.g. acetaminophen) and toxins, which occurs before hepatocyte injury. Direct toxic damage to LSECs also is the initiating event in sinusoidal obstruction syndrome (SOS), in which endothelial cell debris occludes the vascular space, with life-threatening consequences.
LSECs show a number of phenotypic differences from other vascular endothelium as well as other types of liver cells, some of which can be used as LSEC markers. Normal LSECs do not bind the lectin Ulex europaeus and, in most species, do not express factor VIII-related antigen (von Willebrand factor [VWF]). Furthermore, LSECs contain absent to low levels of other molecules characteristically found in vascular endothelium, such as E-selectin, CD31 (PECAM) and CD34, but do express Fc IgG receptors (CD16 and CDw32), CD4, CD14 and aminopeptidase N. CD34-positive LSECs are restricted to a few periportal sinusoids, staining the cell body and processes ( Fig. 1.16A ). The cell bodies of CD34-negative LSEC are immunoreactive for stabilin-2 (stab-2), a HA-binding protein ( Fig. 1.16B, C ). Therefore, CD34 and stab-2 are mutually exclusive markers of LSEC in the normal liver. Under conditions promoting sinusoidal capillarization, a greater proportion of LSECs become CD34-positive.
LSECs exhibit membrane immunoreactivity for intercellular adhesion molecule 1 (ICAM1). The natural ligand for this adhesion molecule, lymphocyte-associated antigen 1 (LFA1), is present on Kupffer cells; this LSEC receptor may therefore be involved in the adhesion of Kupffer cells to the endothelial lining. Upregulation of ICAM1 expression in LSECs may also be important in ‘trapping’ LFA1-positive lymphocytes in inflammatory liver diseases.
There is heterogeneity of LSECs along the acinar axis. In addition to the increased porosity in the perivenular zone previously mentioned, variation in cell size, heterogeneous lectin binding and expression of various receptors, cytoplasmic density, endocytic capacity and surface glycosylation have also been demonstrated. , , ,
In summary, as a fenestrated barrier, LSECs substantially influence the trafficking of molecules and cells between liver parenchyma and blood. They have major functions in hepatic homeostasis, including plasma ultrafiltration, regulation of hepatic microcirculation and vascular tone, inflammation and thrombosis. In addition, they are essential for control of the hepatic immune response. ,
Kupffer cells, present in the lumen of hepatic sinusoids (see Fig. 1.15C ), can be identified with monoclonal antibodies such as CD68. Kupffer cells are specialized liver macrophages, representing the largest population of resident tissue macrophages. They belong to the mononuclear phagocytic system but manifest phenotypic differences which distinguish them from other macrophages. Kupffer cells are of considerable importance in host defense mechanisms; their inflammatory response can be considered as a main mediator in liver injury and repair. Indeed, all of the liver macrophages (resident Kupffer cells and exogenous monocyte-derived macrophages) have an important role in both liver homeostasis and the pathogenesis of various liver diseases, giving opportunity for therapeutic targeting of these cells under appropriate circumstances.
On SEM, Kupffer cells have an irregular stellate shape, and their luminal surface shows many of the structural features associated with macrophages, such as small microvilli, microplicae and sinuous invaginations of the plasma membrane. Kupffer cells bulge into the sinusoidal lumen. Their cell body rests in contact over a more or less large area on the endothelial lining ( Fig. 1.17 ), but they never form junctional complexes with endothelial cells. However, Kupffer cells may be found in gaps between adjacent endothelial cells, and their protoplasmic processes may extend through the larger endothelial fenestrae into the perisinusoidal space of Disse.
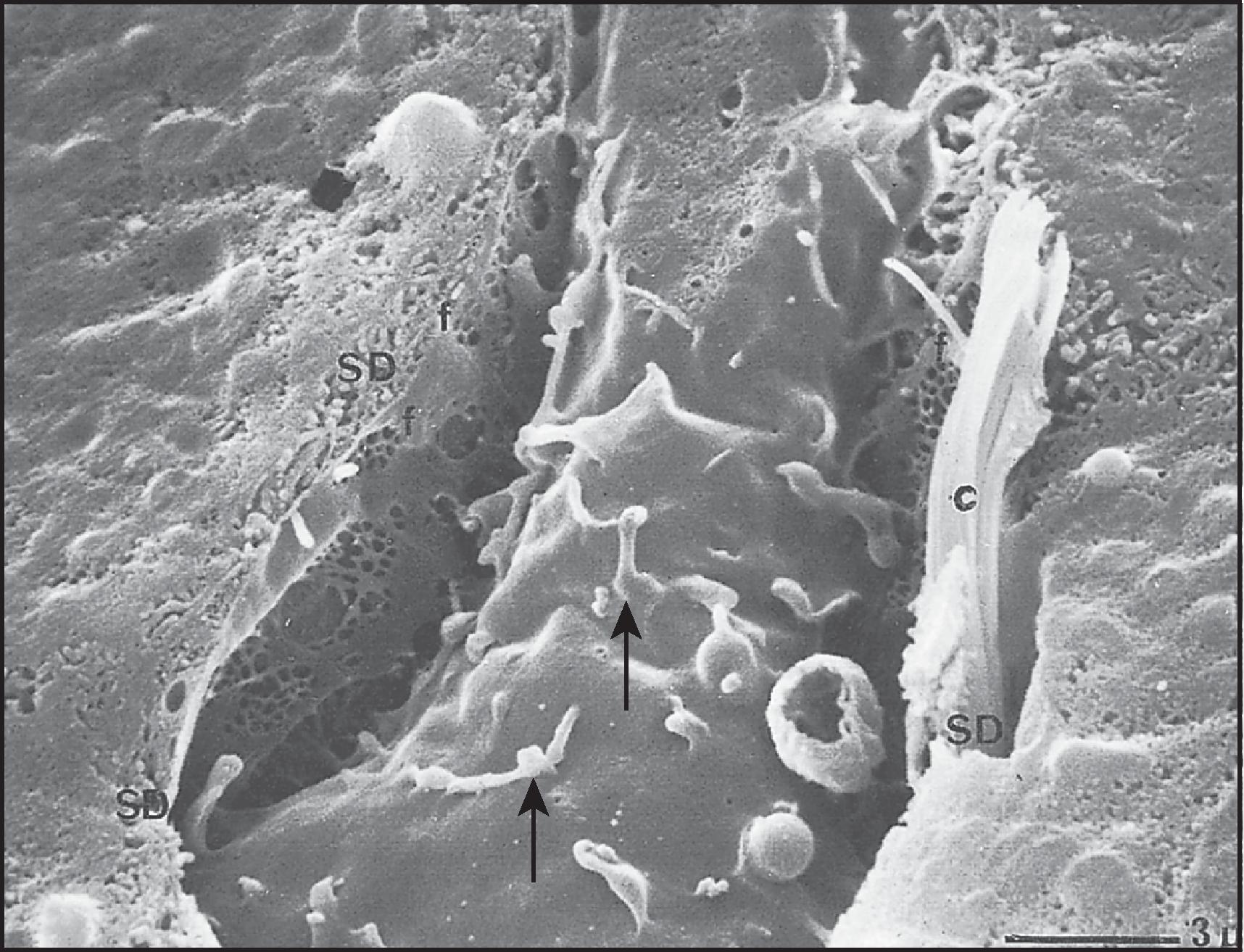
Kupffer cells are more numerous in the periportal sinusoids, and as noted earlier, there is some evidence that, as with hepatocytes and LSECs, Kupffer cells also manifest functional heterogeneity in the lobule, with different subsets distributed through lobular zones. Kupffer cells have been considered to be fixed tissue macrophages, but they appear capable of actively migrating along the sinusoids, both with and against the blood flow. They can migrate into areas of liver injury and into regional lymph nodes. Because of their location and shape, Kupffer cells can interact with blood and cells as they transit.
Kupffer cells contain numerous lysosomes (almost one-quarter of all lysosomes of the liver) and phagosomes, and the cisternae of their ER are rich in peroxidase. Their primary functions include the removal, by ingestion and degradation, of particulate and soluble material from the portal blood, in which they discriminate between ‘self’ and ‘non-self’ particles. They act as scavengers of microorganisms and degenerate normal cells (e.g. effete erythrocytes), circulating tumour cells and various macromolecules. These functions are in part carried out nonspecifically, but Kupffer cells are also involved in the initiation of immunological responses and the induction of tolerance to antigens absorbed from the gastrointestinal tract. The efficiency of this clearance function is shown by the fact that removal of particulate material is limited only by the magnitude of hepatic blood flow; removal of particles may approach single-pass efficiency.
Kupffer cells play a major role in the clearance of gut-derived endotoxin from the portal blood, achieved without the induction of a local inflammatory response. The precise mechanisms involved are not fully understood, but there appears to be finely balanced autoregulation between the release of proinflammatory and inflammatory mediators such as IL-1, IL-6, TNF-α and interferon-γ (IFN-γ) and mediators such as IL-10 that suppress macrophage activation and inhibit their cytokine secretion. The role of Toll-like receptors (TLRs) in this response is considered in other chapters.
Several cytokines, chemokines and reactive nitrogen and oxygen species released by activated Kupffer cells are thought to have local effects, modulating microvascular responses and the functions of hepatocytes and stellate cells. Although Kupffer cells can express class II histocompatibility antigens and can function in vitro as antigen-presenting cells (APCs), they appear to be considerably less efficient at this than macrophages at other sites. Their principal roles in the immune response therefore appear to be antigen sequestration by phagocytosis and clearance of immune complexes.
Kupffer cells exhibit an important plasticity, expressing different phenotypes depending on the local metabolic and immune environment. Therefore, they can express the proinflammatory M1 phenotype or several M2 phenotypes involved in the resolution of inflammation and wound healing. Kupffer cells can play a protective role through their tolerogenic phenotype, as in some drug- and toxin-induced liver injury but can also shift to a pathologically activated state and contribute to chronic inflammation in various liver diseases. Impairment of their activity as scavengers of hepatic blood can contribute to pathogens entering the systemic circulation and thus systemic infection.
There is firm evidence from bone marrow and liver transplant studies showing that Kupffer cells are derived at least in part from circulating monocytes. , However, Kupffer cells are capable of replication, and their local proliferation accounts for a substantial part of the expansion of this cell population in response to liver injury. , Kupffer cells can proliferate in response to T-helper type 2 (Th2) inflammatory signals. Furthermore, Kupffer cells appear in the fetal liver of the mouse before there are circulating monocytes, and evidence indicates that they are derived from primitive macrophages which first appear in the yolk sac. These data suggest that Kupffer cells may have a dual origin.
Liver lymphocytes include cells of the adaptive immune system (T and B lymphocytes) and innate immune system: natural killer (NK) and natural killer T (NKT) cells. NK cells constitute 31% of hepatic lymphocytes and NKT cells, 26%. The liver is thus particularly enriched with cells of the innate immune system compared with other parenchymal organs. While this has immediate value for the liver dealing with foreign antigens released from the gut into the splanchnic circulation, it also means that the liver is well equipped for an immune response to neoantigens expressed within its substance. ,
It has been estimated that an average normal human liver contains approximately 1 × 10 10 lymphoid cells. These lymphocytes are predominantly located within portal tracts but are also found scattered throughout the parenchyma, where they are found in loose luminal contact with Kupffer cells or LSECs. In the peripheral blood, 85% of the lymphocytes are T and B cells, which possess clonotypic antigen-specific receptors. In contrast, up to 65% of lymphocytes within the liver are NK cells, T cells expressing γδ T-cell receptors (TCRs) and T cells expressing NK molecules (NKT cells); clonotypic T and B cells are only a minority of intrahepatic lymphocytes.
Because of their particular location in the space of Disse in the intercellular recess between adjacent hepatocytes, hepatic NK cells were first described as ‘pit cells’ in the rat liver in the 1970s. Recent studies have shown that two distinct NK-cell subsets are present in mouse and human livers: conventional NK cells and liver-resident NK cells. The latter, which are those appearing morphologically as the pit cells, are large granular lymphocytes, usually also in contact with Kupffer and endothelial cells; some lymphocyte microvilli can protrude into the space of Disse. , They present characteristic dense granules, visible on EM, as well as a few other organelles usually concentrated on one side of the cell.
The liver contains the largest number of γδ T cells in the body. , γδ T cells are also found in the skin, gut and respiratory mucosa and in the pregnant uterus and accumulate at sites of infection. They secrete various cytokines and can lyse antigen-bearing target cells. Their precise function in the liver is not yet clear.
Hepatic NKT cells coexpress TCR and NK activating and inhibitory receptors. They can be further subclassified on the basis of their expressing various types of TCRs and various NK receptors. Functional studies have demonstrated that hepatic NKT cells have numerous cytotoxic activities and produce multiple cytokines. Their major role therefore may be to effect local immunological reactions through the production of cytokines.
The relative frequency of the various subpopulations of liver-associated lymphocytes varies between individuals, probably reflecting each individual’s immunological status; this in turn is affected by genetic background and both previous and current antigen exposure. The hepatic environment itself also may influence the distribution of the various subsets. The rich array of subtypes of liver-resident lymphocytes points towards extensive immunosurveillance mechanisms in response to infectious, inflammatory and tumoural liver disease, with direct implications for novel therapies.
Originally identified by Boll and von Kupffer in the 1870s, HSCs were largely ignored until 1951, when Ito described their morphological features on light microscopy. They were subsequently referred to under a variety of terms—Ito cells, hepatic lipocytes, fat-storing cells, stellate cells and para- or perisinusoidal cells. , The now-accepted nomenclature for them is ‘hepatic stellate cells’ (HSCs). Their pathobiology and their role in regulating microcirculation, vitamin A storage and synthesis of ECM components, as well as their contribution to liver inflammation and immunology, have been extensively reviewed.
During embryogenesis, HSCs originate from the mesenchyme of the septum transversum, along with portal tract fibrocytes and perivascular mesenchymal cells. In the normal adult liver, HSCs account for about 5–8% of cells and are regularly spaced along the sinusoids (∼40 µm from HSC nucleus to HSC nucleus). There are about 5–20 HSCs per 100 hepatocytes. Despite their relatively sparse distribution, HSCs have long, thin cytoplasmic processes which have a perisinusoidal distribution in the space of Disse, organized into a sheath surrounding the sinusoid network. Indeed, the close proximity of HSCs with LSECs resembles that of pericytes in capillaries. The cell body of HSCs is often located in the interhepatocytic recess.
HSCs in the normal liver are ‘quiescent’, having received no stimuli to transform to their ‘active’ myofibroblastic state. The relationship of HSCs with other cells in the normal sinusoidal environment is closely related to their normal morphological and functional features, as follows:
The subendothelial processes of HSCs terminate as very thin, thorn-like microprojections which contact the plasma membrane of the hepatocyte microvilli and also the LSECs. Thus the HSCs adhere to the LSECs and hepatocytes, each strongly influencing the behaviour of the other cell types in response to hepatic injury and stimulation of hepatic regeneration.
Autonomic nerve endings in the space of Disse come into contact with HSCs, , and they respond to α-adrenergic stimulation and contract in response to several vasoactive substances, such as prostaglandin, thromboxane A2, ET-1, substance P and angiotensin II. These morphological and functional features support the role of HSCs in the haemodynamic regulation of sinusoidal blood flow.
In their quiescent phenotype, HSCs store vitamin A (80% of the body’s vitamin A) in intracellular lipid droplets. Dietary retinyl esters reach hepatocytes in chylomicron remnants, which pass from the sinusoidal lumen through the endothelial fenestrae and are taken up by hepatocytes. Most of the endocytosed retinol is rapidly transferred to the HSCs for storage. About 75% of HSCs in the normal liver contain lipid droplets, which are intermingled with the usual cytoplasmic organelles and generally abundant cytoplasm. Similar stellate cells, which also share the property of vitamin A storage, exist in the human pancreas, and evidence also indicates a more widespread distribution involving lung, kidney and gut. ,
In accordance with the needs of the host organism, retinol (vitamin A) is released to peripheral target tissues in the form of RBP complexes.
By EM, HSCs are present in the space of Disse, subjacent to LSECs and adjacent to hepatocytes ( Fig. 1.18 ). In normal conditions, HSCs are not readily visualized on light microscopy ( Fig. 1.19A ), but the lipid droplets rich in vitamin A can be demonstrated by fluorescence microscopy of frozen tissue when excitation light of 328 nm is used, or by gold chloride impregnation of tissue during fixation. HSCs can be visualized by GFAP in their quiescent state , ( Fig. 1.19B ), but this staining is not always reproducible; cellular RBP 1 (CRBP1) immunostaining is the best marker to visualize HSCs in their quiescent as well as activated state. When prominent in some pathological conditions such as hypervitaminosis A, HSCs can be visualized in routine histological sections without special stains.
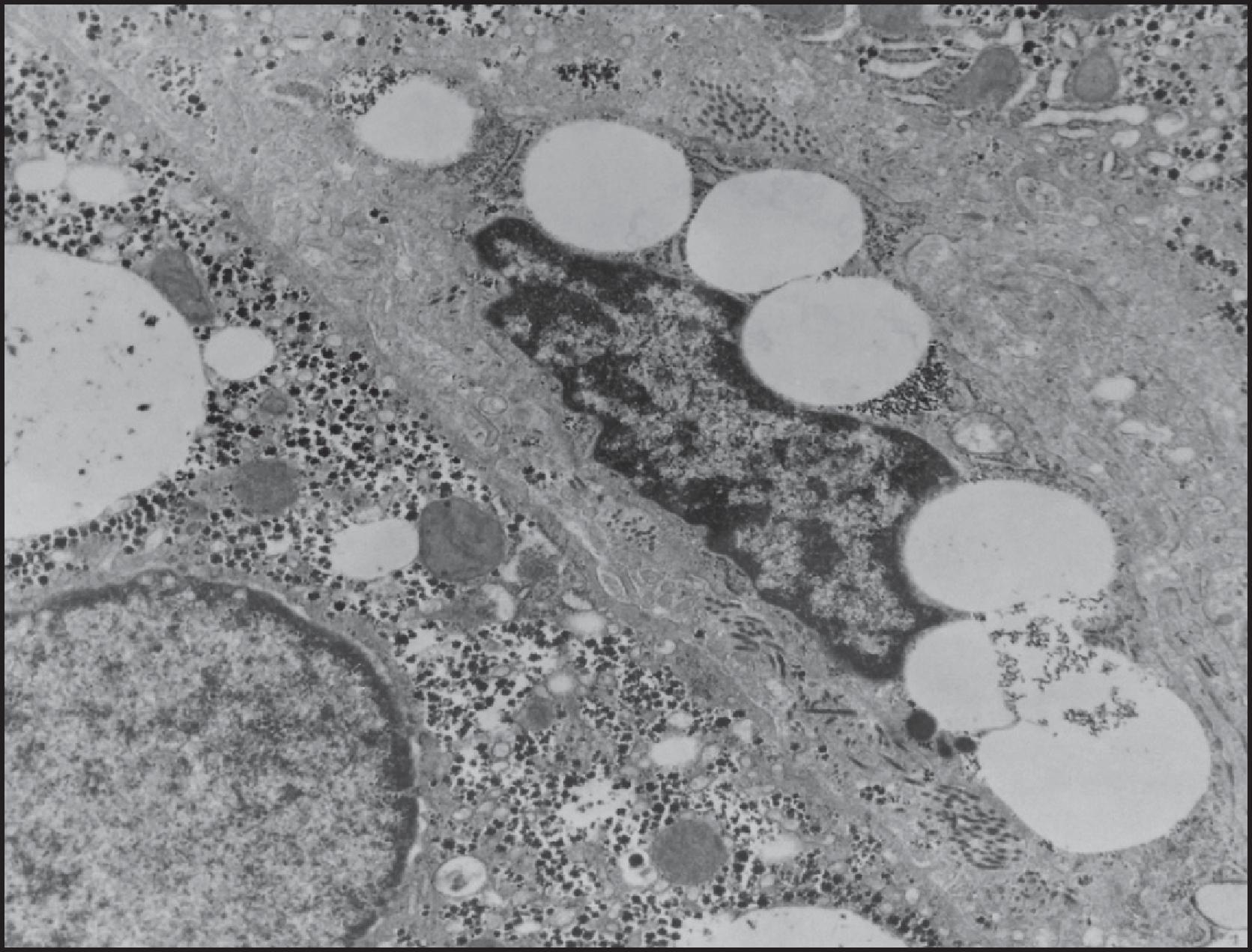
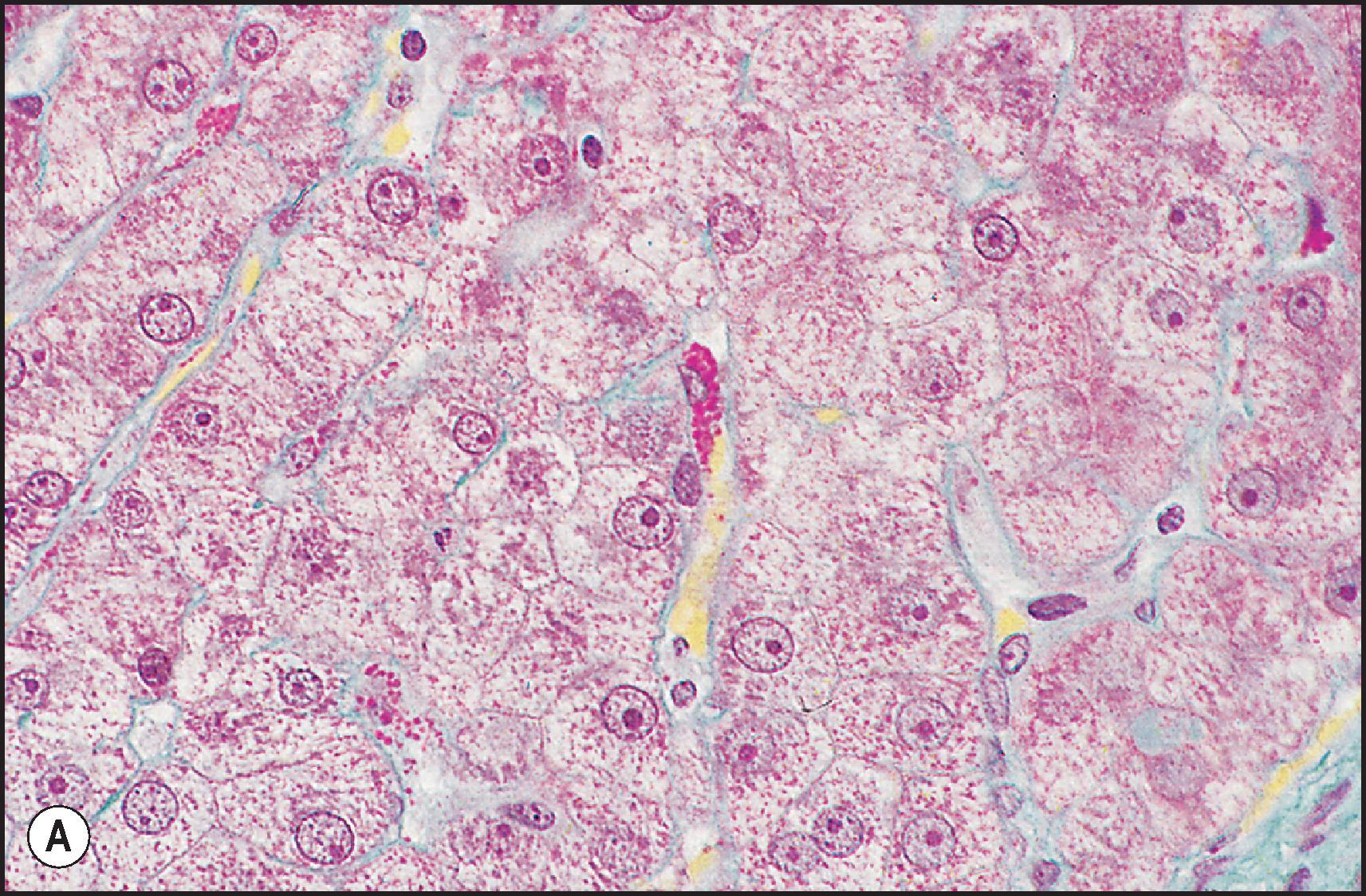
HSCs belong to the myofibroblast family. Although in normal conditions, hepatocytes and LSECs are capable of synthesizing types I, III and IV collagen and fibronectin, , quiescent HSCs are actually the main sinusoidal cells involved in the synthesis of both different collagen types and various glycoproteins (e.g. fibronectin, laminin, tenascin, entactin), as well as metalloproteinases and their inhibitors. Under conditions of stress or injury, HSCs change their phenotype by losing their lipid droplets and acquiring a myofibroblastic phenotype. In such activated condition, a key feature of HSCs is upregulation of α-smooth muscle antigen (SMA), imparting to HSCs a contractile phenotype. , This activation process exhibits marked morphological and functional modifications through a state of ‘transitional cells’ characterized by the progressive decrease of lipids and increase in RER and filamentous skeleton toward the final state of myofibroblastic cell. Secretions of all the ECM components are increased by activated HSCs during the process of liver fibrosis. The mechanisms of HSC activation and the consequences are discussed in the later section on fibrosis.
Recent studies have emphasized the major role of HSCs in liver immunology for several reasons: First, vitamin A represents a key factor in the regulation of immune responses. Second, HSCs can act as antigen presenting cells (APCs). Third, they produce many soluble inflammatory mediators and promote immunosuppressive responses in homeostasis (induction of regulatory T cells, T-cell apoptosis, inhibition of cytotoxic CD8 T cells). Thus, HSC activation has a major role not only in hepatic fibrogenesis but also in regulation of hepatic inflammation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here