Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Stem cells are a potential therapeutic option for the treatment of androgenetic alopecia and other forms of hair loss. However, serious adverse events have been previously reported from the use of stem cells for non dermatologic applications. As such, stem cell therapy is subject to strict regulation by the US Food and Drug Administration.
Adipose tissue is a readily accessible and abundant source of multipotent adipose-derived stem cells and may be processed into stromal vascular fraction. The regenerative effects of stromal vascular fraction are primarily attributed to paracrine signaling between stem cells and hair follicles and the secretion of anti-inflammatory and antiandrogen molecules.
Other stem cell–derived therapies include adipose-derived stem cell–conditioned medium, exosomes, and small molecules that induce lactate production within hair follicle stem cells.
The possible applications of regenerative therapies span every field of medicine and have the potential to significantly improve patient outcomes. The treatment of hair loss is particularly well suited for stem cell–based interventions. Accessible treatment sites allow for minimally invasive or noninvasive approaches, select regenerative therapies require only a modest amount of additional training or equipment, and procedures can be performed on an outpatient basis. Additionally, autologous stem cell–containing regenerative substances are readily harvestable by lipoaspiration or surgical resection ( Pearl 13.1 ).
Adipose tissue is rich in multipotent adipose-derived stem cells. These cells may be concentrated by processing adipose into stromal vascular fraction.
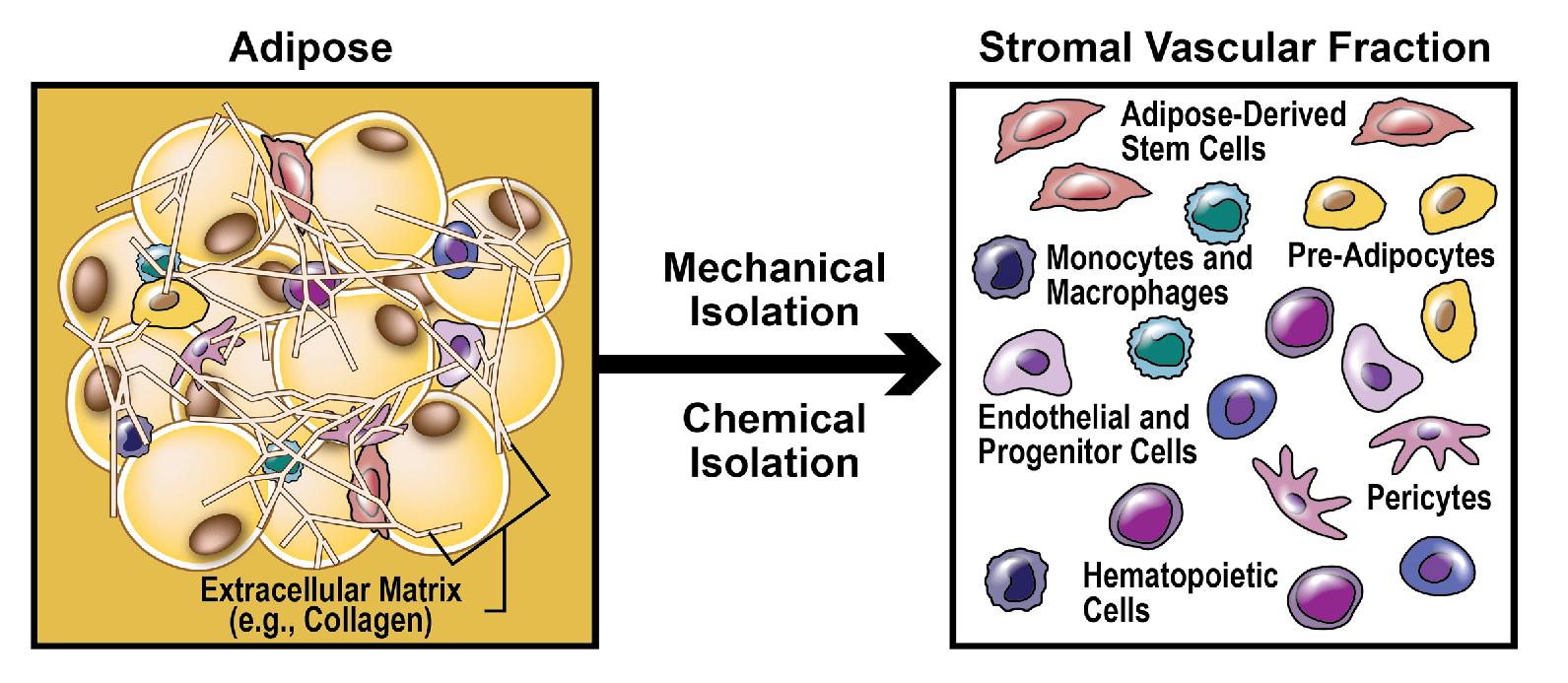
Adipose tissue is an abundant and accessible source of adipose-derived stem cells (ADSCs), a type of mesenchymal stem cell (MSC). These multipotent stem cells can self-renew and also have the capacity to differentiate into other cell types, including adipocytes, keratinocytes, chondrocytes, osteocytes, osteoblasts, vascular and endothelial lineages, hematopoietic cells, hepatocytes, cardiomyocytes, and neurons, depending on culture conditions ( Fig. 13.1 ) (level of evidence: 5). ADSCs may be concentrated by processing adipose tissue either chemically or mechanically into stromal vascular fraction (SVF), which is the substance that remains after removal of adipocytes and connective tissue ( Pearl 13.2 ). In addition to ADSCs, SVF contains other stem cells (e.g., hematopoietic stem cells, pericytes), progenitor cells (e.g., adipocyte progenitors), mature cells (e.g., endothelial cells, erythrocytes, immune cells, smooth muscle cells, fibroblasts), and an abundance of growth factors and other secreted molecules ( Fig. 13.2 ).
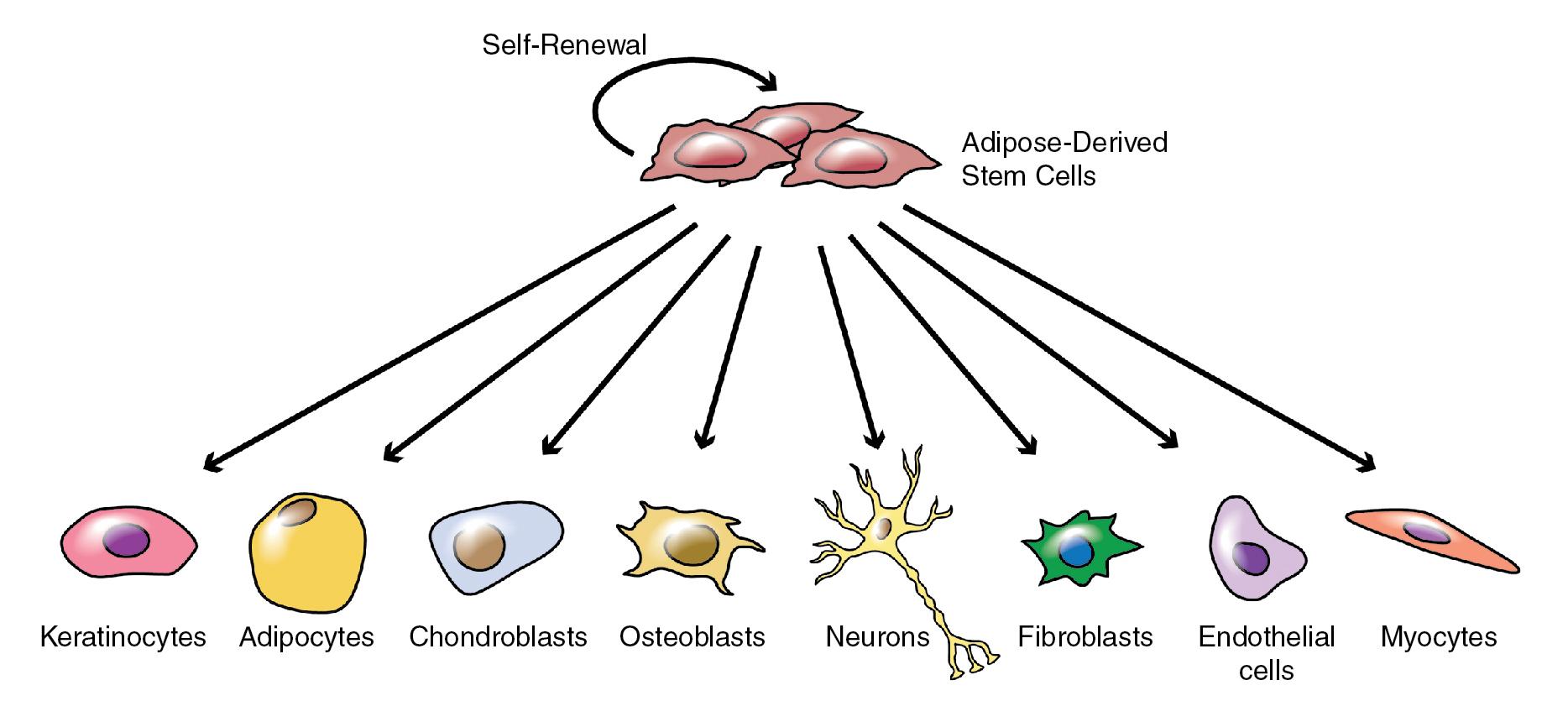
Stromal vascular fraction contains stem cells, progenitor cells, mature cells, growth factors, and other secreted molecules. The potential applications of stromal vascular fraction and other stem cell–based therapies are broad and span specialties other than dermatology.
In the twenty-first century, there has been growing interest in the use of stem cells and stem cell–based modalities for the treatment of various types of alopecia, with a particular focus on androgenetic alopecia (AGA) and alopecia areata (AA). White adipose tissue (WAT) in the dermis is closely associated with hair follicles ( Fig. 13.3 ). The dermal WAT remodels in concert with the hair cycle, thickening around follicles during anagen, and thinning by approximately 50% during the transition from catagen to telogen (level of evidence: 5) ( Pearl 13.3 ). , This synchronous cycling of dermal fat and hair growth is related at least in part to reciprocal signaling between dermal WAT and the dermal papillae and is likely altered in alopecia and other conditions affecting hair growth (e.g., lipodystrophy, obesity).
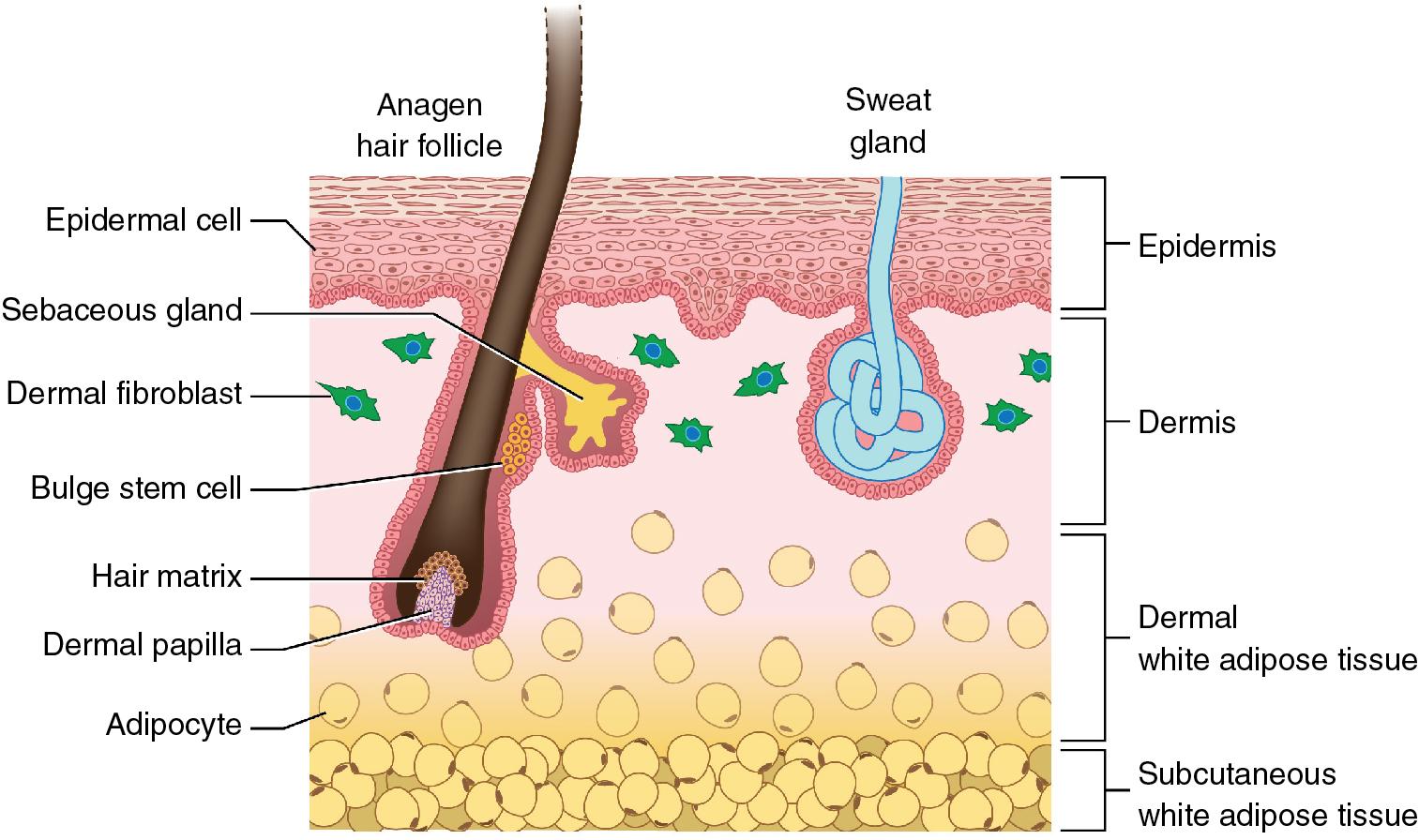
The dermal white adipose tissue rapidly cycles in concert with the hair cycle, and paracrine signaling between the white adipose tissue and dermal papillae appears to be important in regulation of normal hair growth.
As a result, adipocyte progenitors and ADSCs contained within SVF may be particularly important in the treatment of alopecia. Although precise mechanisms are not understood, the regenerative potential of SVF is theorized to arise primarily from paracrine signaling that stimulates regeneration of the host tissue (i.e., hair follicles) and secretion of anti-inflammatory and antiandrogen molecules (level of evidence: 5). , SVF cells secrete platelet derived growth factor (PDGF) and insulin-like growth factor 1 (IGF-1) and express leptin, adiponectin, bone morphogenic protein, and other molecules that regulate hair growth (level of evidence: 5) ( Pearl 13.4 ). Neovascularization and direct tissue formation from differentiation of individual SVF cells (e.g., differentiation into follicular progenitor cells and adipocytes) may also play a role (level of evidence: 5).
The regenerative function of stem cell–based therapies such as stromal vascular fraction in the treatment of alopecia is largely attributed to paracrine signaling between stem cells and hair follicles, as stem cells secrete growth factors that stimulate hair growth, reduce inflammation, and have antiandrogen effects.
The objective of this chapter is to discuss the use of SVF in the treatment of AGA and other nonscarring alopecias. Additional stem cell–based therapies, including ADSC-conditioned medium (ADSC-CM), exosomes, and small molecules that promote activation of hair follicle stem cells, will also be briefly discussed.
At the time of this publication, there are no clinical practice guidelines for the use of SVF or other stem cell therapies in treating alopecia. As a result of scarce high-quality clinical studies, it remains unclear which types of alopecia may respond best to SVF. The majority of ongoing and published clinical trials treat patients with AGA or AA, but studies are limited by sample size, heterogeneity in reported outcomes, and differing treatment techniques (level of evidence: 2b). , As with most interventional therapies for AGA, patients with early hair loss may benefit more than those with severe hair loss (level of evidence: 5) ( Pearl 13.5 ). SVF is not a first-line therapy for AGA, so patient education should focus on the role of less invasive techniques with robust efficacy and safety evidence, such as finasteride, topical minoxidil, or platelet-rich plasma (PRP) (level of evidence: 1a). SVF injections are likely not appropriate for patients who are risk averse or who are not candidates for liposuction (e.g., in patients with severe cardiovascular disease or severe coagulation disorders or in pregnant patients) (level of evidence: 5).
Patients with early androgenetic alopecia may benefit more from regenerative therapies than those with severe hair loss.
It should be noted that, at the time of this publication in the United States, the use of SVF and other stem cell therapies should be restricted to US Food and Drug Administration (FDA)-supervised clinical trials. The FDA has released warnings regarding stem cell therapies, stating they are “concerned that some patients seeking cures and remedies are vulnerable to stem cell treatments that are illegal and potentially harmful.” As of August 2017, the FDA announced increased regulation enforcement and stem cell clinic oversight. This was largely prompted by several notable adverse events that were related to stem cell injection, including bilateral blindness after intravitreal injection of autologous ADSCs by a nurse practitioner without MD or FDA oversight and development of an aggressive spinal cord tumor in a patient who received multiple intrathecal stem cell injections. The FDA urges patients to only undergo stem cell therapy if the treatment is FDA-approved or being studied as part of an FDA-supervised clinical trial with an Investigational New Drug Application (IND). Currently, the only FDA-approved stem cell–based products are hematopoietic progenitor cells indicated for use in patients with hematopoietic disorders. The FDA warns that they may take “administrative and judicial actions” against unapproved use of stem cell products if stem cells are “processed in ways that are more than minimally manipulated.”
Ideally, patients with AGA who are treated with SVF will experience improvement in hair density and hair diameter. The increases in hair density and diameter should be apparent within approximately 6 months and may range from 14%-23% and 11%-24%, respectively. , The proportion of anagen hairs may also increase. Although hair growth may improve after a single treatment, these effects may not persist. Similar to PRP injections, two to three treatments of SVF at approximately 1-month intervals may lead to more noticeable and sustained outcomes.
The degree and duration of improvement is likely dependent on multiple factors, including patient age, etiology and severity of hair loss, and use of adjunctive therapies. Treatment technique is also likely to be important, although sufficiently powered, high-quality clinical trials are required to further elucidate best practices for optimizing outcomes ( Pearl 13.6 ). Current evidence suggests that SVF with low-density ADSCs (0.5 × 10 6 ADSCs/cm 2 ) may be more beneficial than SVF with high-density ADSCs (1 × 10 6 ADSCs/cm 2 ). Also, combination therapy (e.g., SVF with finasteride or topical minoxidil) is likely more effective than SVF alone.
Current evidence suggests that treatment with stromal vascular fraction may lead to increased hair density and hair diameter after approximately 6 months. High-powered, randomized, controlled clinical trials are needed to identify ideal candidates and treatment techniques for stromal vascular fraction.
The treatment techniques described here are intended to be neither comprehensive nor prescriptive, but rather to offer a succinct review of currently available literature. The treating physician should exercise good judgment to care for each patient individually. SVF should be used in an FDA-supervised clinical trial setting, with strict adherence to the trial’s study protocol.
The procedure should be performed by a board-certified physician with sufficient education and training in liposuction and skin surgery such as dermatology or plastic surgery. At present, because SVF is not FDA-approved for the treatment of alopecia, this treatment should be under the purview of an FDA-supervised clinical trial with an IND. Also, as a result of the investigative nature of stem cell therapies, obtaining informed consent is extremely important. The International Society for Stem Cell Research (ISSCR) provides professional standards for consent for stem cell–based interventions but does also urge that such treatments be performed only with FDA oversight.
The preoperative evaluation should include a thorough past medical history, including a history of poor wound healing or abnormal scarring, bleeding abnormalities, immunosuppression, severe infection, prior reactions to surgery and anesthesia, and treatments that the patient has used or is currently using for hair loss. The patient’s medication list should be reviewed, including for medications that affect coagulation or metabolism of local anesthesia. Laboratory studies depend on provider preferences and institution and clinical trial-specific regulations and may include a complete blood count, coagulation studies, and, in women with childbearing potential, a urine pregnancy test. A physical examination should assess for evidence of poor healing or abnormal scarring and evaluate sites appropriate for liposuction, such as the lateral thigh and abdoment.
On the day of treatment, which may take place in an ambulatory setting, photographs of treatment sites should be obtained and used to monitor patient response moving forward. Baseline vital signs should be measured and documented. An oral anxiolytic, sedative, or analgesic may be appropriate, depending on the comfort level of the patient and provider. Parenteral anxiety and pain medications should be used with caution or avoided completely if possible, and general anesthesia is not recommended.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here